Abstract
In this study, we examined the dynamics of inhibitory preparatory processes, using a delayed response task in which a cue signaled a left or right index finger (Experiment 1) or hand (Experiment 2) movement in advance of an imperative signal. In Experiment 1, we varied the duration of the delay period (200, 500, and 900 ms). When transcranial magnetic stimulation (TMS) was applied 100 ms before the imperative, motor evoked potentials (MEPs) elicited in the first dorsal interosseous were strongly inhibited. For delays of 500 ms or longer, this inhibition was greater when the targeted muscle was selected compared with when it was not selected. In contrast, the magnitude of inhibition just after the cue was inversely related to the duration of the delay period, and the difference between the selected and nonselected conditions was attenuated. In Experiment 2, TMS and peripheral nerve stimulation procedures were used during a 300-ms delay period. MEPs in the flexor carpi radialis for both selected and nonselected conditions were inhibited, but without any change in the H-reflex. Taken together, these results reveal the dual influence of temporal constraints associated with anticipation and urgency on inhibitory processes recruited during response preparation.
Keywords: choice task, H-reflex, inhibition, MEP, preparation
Introduction
Our interaction with the environment requires motor decisions depending on the available information. Decisions about hand choice are part of our daily routine (e.g., choosing which hand to use to press a lift button), and engage neural structures of both cortical hemispheres (Swinnen 2002; Duque et al. 2005, 2012; Koch et al. 2006). Transcranial magnetic stimulation (TMS) can be used to assay changes in corticospinal excitability, providing a useful tool to characterize the neural dynamics at play during response selection and preparation. To this end, single-pulse TMS is applied over the motor cortex to elicit motor evoked potentials (MEPs) in a muscle that is either required (selected) or not required (nonselected) for the upcoming response. For example, when the TMS pulse is applied after an imperative signal, MEPs increase in the selected muscle prior to electromyographic (EMG) onset, and decrease in the nonselected muscles, suggesting the operation of a competitive process in response selection (Rossini et al. 1988; Chen et al. 1998; Leocani et al. 2000).
Note, however, that when TMS is applied after the imperative signal, it is difficult to attribute neural changes to response selection and/or response initiation. An alternative approach is to use a delayed response task, in which an informative cue is presented prior to an imperative signal. With a fixed delay period of 900 ms, MEPs are markedly attenuated relative to baseline when the TMS probe is applied 100 ms before the imperative signal (Duque et al. 2010). Moreover, this inhibition is greater when the targeted muscle is selected for the forthcoming response compared with when it is not selected, suggesting some degree of specificity. Duque et al. (2010, 2012) proposed that this delay-period inhibition reflects the operation of 2 separable mechanisms. The first, referred to as impulse control, is hypothesized to help prevent premature movement of the selected effector, and possibly improve the dynamics of response initiation (i.e., rebound inhibition). The second mechanism, referred to as competition resolution, is hypothesized to facilitate response selection through the inhibition of the nonselected effector.
This dual-mechanism hypothesis is based on evidence from a variety of stimulation protocols (Duque et al. 2010). Impulse control is also evident at the spinal level, with the reduction in MEPs accompanied by an attenuation of a spinal reflex associated with the agonist muscle for the forthcoming response (Touge et al. 1998; Duque et al. 2010). In contrast, competition resolution is limited to the supraspinal level, with inhibition of the nonselected muscle evident in MEPs, but not in spinal reflexes. Further evidence that these 2 processes are dissociable comes from a study in which TMS of the primary motor cortex (M1) was coupled with rTMS of premotor or prefrontal cortices (Duque et al. 2012). Dorsal premotor rTMS modulated the MEP responses associated with impulse control, while lateral prefrontal rTMS modulated MEP responses associated with competition resolution.
The excitability changes described above have been observed in a task in which participants are provided with a relatively long delay between the preparatory cue and imperative signal (i.e., >500 ms) and the TMS probe was applied just before the imperative signal. If the TMS pulse is delivered right after the preparatory cue (100 ms post-cue), the MEPs show minimal change, suggesting that response preparation was deferred until near the expected time of the imperative signal (Duque et al. 2010). Other studies have manipulated the duration of the delay period and timing of the TMS pulse (Touge et al. 1998; Davranche et al. 2007; van Elswijk et al. 2007; Duque et al. 2010). Touge et al. (1998) used a delay period of 500 ms. Shortly after the preparatory cue (250 ms post-cue), MEP responses were attenuated in both the selected and nonselected muscles, and H-reflex only in selected muscles. Interestingly, when the delay period was extended from 500 ms to 2 s, no modulation was found in either MEPs or H-reflexes for probes across the delay period. Thus, the recruitment of inhibitory processes may require that the participant can anticipate the onset of the imperative signal, a process that is difficult with a long delay.
To systematically explore the dynamics of inhibitory processes, we designed a pair of experiments in which we varied the duration of the delay period and the timing of the TMS probe. In the first experiment, participants were cued to prepare a movement with the right or left index finger. We used 3 delays (200, 500, and 900 ms) and applied a TMS pulse 100 ms after the preparatory cue or 100 ms before the imperative signal. We measured MEPs in the first dorsal interosseous (FDI) muscle of the left hand, comparing conditions in which this muscle was selected or not selected for the forthcoming response.
We expected to replicate the findings of Duque et al. (2010) with the 900-ms delay period. Namely, that minimal change would be observed in the MEPs with the early probe, while considerable inhibition from impulse control and competition resolution would be observed with the late probe. The 200-ms delay condition creates a situation in which the participants are unable to defer planning processes. If we again see little change in corticospinal excitability in this condition, then we would infer that 100 ms provides insufficient time to recruit inhibitory processes. Alternatively, we might observe inhibition in either the selected, nonselected conditions, or both, suggesting that the recruitment of inhibitory processes is tightly coupled to the anticipated time of the response. For example, inhibition might be seen when the muscle is not selected to respond due to the operation of competition resolution, but not evident when the muscle is part of the selected response due to the impending imperative signal. We included the 500-ms condition as an intermediate value, one in which the delay period affords only a modest extension beyond the time required for selection.
In the second experiment, we complemented the TMS protocol with a peripheral stimulation protocol that provided a measure of excitability changes at the spinal level. Prior work has shown that the monosynaptic H-reflex is suppressed during an extended preparatory period (Brunia et al. 1982; Komiyama and Tanaka 1990; Bonnet et al. 1991; Hasbroucq et al. 1999), even when long-latency reflexes are facilitated (Bonnet and Requin 1982). Relevant to the current work, peripheral stimulation has revealed dissociations in the locus of inhibition for selected and nonselected responses. In the current context, combining TMS and peripheral stimulation methods with different preparatory intervals will provide insight into the dynamics of inhibitory processes at different levels of the motor system.
Materials and Methods
Experiment 1: Corticospinal Excitability
Participants
Twelve right-handed healthy subjects (6 women, mean age 23 ± 6 years) were recruited from the University of California, Berkeley community. Participants were naive to the purpose of the study, provided written informed consent, and were financially compensated for their participation. The protocol was approved by the institutional review board of UC, Berkeley.
Experimental Procedure
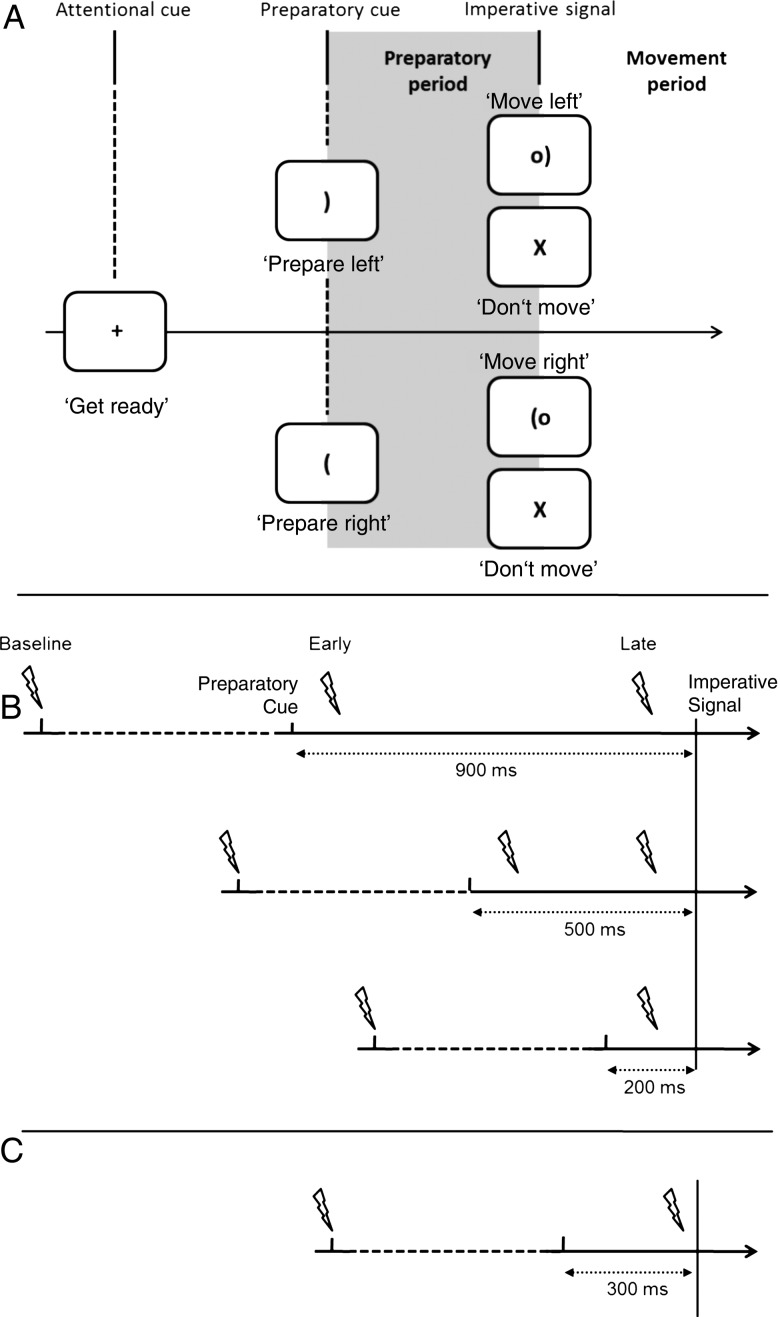
The participants sat in front of a computer screen with both hands resting on their lap, palms down. The task used here has been described as a virtual “soccer game” (see Duque and Ivry 2009). Each trial began with the brief presentation (100 ms) of a fixation marker at the center of the screen. After a blank screen of 600 ms, a preparatory cue appeared at the center position. The cue was a bracket and described as the “soccer goal.” If oriented to the right, the participants should prepare a right hand response; if oriented to the left, the participant should prepare a left hand response. The left index was designated as “selected” when a left finger movement was cued and as nonselected when a right finger movement was cued. After a fixed delay (see below), a circle was added to the display, positioned just outside the bracket. This circle, described as the “ball,” was present for 300 ms and served as the imperative signal (Fig. 1A). Participants were instructed to move their index finger (abduction) “as quickly as possible” after the onset of the imperative signal. On some trials, the cue was replaced by an “X” at the center of the screen (catch trials); participants were instructed to not perform the movement when the “X” stimulus was presented. The catch trials were included to reduce anticipatory responses. The screen remained blank for a 3-s intertrial interval.
Figure 1.
Protocol design and stimulation timings. (A) Participants prepared to move their left or right index finger (Experiment 1) or hand (Experiment 2) following a preparatory cue. Stimulation was delivered during the delay period. (B) There were 3 delay periods in Experiment 1 (900, 500, and 200 ms). For each delay, TMS was administered at the onset of the fixation cross (Baseline), 100 ms after the preparatory cue (Early) or 100 ms before the imperative signal (Late). Note that for the 200 ms delay, early and late TMS correspond to the same time. (C) In Experiment 2, TMS and PNS was applied at baseline or 250 ms after the preparatory cue during a 300-ms delay period.
Three different delay intervals were employed (200, 500, and 900 ms) in separate blocks. Participants completed 10 blocks, 2 with the 200-ms delay and 4 with each of the 500 and 900-ms delays. The difference in the number of blocks was due to the fact that a single TMS timing was used in the 200-ms condition, while 2 TMS timings were used in the 500- and 900-ms conditions (early and late, see below). Each block lasted ∼3 min and the order of the 3 delay periods was counterbalanced. Participants were explicitly informed about the duration of the delay for each block and completed 20 practice trials (no TMS) prior to the start of the set of blocks for a given delay. Within each test block, there were 36 trials, half with left hand cues and half with right hand cues. Of the 18 trials for each hand, 14 were followed by an imperative signal and 4 were catch trials. All trials were presented in random order.
Transcranial Magnetic Stimulation
Corticospinal excitability was assessed by recording MEPs from the FDI of the left hand in response to single-pulse TMS applied over the hand area of the right primary motor cortex. A Magstim 200 stimulator was used to activate a figure-of-eight coil (diameter of wings 70 mm) placed tangentially on the scalp. The handle was oriented toward the back of the head and laterally at a 45° angle, assumed to be approximately perpendicular to the central sulcus.
At the start of each session, the experimenter identified the optimal spot for eliciting MEPs in the left FDI and marked this position on the participant's scalp. The resting motor threshold (rMT) was defined as the minimal TMS intensity required to evoke MEP peak-to-peak amplitudes of ∼50 µV in the targeted muscle on 5 of 10 consecutive trials. During the main phase of the experiment, the TMS intensity was set to 120% of the participant's rMT.
In each block, a TMS pulse was applied on 30 of the 36 trials. The pulse was applied at onset of the fixation marker on 10 of these 30 trials, providing a within-block baseline measure of excitability. The other 20 pulses were applied during the delay period, either 100 ms after the onset of the preparatory cue (early; n = 10) or 100 ms prior to the onset of the imperative signal (late; n = 10). In the 200-ms condition, the early and late timings are identical (Fig. 1B).
EMG Recording
EMG activity was recorded by surface electrodes (Delsys, Inc.) placed over the left and right FDI. EMG data were collected for 3 s on each trial, starting 200 ms before the onset of the fixation point. The EMG signals were amplified and bandpass filtered online (50–2000 Hz), and digitized at 2000-Hz for offline analysis. EMG signals from left FDI were used to measure MEPs, defined as the peak-to-peak amplitude difference in the EMG signal elicited by the TMS pulse. Trials with background EMGRMS (root mean square) activity above 0.01 mV in either hand during a 100 ms window preceding the TMS pulse were excluded from the analysis (21 of 4320 trials).
Experiment 2: Corticospinal and Spinal Excitability
Participants
A total of 13 right-handed healthy participants (11 men, aged 28 ± 7 years) were recruited from the University of Burgundy (Dijon, France). The protocol was approved by the University's institutional review board.
Experimental Procedure
The general structure of Experiment 2 was similar to that of Experiment 1 with 3 notable changes (Fig. 1C). First, in addition to measuring TMS-elicited MEPs, we also used peripheral nerve stimulation (PNS) to measure changes in spinal excitability. Since reflexive responses tend to be variable in intrinsic hand muscles (Mazzocchio et al. 1995), we switched to a wrist response for the choice RT task, allowing us to measure H-reflexes in the flexor carpi radialis (FCR) following median nerve stimulation. Second, the H-reflexes and MEPs were recorded from the FCR muscle of the right hand. Through a comparison with Experiment 1, we could assess laterality differences in preparatory processes, although we do so only qualitatively given the various methodological differences. Third, we only used the short delay period since our main focus here was on the recruitment of inhibitory processes when the preparatory interval is brief. Because modulation of H-reflexes in choice RT tasks has been shown to require 250 ms (see Touge et al. 1998), we extended the short delay interval to 300 ms.
All participants completed a block of trials with the PNS protocol. Eight of the 13 participants were then enlisted to complete a second block of trials, this time with the TMS protocol. Each block was composed of 60 trials, half of which involved the right hand cue and half of which involved the left hand cue. For 44 trials, the cue was followed by the soccer ball imperative signal and participants were required to flex the cued wrist as fast as possible. The other 16 trials were catch trials in which the cue was followed by an “X” and participants were instructed to not perform the cued movement. The test block was preceded by a practice block of 20 trials.
Peripheral Nerve and Transcranial Magnetic Stimulation
To elicit H-reflexes in right FCR, the right median nerve was electrically stimulated by 2 electrodes connected to a Digitimer stimulator (Model DS7). The intensity was adjusted on an individual basis to produce an H-reflex with either no preceding M-wave, or an H-reflex that had the smallest and most constant M-wave component (see Maffiuletti et al. 2000). The range of intensities was 1.3–7.4 mA (mean = 4.0 ± 1.6 mA). For participants completing the TMS protocol, the rMT was defined as in Experiment 1, but with the coil positioned to optimize MEPs from right FCR. The stimulator intensity ranged from 43 to 74 of maximum stimulator output (mean = 58 ± 11%).
We used only a single delay period of 300 ms, fixing the stimulation time (for TMS or PNS) at 250 ms, that is, 50 ms before the imperative signal. As noted above, we opted to increase the short delay to 300 ms and probe 250 ms after the preparatory cue (relative to the shorter timings used in Experiment 1) to maximize the opportunity to detect differences in reflex modulation between trials when right FCR was selected or nonselected for the forthcoming response.
EMG Recording
EMG activity was recorded by surface electrodes placed over the right and left FCR. EMG data were collected for 2.1 s on each trial, starting 100 ms prior to the stimulation. The EMG signals were amplified and bandpass filtered online (50–2000 Hz, Biopac Systems, Inc.), and digitized at 2000 Hz for offline analysis. Trials with EMGRMS above 10 µV were discarded (52 of 2688 trials).
Data and Statistical Analysis (Experiments 1 and 2)
Reaction time (RT) was defined as the interval between the presentation of the imperative signal and the point at which the EMGRMS in the appropriate effector was 2.5 times above the baseline level. MEPs and H-reflexes were measured as the peak-to-peak amplitude of the response elicited after the stimulation. Mean values for RT, MEP and H-reflex were calculated for each participant in each experimental condition. The Shapiro-Wilk test was used to evaluate if the observed values were normally distributed. This test revealed a normal distribution for the MEPs (P > 0.05), but not for the H-reflexes and RTs (P < 0.05).
In Experiment 1, RT was analyzed with a Friedman ANOVA to assess the effects of “effector” (selected: left index, nonselected: right index) and “pulse timing” (baseline, early, late) for each delay period separately. Wilcoxon tests with paired values were used for post hoc analysis. We used the same statistical tests with averaged RTs to assess the effect of “delay” (200, 500, and 900 ms). For the MEP data, we first tested, by means of a within-subject one-way ANOVA, whether MEP amplitude at baseline varied across the 3 delay conditions. The MEP amplitudes were then normalized relative to the baseline and converted to percentage scores: (Condition/Baseline − 1) × 100. In this format, values <0 are indicative of inhibition. We used one-sample t-tests (with Bonferroni correction for multiple comparisons) to test if the percentage score in each condition was significantly different from zero, a probe on whether corticospinal excitability had changed from baseline. Normalized MEP values were also submitted to a three-way repeated measure ANOVA with effector (selected, nonselected), delay (200, 500, and 900), and pulse timing (early, late) as within-subject variables. Note that this design is not balanced since there is only one pulse timing in the 200-ms delay condition (100 ms after preparatory cue is the same as 100 ms before the imperative signal). To compensate for this imbalance, the data were randomly sampled without replacement to create 2 distributions, one for the “early” pulse condition and one for the “late” pulse condition. Because these 2 distributions were derived from the same sample, they should not differ. Moreover, this method is conservative in that the derived distributions will have larger variability than the single distribution.
In Experiment 2, RT was analyzed with a Friedman ANOVA to assess the effects of effector (selected: right hand, nonselected: left hand), and pulse timing (baseline, delay) for each type of stimulation (TMS and PNS). Wilcoxon tests with paired values were used for post hoc analysis. MEP and H-reflex amplitudes were normalized relative to the baseline and converted to percentage scores. One-sample t-tests (with Bonferroni correction for multiple comparisons) were used to assess changes in corticospinal excitability relative to baseline. To evaluate differences in the effector condition (selected, nonselected), we used a paired t-test. To evaluate the effects of peripheral stimulation, we used nonparametric tests with the normalized H-reflex values, since these data violated normality. One-sample Wilcoxon-ranked tests were used to test if the percentage scores in each effector condition were significantly different from zero. Finally, Wilcoxon tests with paired samples were used to examine differences between the selected and nonselected conditions.
For all statistical analyses, the alpha value was set at 0.05. The data are presented as mean values (±standard deviation).
Results
Experiment 1
Experiment 1 was designed to examine the changes in corticospinal excitability at different time points during the preparatory period and the effect of the duration of the preparatory period. To this end, we measured MEPs at an early and late point within delay periods of varying duration, comparing these with a baseline state.
Behavior
Behavioral data were evaluated to examine the effect of the different delay periods and TMS stimulation on RTs. Within each of the 3 delay periods, there was no effect of RT as a function of the factors effector (selected and nonselected) and pulse timing (baseline, early, and late) (for all, P > 0.27). But there was an effect of the delay period (χ2 = 11.16, P = 0.003). The mean RTs for the 200, 500, and 900 ms conditions were 288 (±58), 270 (±59), and 276 (±66) ms, respectively. RT was significantly slower in the 200-ms condition compared with the 500-ms condition (Z = 3.05, P = 0.002) and tended to be slower compared with the 900-ms condition (Z = 1.64, P = 0.099). The participants were also more likely to respond on catch trials when the preparatory period was shortened. Across the 3 delays, an increase in the EMG (and in some cases, overt movement) was evident on 13.02 ± 14.46, 12.24 ± 13.22, and 8.33 ± 8.87% of the catch trials for the 200, 500, and 900 ms delay conditions, respectively.
Corticospinal Excitability: MEPs
MEP amplitudes did not differ across the 3 baseline conditions (F2,22 = 2.66, P = 0.09; on average:1.36 ± 0.60, 1.51 ± 0.74, and 1.51 ± 0.64 mV for the 200-, 500-, and 900-ms delay, respectively). We then calculated changes in corticospinal excitability for each condition, relative to its own baseline, and tested whether the normalized MEP values were different from zero (correcting for multiple comparisons).
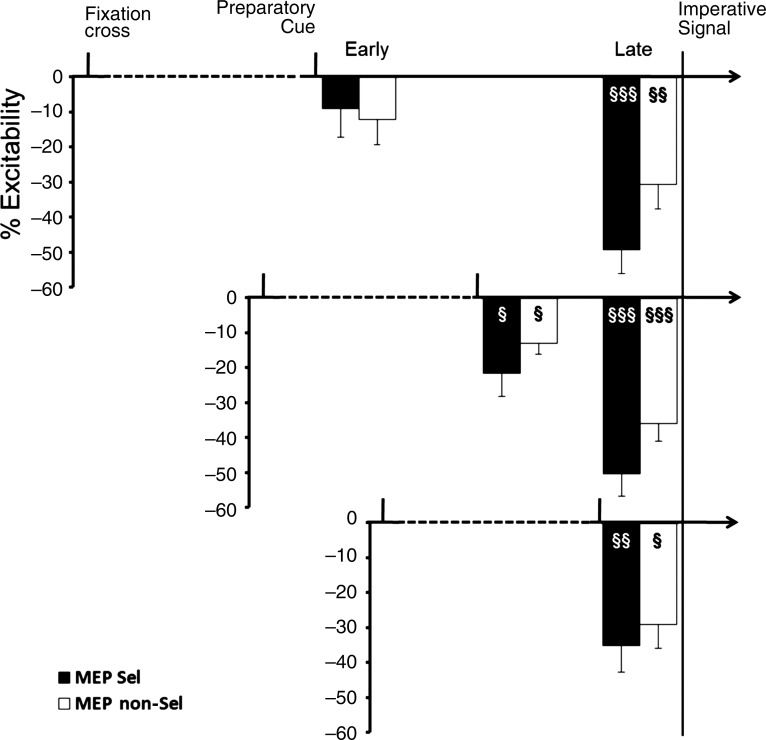
When the TMS pulse was applied 100 ms prior to the imperative signal (late pulse), there was a significant decrease in the mean MEP amplitude relative to baseline in all the delay conditions (Fig. 2; all P < 0.01). The results were different when the TMS pulse was applied 100 ms after the preparatory cue (early pulse), with the magnitude of suppression increasing when the delay periods became shorter. The mean MEP values were significantly lower than baseline for the 200-ms and the 500-ms delay conditions, an effect observed for both effectors (all 4 values, P < 0.05). However, for the 900-ms delay, the MEP values were not significantly different from baseline for either the selected (P = 0.89) or nonselected (P = 0.17) condition, although both values were negative relative to baseline.
Figure 2.
Timeline of corticospinal excitability (Experiment 1). Upper chart: 900-ms delay period. Middle chart: 500-ms delay period. Lower chart: 200-ms delay period. Data are presented as a percentage change from baseline (TMS at onset of fixation). Early and late TMS correspond to 100 ms after preparatory cue and 100 ms prior to imperative signal, respectively. Black bars are from trials in which the left finger was selected (Sel) and white bars are from nonselected trials (NSel). Error bars correspond to standard errors. §P < 0.05, §§P < 0.01, §§§P < 0.001: different from 0.
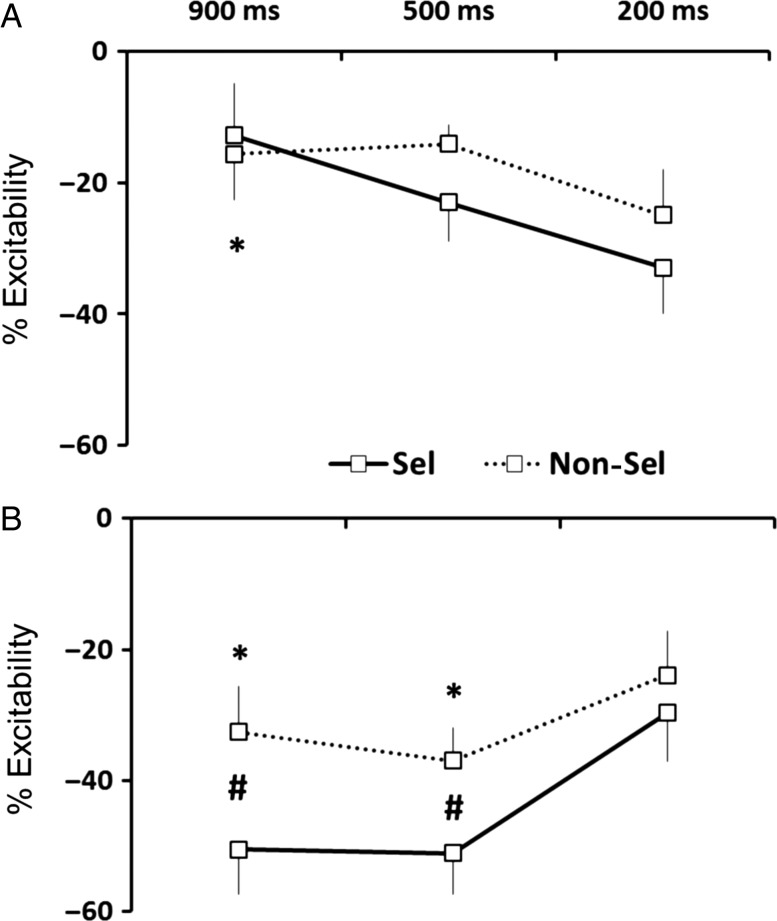
The comparison of normalized MEP amplitudes between the conditions showed significant main effects of pulse timing (F1,11 = 6.68, P = 0.02) and effector (F1,11 = 22.72, P < 0.001). There was no main effect of delay (F2,22 = 0.40, P = 0.67). However, there were 2 two-way interactions, pulse timing × effector (F1,11 = 5.86, P = 0.03) and pulse timing × delay (F2,22 = 11.72, P < 0.001). Post hoc analyses revealed that MEP suppression was stronger when the effector was selected for the forthcoming response compared with the nonselected condition, but only for the late pulse timing (P < 0.001). Moreover, this suppression was greater for late pulse timing compared with early pulse timing, but only for the 500-ms and 900-ms delay conditions (both, P < 0.001). There was also a significant three-way interaction of delay × pulse timing × effector (F2,22 = 3.95, P = 0.03). The higher order interaction stemmed from 2 effects. First, the magnitude of MEP reduction moved in opposite directions for the 2 probes. For the early probe, the suppression increased as the delay period became shorter (Fig. 3A). In contrast, for the late probe, suppression increased as the delay period became longer (Fig. 3B). Second, the degree of MEP suppression for early probes did not differ between the selected and nonselected trials for all 3 of the delay periods (all P > 0.05). However, for late probes, suppression was greater when the left index finger was selected compared with nonselected when the delay period was at least 500 ms in duration (Fig. 3B; in long and medium delays, P < 0.05; in short delay, P > 0.05).
Figure 3.
Dynamics of inhibition in Experiment 1 when TMS was triggered (A) 100 ms after the preparatory cue (Early Timing) and (B) 100 ms prior to imperative signal (Late Timing). Error bars correspond to standard errors. *P < 0.05 different from 200-ms delay period. #P < 0.05 difference between selected and nonselected.
In sum, the results of Experiment 1 revealed the flexible recruitment of inhibitory processes, as a signature of MEP suppression. With a long preparatory interval (900 ms cue), the results were consistent with earlier observations (Duque and Ivry 2009; Duque et al. 2010): MEPs were reduced just prior to the imperative signal and this effect was greater when the probed muscle was the agonist for the forthcoming response. With this long delay, inhibition was modest (and not significant) 100 ms after the preparatory cue. When the delay period was shortened and thus, preparatory processes hastened, inhibitory effects became prominent at the early probe. Indeed, when there was only 200 ms between the preparatory cue and the imperative signal, pronounced inhibition was observed 100 ms after the preparatory cue (or 100 ms before the imperative signal), and this inhibition was independent of whether the targeted muscle was selected or not selected for the forthcoming response.
Experiment 2
In the short delay condition of Experiment 1, there was a trend for inhibition to be larger when the muscle was the agonist for the selected response. This difference has, in prior reports, provided one piece of evidence that separate inhibitory processes are recruited to regulate the control of a selected response and a nonselected response. In one study (Duque et al. 2010), a second dissociation was obtained by comparing these 2 conditions with central and peripheral stimulation protocols. We applied that same strategy here, using TMS as a probe on overall corticospinal excitability and PNS to probe changes that are manifest at the spinal level. We set the short delay period to 300 ms and applied either a TMS or PNS pulse 250 ms after the preparatory cue given prior work indicating that changes in spinal excitability are only evident 250 ms after a preparatory cue (Touge et al. 1998).
Reaction Time
TMS stimulation did not affect RT (χ2 = 1.05, P = 0.78). For the right hand, RTs were 322 ± 31 ms (TMS at baseline) and 326 ± 61 ms (TMS during delay periods), while for left hand RTs were 329 ± 45 and 327 ± 53 ms, respectively. Similarly, PNS did not affect RTs (χ2 = 5.03, P = .17). For the right hand, RTs were 319 ± 54 ms (TMS at baseline) and 295 ± 54 ms (TMS during delay periods), while for the left hand, RTs were 309 ± 51 and 304 ± 45 ms, respectively.
Corticospinal Excitability: MEPs
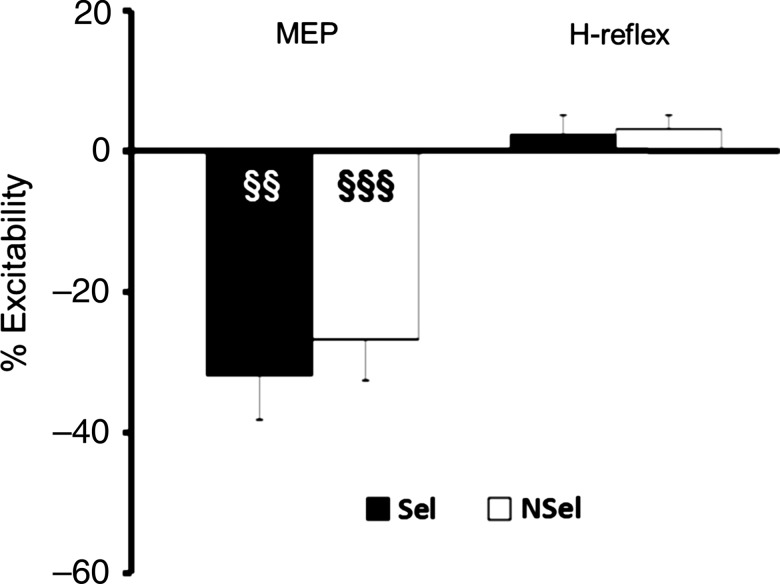
The TMS results were similar to those from the short delay period in Experiment 1 (Fig. 4, left; and Fig. 5A for typical recording). Marked inhibition of the MEPs was observed when the right hand was cued (t(7) = −4.71, P = 0.002) or not cued (t(7) = −4.90, P = 0.001) for the forthcoming response. Numerically, the effect was slightly larger in the selected condition (−32 ± 19%) compared with the nonselected condition (−28 ± 16%), but this difference was not significant (t(7) = −0.80, P = 0.44).
Figure 4.
Measure of corticospinal and spinal excitability (Experiment 2). TMS and PNS, eliciting MEPs and H-reflexes, respectively, were applied 250 ms after the preparatory cue. Values are represented as percentage of baseline. Black bars are for trials in which the right hand was selected (Sel) and white bars are from nonselected trials (NSel). Error bars represent standard errors. §§P < 0.01, §§§P < 0.001: different from 0.
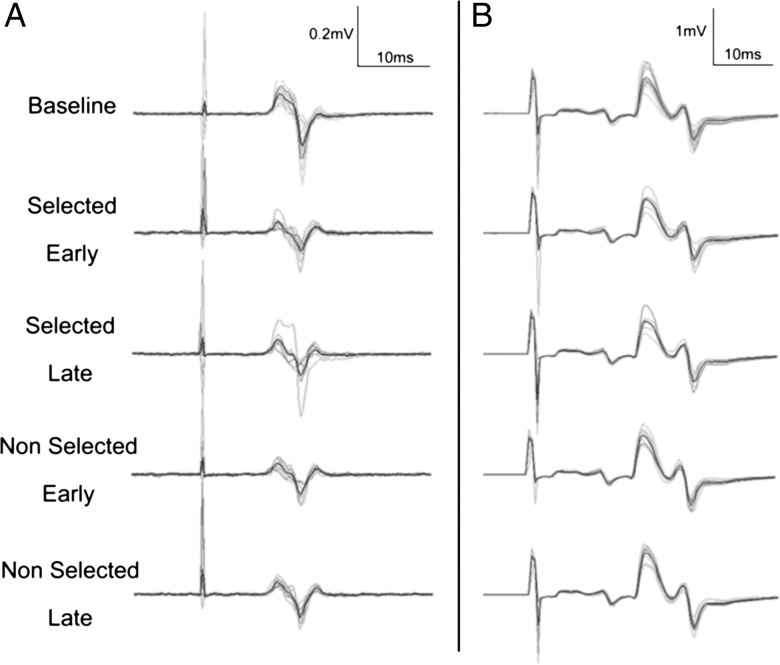
Figure 5.
Typical recording of MEPs (A) and H-reflexes (B) in Experiment 2. Black line represents the average and gray lines all the trials.
Spinal Excitability: H-reflex
The PNS results suggest that, with the short delay, there was no modulation of excitability at the spinal level. Relative to baseline (mean amplitude: 0.91 ± 0.74 mV), the H-reflex response showed a small increase in magnitude when the right wrist was selected (2.52 ± 13.48%) or not selected (3.31 ± 6.73%) for the forthcoming response. Neither effect approached significance (P > 0.05) nor did the 2 values differ (Z = 0.59, P = 0.55; Fig. 4, right; and Fig. 5B for typical recording). When considered in light of the MEP data, it appears that the effects of inhibitory processes engaged with short delay periods are limited to supraspinal mechanisms.
Discussion
In this study, we used variable delay periods to investigate the dynamics of corticospinal and spinal activation during the preparation of a response involving a choice between the 2 index fingers (Experiment 1) and the 2 hands (Experiment 2). We found that the pattern of inhibition varied as a function of the duration of the delay period. During short delay periods (200 and 300 ms), corticospinal, but not spinal, excitability decreased immediately after the preparatory cue, independent of the forthcoming response (right or left side). In contrast, during delays of 500 or 900 ms, inhibition was attenuated immediately after the preparatory cue, increased right before the imperative signal, and was larger when the probed muscle was the agonist for the forthcoming response.
Temporal Constraints on the Recruitment of Inhibitory Processes in Response Preparation
The results with a long preparatory period are consistent with previous results (Touge et al. 1998; Duque et al. 2010). Corticospinal excitability was modulated over preparatory delays of 500 and 900 ms with MEPs showing substantial inhibition as the imperative signal approached. Moreover, the amount of inhibition was greater when the targeted effector was selected as the agonist of the forthcoming response compared with when it was not selected. This pattern is consistent with the idea of 2 inhibitory mechanisms, referred to in previous work as impulse control and competition resolution. Impulse control is hypothesized to prevent premature initiation of the selected action, and competition resolution is hypothesized to prevent execution of the nonselected response. The early probes with these long delays reveal that these inhibitory processes emerge late in the preparatory period. In the 900-ms delay condition, the MEPs were not significantly attenuated, relative to baseline, when the TMS probe was applied 100 ms after the preparatory cue. In the 500-ms condition, the MEPs were inhibited at the early probe, although the magnitude of inhibition was lower than for the late probe and similar for when the left index finger was selected and not selected for the forthcoming response. The overall pattern here is consistent with results showing that motor responses can be prematurely triggered in response to acoustic startle-like stimuli, suggesting that response preparation is a progressive process (Carlsen et al. 2011). These findings suggest that the magnitude of inhibition parallels the state of motor planning.
The short delay periods in Experiment 1 (200 ms) and Experiment 2 (300 ms) provided the strongest demonstration of the flexible manner in which inhibition is recruited. Here, the MEPs were rapidly attenuated relative to baseline, reaching a level of inhibition comparable with that seen with the late pulses in the longer delay conditions. Interestingly, the level of inhibition was similar when the targeted muscle had been cued (selected) or not cued (nonselected) for the forthcoming response. Moreover, we did not observe a change in the magnitude of the H-reflex in Experiment 2, suggesting that changes in excitability during short preparatory periods are mainly supraspinal.
We can consider several hypotheses that would be consistent with the inhibitory effects observed in the short delay conditions. First, given that the instructions emphasized response speed, the onset of the cue might trigger the rapid engagement of preparatory processes for both left and right finger responses, with the identification of the cue requiring additional processing time. An inhibitory process might be recruited to ensure that these initial activations do not produce premature movement, the process we have referred to as impulse control. However, in our earlier work, one signature of impulse control had been inhibition of spinal excitability (H-reflex suppression), an effect only observed when the targeted finger was selected for the forthcoming response (Duque et al. 2010). With the short delay, we did not observe a change in the magnitude of the H-reflex response. Future work will be required to address whether the absence of an H-reflex response here is due to the use of a short preparatory period in which there is little time for response selection prior to the imperative or some other factors. For example, given our experimental design, participants could predict the onset of the imperative signal as they were aware of the delay duration for each block. With a mixed-delay design, participants would most likely prepare as early as possible, providing an alternative way to probe the dynamics of preparatory processes at various levels of the system.
Second, the pattern of inhibition observed at short delays could be the signature of a competition resolution mechanism. With the onset of the preparatory cue, evidence begins to accrue for each candidate response, with each candidate inhibiting alternative representations as part of this competition. By this view, our early probes are occurring at a time point where the balance of activation has not shifted to the eventual winner of this competition. The behavioral data are consistent with the hypothesis that response selection is relatively incomplete in the short delay condition. Not only were reaction times longer, but there was also a tendency for participants to make (partial) responses on the catch trials. It would be interesting to examine MEP changes in a short delay condition in which selection is not required (e.g., simple reaction time task). The competition resolution hypothesis would predict that inhibition would be attenuated or absent with short delay periods. We note that this early inhibition was not present in the long delay condition. This observation suggests that the rate of the selected process is modulated by the amount of time provided for preparation.
Third, the early inhibition might reflect a more generic process, one that is recruited when there is a tension between the requirements to respond quickly but discrimination is not complete. Such a process would help to avoid anticipatory responses before selection has been completed. This hypothesis would also be consistent with the longer RTs observed in the short delay-period conditions.
Our focus has been on preparatory processes triggered by the cue. An alternative way to consider the data is with respect to the imperative signal. It is interesting to note that, for the nonselected hand, the magnitude of inhibition was similar in all 3 conditions when assessed 100 ms prior to the imperative. In contrast, inhibition of the selected hand was higher at this time point in the 2 longer delay conditions. This pattern may suggest that inhibition of the nonselected hand occurs relative to the time of the imperative signal, perhaps in anticipation of this signal. Future studies that involve multiple stimulation timings with variable delay periods would help reveal the dynamics of this inhibition in terms of how it is constrained by the duration of the preparatory period, anticipation of the imperative, or an interaction of these factors.
Neural Mechanisms for Preparatory Inhibition
Separate lines of evidence point to possible neural mechanisms associated with inhibitory processes recruited during action selection and response initiation. The dorsal premotor cortex (PMd) has been implicated in the selection and implementation of action plans (Grafton et al. 1998; Cavina-Pratesi et al. 2006; Hoshi and Tanji 2006; Terao et al. 2007). Specifically, PMd neurons fire in a selective manner during a delay period following a preparatory cue, and this activity is hypothesized to carry information about the future actions (Cisek and Kalaska 2005; Churchland et al. 2006). While the output of PMd has a strong influence over primary motor cortex, it has also been shown that PMd modulates excitability within spinal motor circuitry (Bizzi et al. 2000), including through the excitation of inhibitory interneurons (Fetz 1999; Prut and Fetz 1999; Fetz et al. 2002). Transient disruption of PMd reduces inhibition of the selected response, consistent with the idea that PMd is part of a circuit associated with impulse control (Duque et al. 2012). That is, as part of its contribution to preparatory activity, PMd may suppress spinal excitability associated with the selected response. In the current study, we failed to observe depression of the H-reflex with short delay periods. Although caution is required when considering null effects, these data would suggest that a PMd-spinal pathway is not sufficiently engaged to account for early inhibitory effects when preparatory time is limited.
Alternatively, inhibition with short delays might reflect the engagement of inhibitory processes associated with competition resolution. Repetitive TMS over the lateral prefrontal cortex (LPF) reduced inhibition in the representation of all task-relevant effectors (Duque et al. 2012). This effect is consistent with the idea that selection entails a competitive process, such that representations vie for selection through their accrual of activation and suppression of alternative representations (Gold and Shalden 2007; Cisek and Kalaska 2010; but see Brown and Healthcote 2008). This competition may be engaged during the short delay trials here; indeed, the short delays would place a premium on rapid selection.
It is also possible that the inhibition at short delays reflects a more generic inhibitory process. Signatures of widespread inhibition of the motor system have been observed when canceling an ongoing motor response (Badry et al. 2009; Cai et al. 2012; Greenhouse et al. 2012; Majid et al. 2012; Wessel and Aron 2013). A similar mechanism may operate following a preparatory cue to facilitate the selection processes, possibly by focusing on task-relevant representations. To date, the neural locus of such generic signals has focused on a cortical–subcortical network spanning the right inferior LPF, pre-SMA, and the subthalamic nucleus (e.g., Aron 2007) that is recruited when volitional control is required (i.e., during the stop signal tasks). It remains to be seen if this inhibitory control network is also recruited to facilitate the preparation of motor responses.
Physiological data from various methodologies provide a more complete picture of the preparatory mechanisms at play in delayed response tasks. Depending on the tuning properties of single neurons, cellular activity may increase or decrease during a delay period (e.g., Riehle and Requin 1989; Bastian et al. 2003; Cisek and Kalaska 2005), although these fluctuations do not map in any direct manner to the operation of excitatory and inhibitory mechanisms (e.g., Kaufman et al. 2010). Neuroimaging studies generally reveal increased motor cortical activity during delay periods, although an increase in these hemodynamic signals may reflect the operation of facilitatory, inhibitory, or both types of processes (see Rushworth et al. 2009; Cisek and Kalaska 2010). TMS and rTMS paradigms provide evidence for the parallel engagement of inhibitory and facilitatory processes. For example, one can observe reductions in corticospinal and spinal excitability even when, concurrently, there is reduced intracortical inhibition, a measure taken to reflect an increase in local excitability (Duque and Ivry 2009).
The overall picture suggests a transient decrease in motor excitability prior to the imperative stimulus after which corticospinal excitability rapidly increases to initiate the planned response (Hasbroucq et al. 1997; Coxon et al. 2006; van den Hurk et al. 2007; Sinclair and Hammond 2008; Duque and Ivry 2009; Labruna et al. 2014). The selection processes inherent in choice tasks may recruit various cortical and spinal mechanisms to modulate the readiness state of the system (Touge et al. 1998; Hasbroucq et al. 1999; Duque et al. 2010). The current results provide a window into these dynamics, showing that the dip in excitability during the preparatory period is influenced by the time afforded for movement preparation.
Summary
In conclusion, the current results provide compelling evidence of the operation of inhibitory processes in delayed response tasks, inhibition that is strongly modulated by the duration of the preparatory period. Across the 2 experiments, we observed similar patterns when TMS was applied over either hemisphere and for different effectors of the upper limbs (finger and hand muscles). In line with previous work (Touge et al. 1998; Duque et al. 2010), inhibition increased progressively from the preparatory cue to the imperative signal, becoming larger when the effector was selected for the forthcoming response with longer delay periods. Most interesting, the recruitment of inhibitory mechanisms is hastened with short delay periods, a pattern that would superficially appear counter-productive.
Funding
This work was supported by Grants NS0085570 and NS074917 from the National Institute of Health (USA).
Notes
We thank Cyril Sirandré, who helped build the technical support for Experiment 2. Conflict of Interest: None declared.
References
- Aron AR. 2007. The neural basis of inhibition in cognitive control. Neuroscientist. 13:214–228. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. 2009. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 120:1717–1723. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schöner G, Riehle A. 2003. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 18:2047–2058. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Tresch MC, Saltiel P, d'Avella A. 2000. New perspectives on spinal motor systems. Nat Rev Neurosci. 1:101–108. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Requin J. 1982. Long loop and spinal reflexes in man during preparation for intended directional hand movements. J Neurosci. 2:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M, Requin J, Stelmach GE. 1991. Changes in electromyographic responses to muscle stretch, related to the programming of movement parameters. Electroencephalogr Clin Neurophysiol. 81:135–151. [DOI] [PubMed] [Google Scholar]
- Brown SD, Healthcote A. 2008. The simplest complete model of choice response time: linear ballistic accumulation. Cogn Psychol. 57:153–178. [DOI] [PubMed] [Google Scholar]
- Brunia CH, Scheirs JG, Haagh SA. 1982. Changes of Achilles tendon reflex amplitudes during a fixed foreperiod of four seconds. Psychophysiol. 19:63–70. [DOI] [PubMed] [Google Scholar]
- Cai W, George JS, Verbruggen F, Chambers CD, Aron AR. 2012. The role of the right presupplementary motor area in stopping action: two studies with event-related transcranial magnetic stimulation. J Neurophysiol. 108:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. 2011. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Behav Rev. 35:366–376. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Köhler S, Obhi SS, Marzi CA, Goodale MA. 2006. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci. 26:2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. 1998. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 44, 317–325. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. 2006. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 26:3697–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. 2005. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 45:801–814. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. 2010. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 33:269–298. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. 2006. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 95:3371–3383. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. 2007. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 25:3766–3774. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. 2009. Role of corticospinal suppression during motor preparation. Cereb Cortex. 19:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. 2012. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 32:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. 2010. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 30:3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. 2005. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 15:588–593. [DOI] [PubMed] [Google Scholar]
- Fetz EE. 1999. Response properties of spinal interneurons in awake, behaving primates. Pain. 6:S55–S60. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S. 2002. Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev. 40:53–65. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shalden MN. 2007. The neural basis of decision making. Annu Rev Neurosci. 30:535–574. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA. 1998. Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J Neurophysiol. 79:1092–1097. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. 2012. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J Neurophysiol. 107:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. 1997. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 5:185–192. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. 1999. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 124:33–41. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. 2006. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 95:3596–3616. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Santhanam G, Yu BM, Afshar A, Ryu SI, Shenoy KV. 2010. Roles of monkey premotor neuron classes in movement preparation and execution. J Neurophysiol. 104:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. 2006. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 26:7452–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Tanaka R. 1990. The differences in human spinal motoneuron excitability during the foreperiod of a motor task. Exp Brain Res. 79:357–364. [DOI] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. 2014. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci. 26:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. 2000. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 123:1161–1173. [DOI] [PubMed] [Google Scholar]
- Maffiuletti NA, Martin A, Van Hoecke J, Schieppati M. 2000. The relative contribution to the plantar flexor torque of the soleus motor units activated by the H-reflex and M response in human. Neurosci Lett. 288:127–130. [DOI] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. 2012. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex. 22:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Rossi A. 1995. Distribution of Ia effects onto human hand muscle motoneurones as revealed using an H-reflex technique. J Physiol. 489:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. 1999. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 401:590–594. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. 1989. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol. 61:534–549. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. 1988. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 458:20–30. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Mars RB, Summerfield C. 2009. General mechanisms for making decisions? Curr Opin Neurobiol. 19:75–83. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. 2008. Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res. 186:385–392. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. 2002. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 3:348–359. [DOI] [PubMed] [Google Scholar]
- Terao Y, Furubayashi T, Okabe S, Mochizuki H, Arai N, Kobayashi S, Ugawa Y. 2007. Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci. 19:1556–1573. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. 1998. Reduced excitability of the corticospinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 109:489–495. [DOI] [PubMed] [Google Scholar]
- van den Hurk P, Mars RB, van Elswijk G, Hegeman J, Pasman JW, Bloem BR, Toni I. 2007. Online maintenance of sensory and motor representations: effects on corticospinal excitability. J Neurophysiol. 97:1642–1648. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. 2007. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 19:121–131. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. 2013. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J Neurosci. 33:18481–18491. [DOI] [PMC free article] [PubMed] [Google Scholar]