Abstract
The prevalence of adolescent obesity has increased dramatically over the past three decades, and research has documented that the number of television shows viewed during childhood is associated with greater risk for obesity. In particular, considerable evidence suggests that exposure to food marketing promotes eating habits that contribute to obesity. The present study examines neural responses to dynamic food commercials in overweight and healthy-weight adolescents using functional magnetic resonance imaging (fMRI). Compared with non-food commercials, food commercials more strongly engaged regions involved in attention and saliency detection (occipital lobe, precuneus, superior temporal gyri, and right insula) and in processing rewards [left and right nucleus accumbens (NAcc) and left orbitofrontal cortex (OFC)]. Activity in the left OFC and right insula further correlated with subjects' percent body fat at the time of the scan. Interestingly, this reward-related activity to food commercials was accompanied by the additional recruitment of mouth-specific somatosensory-motor cortices—a finding that suggests the intriguing possibility that higher-adiposity adolescents mentally simulate eating behaviors and offers a potential neural mechanism for the formation and reinforcement of unhealthy eating habits that may hamper an individual's ability lose weight later in life.
Keywords: action observation, advertising, fMRI, food, obesity
Introduction
Obesity is a key public health problem in the United States of America and has become progressively more prevalent in the past 3 decades (Ogden et al. 2014; National Center for Health Statistics, 2012). At the same time, many aspects of food consumption have changed—greater use of prepared food products in the home and greater utilization of restaurants. Corporate fast food restaurants appeared in the 1950s, before the obesity epidemic but have expanded greatly since then. Today, dozens of national chains compete intensively on food price and portion size. Nowhere is this competition better illustrated than in television advertising for these products, where children view up to 13 food ads per hour of programming (Dembek et al. 2013).
Prior behavioral work has demonstrated relationships between adolescents' receptivity to food commercials and body mass index (BMI) (McClure et al. 2013) and the amount of snacking following food ad viewing (Halford et al. 2004). Neuroimaging studies exploring the relationship between food cue-reactivity and obesity in adults have consistently identified a putative network of reward regions including the ventral striatum, insula, and regions of the orbitofrontal cortex (OFC) (Rothemund et al. 2007; Stoeckel et al. 2008; Bruce et al. 2010; Stice et al. 2011; Dimitropoulos et al. 2012; Wagner et al. 2013) as well as regions related to visual attention (McCaffery et al. 2009; Martin et al. 2010), and somatosensory processing (Stice et al. 2011). Moreover, food cue-reactivity in reward and attention regions have been linked to future weight gain (Demos et al. 2012; Yokum et al. 2012), trait impulsivity (Kerr et al. 2014), self-reported craving (Kober et al. 2010), giving in to cravings (Lopez et al. 2014), diet violations (Demos et al. 2011), diet failures (Murdaugh et al. 2012), as well as dieting status and weight loss strategies (Bruce et al. 2012; Bruce et al. 2014). Although the majority of these studies have been conducted in adults, adolescence is often characterized by heightened sensitivity to reward cues, potentially leading to increases in risky behaviors (Fareri et al. 2008; Casey 2014), thus providing further motivation for investigating this relationship in an adolescent population.
Although much of this work has been conducted using static pictures of appetizing foods, recent work by Gearhardt et al. (2013) has extended food cue-reactivity findings in adolescents to dynamic food commercials. Because food commercials are specifically designed to entice consumption of the advertised product, these cues may be particularly powerful motivators of eating behavior. Additionally, dynamic reward cues like food commercials may capitalize on and reinforce well-established, automatic habits through mimicry and observational learning. Behavioral mimicry studies in adults have shown that the behavior of a confederate can impact the viewer's own behaviors (Chartrand and Bargh 1999), and other work has more directly tied this phenomenon to eating behaviors (Johnston 2002).
Brain imaging research on action observation has shown that observing others perform goal-directed actions recruits a putative action–observation network, which includes lateral frontal (IFG), motor, premotor, supplementary motor, somatosensory regions, the intraparietal lobule, the superior parietal lobule, and the intraparietal cortex (IPS) (Caspers et al. 2010).
Much like eating, smoking is another highly reinforced and automatic behavior, and our prior work has shown that smokers activate both reward (left OFC) and action-observation regions (left IPS and IFG) more so than nonsmokers when viewing dynamic depictions of others smoking (Wagner et al. 2011). The present study sought to examine the neural responses to food commercials in order to better understand the relationship between real-world food advertising and adolescent obesity. Based on our previous work, we hypothesized that adolescents would demonstrate greater activity for food commercials in regions involved in reward and that recruitment would correlate with individual differences in adiposity. An open question was whether high-adiposity adolescents would additionally recruit brain regions that are commonly activated in studies of action observation, a finding that might suggest high-adiposity adolescents are more likely to simulate eating when observing others eat.
Methods
Subjects
Forty right-handed adolescents (20 female and 20 male) between the ages of 12 and 17 (mean age = 14.3 years) were recruited locally through the Children's Hospital at Dartmouth Hitchcock Medical Center, based on their BMI percentiles. We obtained permission from the IRB to conduct a limited search of the electronic medical records to identify adolescents in the pediatric practice whose BMI was ≥95th percentile (obese) and whose BMI was between the 40th and the 59th percentile (healthy weight). An opt-out letter was sent to the parents of all of these adolescents from the physicians in the practice informing them of the study, after which we proactively called them to invite to participation. Participants were matched for age and gender. Enrolled adolescents and their parents were consented verbally, and participants were unaware that they had been recruited based on BMI. All procedures were approved by the Committee for the Protection of Human Subjects at Dartmouth College. Due to excessive movement in two subjects and technical problems with data collection in a third subject, 37 participants were included in the final analyses. For these subjects (20 female and 17 male), the mean age was 14.4 years (s.d. = 1.3 years; range = 12–16). Of these subjects, mean BMI for adolescents recruited as obese (n = 18) was 33.2 (s.d. = 2.51) and mean BMI for adolescents recruited as healthy weight (n = 19) was 20.15 (s.d. = 2.05) (Table 2). Across all subjects (n = 37), mean BMI was 26.49 (s.d. = 6.99) and the range was 16.5–37.7. One subject recruited as healthy weight met national criteria for being overweight (>85th percentile) on the day of the scan.
Table 2.
Demographic characteristics of participants. Means and standard deviations for BMI and body fat percentages within obese and healthy-weight recruited groups and across all subjects
| BMI (mean) | BMI (s.d.) | Body fat percent (mean) | Body fat percent (s.d.) | |
|---|---|---|---|---|
| Obese | 33.20 | 2.51 | 42.54 | 8.27 |
| Healthy weight | 20.15 | 2.05 | 20.15 | 9.83 |
| All subjects | 26.49 | 6.99 | 31.04 | 14.47 |
Although participants were recruited based on an obesity metric available to us prior to the scan session (BMI), percent body fat was collected on the day of the scan as an additional measure of individual adiposity. Mean percent body fat for adolescents recruited as obese was 42.54 (s.d. = 8.27), and mean percent body fat for adolescents recruited as healthy weight was 20.15 (s.d. = 9.83) (Table 2). Mean percent body fat across all subjects was 31.04 (s.d. = 14.47), and the range was 8.3–52.5%.
Stimuli
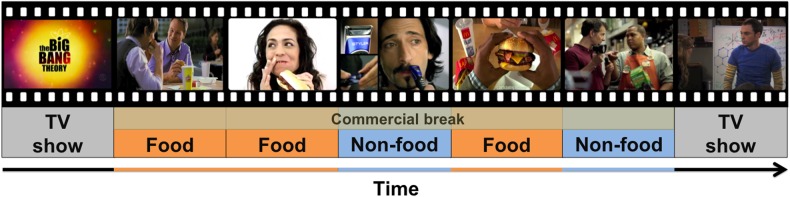
Twelve food and 12 non-food high-resolution commercials were matched for length (mean food commercial = 28.4 s; mean control commercial = 28.9 s) (Table 1). Commercials were selected based on quality, relevance to the age group, and publication date. Non-food commercials were included as a comparison to account for low-level visual properties inherent to processing dynamic scenes. A separate cohort of 28 adolescents (mean age = 12.61 years; s.d. = 1.71 years) rated a randomized subset the commercials (mean number of commercials viewed = 6.07, s.d. = 0.99) on interest (“how interesting do you think this commercial is?”) and excitement (“how exciting do you think this commercial is?”) on a sliding scale from 0 to 1. Ratings of interest for the food commercials (mean = 0.395, s.d. = 0.202) and ratings of interest for the neutral commercials (mean = 0.403, s.d. = 0.154) did not significantly differ (t(27) = −0.548, P = 0.808). Similarly, ratings of excitement for the food commercials (mean = 0.375, s.d. = 0.191) and ratings of interest for the neutral commercials (mean = 0.390, s.d. = 0.134) did not significantly differ (t(27) = −0.410, P = 0.690). During scanning, commercials were presented in a pseudo-randomized order so that no more than two commercials in a condition or 2 commercials of the same brand appeared in subsequence. These commercials were embedded as four “commercial breaks” into an episode of a popular age-appropriate television show, The Big Bang Theory (Fig. 1). Each commercial break consisted of six commercials, or ∼2.8 min (67 TRs) of commercial time.
Table 1.
Food and non-food commercials used in scanning paradigm
| Brand | Product | Name/description | Duration (s) |
|---|---|---|---|
| Food commercials | |||
| McDonald's | Quarter pounder | Made with 100% beef | 15 |
| McDonald's | Double quarter pounder | It adds character | 30 |
| McDonald's | Angus third pounder | Eyes on the road | 30 |
| McDonald's | McRib | McRib is back | 30 |
| McDonald's | Chicken Nuggets | Slams even dunkier | 30 |
| McDonald's | Chipotle BBQ bacon angus | Angus axiom #43 | 30 |
| Wendy's | 99-cent menu | My 99: Drive through | 30 |
| Wendy's | 99-cent menu | My 99: Skate park | 28 |
| Wendy's | Chicken sandwich | Slap in the face | 30 |
| Dunkin Donuts | Breakfast sandwiches | Adventure runs on Dunkin | 29 |
| KFC | $5 meal | Today is a KFC day | 30 |
| Pizza Hut | Big Italy pizza | Big Italy | 29 |
| Non-food commercials | |||
| Lowe's | Store sale event | Great American fix-up | 15 |
| Gillette | Fusion ProGlide Styler | Masters of Style | 30 |
| Quicken Loans | Retail mortgage | Who do you think I am? | 30 |
| Tide | Tide laundry detergent | Hoodies & Cargo shorts | 31 |
| Chevrolet | Volt | Volt owners: gas stations | 31 |
| Toyota | Camry, Corolla, Priux | #1 for everyone sales event | 26 |
| Gain | Gain laundry detergent | Revolving door | 32 |
| Sprint | Cellular phone data plan | Truly unlimited data | 31 |
| Simple Green | All purpose cleaner | I got that | 30 |
| Verizon | 4G LTE | Bad idea | 30 |
| Johnson's | Head-to-toe wash | Nice work | 31 |
| Farmer's | Insurance | University of Farmers: Maze | 30 |
Figure 1.
Study design. Subjects viewed episode of The Big Bang Theory with food and control (non-food) commercials embedded throughout as typical commercial breaks.
Procedure
Subjects were naïve to the purpose of the experiment and were simply told that the study was aimed at understanding the brain's response to viewing television shows. Subjects were asked not to eat food or to consume any caffeinated beverages for the 2 h prior to their study appointment. Before scanning, subjects were weighed using a Tanita scale (model TBF-300A Arlington Heights), which uses bioelectric impedance analysis to determine body composition and has been shown to be a reliable measure of body fat (Jebb et al. 2007). Consistent with our cover story, subjects were asked to report how many TV shows they watch per week on average.
During scanning, subjects watched a 19-min episode of The Big Bang Theory. Food and non-food commercials were pseudo-randomized and embedded into the natural commercial breaks of the episode. The TV show and commercials were presented with SuperLab 4.5 software (Cedrus Corporation). Participants were given no overt task instructions and were allowed to passively view the TV show and commercials. Echo-planar images (EPIs) were acquired during commercial presentations and reference scans, and structural images were acquired during the TV show presentation. In total, 12 food and 12 control (non-food) commercials were presented over four “commercial breaks.”
Image Acquisition
All scanning was performed on a 3.0T Philips Achieva MRI fit with a 32-channel SENSE (Sensitivity Encoding) headcoil. Structural images were obtained using a T1-weighted MP-RAGE protocol (TR = 9.9 ms; TE = 4.6 ms; flip angle = 8°; 1 × 1 × 1 mm3 voxels). Functional images were acquired using a T2*-weighted EPI protocol (TR = 2500 ms; TE = 35 ms; flip angle = 90°; 3 × 3 × 3 mm3 voxels; sense factor of 2). Four functional runs were collected (67 TRs each) for each participant.
Image Preprocessing
All imaging preprocessing and subsequent analyses were conducted in SPM8 (Wellcome Department of Cognitive Neurology) in conjunction with a suite of tools for preprocessing and analysis (https://github.com/ddwagner/SPM8w). Functional images were slice-time-corrected and realigned to account for temporal differences in slice acquisition and head motion, respectively. Resulting volumes were spatially normalized to the ICBM 152 template brain (Montreal Neurological Institute) and spatially smoothed using an 8-mm (FWHM) Gaussian kernel.
Data Analysis
Task conditions and covariates of no interest were convolved with a canonical hemodynamic response function and included in a general linear model to determine neural responses to food and non-food commercials. Nuisance regressors included 6 motion parameters, the session mean, and a linear trend to account for low-frequency scanner drift. The resulting subject-level contrasts of FOOD > NON-FOOD commercials were entered into a second-level random-effects analysis. This produced a group-level statistical parametric map that represented the overall changes in neural activity for FOOD > NON-FOOD commercials across subjects. The group-level contrast map was thresholded at P < 0.005 and cluster corrected to account for multiple comparisons using 5000 Monte Carlo simulations. These simulations estimated a minimum cluster size of 173 voxels.
Given our a priori hypothesis that reward-processing regions would correlate with adiposity, region-of-interest (ROI) analyses were performed on the nucleus accumbens (NAcc), the OFC, and the insula. Left and right NAcc ROIs were defined anatomically using the automatic segmentation tool (aseg) in FreeSurfer (Fischl 2004) to create a probabilistic mask from anatomical MPRAGE scans collected on all subjects. Voxels that were present in at least 75% of all subjects' segmented NAcc regions were included in the ROI. The FOOD > NON-FOOD commercials contrast (P < 0.05, corrected based on a cluster extent threshold of 913 voxels estimated with 5000 Monte Carlo simulations) was used to identify cortical reward ROIs. ROI selection in this case is unbiased with respect to body fat (Kriegeskorte et al. 2009; Vul et al. 2009), as ROIs were defined using an independent contrast that did not correlate signal change with body fat. Three cortical reward ROIs (10-mm spheres centered on the peak activation) were identified from this contrast, the left OFC (−6, 42, −12), right OFC (27, 36, −24), and right insula (39, −6, 3). All ROIs were interrogated for outliers (i.e., individuals whose activity was > two standard deviations from the mean activation of the ROI), and the resulting correlations with percent body fat, BMI, and TV viewing were conducted on each ROI after removal of outliers.
In order to identify additional brain regions that were more active when viewing food commercials as a function of percent body fat, an exploratory whole-brain regression was performed. Each subject's FOOD > NON-FOOD contrast was entered into a regression analysis using individual body fat percentage as a covariate. Age and gender were included in this model to account for variance in body fat percentages for males and females of different ages (Rosner et al. 1998; Blaak 2001). Resulting statistical maps for the exploratory whole-brain analyses were thresholded using a more stringent threshold (P < 0.001) and were cluster corrected to a minimum extent of 74 voxels to account for whole-brain multiple comparison based on 5000 Monte Carlo simulations.
Data Visualization
All fMRI results were visualized in Connectome Workbench Version 0.85 (Marcus et al. 2010; Marcus et al. 2011) available from http://www.humanconnectome.org/connectome/connectome-workbench.html. Cortical surface results were mapped onto the Conte69 mid-thickness surfaces (Van Essen et al. 2012).
Results
Behavioral Results
Adolescents reported watching an average of 5 h of TV shows per week (s.d. = 3.05, range = 1–13 h). The number of reported TV viewing significantly correlated with subjects' BMI (r = 0.49, P < 0.005) and percent body fat (r = 0.41, P < 0.05). BMI was also correlated with percent body fat (r = 0.85, P < 0.0001).
Imaging Results
Food Versus Non-Food Commercials
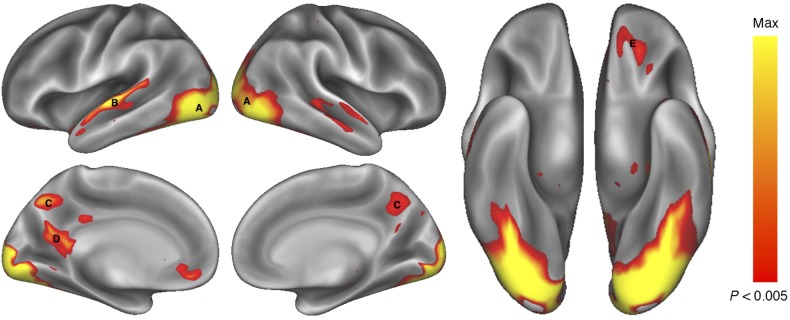
A random-effects analysis identified several regions that showed greater activation during food commercials compared with non-food commercials (Fig. 2). In particular, the left OFC, occipital lobe, bilateral regions of the superior and middle temporal gyri, and the posterior cingulate gyrus all demonstrated significantly greater activation in response to FOOD commercials than to NON-FOOD commercials (P < 0.05, corrected; Table 3).
Figure 2.
Brain regions showing greater activity when viewing FOOD commercials than NON-FOOD commercials. Activations (P < 0.005, 173 contiguous voxels) are displayed on an inflated rendering of the cortical surface (Marcus et al. 2010; Marcus et al. 2011). Greater activation for FOOD commercials was observed in a number of occipital regions (A) extending from the occipital pole through the fusiform gyrus, the left superior and middle temporal gyrus (B), the precuneus (C and D), and the left orbital frontal cortex (E).
Table 3.
Regions that were significantly more active (P < 0.05, corrected) for FOOD > NON-FOOD commercials
| Region | Coordinates (MNI) |
Volume (mm3) | Peak T | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Occipital lobe | 12 | −102 | 12 | 52 785 | 13.19 |
| R Medial temporal gyrus | 69 | −6 | −6 | 3339 | 5.67 |
| L Superior temporal gyrus | −69 | −18 | 0 | 2997 | 6.85 |
| L Precuneus | −6 | −48 | 15 | 1188 | 4.67 |
| L Orbitofrontal cortex | −12 | 54 | −27 | 747 | 4.29 |
A priori ROI Analyses
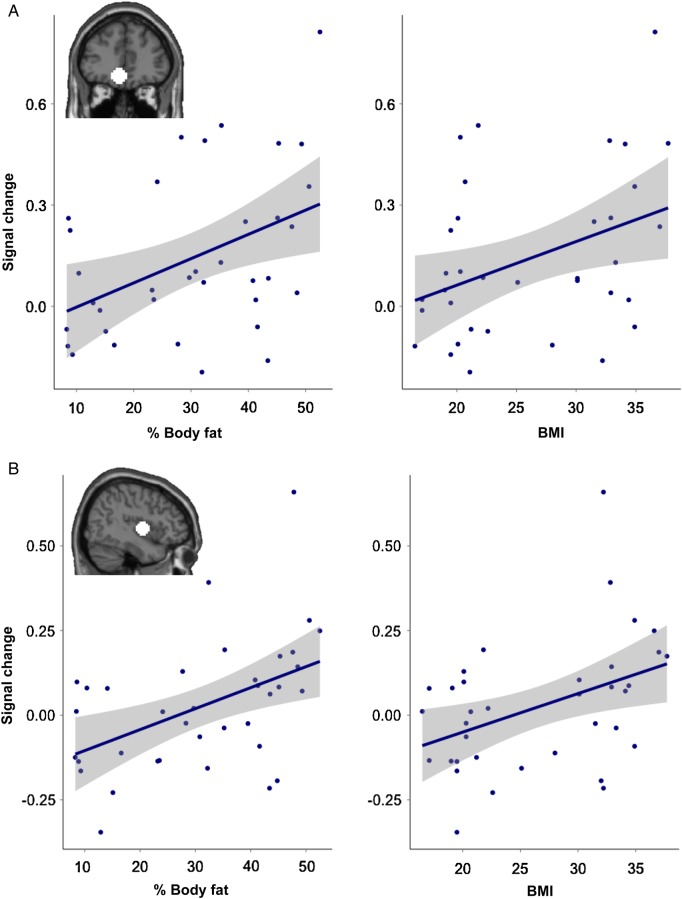
Given our a priori hypothesis, we investigated the activation observed within anatomically defined left and right NAcc ROIs, and the left and right OFC and right insula ROIs identified in the FOOD > NON-FOOD contrast. Estimates of signal change for FOOD > NON-FOOD commercials within the left OFC and right insula correlated with participants' percent body fat (left OFC; r = 0.43, P < 0.01 right insula; r = 0.38, P < 0.05) (Fig. 3). Because body fat correlated with both BMI and TV viewing, we also correlated left OFC and right insula activity with these measures across subjects. Activity in left OFC and right insula also correlated with BMI (left OFC; r = 0.38, P < 0.05 right insula; r = 0.41, P < 0.05) but did not correlate with the amount of TV viewing (left OFC; r = 0.04, P = 0.84, right insula; r = 0.25, P = 0.14). Activity in the right OFC did not correlate with body fat (r = 0.25, P = 0.16), BMI (r = 0.24, P = 0.17), or TV watching (r = 0.01, P = 0.93).
Figure 3.
Regions correlating with percent body fat. (A) The magnitude of response to food commercials in a region of the left orbitofrontal cortex, defined by the FOOD > NON-FOOD contrast, correlated with percent body fat (r = 0.43, P < 0.01) and BMI (r = 0.38, P < 0.05). (B) The magnitude of response to food commercials in a region of the right insula, defined by the FOOD > NON-FOOD contrast, correlated with percent body fat (r = 0.38, P < 0.05) and BMI (r = 0.41, P < 0.05).
Although activity in left and right NAcc was greater for FOOD than NON-FOOD commercials (left: t(35) = 3.9, P < 0.0005; right: t(35) = 3.2, P < 0.005), it did not correlate with body fat, BMI, or TV watching (P > 0.05). We did not observe significant activations in the left insula in the FOOD > NON-FOOD contrast, and so this region was not interrogated further.
Exploratory Whole-Brain Regression Analysis
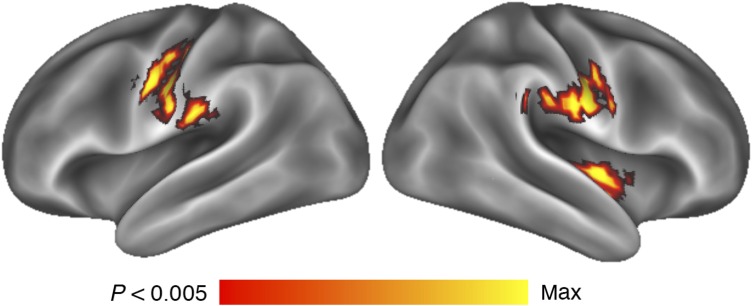
To identify additional brain regions that correlated with percent body fat, an exploratory whole-brain regression analysis was conducted by correlating FOOD > NON-FOOD signal change with body fat on a voxel-by-voxel basis. Results revealed regions that significantly correlated between the FOOD > NON-FOOD commercials and percent body fat (P < 0.05, corrected; Fig. 4 and Table 4). Bilateral sensorimotor cortices along the pre- and post-central gyri and bilateral central sulci and a region of the right insula/posterior opercula demonstrated this correlation. Finally, a region in the posterior cerebellum demonstrated greater activation with increases in percent body fat.
Figure 4.
Whole-brain response to FOOD commercials covaried with percent body fat, accounting for age and gender. Activations are overlayed on an inflated representation of the cortical surface (Marcus et al. 2010; Marcus et al. 2011). Activations were observed in bilateral regions of sensorimotor cortices along the pre- and post-central gyri and bilateral central sulci and a region of the right insula/posterior opercula.
Table 4.
Regions that significantly correlated (P < 0.05, corrected) with increases in percent body fat in response to food commercials
| Region | Coordinates (MNI) |
Volume (mm3) | Peak T | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| L Central sulcus | −54 | −6 | 33 | 2097 | 5.08 |
| R Central sulcus | 57 | −12 | 30 | 1593 | 5.20 |
| Cerebellum | −6 | −93 | −33 | 936 | 4.51 |
| R Insula | 39 | −12 | 0 | 891 | 5.15 |
Since BMI is a widely used metric for determining obesity status, whole-brain responses to food commercials were similarly regressed with BMI (again accounting for age and gender). In doing so, only the right sensorimotor region demonstrated this relationship at the threshold used for the percent body fat regression (P < 0.05, corrected). However, this region was recruited to a lower extent (101 voxels for BMI vs. 184 voxels for percent body fat). No other regions were significantly correlated with BMI.
Discussion
The present study contributes to our growing understanding of the influence of naturalistic, dynamic food commercials on neural activity and eating behavior in adolescents. The extension of such content to the study of appetitive behaviors here and elsewhere (Gearhardt et al. 2013) may serve to better understand the full complement of activations associated with healthy and unhealthy eating habits. Across all subjects, food commercials more strongly activated the OFC, insula, and NAcc, regions consistently activated in reward processing and encoding valuation (Rothemund et al. 2007; Cloutier et al. 2008; Stoeckel et al. 2008; Bruce et al. 2010; Stice et al. 2011; Wagner et al. 2011; Demos et al. 2012; Dimitropoulos et al. 2012; Simmons, Rapuano, Ingeholm, et al. 2013). This finding supports our hypothesis that food commercials engage reward-related regions of the brain more strongly than non-food commercials and is consistent with previous studies (Gearhardt et al. 2013). Additionally, regions within the occipital lobe, the left and right superior and middle temporal gyrus, and the posterior cingulate were all significantly more active for the food commercials compared with non-food commercials. The greater activation of these regions may reflect greater attention and saliency detection for the food commercials, which is also consistent with earlier work (Gearhardt et al. 2013).
Of particular interest, the greater left OFC and right insula activity to food commercials additionally correlated with adolescent adiposity and was accompanied by the additional recruitment of sensorimotor regions in high-adiposity adolescents. Although BMI is commonly used as a proxy for obesity classification, as was used in Gearhardt et al. (2013), the present study capitalized on an additional measure (body fat) to characterize obesity status as this metric has been argued to provide a more accurate measure of physical health (Shah and Braverman 2012; Ahima and Lazar 2013), particularly in adolescents (Widhalm et al. 2001; Freedman et al. 2005). In the present study, the whole-brain regression revealed a more robust correlation between body fat and sensorimotor and insula activity than did BMI. No regions were significantly correlated with age and gender alone, suggesting the findings reported here are driven by percent body fat. Collectively, these findings suggest that correlating brain activity with body fat may offer a more complete picture of individual differences in neural activity and their relationship to obesity than BMI alone.
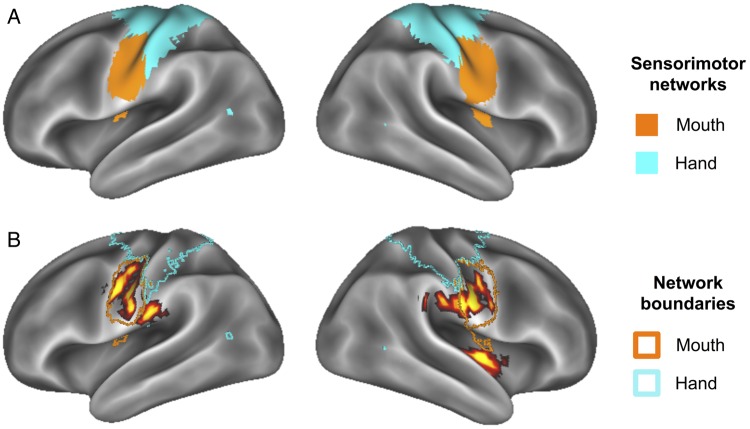
It is interesting to note that the peak voxels within these bilateral sensorimotor activations observed here have previously been reported in fMRI studies (within 6 mm) examining lip, tongue and jaw movements (Funk et al. 2008; Grabski et al. 2012), mastication (Takahashi et al. 2007), and swallowing (Lowell et al. 2008). Further, a PET study has demonstrated greater resting metabolic activity in oral somatosensory cortex in obese subjects relative to healthy-weight subjects (Wang et al. 2002). Figure 5 shows the overlap between our sensorimotor activations and subregions of sensorimotor systems as defined by resting-state functional connectivity MRI (rs-fcMRI; Power et al. 2011) (Fig. 5a). When overlaid in this fashion, correlated food-commercial activity with body fat was localized to the “mouth” sensorimotor network with little crossover into the “hand/body” sensorimotor network (Fig. 5b).
Figure 5.
(A) Functionally defined sensorimotor networks via resting-state connectivity (Power et al. 2011) provide evidence for separable mouth and hand (body) subnetworks. (B) Activity from (A) overlaid on network boundaries from Power et al. (2011) demonstrates specificity of activity to “mouth” sensorimotor network.
Additionally, the right insula demonstrated a similar correlation with body fat. This region spanned mid-insula and extended into posterior insular cortex. Previous studies have reported the mid-insula to be involved in gustatory processing (Veldhuizen et al. 2011; Simmons, Rapuano, Kallman, et al. 2013). When considered in this context, the present findings may also suggest that higher-adiposity adolescents activate taste representations when viewing food commercials. Moreover, the posterior insula is considered to be directly associated with processing somatosensory information (Ostrowsky 2002; Craig 2003), and the functional and structural connectivity of these regions have more recently been identified (Cauda et al. 2011; Jakab et al. 2012). The extension into posterior insula reported here suggests that this region may be representing an integration of mouth somatosensory and gustatory information that is more highly activated when high-adiposity adolescents view food commercials.
Collectively, these findings suggest that higher-adiposity adolescents more strongly recruit oral somatomotor and gustatory regions pertinent to eating behaviors while viewing food commercials, in comparison with their lower-adiposity counterparts. Previous studies investigating action observation have located neurons responsive both to the observation and execution of goal-directed actions, commonly termed mirror neurons (Gallese 1998). Such neurons have been defined in primate motor-related cortical areas in response to performing actions or viewing others perform an action (Kohler et al. 2002). Further, ingestive mirror neurons have been identified in similar motor regions in monkeys exhibiting eating behaviors or while watching other monkeys eat (Ferrari et al. 2003) and have more recently been identified in human somatosensory cortex in response to touch or viewing others being touched (Keysers et al. 2004). The greater recruitment of sensorimotor and insula cortices associated with eating in high-adiposity adolescents suggests the intriguing possibility that these individuals mentally simulate eating behavior in response to viewing food commercials, which may then contribute to the enactment of the behavior itself. Although speculative, dynamic reward cues such as food commercials, in addition to being evaluated as more rewarding in high-adiposity adolescents, may reinforce well-established, automatic eating habits through mimicry and observational learning. To the extent that such recruitment serves to establish eating habits and patterns, the present results offer a potential neural mechanism that may interfere with an overweight or obese adolescent's future attempts to eat less and to lose weight later in life. Perhaps, more encouragingly, the present findings may also provide clues for intervention strategies aimed at promoting healthy, long-term eating habits.
Limitations
The adolescents in this study reported watching an average of 5 h of TV per week. This statistic is low compared with national survey data reporting up to 4 h of TV viewing a day (Rideout et al. 2010) and suggests that the present study may be underpowered in correlating reward cue-reactivity with TV watching (which was reported as non-significant herein). Future studies may aim to include participants that more closely represent the national average in terms of media use and other possible confounding variables (e.g., socioeconomic status).
The present study also utilized percent body fat as a measure of individual adiposity, determined via bioelectric impedance. The validity of this measure has previously been challenged (Talma et al. 2013) and should therefore be interpreted with some caution. However, others have suggested body fat measurements to be superior to BMI when examining individual differences (Ode et al. 2007; Shah and Braverman 2012; Ramel et al. 2013). Given that our findings were largely consistent across both BMI and body fat metrics, we believe that a complete reporting of both measurements is worthwhile while the field resolves these assessment methodologies. In adolescents, it is possible that pubertal status may influence percent body fat measurements, and this was not assessed in the current study. Future studies relating obesity metrics to neural responses may wish to consider alternative strategies for measuring adiposity and accounting for individual variability within this measure.
Funding
This work was supported by the UMass/Dartmouth/Vermont Cancer Centers Collaborative Research Program Grant Initiative, the National Institute on Drug Abuse (grant number R01DA022582), the National Science Foundation Graduate Research Fellowship (grant number DGE-1313911 to K.M.R.) and the William H. Neukom 1964 Institute for Computational Science at Dartmouth College (to J.F.H.).
Notes
The authors thank Courtney Rogers for assistance with recruiting and scanning participants. Conflict of Interest: None declared.
References
- Ahima RS, Lazar MA. 2013. Physiology. The health risk of obesity--better metrics imperative. Science. 341:856–858. [DOI] [PubMed] [Google Scholar]
- Blaak E. 2001. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 4:499–502. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Bruce JM, Ness AR, Lepping RJ, Malley S, Hancock L, Powell J, Patrician TM, Breslin FJ, Martin LE et al. 2014. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity (Silver Spring). 22:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. 2010. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 34:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM, Savage CR. 2012. Changes in brain activation to food pictures after adjustable gastric banding. Surg Obes Relat Dis. 8:602–608. [DOI] [PubMed] [Google Scholar]
- Casey BJ. 2014. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 66:295–319. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. 2010. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. 2011. Functional connectivity of the insula in the resting brain. Neuroimage. 55:8–23. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. 1999. The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol. 76:893–910. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. 2008. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci. 20:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 2003. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 13:500–505. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. 2012. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 32:5549–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Heatherton TF. 2011. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J Cogn Neurosci. 23:1952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembek CR, Harris JL, Schwartz MB. 2013. Rudd Report. Where children and adolescents view food and beverage ads on TV: Exposure by channel and program. Rudd Center for Food Policy & Obesity. [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. 2012. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 58:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR. 2008. Reward-related processing in the human brain: developmental considerations. Dev Psychopathol. 20:1191–1211. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. 2003. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 17:1703–1714. [DOI] [PubMed] [Google Scholar]
- Fischl B. 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. 2005. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond). 29:1–8. [DOI] [PubMed] [Google Scholar]
- Funk M, Lutz K, Hotz-Boendermaker S, Roos M, Summers P, Brugger P, Hepp-Reymond M-C, Kollias SS. 2008. Sensorimotor tongue representation in individuals with unilateral upper limb amelia. Neuroimage. 43:121–127. [DOI] [PubMed] [Google Scholar]
- Gallese V. 1998. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 2:493–501. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. 2013. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci. 9(7):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz J-L, Vallée N, Tropres I, Baciu M, Le Bas J-F, Sato M. 2012. Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp. 33:2306–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. 2004. Effect of television advertisements for foods on food consumption in children. Appetite. 42(2):221–225. [DOI] [PubMed] [Google Scholar]
- Jakab A, Molnár PP, Bogner P, Béres M, Berényi EL. 2012. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. 25:264–271. [DOI] [PubMed] [Google Scholar]
- Jebb SA, Cole TJ, Doman D, Murgatroyd PR, Prentice AM. 2007. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr. 83:115–122. [DOI] [PubMed] [Google Scholar]
- Johnston L. 2002. Behavioral mimicry and stigmatization. Soc Cogn. 20:18–35. [Google Scholar]
- Kerr KL, Avery JA, Barcalow JC, Moseman SE, Bodurka J, Bellgowan PSF, Simmons WK. 2014. Trait impulsivity is related to ventral ACC and amygdala activity during primary reward anticipation. Soc Cogn Affect Neurosci. 10(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton J-L, Fogassi L, Gallese V. 2004. A touching sight. Neuron. 42:335–346. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. 2010. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 107:14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. 2002. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 297:846–848. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 12:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. 2014. Neural predictors of giving in to temptation in daily life. Psychol Sci. 25:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. 2008. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 42:285–295 (accessed 18 January 2014). Available from: URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2556067&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Fotenos AF, Csernansky JG, Morris JC, Buckner RL. 2010. Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J Cogn Neurosci. 22:2677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, Van Essen DC. 2011. Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. 2010. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring). 18:254–260. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, Wing RR. 2009. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 90:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure AC, Tanski SE, Gilbert-Diamond D, Adachi-Mejia AM, Li Z, Li Z, Sargent JD. 2013. Receptivity to television fast-food restaurant marketing and obesity among U.S. Youth Am J Prev Med. 45:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, Weller RE. 2012. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 59:2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. 2012. Health, United States, 2011: with special features on socioeconomic status and health. Department of Health and Human Services. [PubMed] [Google Scholar]
- Ode JJ, Pivarnik JM, Reeves MJ, Knous JL. 2007. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc. 39:403–409. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K. 2002. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 12:376–385. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL et al. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel A, Halldorsson TI, Tryggvadottir EA, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. 2013. Relationship between BMI and body fatness in three European countries. Eur J Clin Nutr. 67:254–258. [DOI] [PubMed] [Google Scholar]
- Rideout VJ, Foehr UG, Roberts DF. 2010. Generation M2: media in the lives of 8- to 18-year-olds. Henry J. Kaiser Family Foundation.
- Rosner B, Prineas R, Loggie J, Daniels SR. 1998. Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr. 132:211–222. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. 2007. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 37:410–421. [DOI] [PubMed] [Google Scholar]
- Shah NR, Braverman ER. 2012. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, Martin A. 2013. The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain Struct Funct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Avery JA, Hall KD, Martin A. 2013. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. 16:1551–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. 2011. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 31:4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. 2008. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 41:636–647. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Miyamoto T, Terao A, Yokoyama A. 2007. Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience. 145:791–794. [DOI] [PubMed] [Google Scholar]
- Talma H, Chinapaw MJM, Bakker B, HiraSing RA, Terwee CB, Altenburg TM. 2013. Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes Rev. 14:895–905. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. 2012. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 22:2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN. 2011. Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp. 32:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. 2009. Puzzlingly high correlations in fmri studies of emotion, personality, and social cognition. Perspect Psychol Sci. 4:274–290. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. 2013. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychol Sci. 24:2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Dal Cin S, Sargent JD, Kelley WM, Heatherton TF. 2011. Spontaneous action representation in smokers when watching movie characters smoke. J Neurosci. 31:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Felder C, Fowler JS, Levy AV, Pappas NR, Wong CT, Zhu W, Netusil N. 2002. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport. 13:1151–1155. [DOI] [PubMed] [Google Scholar]
- Widhalm K, Schönegger K, Huemer C, Auterith A. 2001. Does the BMI reflect body fat in obese children and adolescents? A study using the TOBEC method. Int J Obes Relat Metab Disord. 25:279–285. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. 2012. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond). 36:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]