Abstract
A subanesthetic dose of the noncompetitive N-methyl-d-aspartate receptor antagonist ketamine is known to induce a schizophrenia-like phenotype in humans and nonhuman primates alike. The transient behavioral changes mimic the positive, negative, and cognitive symptoms of the disease but the neural mechanisms behind these changes are poorly understood. A growing body of evidence indicates that the cognitive control processes associated with prefrontal cortex (PFC) regions relies on groups of neurons synchronizing at narrow-band frequencies measurable in the local field potential (LFP). Here, we recorded LFPs from the caudo-lateral PFC of 2 macaque monkeys performing an antisaccade task, which requires the suppression of an automatic saccade toward a stimulus and the initiation of a goal-directed saccade in the opposite direction. Preketamine injection activity showed significant differences in a narrow 20–30 Hz beta frequency band between correct and error trials in the postsaccade response epoch. Ketamine significantly impaired the animals' performance and was associated with a loss of the differences in outcome-specific beta-band power. Instead, we observed a large increase in high-gamma-band activity. Our results suggest that the PFC employs beta-band synchronization to prepare for top–down cognitive control of saccades and the monitoring of task outcome.
Keywords: beta-band, gamma-band, ketamine, local field potential, performance monitoring, prefrontal cortex
Introduction
Schizophrenia is a debilitating neuropsychiatric disease affecting nearly 1% of the world's population, yet its underlying neural mechanisms remain poorly understood. An old theory (Wernicke 1906) that has gained traction in recent years is the notion that schizophrenia is not caused by focal brain alterations but by a dysconnectivity in communication between brain regions (Friston 1998; Phillips and Silverstein 2003; Stephan et al. 2006).
Recent functional connectivity studies have shown that the acute phases of the illness are associated with dysconnectivity of the prefrontal cortex (PFC) (Anticevic et al. 2015), a key region for cognitive function in primates (Miller and Cohen 2001). Similar changes in functional connectivity were observed in healthy volunteers after subanesthetics doses of the noncompetitive N-methyl-d-aspartate (NMDA) glutamate receptor antagonist, ketamine, which also induces a transient schizophrenia-like behavioral phenotype (Krystal et al. 1994; Adler et al. 1999).
Findings from electroencephalogram (EEG) recordings in schizophrenia patients support the hypothesis of a dysconnectivity in communication between brain regions, which is known to be mediated by neural oscillations (Salinas and Sejnowski 2000, 2001; Tiesinga and Sejnowski 2004; Womelsdorf et al. 2014). The most prominent findings are an increase in gamma-band EEG activity (>30 Hz) (Barr et al. 2010; Sun et al. 2011) and a decrease in beta-band EEG activity (15–30 Hz) (Krishnan et al. 2005; Uhlhaas et al. 2006; Hirano et al. 2008).
While EEG recordings in nonhuman primates have shown that subanaesthetics doses of ketamine reduce mismatch-negative and P3a event-related potentials (Gil-da-Costa et al. 2013), it is unknown whether ketamine also alters oscillatory potentials in the PFC. Here we investigated the effects of a subanesthetic dose of ketamine on outcome-related local field potentials (LFPs) in the macaque PFC during an antisaccade task, which requires subjects to suppress a saccade towards a flashed peripheral stimulus instead to generate a saccade to the opposite direction (Munoz and Everling 2004). Poor antisaccade task performance is a potential biomarker for schizophrenia (Crawford et al. 1998; Hutton and Ettinger 2006) and has also been found in nonhuman primates following ketamine administration (Condy et al. 2005; Skoblenick and Everling 2012, 2014).
Materials and Methods
Animals
The experiments were performed in accordance with the Canadian Council of Animal Care policy on the use of laboratory animals and all procedures were approved by the Animal Use Subcommittee of the University Western Ontario Council on Animal Care. For this study, 2 male rhesus monkeys (Macaca mulatta) weighing 5 kg (Monkey T) and 7 kg (Monkey O) performed the behavioral tasks. The animals were implanted with a recording chamber located above their lateral PFC and a plastic head restraint, as previously described (Johnston and Everling 2006). Postsurgical treatments included analgesics, prophylactic antibiotics, and oversight by the university veterinarian. Following surgical recovery, animals had cranial MR imaging to obtain anatomical localization of the recording chambers (Fig. 1B).
Figure 1.
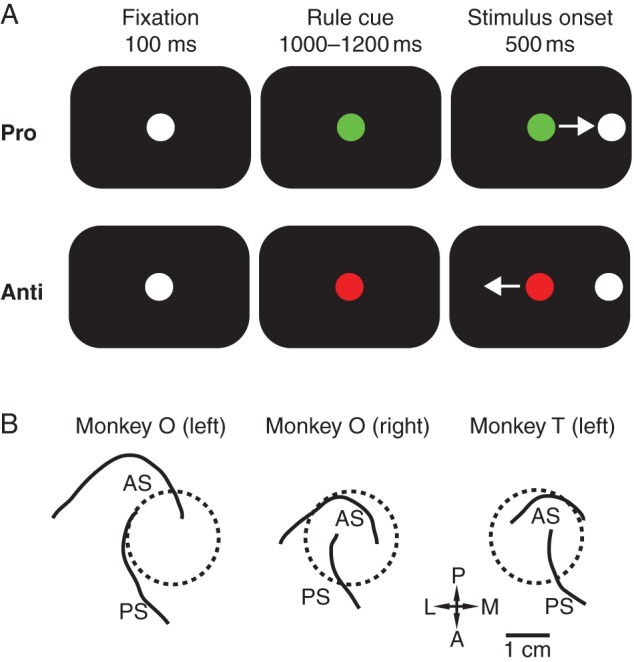
Task and recording locations. (A) Organization of antisaccade task. Monkey O was trained with a green rule cue indicating a prosaccade trial and a red rule cue indicating an antisaccade trial. The colors were inversed for Monkey T. After a correct saccade, the animal was rewarded and the screen was blanked for 500 ms before the fixation cue appeared again. (B) Reconstruction of chamber placements. Monkey O had bilateral recording chamber implantation and had 2 sessions recorded from the right hemisphere and 3 sessions recorded from the left hemisphere. Monkey T had all 3 sessions recorded from his unilateral left hemisphere chamber. as, arcuate sulcus; ps, principal sulcus; P, posterior; A, anterior; L, lateral; M, medial.
Behavioral Task
Both animals were trained to perform the antisaccade task to receive a liquid reward, as described previously by our laboratory (Johnston and Everling 2011; Skoblenick and Everling 2012). Briefly, the animal was enclosed in a light and sound-attenuated chamber with their head restrained and oriented toward a computer monitor displaying the task. Each trial began with a fixation stimulus, which was replaced with a rule cue after fixation for 100 ms. Monkey O was trained that a green-colored rule cue indicated a prosaccade task and a red-colored cue indicated an antisaccade task. The color rules were reversed for training Monkey T. After a randomized 1000–1200 ms instruction period, a white, circular, peripheral stimulus appeared either 8° to the left or right of the central fixation cue. Prosaccade trials required the animal look at this stimulus, while antisaccade trials required the animal to look toward a blank location on the screen diametrically opposite to the stimulus' position. Following correct trials, a liquid reward was delivered to the monkey's mouth through a sipping tube immediate after a saccade to the correct target location. Eye gazes in any direction other than toward or away from the stimuli terminated the trial immediately. Following the completion of a trial, the screen was blanked and a new trial began after a 500-ms intertrial interval (Fig. 1A). Trials were presented in a randomized order such that pro- and antisaccade trials occurred equally as often. Owing to the minimal amount of erroneous prosaccade trials in the preketamine condition, analyses between correct and error trials were performed exclusively on the antisaccade condition. The animal's eye position was recorded and digitized at either 120 Hz using an ISCAN infrared pupillary tracking system for Monkey O (ISCAN, Woburn, MA, USA) or at 1000 Hz using an Eyelink 1000 infrared pupillary tracking system for both Monkey O and Monkey T (SR Research, Mississauga, ON, Canada).
Recording
The setup for multielectrode recording sessions began a day before the experimental session with the installation of the semichronic multielectrode grid (Neuronitek, London, ON, Canada) into the recording chamber. The 36 tungsten electrodes (FHC, Bowdoin, ME, USA) were lowered through a silicone membrane and into the monkey's lateral PFC, providing 32 recording channels and 4 reference channels. Each electrode was lowered manually using a micro-screwdriver until background activity was observed on a maximal number of recording channels. The animal was returned to its cage until the next day so that the electrodes had time to settle in the cortical tissue. On an experimental session day, the animal was returned to the sound-attenuated experimental chamber and the electrode grid was connected to a head stage and amplifying unit. Neuronal spiking activity and LFP activity from each channel were combined with performance and eye-tracking data in a multiacquisition processor (MAP) system (Plexon, Dallas, TX, USA) and sorted offline using 2D and 3D principal component analysis. Subsequent recording days only required reconnecting the head stage and a minimal adjustment to each electrode's depth before spiking neuronal activity was observed. The multielectrode grid was left implanted for 2 weeks, after which it was removed for cleaning and sterilization of the recording chamber. The LFP data were recorded simultaneously with single unit activity that was analyzed and reported separately (Skoblenick and Everling 2014).
Drug Administration
Each session began with a 15-min preinjection period, during which the animal performed 200–250 trials with accompanying PFC recordings. The animals both made on average <15% errors on both pro- and antisaccade trials. At the 15 min mark, the experimental paradigm was paused briefly and the animal was given a 0.4 mg/kg intramuscular injection of ketamine diluted in saline to 0.4 mL into their right triceps brachii muscle. The experimental booth was closed and the experimental task resumed. In total, the injection process introduced less than a 20-s pause and the animal began performing the task again immediately. Both animals had their dose titrated between 0.2 and 1.0 mg/kg to optimize a dose that elicited a behavioral deficit but had no appreciable anesthetic effect (Condy et al. 2005). The animals' behavior continued at baseline performance levels for approximately 5 min before the behavioral effects of the ketamine injection became apparent. Sessions would then continue for another 45 min to monitor the effects of ketamine. Control experiments with injections of saline did not produce any significant changes between pre- and postinjection periods in the animals' behavioral performance or neuronal activity. Experimental sessions with ketamine were spaced at least 3 days apart to ensure there were no cumulative effects of the drug or changes to the preinjection baseline activity (Pouget et al. 2010).
Data Analysis
Electrophysiological data were analyzed using custom scripts for Matlab (Mathworks) that made use of the FieldTrip toolbox (http://fieldtrip.fcdonders.nl/) developed at the Donders Institute for Brain, Cognition and Behaviour (Oostenveld et al. 2011). For LFP analysis, the continuous analog signal was divided into discrete trials using event time markers provided by the Plexon MAP system. Data were filtered with a low-pass filter at 150 Hz and line noise was removed at 60 and 120 Hz using a discrete Fourier transform (see (Womelsdorf et al. 2006)). Z-score thresholding, and independent component analysis were used to detect and discard any additional mechanical artifacts in the analog signal. To remove the reward artifact, the data were first downsampled to 1/100th the sampling frequency after which component analysis was run to manually identify those components that contained the artifacts. The component analysis was then reperformed on the original data without downsampling to remove the artifact components from the final dataset (Supplementary Fig. 1). To determine time-locked LFP power, frequency analysis was performed using the multitaper method with a discrete prolate spheroidal sequence taper set around a 0.667-s window every 50 ms for the low-frequency range (1–60 Hz) and a 0.33-s window every 50 ms for the high-frequency range (40–150 Hz). The LFP data for each trial were normalized to the oscillatory activity on the same channel during the preceding intertrial interval (500-ms preceding fixation onset) resulting in a Z-score that could be compared between channels, sessions, and animals. There was no significant difference between the pre- and postketamine baseline power spectrums (Supplementary Fig. 2). Statistical analysis on the resulting time–frequency–LFP power maps used a nonparametric cluster-based analysis that created a T-value map for the significance level between 2 conditions and highlighted time–frequency epochs with statistical significance and corrected for multiple comparisons across time and frequency bins (Maris and Oostenveld 2007; Womelsdorf et al. 2010). Frequency data were divided into 0.33-Hz bins, while time data were measure in 0.1-s bins. The bootstrapping analyses were performed against 10 000 permutations of the data to create the significance maps. Neuronal spiking activity was analyzed as previously described (Skoblenick and Everling 2012) and analyses into the signal-to-noise ratio differences between correct and error conditions are previously discussed in Skoblenick and Everling (2014).
Results
Data were collected in the dorsolateral PFC (Fig. 1B) during 8 testing sessions (5 with Monkey O and 3 with Monkey T) yielding 158 LFP channels and 215 neurons for analysis. Channels without usable signals were removed from the analysis. Behavioral effects for this dataset have been described previously (Skoblenick and Everling 2014). We limited the analysis to antisaccade trials, as the animals made no or very few errors on prosaccade trials before ketamine as previously reported (Skoblenick and Everling 2014).
Prefrontal Cortex Exhibits Outcome-Dependent Beta-Band LFP Activity
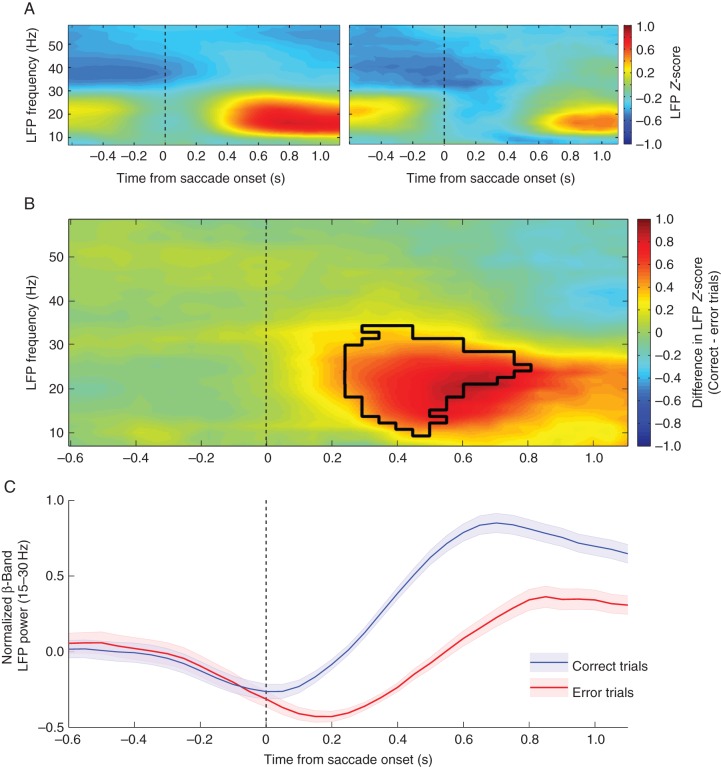
To examine how the beta-band range of LFP activity may be involved in a task requiring explicit cognitive control, we recorded the LFP signal in 2 monkeys. After lower frequency analysis was performed (1–60 Hz), a clear time–frequency epoch emerged with band-limited power modulation following the animal's saccade response to the trial stimulus. The response occurred most strongly between 15 and 30 Hz, corresponding to beta-band oscillations. Furthermore, this response appeared to be outcome-specific, showing a stronger effect following trials in which the animal made a correct response (Fig. 2). After the effect was visualized the statistical strength of these findings were tested with a cluster-based analysis (see Materials and Methods). The resulting T-score map highlighted a cluster between 0.3 and 0.75 s after saccade onset that showed a significant difference (P < 0.05, multiple comparison corrected) in the LFP activation between correct and error trials. To better illustrate the evolution of this difference, the mean LFP power of the relevant oscillatory range (15–30 Hz) was plotted as mean ± SEM over the course of the trial (Fig. 2B).
Figure 2.
Performance-selective differences in beta-band LFP power. (A) Heatmaps displaying the trial time from saccade onset (x-axis) and frequencies (y-axis) for correct (left) and error (right) responses. The color bar indicates the mean LFP z-score value for trials with that outcome. (B) The larger heatmap displays the difference between the mean activity following a correct trial versus an error trial. The color bar indicates the z-score value obtained from (correct trials – error trials) baseline normalized z-score. Positive values indicate stronger activity during correct trials while negative values show time–frequency points with stronger activity for error trials. The black outline indicates the region in which statistical significance was found via cluster-based analysis. (C) Mean Z-score ± SEM for the beta-band frequency range (15–30 Hz) aligned on saccade onset for correct (blue) and error (red) trials.
Ketamine Reduces Performance Selectivity of Beta-Band LFP Activity
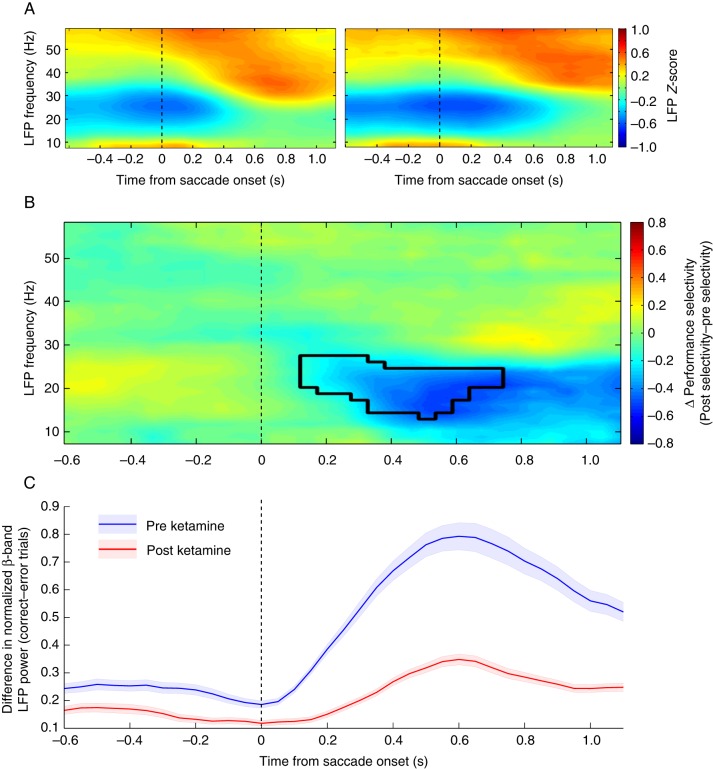
After observing the selectivity in the beta-band response following task completion, we next analyzed how these changes were affected by the administration of a subanesthetic dose of ketamine. Figure 3 shows that ketamine decreased performance selectivity in the beta-band such that no significant difference was found between correct and error trials in the beta-band (Fig. 3A). The overall beta-band activity was decreased (preketamine mean Z-score: 0.132 ± 0.267, postketamine mean Z-score: −0.278 ± 0.205, P < 0.001) to the detriment of the correct-selective epoch beginning 200 ms after saccade onset. A nonsignificant increase in activity in the 40-Hz range was also noted after ketamine administration, and this 40 Hz activity showed no selectivity between correct and error trials. The differences in (correct – error) LFP power were compared for statistical significance and the cluster-based analysis once again found a time–frequency epoch significantly different (P < 0.05, multiple comparison corrected) between pre- and postketamine conditions (Fig. 3B). The evolution of this change in selectivity over the course of the trial was also plotted as mean power (15–30 Hz) ± SEM to illustrate how pre- and postketamine conditions differed (Fig. 3C). Power spectrograms also highlight the beta-band selectivity before ketamine administration and the changes within this range caused by drug administration (Supplementary Fig. 3).
Figure 3.
Difference in performance selectivity following ketamine administration. (A) Heatmaps for correct (left) and error (right) responses following ketamine administration with trial time from saccade onset (x-axis) and frequencies (y-axis). The color bar indicates the mean LFP z-score for the corresponding trial outcome. (B) Heatmap displays the trial time from saccade onset (x-axis) and frequencies (y-axis) with the color bar indicating the difference in absolute selectivity calculated as (absolute difference between correct and error trials after ketamine) – (absolute difference between correct and error trials before ketamine). Thus, a positive value indicates a time–frequency area in which the difference between correct and error trials was greater after ketamine injection, whereas a negative value indicates the performance selectivity was greater before ketamine. The epoch found to be statistically significant through cluster-based analyses is outlined in black. (C) Mean difference between correct and error trials ± SEM in the beta-band range (15–30 Hz) is plotted for both preketamine values (blue) and postketamine values (red).
No Significant Gamma-Band Selectivity
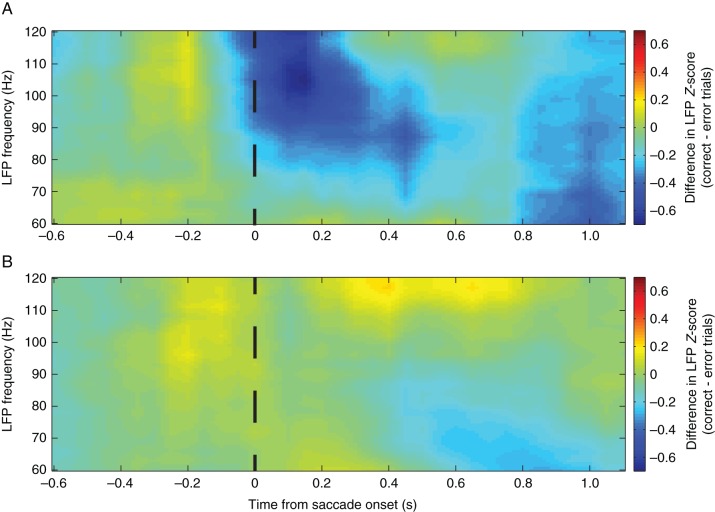
With the gamma-band of oscillatory activity also implicated in schizophrenia, we next examined how the higher frequencies responded to the task before and after ketamine administration. Difference maps were generated between (correct – error) task outcomes and a clearly defined error selectivity before the administration of ketamine was visible; however, cluster-based comparisons between the correct and error heatmaps did not reach significance in any relevant areas (Fig. 4). Overall mean gamma power (40–120 Hz) during the entire trial (0.6 s before stimulus onset to 1.0 s after stimulus onset) was significantly increased following ketamine administration (preketamine mean Z-score = −0.1584 ± 0.3521 postketamine mean Z-score = 0.8535 ± 0.5539, P < 0.000001 Student's t-test).
Figure 4.
Outcome-specific heat maps for gamma-band frequencies. The mean difference between correct and error trials for preketamine trials (A) and postketamine trials (B) are displayed, with positive values indicating stronger activity for correct trials and negative values showing stronger activity for error trials. No significance was found through the cluster-based analyses for the preketamine selectivity map; however, overall gamma-band activity was significant increased between preketamine and postketamine values.
Gamma-Band Activity Follows Neural Spiking Patterns
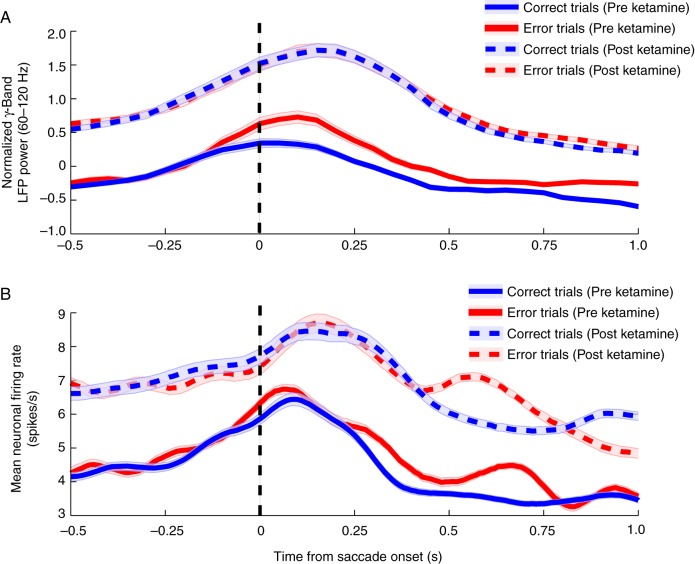
Lastly, we found that the temporal evolution of gamma-band LFP activation (Fig. 5A) was similar to the temporal evolution of neural spiking activity (Fig. 5B) independent of ketamine administration. By comparing the mean LFP power in the high-gamma range (60–120 Hz) across the duration of a trial to the mean firing rates, we found correlations between these 2 measurements. Indeed, preketamine high-gamma LFP power follows a very similar pattern of evolution and peak time to the average spiking activity of neurons in the PFC. Further validation of these parallels can be observed postketamine. Significant correlations of LFP power with the mean neuronal spiking rate were evident in the preketamine trials (correct trials: r = 0.58, P < 0.001; error trials: r = 0.84, P < 0.0001) as well as in the postketamine trials (correct trials: r = 0.37, P < 0.05; error trials: r = 0.87, P < 0.0001).
Figure 5.
Gamma-band LFP power and corresponding neuronal firing rates. (A) Mean high-gamma-band (60–120 Hz) activity ± SEM is plotted contrasting both preketamine (solid lines) correct trials (blue) and error trials (red) with postketamine (dashed lines) values. The pattern of activity is notably similar to the mean spiking activity simultaneously measured from neurons in the PFC (B) in both their peak activity epoch and their response to ketamine administration.
Discussion
This experiment set out to determine if the LFP events observed during tasks that challenge a subject's cognitive control over behavior may also be measured in the antisaccade task. Furthermore, we wanted to test if these events are altered by ketamine in a manner similar to the aberrant oscillatory activity found in human patients with schizophrenia (Uhlhaas and Singer 2010, 2013). The most prominent findings emerged in the beta frequency range (15–30 Hz) and were sensitive to the outcome of the trial. The PFC in conjunction with the anterior cingulate cortex has been shown to have an important role in performance monitoring during tasks requiring the cognitive suppression of automatic responses (MacDonald et al. 2000; Swick and Turken 2002; Ridderinkhof et al. 2004; Walton et al. 2004; Rothe et al. 2011; Shen et al. 2015). Our data show that the beta-band may be involved in this feedback monitoring, as the timing occurs only after the animal's response has been made and is dependent on whether or not the trial was performed correctly.
Alternatively, the beta-band range of oscillations has been demonstrated in other brain regions to have a very strong impact on motor control (Androulidakis et al. 2006; Swann et al. 2009). Increased beta activity decreases the ability of a subject to initiate a new movement or to disengage from a current action (Gilbertson et al. 2005; Pogosyan et al. 2009). It has also been shown in human EEG studies to increase in tasks during which a muscle must maintain a specific action against an unpredictable amount of force (Androulidakis et al. 2007). Thus, the beta activity we observe in the PFC may reflect the coordination with the saccade system to hold the eye's position at the correct location. This maintenance at a correct location has been hypothesized to allow the organism to gain more information and increase attention to a desirable outcome or to process the stimuli that resulted in a reward (Engel and Fries 2010; Buschman et al. 2012; Salazar et al. 2012; Womelsdorf et al. 2014). Incorrect trials, with their decreased beta-band activity, are associated with a quicker disengagement from the peripheral stimulus possibly due to its unrewarding properties.
The introduction of ketamine to this pathway appears to disrupt the outcome-specific signal causing the animals to no longer display beta-band activity that is selective for correct trials. The beta signal is elicited in the same epoch for both correct and error trials, indicating that the animal either does not recognize the trial was erroneous or that the PFC is no longer able to send outcome-specific efferent signals to downstream locations. These results corroborate our previous findings with regard to a change in the animal's postresponse reaction time (Skoblenick and Everling 2014). In those studies, we discovered that in the preketamine state, the animal's gaze would remain fixed on the target location longer after making a correct pro- or antisaccade when compared with error trials. After ketamine, the animal's gaze lingered much longer on the stimulus for an incorrect antisaccade trial, similar to their postresponse reaction after making a correct prosaccade.
This study showed that after ketamine administration, the beta-band response was similar for both correct and error trials with an overall decrease relative to baseline. This supports the hypothesis that ketamine creates an unstable motor state manifested as a decreased in beta-band power, just as increased beta-band power reflects the focused maintenance of a motor state such as ocular fixation at a target location (Engel and Fries 2010; Womelsdorf et al. 2014). Other studies of the PFC have shown the region's importance in response suppression (Narayanan et al. 2006, 2013). The overall depressed beta-band state in the PFC following ketamine administration may manifest as an inability to engage a preparatory set or motor state, leading to increased response times and indiscriminant response selection (Stoet and Snyder 2006).
Evidence for this beta-band influence on behavior has also been demonstrated in hypo-dopaminergic states such as Parkinson's disease (Limousin et al. 1995; Brown 2003). It is suspect that the increased beta-band activity observed in Parkinson's patients may contribute to the decreased ability to initiate motor movements based on internal or cognitive cues (Kuhn et al. 2004, 2008; Chen et al. 2007). This epoch-specific increase in beta-band activity may also correspond with task-specific prefrontal hypo-dopaminergic states observed in schizophrenia (Dworkin and Opler 1992; Abi-Dargham and Moore 2003; Stone et al. 2007). Additionally, we found that the beta power was decreased, which corresponds both with increased PFC dopamine release caused by ketamine (Lorrain et al. 2003) and the resting-state beta power decrease in schizophrenia (Krishnan et al. 2005; Uhlhaas et al. 2006). Combining these findings reinforces the critical interplay between the glutamatergic and dopaminergic systems in schizophrenia and its associated animal models.
Recently, ketamine has shown promising results for the relief of treatment-resistant major depressive disorder (Aan Het Rot et al. 2012). These effects may be also be modulated through ketamine's influence on LFPs, as deep brain stimulation experiments show that an increase in gamma-band activity correlate with an amelioration of depression-like symptoms, similar to ketamine's effect on gamma-band activity (Gazit et al. 2015).
Other psychoactive drugs have also been found in to produce changes in the LFPs of the rodent PFC. Exposure to the cannabinoid receptor analog WIN during adolescence has produced changes in the PFC GABAergic transmission and beta-band oscillatory activity (Cass et al. 2014), while phencyclidine can disrupt the delta-band activity in this region (Kargieman et al. 2007). Additionally, multiple classes of psychoactive drugs can increase the gamma-band activity in this region while disrupting the spike-field coherence (Wood et al. 2012).
Gamma-band oscillations have been studied extensively in schizophrenia for many years as well but have been debated whether they are increased or decreased in the disease (Kocsis et al. 2013). NMDA antagonism in humans (Hong et al. 2010) and animal models of schizophrenia (Pinault 2008; Kocsis 2012; Moran et al. 2015) have demonstrated an increase in gamma-band activity, whereas human schizophrenia studies showed a decrease in gamma-band signal (Gandal et al. 2012). It is only recently that upon revisiting the human schizophrenia data, 2 trends emerged: there appears to be an overall increase in gamma activity resulting in more background noise and thus a relatively decreased signal (Kikuchi et al. 2011; Spencer 2011; Suazo et al. 2014); and that antipsychotic medications decrease the gamma-band activity (Jones et al. 2012; Anderson et al. 2014).
Our study found for the first time that there was indeed an overall increase in gamma-band activity following ketamine administration in nonhuman primates. This increase mirrored the increase in spiking activity that we had previously described (Skoblenick and Everling 2012). The increased spiking activity was found to be reducing the signal-to-noise ratio of task-specific neurons in the PFC because of their increased activity due to ketamine administration (Jackson et al. 2004). It may be that the increase in gamma-band activity we are observing in the primate PFC may be another manifestation of this increase in background noise and a loss in an additional dimension of information conveyance. Furthermore, these similarities lend credence to the idea that oscillatory activity in this high-gamma range may be reflective of the net spiking activity of nearby neurons in the macaque brain (Ray et al. 2008; Whittingstall and Logothetis 2009). Additional investigation is required to support this idea but this opens the possibility to measure gamma-band power as a proxy measurement for spiking activity.
These findings show that ketamine reduces band-limited activation component in the beta and enhances the gamma frequency range. Whether this performance-related outcome signal is specific to the antisaccade task cannot be unequivocally ruled out in our study because of the use of a single task. However, we speculate that the outcome-specific signals in the PFC that we found to be modified and almost abolished with ketamine are linked to the behavioral decline and failure to adjust performance during the task. Future studies will identify whether the NMDA-mediated outcome evaluation signals are important to adjust behavior across different cognitive demanding tasks.
In summary, beta-band activity appears to play an important role in the PFC for determining how to respond to correct and erroneous trial outcomes. This performance-sensitive frequency and time-locked pattern is vulnerable to disruption in the ketamine model of schizophrenia. The NMDA antagonist removes differentiation between correct and error trial outcomes following saccade responses. In addition, ketamine also increased the overall activity in the gamma-band range, potentially reflecting decreased cognitive control in the PFC and similar increases in background noise as observed in the neuronal spiking activity. These results further validate the neural similarities between humans with schizophrenia and the ketamine model of schizophrenia in nonhuman primates.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This research was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Ontario Mental Health Foundation to S.E. and a CIHR Canadian graduate scholarship to K.J.S.
Supplementary Material
Notes
We are grateful to J. Gati for help in MR imaging. Conflict of Interest: None declared.
References
- Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ. 2012. Ketamine for depression: where do we go from here? Biol Psychiatry. 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Moore H. 2003. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 9:404–416. [DOI] [PubMed] [Google Scholar]
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. 1999. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 156:1646–1649. [DOI] [PubMed] [Google Scholar]
- Anderson PM, Pinault D, O'Brien TJ, Jones NC. 2014. Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in cortical gamma oscillations. Int J Neuropsychopharmacol. 17:1895–1904. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Gilbertson TP, Brown P. 2006. Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. Eur J Neurosci. 24:3299–3304. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Yarrow K, Litvak V, Gilbertson TP, Brown P. 2007. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur J Neurosci. 25:3758–3765. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT et al. 2015. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 77:569–580. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Tran LC, Chen R, Fitzgerald PB, Daskalakis ZJ. 2010. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 121:146–152. [DOI] [PubMed] [Google Scholar]
- Brown P. 2003. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 18:357–363. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. 2012. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 76:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. 2014. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry. 19:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Litvak V, Gilbertson T, Kuhn A, Lu CS, Lee ST, Tsai CH, Tisch S, Limousin P, Hariz M et al. 2007. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson's disease. Exp Neurol. 205:214–221. [DOI] [PubMed] [Google Scholar]
- Condy C, Wattiez N, Rivaud-Pechoux S, Gaymard B. 2005. Ketamine-induced distractibility: an oculomotor study in monkeys. Biol Psychiatry. 57:366–372. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. 1998. Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. Am J Psychiatry. 155:1703–1710. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Opler LA. 1992. Simple schizophrenia, negative symptoms, and prefrontal hypodopaminergia. Am J Psychiatry. 149:1284–1285. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 20:156–165. [DOI] [PubMed] [Google Scholar]
- Friston KJ. 1998. The disconnection hypothesis. Schizophr Res. 30:115–125. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. 2012. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 62:1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit T, Friedman A, Lax E, Samuel M, Zahut R, Katz M, Abraham L, Tischler H, Teicher M, Yadid G. 2015. Programmed deep brain stimulation synchronizes VTA gamma band field potential and alleviates depressive-like behavior in rats. Neuropharmacology. 91:135–141. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD. 2013. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci USA. 110:15425–15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. 2005. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 25:7771–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, Kanba S, Onitsuka T. 2008. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 28:4897–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, Lahti AC. 2010. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 35:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. 2006. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 43:302–313. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. 2004. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 101:8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Everling S. 2011. An approach to understanding the neural circuitry of saccade control in the cerebral cortex using antidromic identification in the awake behaving Macaque Monkey Model. In: Lane EL, Dunnett SB, editors. Animal models of movement disorders. New York (NY): Humana Press; p. 161–181. [Google Scholar]
- Johnston K, Everling S. 2006. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci. 26:12471–12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Reddy M, Anderson P, Salzberg MR, O'Brien TJ, Pinault D. 2012. Acute administration of typical and atypical antipsychotics reduces EEG gamma power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in gamma power. Int J Neuropsychopharmacol. 15:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. 2007. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci USA. 104:14843–14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Hashimoto T, Nagasawa T, Hirosawa T, Minabe Y, Yoshimura M, Strik W, Dierks T, Koenig T. 2011. Frontal areas contribute to reduced global coordination of resting-state gamma activities in drug-naive patients with schizophrenia. Schizophr Res. 130:187–194. [DOI] [PubMed] [Google Scholar]
- Kocsis B. 2012. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 71:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Brown RE, McCarley RW, Hajos M. 2013. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther. 19:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O'Donnell BF. 2005. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 116:614–624. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 51:199–214. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI et al. 2008. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 28:6165–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. 2004. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 127:735–746. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. 1995. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 345:91–95. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. 2003. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 117:697–706. [DOI] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 288:1835–1838. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 164:177–190. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ. 2015. Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology. 40:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 5:218–228. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. 2013. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 16:1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. 2006. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 139:865–876. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. 2003. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 26:65–82; discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Pinault D. 2008. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 63:730–735. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. 2009. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 19:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget P, Wattiez N, Rivaud-Pechoux S, Gaymard B. 2010. Rapid development of tolerance to sub-anaesthetic dose of ketamine: an oculomotor study in macaque monkeys. Psychopharmacology (Berl). 209:313–318. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. 2008. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 28:11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. 2004. The role of the medial frontal cortex in cognitive control. Science. 306:443–447. [DOI] [PubMed] [Google Scholar]
- Rothe M, Quilodran R, Sallet J, Procyk E. 2011. Coordination of high gamma activity in anterior cingulate and lateral prefrontal cortical areas during adaptation. J Neurosci. 31:11110–11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RF, Dotson NM, Bressler SL, Gray CM. 2012. Content-specific fronto-parietal synchronization during visual working memory. Science. 338:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. 2001. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. 2000. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J Neurosci. 20:6193–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Ardid S, Kaping D, Westendorff S, Everling S, Womelsdorf T. 2015. Anterior cingulate cortex cells identify process-specific errors of attentional control prior to transient prefrontal-cingulate inhibition. Cereb Cortex. 25:2213–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoblenick K, Everling S. 2012. NMDA antagonist ketamine reduces task selectivity in macaque dorsolateral prefrontal neurons and impairs performance of randomly interleaved prosaccades and antisaccades. J Neurosci. 32:12018–12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoblenick K, Everling S. 2014. N-methyl-d-aspartate receptor antagonist ketamine impairs action-monitoring activity in the prefrontal cortex. J Cogn Neurosci. 26:577–592. [DOI] [PubMed] [Google Scholar]
- Spencer KM. 2011. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 5:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. 2006. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 59:929–939. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. 2006. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 31:1675–1681. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. 2007. Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol. 21:440–452. [DOI] [PubMed] [Google Scholar]
- Suazo V, Diez A, Montes C, Molina V. 2014. Structural correlates of cognitive deficit and elevated gamma noise power in schizophrenia. Psychiatry Clin Neurosci. 68:206–215. [DOI] [PubMed] [Google Scholar]
- Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, Daskalakis ZJ. 2011. Gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 1413:98–114. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. 2009. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 29:12675–12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Turken AU. 2002. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 99:16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga PH, Sejnowski TJ. 2004. Rapid temporal modulation of synchrony by competition in cortical interneuron networks. Neural Comput. 16:251–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. 2006. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 26:8168–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 11:100–113. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2013. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci. 15:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. 2004. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 7:1259–1265. [DOI] [PubMed] [Google Scholar]
- Wernicke C. 1906. Grundrisse der Psychiatrie. Leipzig, Germany: Thieme. [Google Scholar]
- Whittingstall K, Logothetis NK. 2009. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron. 64:281–289. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. 2006. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 439:733–736. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. 2010. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci USA. 107:5248–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P. 2014. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nat Neurosci. 17:1031–1039. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. 2012. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 32:3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.