Abstract
Posterior parietal cortex (PPC) of prosimian galagos includes a rostral portion (PPCr) where electrical stimulation evokes different classes of complex movements from different subregions, and a caudal portion (PPCc) where such stimulation fails to evoke movements in anesthetized preparations ( Stepniewska, Fang et al. 2009). We placed tracer injections into PPCc to reveal patterns of its cortical connections. There were widespread connections within PPCc as well as connections with PPCr and extrastriate visual areas, including V2 and V3. Weaker connections were with dorsal premotor cortex, and the frontal eye field. The connections of different parts of PPCc with visual areas were roughly retinotopic such that injections to dorsal PPCc labeled more neurons in the dorsal portions of visual areas, representing lower visual quadrant, and injections to ventral PPCc labeled more neurons in ventral portions of these visual areas, representing the upper visual quadrant. We conclude that much of the PPCc contains a crude representation of the contralateral visual hemifield, with inputs largely, but not exclusively, from higher-order visual areas that are considered part of the dorsal visuomotor processing stream. As in galagos, the caudal half of PPC was likely visual in early primates, with the rostral PPC half mediating sensorimotor functions.
Keywords: behavior, intraparietal cortex, motor areas, somatosensory cortex, visual areas
Introduction
The posterior parietal cortex (PPC) of primates, located between anterior parietal somatosensory areas and visual areas of temporal and occipital cortex, plays an important role in planning and producing goal-directed movements. In order to relate parts of the body to objects in space, PPC integrates sensory information from visual, somatosensory, vestibular, and auditory modalities (Hyvarinen 1982; Andersen et al. 1997; Cohen and Andersen 2002; Sereno and Huang 2014). The visual contribution to PPC comes from a number of visual areas of the dorsal stream that are especially involved in processing information about visual motion; for example, areas of the middle temporal (MT) region, MT-complex (e.g., Felleman and Kaas 1984; Kaas and Morel 1993), dorsomedial visual area, DM (e.g., Baker et al. 1981; Lui et al. 2006; Kaskan et al. 2010), and early visual areas, V2 and V3 (e.g., Kaskan et al. 2009). As PPC is a key region of the dorsal stream of sensory processing for action, there has been a great deal of interest in its functional organization, mostly in macaque monkeys (e.g., Andersen and Buneo 2002; Orban et al. 2006), but also in humans (e.g., Shikata et al. 2008; Hinkley et al. 2009; for review, see Grefkes and Fink 2005). Evidence from macaque monkeys suggests that PPC is subdivided into a number of parallel circuits devoted to different actions, such as the parietal reach region, PRR, for reaching (Batista et al. 1999), the lateral intraparietal area, LIP, for directing gaze toward a site of interest (Colby et al. 1996), the anterior intraparietal area, AIP, for grasping and manipulating objects (Sakata et al. 1995), and the ventral intraparietal area, VIP, for defensive behavior (Cooke et al. 2003).
Importantly for the present study, our microstimulation studies in galagos (Stepniewska et al. 2005; Stepniewska, Fang et al. 2009) and New World monkeys (Gharbawie et al. 2011a) have shown the existence of a number of functionally distinct regions where electrical stimulation evokes different types of complex actions such as reaching, grasping, or defensive movements. Subdivisions of rostral, but not caudal PPC (PPCr and PPCc, respectively), can be identified by the different movement patterns they produce when stimulated with long (0.5 s) trains of electrical pulses. These PPCr subdivisions integrate different combinations of inputs from visual and higher-order somatosensory and auditory areas, to create outputs to premotor (PM) and primary motor (M1) areas of frontal cortex (Stepniewska, Cerkevich et al. 2009). While somatosensory and auditory inputs reach PPCr directly, visual information to PPCr is sent mostly via connections with PPCc, as connections from visual areas seem to be largely restricted to the caudal PPC region (Krubitzer and Kaas 1993; Beck and Kaas 1998a,1998b; Collins et al. 2001; Fang et al. 2005), and PPCc is interconnected with the PPCr. However, little is known about functional organization of PPCc in prosimian galagos or New World monkeys. In our experiments, electrical stimulation fails to elicit movements in this caudal PPC region. It is presently uncertain if PPCc has a number of functional divisions, as does PPCr, each with different patterns of connections with dorsal stream visual areas, or few functional divisions, and few dominant patterns of cortical connections. Thus, determining the anatomical connections of PPCc with PPCr, motor, as well as occipital and temporal visual cortical areas is critical to understanding the neural mechanisms responsible for the production of appropriate motor behaviors based on visual information.
To study PPC connections, we chose the prosimian galagos, which are considered to most closely resemble early primates (Kaas et al. 2011). The galago brain is relatively small given its body size when compared with anthropoid primates (Jerison 1973), but the presence and arrangement of cortical areas reflects a typical primate pattern (Fig. 1A). Moreover, galago brains have just a few shallow fissures (Jerison 1973), so cortical areas including PPC are mostly exposed on the brain surface. Thus, cortical areas are easily accessible for electrical stimulation and tracer injections. Results indicating the functional organization and cortical connections of the rostral half of PPC in galagos have been previously published (Stepniewska, Fang et al. 2009; Stepniewska, Cerkevich et al. 2009; see also Fang et al. 2005). Here we focused on the connections of the caudal (visual) half of PPC. By studying connections of particular parts of PPCc with visual areas of known retinotopic organization (e.g., V2, V3, DM, or MT), we gained information about the large-scale functional organization of PPC. Overall, the present results, in conjunction with those obtained previously from PPCr (Stepniewska, Fang et al. 2009; Stepniewska, Cerkevich et al. 2009) and relevant results from other primates, provide insights into the organization of PPC in early primates. Preliminary results concerning caudal PPC connections have been published in an abstract form (Stepniewska et al. 2010; see also Fang et al. 2005).
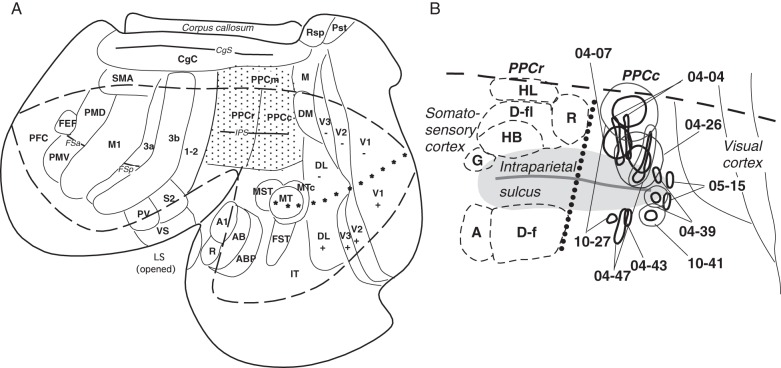
Figure 1.
The location of injection sites in a standardized view of PPC. (A) The location of PPC (shaded with dots) shown in relation to other cortical areas in the left hemisphere of a galago (Otolemur garnetti) brain. Motor areas are based on Wu et al. (2000) and Fang et al. (2005), somatosensory areas are from Wu and Kaas. (2003), visual areas are from Lyon and Kaas (2002a), and auditory areas are from Kaas and Hackett (2000). Cortical areas are marked as follows. M1, primary motor cortex; PMD and PMV, dorsal and ventral premotor areas, SMA, supplementary motor area; FEF, frontal eye field; S1 and S2, primary and secondary somatosensory areas; 1-2, somatosensory areas 1 and 2; PV, ventral somatosensory area; V1, primary visual area; V2, second visual area; V3, third visual area; DL, dorsolateral visual area; DM, dorsomedial visual areas; M, medial area; MT, middle temporal area; MTc, middle temporal crescent area; MST, middle superior temporal area; FST, fundal superior temporal area; Rsp, retrosplenial cortex; Pst, area prostriata; IT, inferotemporal area; A1, primary auditory cortex; R, rostral primary auditory area; AB, auditory belt. Sulci are marked as follows. LS,– lateral sulcus; IPS, intraparietal sulcus; STS, superior temporal sulcus; FSa, frontal anterior sulcus; FSp, frontal posterior sulcus. The horizontal meridian is marked with small stars. The cortical region around the intraparietal sulcus is magnified in B. (B) The location of injection sites outlined by thick (core of injection) and thin (halo of injection) lines in the caudal half of PPC in all studied galagos. The border between responsive to ICMS rostral PPC (PPCr) and nonresponsive to ICMS caudal PPC (PPCc) is marked by dotted line. A total of 13 injections were made in the nonresponsive to microstimulation PPCc. Six injections were made in dorsal PPCc and 5 injections were made in ventral PPCc. Two small injections were made at the caudal tip of IPS (case 05-15), and they were not included in quantification of results (see Fig. 6). In PPCr note the approximate locations of complex movement domains outlined with dashed lines. Above IPS, G, grasp; HB, hand to body; D-fl, forelimb defensive; R, reach; HL, hindlimb. Below IPS, A, face aggressive; D-f, face defensive.
Materials and Methods
Nine adult galagos (Otolemur garnetti) of both sexes were used in this study (Table 1). In most of these animals, we used intracortical microstimulation (ICMS) with long (500 ms) trains of electrical pulses to find the border between responsive and unresponsive PPC sectors as well as to delineate functional zones in the PPCr (for more details, see Stepniewska, Fang et al. 2009). All experiments were carried out according to guidelines of the National Institutes of Health Guide for the care and use of laboratory animals and were approved by the Vanderbilt University Animal Care and Use Committee.
Table 1.
Location of tracer injections in experimental cases
| Case # | PPC caudal |
PPC rostral | ICMS results | |
|---|---|---|---|---|
| Dorsal | Ventral | |||
| 04-04 | FE | – | FR, BDA | + |
| 10-41 | – | DY | FE | – |
| 04-39 | BDA | FB | FR, FE | + |
| 04-47 | FR | BDA | FE | – |
| 10-27a | DY | FR | BDA | + |
| 04-07a | CTB | – | FR, FE | + |
| 04-26a | FB | – | BDA, FR | – |
| 04-43 | – | BDA | – | – |
| 05-15 | FR, BDA | WGA-HRP,FE | + | |
aIn these cases connections of rostral and caudal PPC were compared.
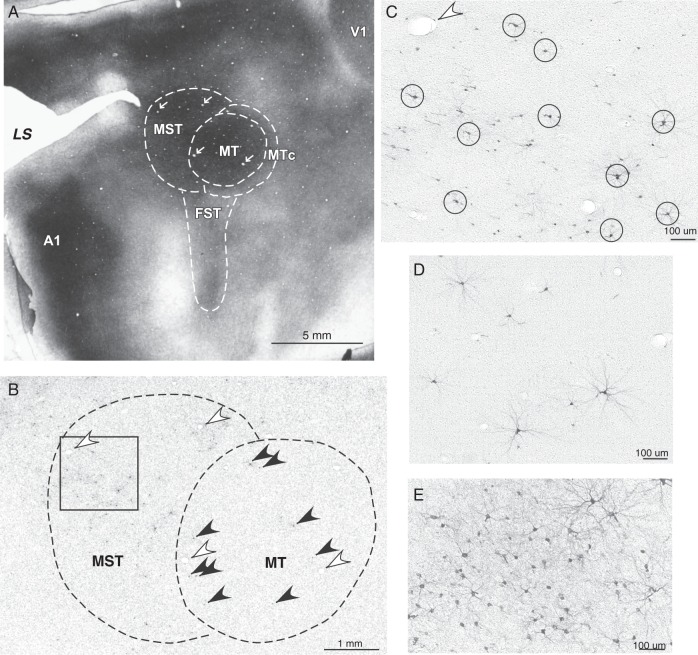
Surgeries were performed under aseptic conditions as described in Stepniewska, Fang et al. 2009. After exposing portions of the left hemisphere, we stimulated the cortex around the intraparietal sulcus (IPS) with microelctrodes to identify the posterior border of responsive to stimulation region of PPC as well as its cortical zones engaged in different motor behaviors. Then we placed injections of different tracers (wheat germ agglutinin conjugated to horseradish peroxidase, WGA-HRP, biotinylated dextran amine, BDA, cholera toxin subunit B, CTB, fluororuby, FR, fluoroemerald, FE, fast blue, FB and diamidino yellow, DY) into various locations in caudal PPC that were unresponsive to stimulation, and in different functional zones within rostral PPC. After the injections were made, the cortex was covered with gelfilm, the skull closed with a cap made of dental acrylic, and the skin sutured. Animals received antibiotics and analgesics and were carefully monitored during recovery from anesthesia. After 5–12 days, some of the galagos were anesthetized for further ICMS mapping, followed by a lethal dose of pentobarbital. When a-reflexive, they were perfused through the heart. Other galagos with previous injections of tracer did not receive additional microstimulation mapping, and after the tracer transport time, were directly anesthetized with a lethal dose of pentobarbital and perfused. The brain was first perfused with phosphate buffered saline, followed by 2–4% paraformaldehyde, and then 2% paraformaldehyde with 10% sucrose (see Stepniewska, Fang et al. 2009). The brain was removed, and the cortex was separated from the brainstem (except case 05-15), manually flattened (see Stepniewska et al. 2005) and stored overnight in 30% sucrose in phosphate buffer at 4°C. The flattened cortical tissue was cut parallel to the surface at 40–50 μm. In case 05-15, the whole brain was cut in the coronal plane to reveal the laminar distribution of labeled cortical neurons. A series of unstained sections was mounted on slides for the examination of neurons labeled with fluorescent markers. The other series of sections were processed to reveal neurons labeled with other tracers [e.g., WGA-HRP (Gibson et al. 1984); BDA (Veenman et al. 1992); CTB (Bruce and Grofova 1992)] or cortical architecture [cytochrome oxidase, CO (Wong-Riley 1979); myelinated fibers (Gallyas 1979, Fig. 2A)] and then mounted. Labeled neurons were plotted at resolution 16× or 20× with a Leitz microscope coupled to an X, Y encoder and a Macintosh computer running Igor Pro Software (Igor Pro 3.14, Wave Metrics, Inc.). In few instances, when it was necessary to better identify weakly labeled cells, we used higher resolution (40×). Cells labeled with DY and FB were visualized with fluorescence illumination passed through a 360-nm wavelength filter. FR-labeled cells were visualized with a 530- to 560-nm wavelength filter, and FE-labeled cells with a 495–520-nm filter. BDA and CTB-labeled cells were visualized under bright-field, and WGA-HRP-labeled cells were visualized under dark-field illumination. In cases with BDA injections, in addition to labeled neurons (Fig. 2C–E), the regions containing labeled terminals were marked as well. For each case with flattened cortex, the injection sites and label were drawn from 3–5 individual sections representing different cortical layers. In the case cut coronally (05-15), the whole series of coronal sections was drawn. Individual drawings were then aligned using blood vessels and other anatomical landmarks for local alignment (Fig. 2A,B), and a surface view of the cortex with labeled neurons was reconstructed. Patterns of connections were related to the microstimulation maps of motor regions (see Stepniewska, Fang et al. 2009), and to architectonic boundaries of cortical areas that were revealed in adjacent brain sections processed for myelinated fibers or cytochrome oxidase. The boundaries of frontal motor areas M1, PMD, and PMV (see Table 2 for abbreviations), as well as rostral PPC were based on microelectrode stimulation experiments (for more details see Fang et al. 2005 and Stepniewska, Fang et al. 2009). The primary somatosensory (S1), auditory (A1) and visual (V1) areas, as well as MT and middle superior temporal (MST) visual areas, were distinguished by their dense CO and myelin staining (see Fig. 2A). The locations and boundaries of other cortical areas were estimated using previously established relationships to well defined cortical areas. The architecture of PPC was quite uniform in myelin and CO preparations, and did not reflect the physiological or connectional differences between the PPCr (responsive to ICMS) and PPCc (unresponsive to ICMS) evident in our study. Reconstructions of surface-view distributions of labeled neurons relative to cortical areas were created using Canvas v8.0 software installed on an iMac computer. In all cases, we quantified the number of labeled cells in all identified cortical areas, and calculated their density for each area. The output from Igor Pro was digitized and imported into Matlab. Because Igor Pro identifies and labels cell position with a circular identifying mark, the number of labeled cells in each region of interest (shown in Table 3) was calculated using a custom routine based on the circular Hough transform (Atherton and Kerbyson 1999). The circular Hough transform is one of the more common and robust techniques designed to identify and count the number of circles/dots within an image.
Figure 2.
Photomicrographs of brain sections cut parallel to the surface of flattened cortex in the middle temporal region in case 04-39. (A) Section stained for myelinated fibers with dark MT and MST, and lighter FST. The proposed boundaries of these areas and MTc are indicated. Primary auditory cortex (A1) and visual cortex (V1) are also densely myelinated. (B) Section through MT and MST processed for BDA with inset magnified in C. Note single BDA-labeled neurons (black arrows) in MT. C shows neurons labeled in MST; some of the largest and darkly labeled neurons are circled. White arrows in A, B mark corresponding blood vessels. D, E show sparse and dense distributions of labeled neurons in MST and dorsal V2, respectively.
Table 2.
Abbreviations
| A1, primary auditory area |
| AB, auditory belt |
| ABP, auditory parabelt |
| AIP, anterior intraparietal area |
| BDA, biotinylated dextran amine |
| CgC, cingulate cortex |
| CgS, cingulate sulcus |
| CTB, cholera toxin, subunit B |
| DL, dorsolateral visual area |
| DM, dorsomedial visual area |
| DY, diamidino yellow |
| FB, fast blue |
| FE, fluoroemerld |
| FEF, frontal eye field |
| FR, fluororuby |
| FSa, frontal sulcus, anterior |
| FSp, frontal sulcus, posterior |
| FST, fundal superior temporal visual area |
| ICMS, intracortical microstimulation |
| IPS, intraparietal sulcus |
| IT, inferotemporal cortex |
| ITc, inferotemporal cortex, caudal |
| LS, lateral sulcus |
| M, medial area |
| M1, primary motor cortex |
| MST, middle superior temporal visual area |
| MT, middle temporal visual area |
| MTc, middle temporal area, crescent |
| PFC, prefrontal cortex |
| PM, premotor cortex |
| PMD, dorsal premotor area |
| PMDc, dorsal premotor area, caudal |
| PMDr, dorsal premotor area, rostral |
| PMV, ventral premotor area |
| PPC, posterior parietal cortex |
| PPCc, posterior parietal cortex, caudal |
| PPCm, posterior parietal cortex, medial |
| PPCr, posterior parietal cortex, rostral |
| Pst, area prostriata |
| PV, ventral parietal area |
| R, rostral primary auditory area |
| Rsp, retrosplenial cortex |
| SMA, supplementary motor area |
| ST, superior temporal area |
| STS, superior temporal sulcus |
| S1 (or 3b), primary somatosensory area |
| S2, secondary somatosensory area |
| V1, primary visual area |
| VS, ventral somatosensory area |
| V2, second visual area |
| V2d, second visual area, dorsal |
| V2v, second visual area, ventral |
| V3, third visual area |
| V3d, third visual area, dorsal |
| V3v, third visual area, ventral |
| WGA-HRP, wheat germ agglutinin conjugated to horseradish peroxidase |
| 1-2, somatosensory areas 1 and 2 |
| 3a, somatosensory area 3a |
| 3b (or S1), primary somatosensory area |
Table 3.
Distribution of cells labeled in cortical areas after tracer injections into dorsal and ventral PPCc
| Dorsal injections |
Ventral injections |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 04-07 | 04-04 | 04-26 | 04-39 | 04-47 | 10-27 | 04-39 | 04-43 | 04-47 | 10-27 | 10-41 | |
| CTB | FE | FB | BDA | FR | DY | FB | BDA | BDA | FR | DY | |
| 10 482 | 2154 | 6815 | 6853 | 5646 | 6897 | 2424 | 3605 | 3416 | 600 | 4698 | |
| PFC | 285 | 12 | – | 1 | 2 | 62 | – | 1 | 1 | – | 20 |
| FEF | 133 | 8 | 3 | 16 | 12 | 58 | – | – | 5 | 6 | 44 |
| PMD | 297 | – | – | 2 | 50 | 159 | – | – | 55 | – | 34 |
| PMV | 99 | – | 1 | – | 1 | 52 | – | – | 3 | – | – |
| M1 | 2 | – | – | 1 | 1 | 5 | 1 | – | 12 | – | – |
| 3a | 21 | – | – | – | – | – | – | – | 3 | – | – |
| 3b | 38 | 1 | – | – | 2 | – | – | 1 | 3 | – | – |
| 1-2 | 42 | – | – | – | 2 | – | – | 3 | 6 | – | – |
| S2-PV | – | – | – | – | 5 | – | 1 | 2 | 1 | – | – |
| CgC | 500 | 46 | 396 | 408 | 191 | 1084 | 1 | 48 | 29 | 31 | 163 |
| PPCr | 1594 | 11 | 692 | 527 | 217 | 689 | 8 | 710 | 290 | 7 | 127 |
| PPCc | 3587 | 1479 | 2712 | 2262 | 2345 | 2694 | 1143 | 873 | 1796 | 416 | 2553 |
| PPCm | 1385 | 28 | 325 | 328 | 104 | 529 | – | 40 | 15 | 15 | – |
| MST | 57 | 61 | 131 | 194 | 220 | – | 5 | 320 | 102 | 3 | 1 |
| MT | 4 | 48 | 36 | 37 | 13 | – | 11 | 51 | 79 | 14 | 11 |
| FST | 41 | – | 3 | 27 | 7 | 12 | 23 | 40 | 28 | – | 33 |
| DM | 225 | 18 | 552 | 393 | 298 | 371 | 30 | 62 | 124 | 23 | 290 |
| M | 97 | 2 | 157 | 269 | 22 | 277 | – | – | 9 | 6 | – |
| DL | 592 | 186 | 81 | 471 | 595 | 24 | 403 | 425 | 403 | 23 | 690 |
| V3d | 300 | 46 | 691 | 477 | 500 | 373 | 10 | 2 | 41 | 10 | 2 |
| V3v | 6 | – | 24 | 33 | 2 | 6 | 29 | 147 | 13 | 2 | 7 |
| V2d | 378 | 187 | 696 | 614 | 997 | 82 | 8 | – | 40 | 6 | – |
| V2v | 5 | – | 19 | 61 | 8 | – | 16 | 242 | 121 | 2 | 8 |
| V1 | 30 | 2 | 7 | 30 | 3 | – | 14 | 5 | 22 | – | 1 |
| ST | 702 | 14 | 197 | 168 | 12 | 1 | 27 | 198 | 76 | – | 87 |
| Rsp | 9 | 1 | 2 | 433 | 11 | 194 | – | – | 2 | 5 | – |
| ITc | 52 | 4 | 90 | 101 | 26 | 225 | 694 | 435 | 137 | 32 | 627 |
Note: The upper rows sequentially list case number of each galago, tracer injected, and total number of cells labeled in the areas from each injection. The cortical areas are listed in the first column, and successive columns contain the number of cells labeled in each area. The densest concentration of labeled cells for each injection is listed in bold, and 2 other regions with the next highest numbers of labeled neurons are underlined. ITc might involve cortex of ventral wall; Rsp, retrosplenial cortex + area prostriata; ST, cortex between MST-FST and A1 (might include belt and parabelt auditory regions).
Results
The posterior parietal region (PPC) in galagos involves cortex on the dorsolateral surface around the horizontally running IPS, with some of the cortex buried in the sulcus and some extending onto the medial wall (Fig. 1). In our previous study, we defined the pattern of cortical connections of the anterior half of PPC, which when microstimulated produced complex meaningful movements (Stepniewska, Fang et al. 2009; Stepniewska, Cerkevich et al. 2009). The PPC sites associated with the same movement were grouped together forming functional domains characterized by a specific movement. Each domain had a distinct pattern of cortical connections. A hand-to-mouth movements domain in anterior PPCr was connected mostly to the forelimb representation in motor (PM, M1), and somatosensory areas (3a, 1-2 and areas in the lateral sulcus [LS]). The more posterior defensive and reaching domains had additional connections with nonprimary visual areas (V2, V3, DL, DM, MST). These differences in connections identify parts of cortical networks that mediate different motor behaviors.
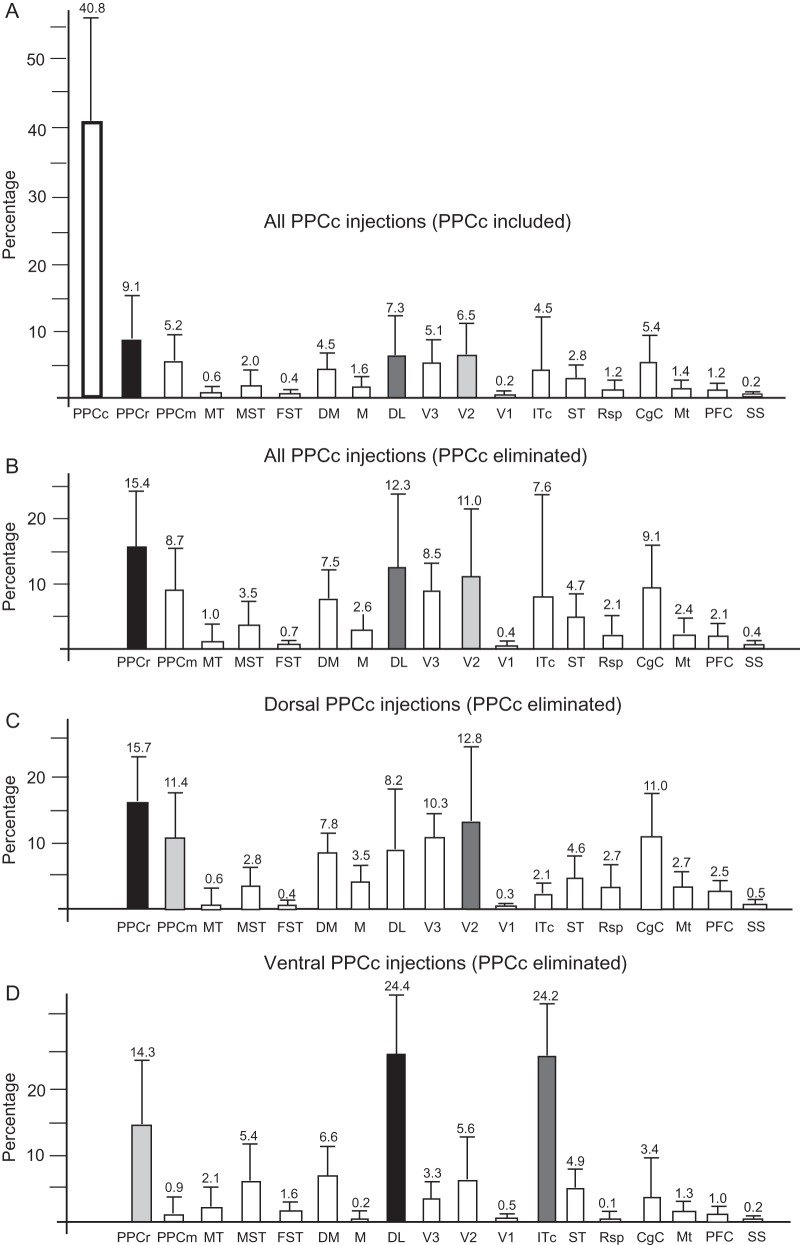
In the present study, we defined the patterns of ipsilateral cortical connections of the caudal portion of PPC (PPCc), in which ICMS fails to elicit a movement in anesthetized animals, and we compared this pattern with the pattern of connections of movement-related PPCr. Results presented here are based on 13 tracer injections to PPCc and 13 tracer injections into movement domains in PPCr in 9 galagos (Table 1). The locations of PPCc injections in a standard view of the PPC are indicated in Figure 1B. Six injections were placed dorsal to the IPS, and 5 were placed ventral to IPS, just below the sulcus. Two small injections were placed at the very caudal tip of the IPS. The placement of these injections and the resulting distributions of labeled neurons (and axon terminals) in ipsilateral cortex of individual cases are shown in a dorsal view of the flattened cortex in Figures 3–5 and 7–9. Here we provide a description of the patterns of cortical connections of the dorsal and ventral regions of PPCc, and then we compare these patterns with patterns of PPCr connections. In all cases with dorsal and ventral injections most of the labeled neurons (on average ∼40%, see Fig. 6A) were found in PPCc regions surrounding the injection site. In each case, the percentage of cells labeled in other areas was calculated after labeled neurons in PPCc were excluded (see Table 4), and the proportions of labeled neurons shown throughout Results (see below) refer to those values.
Figure 3.
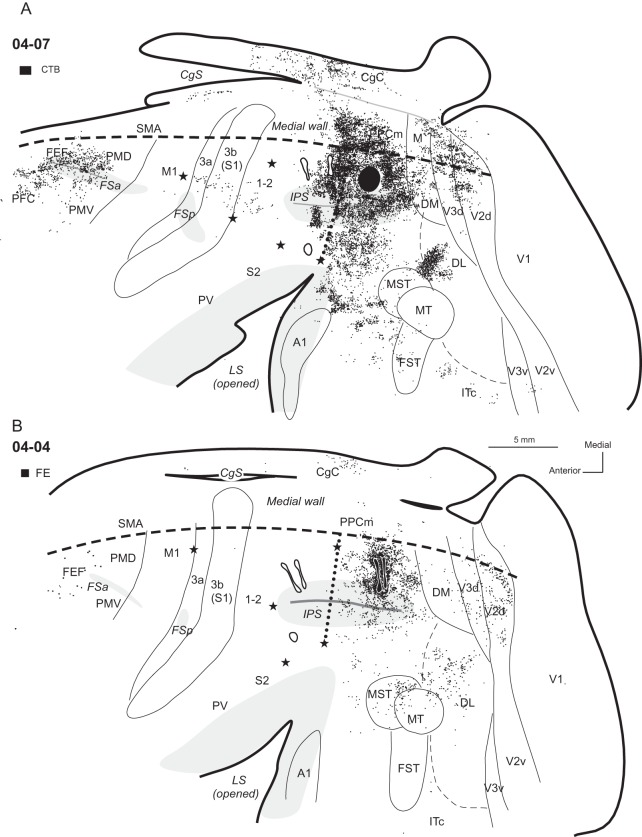
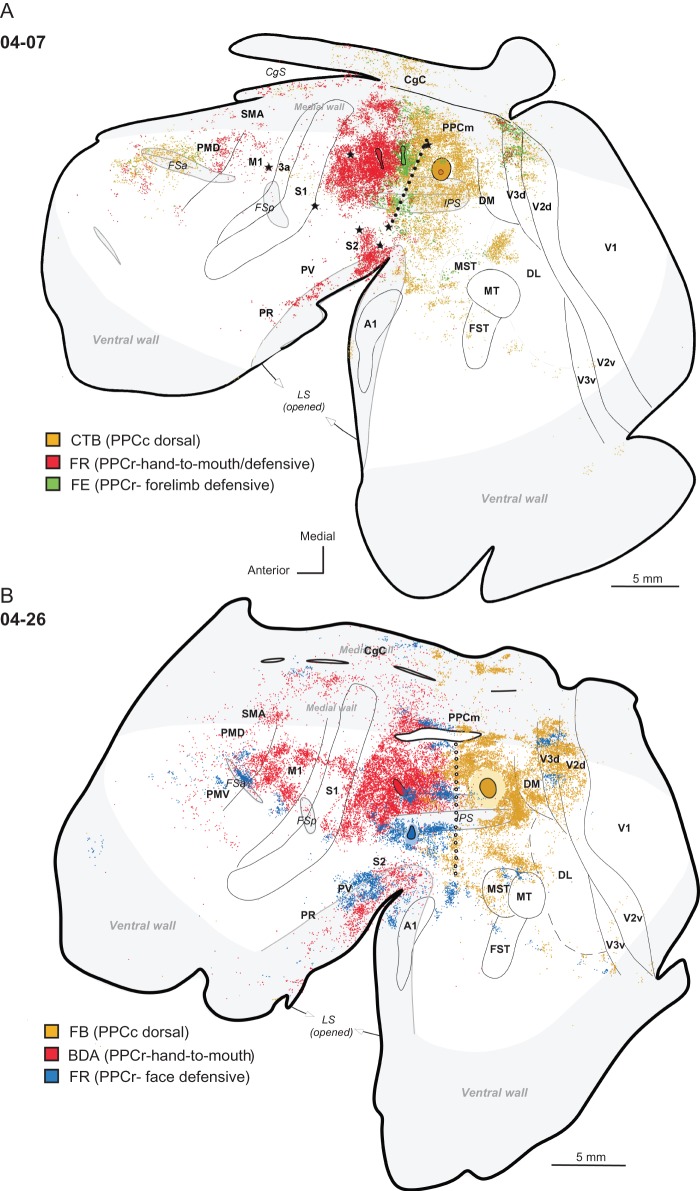
Distributions of labeled neurons after injections (solid black shapes) in the dorsal part of PPCc, shown on an outline of flattened cortex in cases (A) 04-07 and (B) 04-04. Dots indicate the locations of labeled cells. Each dot represents one labeled cell. The locations of tracer injections in the rostral PPC (outlined in black) are also marked for reference. For comparisons of PPCc and PPCr connectivity patterns see Figures 10 and 11. Thin lines indicate the approximate borders of areas defined by physiological mapping and patterns of CO and myelination. Thick lines outline the section or indicate breaks in the section due to sulci. Opened sulci are marked with light gray. Stars mark electrolytic lesions. For the detailed microstimulation maps of these cases see Figure 2A,B in Stepniewska, Fang et al. (2009). The thick dashed lines mark borders between dorsolateral and medial aspects of the hemisphere. Dotted line shows PPCr/PPCc border. CTB injection in case 04-07 and the FE injections in case 04-04 produced similar results, although the label in case 04-07 was much denser. Thus, the densest label was found in the adjoining parts of PPCc and in PPCr, medial PPC (PPCm), and extrastriate visual areas V2, V3, DM, and DL. Less dense label was found in the caudal CgC on the medial wall, MST, FST, MT, and cortex anterior to these areas on the lateral surface of the brain. Frontal and prefrontal areas were also labeled.
Figure 5.
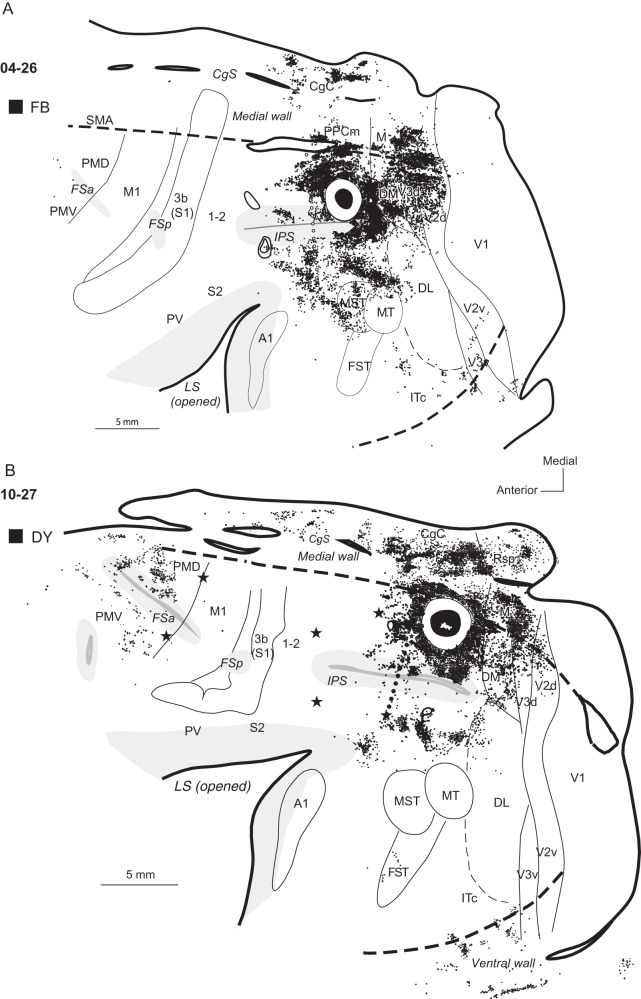
Distributions of neurons labeled after tracer injections in dorsal PPCc in cases (A) 04-26 and (B) 10-27. Most of the neurons labeled by large injections of FB and DY were in extrastriate visual areas of the dorsal half of the hemisphere. Note also the much denser distribution of labeled neurons in the CgC and PMD after a more medial injection in case 10-27 in comparison to case 04-26 with a more lateral injection. Conventions are the same as in Figures 1, 3, and 4.
Figure 7.
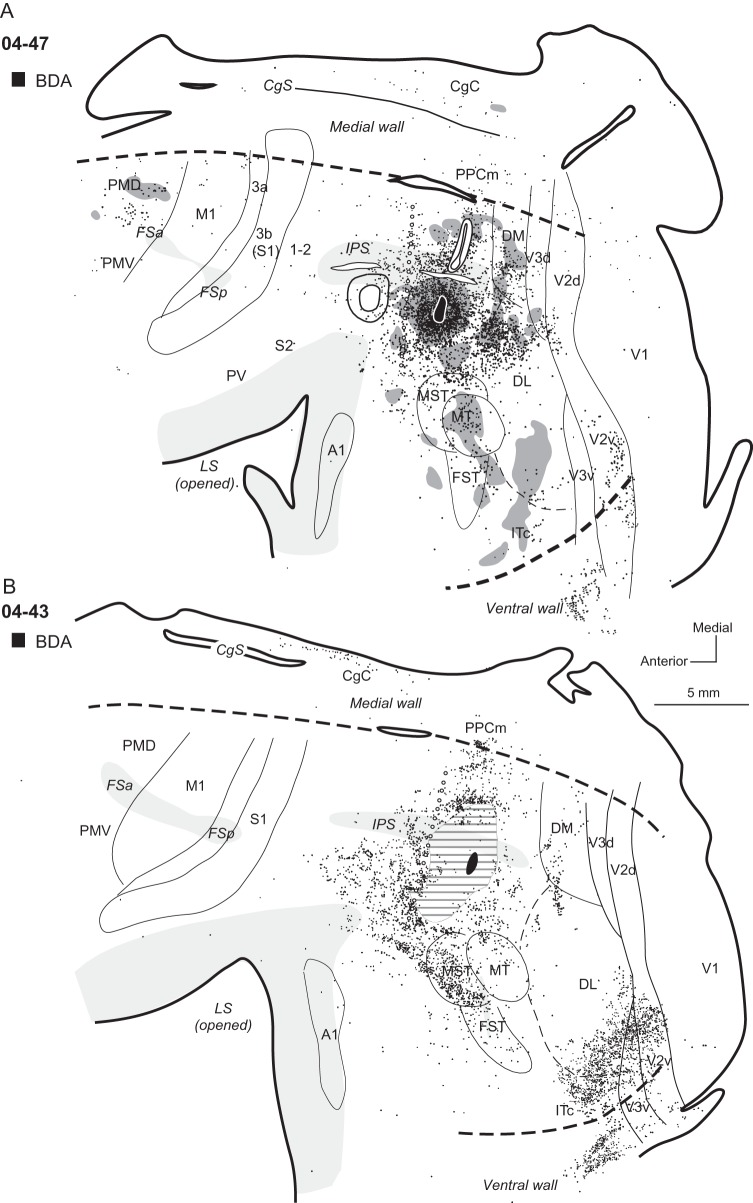
Distributions of labeled neurons after injections in the middle portion of ventral PPCc in cases (A) 04-47 and (B) 04-43. The pattern of horizontal lines around the injection site in (B) marks the region of damaged cortical tissue, which did not allow for accurate location of label. Note that patterns of connections are similar in both cases, but different from cases with dorsal PPCc injections. Thus, the labeled neurons concentrate in PPCc and ventral PPCr, as well as DL, DM, and mostly ventral (not dorsal) parts of V2 and V3. Labeled neurons are also in ITc, and they are much denser in case 04-43. Neurons labeled in premotor cortex are found in case 04-47, but not in case 04-43. In case 04-47, labeled axon terminals are present in premotor cortex and in extrastriate cortical areas, where the terminals mix with labeled neuron cell bodies. Conventions are the same as in Figures 1, 3, and 4.
Figure 9.
Distributions of neurons labeled after tracer injections in cases (A) 04-39 and (B) 10-41. In both cases, tracers were injected in the ventral PPCc. Note that labeled neurons outside PPC are most densely distributed in the visual areas of the lower half of the hemisphere, especially in DL and ITc. Some sparsely distributed labeled neurons were also in areas DM, V2, and V3. In case 10-41 with larger injections, additional patches of labeled neurons were in PMD, FEF, and CgC. Conventions are the same as in Figures 1, 3, and 4.
Figure 6.
Summaries of the relative distributions of labeled cells in cortical areas after PPCc injections (excluding case 05-15). (A) Distribution of labeled cells in all areas after all PPCc injections. (B) Distribution of labeled cells in all cortical areas except PPCc after all injections. (C,D) Distribution of labeled cells in cortical areas except PPCc, after injections in dorsal (C) and ventral (D) PPCc. The mean percentage of labeled cells (number above each column) and corresponding standard errors of the mean were calculated from the distributions presented in Table 3. Black, dark gray, and light gray columns correspond to areas with the highest percentage of labeled cells. The superior temporal (ST) region between auditory cortex and MT complex might include the auditory belt and parabelt. ITc might involve cortex of ventral wall; Mt, motor areas (PMD, PMV, and M1); PFC, prefrontal cortex including FEF; SS, somatosensory areas (3a, 3b, 1-2, S2-PV).
Table 4.
Percentage of cells labeled in cortical areas after PPCc injections (PPCc eliminated)
| Dorsal injections |
Ventral injections |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 04-07 | 04-04 | 04-26 | 04-39 | 04-47 | 10-27 | 04-39 | 04-43 | 04-47 | 10-27 | 10-41 | |
| CTB | FE | FB | BDA | FR | DY | FB | BDA | BDA | FR | DY | |
| 6895 | 675 | 4103 | 4591 | 3301 | 4203 | 1281 | 2732 | 1620 | 184 | 2145 | |
| PFC | 4.1 | 1.7 | – | 0 | 0.1 | 1.5 | – | 0 | 0.1 | – | 0.9 |
| FEF | 1.9 | 1.2 | 0.1 | 0.3 | 0.4 | 1.4 | – | – | 0.3 | 3.3 | 2.1 |
| PMD | 4.3 | – | – | 0 | 1.5 | 3.8 | – | – | 3.4 | – | 1.6 |
| PMV | 1.4 | – | 0 | – | 0 | 1.2 | – | – | 0.2 | – | – |
| M1 | 0 | – | – | 0 | 0 | 0.1 | 0.1 | – | 0.7 | – | – |
| 3a | 0.3 | – | – | – | – | – | – | – | 0.2 | – | – |
| 3b | 0.6 | 0.1 | – | – | 0.1 | – | – | 0 | 0.2 | – | – |
| 1-2 | 0.6 | – | – | – | 0.1 | – | – | 0.1 | 0.4 | – | – |
| S2-PV | – | – | – | – | 0.2 | – | 0.1 | 0.1 | 0.1 | – | – |
| CgC | 7.3 | 6.8 | 9.6 | 8.9 | 5.8 | 25.8 | 0.1 | 1.8 | 1.8 | 16.8 | 7.6 |
| PPCr | 23.1 | 1.6 | 16.9 | 11.5 | 6.6 | 16.4 | 0.6 | 26.0 | 18.0 | 3.8 | 5.9 |
| PPCm | 20.1 | 4.1 | 7.9 | 7.1 | 3.2 | 12.6 | – | 1.5 | 0.9 | 8.2 | – |
| MST | 0.8 | 9.0 | 3.3 | 4.2 | 6.7 | – | 0.4 | 11.7 | 6.3 | 1.1 | 0 |
| MT | 0.1 | 7.1 | 0.9 | 0.8 | 0.4 | – | 0.9 | 1.9 | 4.9 | 7.6 | 0.5 |
| FST | 0.6 | – | – | 0.6 | 0.2 | 0.3 | 1.8 | 1.5 | 1.7 | – | 1.5 |
| DM | 3.3 | 2.7 | 13.5 | 8.6 | 9.0 | 8.8 | 2.3 | 2.3 | 7.7 | 12.5 | 13.5 |
| M | 1.4 | 0.3 | 3.8 | 5.9 | 0.7 | 6.6 | – | – | 0.6 | 3.3 | – |
| DL | 8.6 | 27.5 | 2.0 | 10.3 | 18.0 | 0.6 | 31.5 | 15.6 | 24.9 | 12.5 | 32.2 |
| V3d | 4.4 | 6.8 | 16.8 | 10.4 | 15.1 | 8.9 | 0.8 | 0.1 | 2.5 | 5.4 | 0.1 |
| V3v | 0.1 | – | 0.6 | 0.7 | 0.1 | 0.1 | 2.3 | 4.1 | 0.8 | 1.1 | 0.3 |
| V2d | 5.5 | 27.7 | 17.0 | 13.4 | 30.2 | 2.0 | 0.6 | – | 2.5 | 3.2 | – |
| V2v | 0.1 | – | 0.5 | 1.3 | 0.2 | – | 1.2 | 8.9 | 7.5 | 1.1 | 0.4 |
| V1 | 0.4 | 0.3 | 0.2 | 0.7 | 0.1 | – | 1.1 | 0.2 | 1.4 | – | 0 |
| ST | 10.2 | 2.1 | 4.8 | 3.7 | 0.4 | 0 | 2.1 | 7.2 | 4.7 | – | 4.1 |
| Rsp | 0.1 | 0.1 | 0 | 9.4 | 0.3 | 4.6 | – | – | 0.1 | 2.7 | – |
| ITc | 0.8 | 0.6 | 2.2 | 2.2 | 0.8 | 5.4 | 54.2 | 15.9 | 8.5 | 17.4 | 29.2 |
Note: The upper rows sequentially list case number of each galago, tracer injected, and total number of cells labeled in the areas from each injection. The cortical areas are listed in the first column, and successive columns contain the percentage of cells labeled in each area. The densest concentration of labeled cells for each injection is listed in bold, and the region with the successive highest number of labeled neurons is underlined. 0 marks % of cells below 0.1%. Other conventions as in Table 3.
Connections of Dorsal PPCc
Six injections of tracers were placed in various locations within dorsal PPCc, in cortex above the IPS, and corresponding to the posterior half of area 7d of Preuss and Goldman-Rakic (1991a) (Fig. 1B and Table 1). Most injections were large and involved both dorsolateral (7d-l) and dorsomedial (7d-m) subareas, although in some cases the injection cores were restricted to only one of these subareas (e.g., 7d-l in case 04-39 or 7d-m in case 10-27).
In case 04-07, a CTB injection was placed just caudal to the PPCr/PPCc border, as defined by ICMS (Fig. 3A). The injection core did not involve the cortex in the IPS. This injection densely labeled adjoining parts of the PPCr (23.1% of labeled cells), where electrical stimulation evoked defensive movements (see Stepniewska, Fang et al. 2009). This region of densely packed labeled neurons spread medially to include cortex on the medial wall of the cerebral hemisphere. Medial PPC (PPCm), likely a homolog of area 7m/PGm in macaques contained 20.1% of labeled cells, and cingulate cortex (CgC) contained 7.3% of such cells. The labeled neurons also spread laterally onto both banks of the IPS, as well as onto the ventral PPC below the sulcus. In the cortex below the IPS, label was found in the adjacent PPCr region. Posterior to the injection site, less densely packed labeled cells extended through area DM (3.3%) and medial area (M, 1.4%), dorsal V3 (4.4%), and dorsal V2 (5.5%). In dorsal V3 and V2, labeled neurons were arranged in bands, possibly reflecting the band-like modular organization of these areas (Cusick and Kaas 1988; Collins et al. 2001; Fan et al. 2012). Only a few neurons were labeled in ventral V3 and V2 (total 0.2%) and a small proportion of neurons were labeled in dorsal V1 (0.4%). There was also a large focus of labeled neurons in dorsal DL (8.6%), as well as number of a smaller foci between MT-MST and the LS (ST, 10.2%). These smaller foci were in the relative locations of areas identified in macaques as parts of the auditory belt and possibly parabelt, as well as a polysensory area, STP (Kaas and Hackett 2000). Area MT was almost completely free of label (0.1%), and areas MST and fundal superior temporal (FST) together contained 1.4% of labeled neurons. A few patches of labeled neurons were in caudal inferotemporal cortex (ITc, 0.8%). In the frontal cortex, a distribution of labeled neurons included the frontal eye field (FEF), the rostral part of dorsal premotor cortex (PMD), and ventral premotor cortex, PMV (Wu et al. 2000; Fang et al. 2005), where together 7.6% neurons were labeled. The zone of labeled neurons extended anteriorly into prefrontal cortex (PFC) with 4.1% of labeled neurons. Other motor areas, such as M1 and supplementary motor area (SMA), were free (SMA) or almost free (M1) of label. Somatosensory areas, portions of 3a, 3b (S1), and 1-2 representing the forelimb (Wu and Kaas 2003) contained small amounts of labeled neurons (total 1.5%), but areas S2 or PV in the LS were unlabeled.
A comparable pattern of connections was observed in case 04-04 with 2 FE tracer injections in the dorsal half of PPCc (Fig. 3B), although about 5 times fewer neurons were labeled in this case. The injections were placed a little more caudally in PPC and the core from these 2 smaller and closely positioned injections extended a little more dorsally and ventrally in comparison to the CTB injection in case 04-07. The major focus of labeled neurons in 04-04 covered PPCc surrounding the injection site, and expanded anteriorly toward (but did not include) PPCr. The distribution of labeled neurons spread into the IPS covering both banks of the sulcus, but remained restricted to its caudal half. Some neurons were labeled in medial PPC (PPCm, 4.1%). Posteriorly, the adjacent area DM and especially area M were poorly labeled (2.7 and 0.3%, respectively), but dorsal parts of both V3 and V2 contained numerous labeled neurons (6.8 and 27.7%, respectively). Labeled cells were arranged in stripes spanning the width of these areas, as in case 04-07, but their location in case 04-04 was more lateral than in case 04-07. Only two neurons were labeled in V1. More neurons were labeled in the superior temporal cortex in this case than in case 04-07, and MST contained more labeled neurons (9%) than MT (7.1%) or the cortex between IPS and MST. The dorsal part of DL was labeled as well (27.5%). CgC contained 2 small groups of labeled neurons, with the caudal group containing more cells than the rostral group (together 6.8%). The frontal areas PMD and FEF contained much less labeled neurons in case 04-04 (1.2%) than in case 04-07 (6.2%). No labeled cells were found in M1, SMA, or in somatosensory areas within the LS, and anterior parietal cortex contained few labeled cells (0.1%).
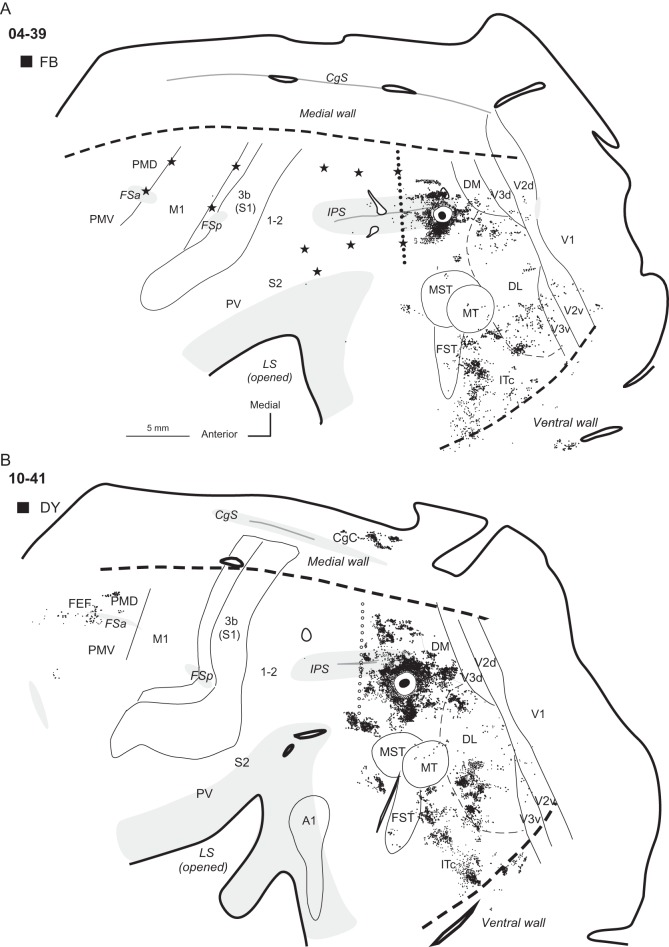
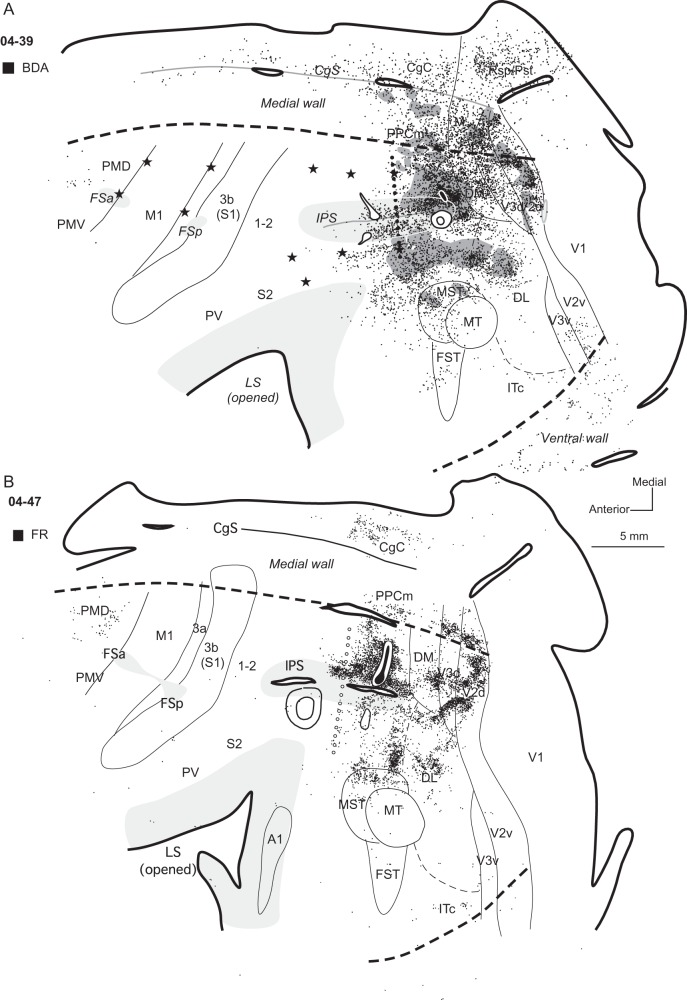
Three other injections were placed in a more caudal location within dorsal PPCc relative to the 2 cases described above. The core of all 3 injection sites involved more (04-47 Fig. 4B) or less (04-39 Fig. 4A; 04-26, Fig. 5A) of the dorsal bank of the IPS (Fig. 1B), and all these injections resulted in similar patterns of label, so results were averaged for these 3 cases. As in the cases described above, label was mostly concentrated in the dorsal half of the hemisphere, labeling a majority of neurons in visual areas DM (∼10%), dorsal V3 (∼14%), and dorsal V2 (∼20%) (Fig. 2D), as well as in other cortical areas on the medial surface. PPCm and CgC contained about 6% of labeled neurons, and less label was concentrated in area M (∼3.5%) and retrosplenial cortex (Rsp, ∼3.2%) (see Table 4). In case 04-39 with a BDA injection, neurons labeled in these areas were mixed with labeled axon terminals. Many labeled neurons were found in the dorsal bank of the IPS, but in case 04-47 (Fig. 4B), where the core of the injection encroached upon the dorsal bank of the IPS, the distribution of labeled neurons was denser. In all 3 cases the distribution of labeled neurons extended beyond the PPCr–PPCc border, but was limited to about the posterior third of PPCr (see especially Fig. 4A, case 04-39 with electrolytic lesions at PPCr/PPCc border), where 11.7% of labeled neurons were present. Fewer labeled neurons were in the ventral bank of the IPS, and they were mostly in the caudal half of the sulcus. Cortex below the IPS (ventral PPC) was densely labeled, and in case 04-39 BDA-labeled cells in this location were mixed with anterogradely labeled axon terminals. Numerous patches of labeled neurons and terminals were found in dorsal DL and MST, and a fewer labeled cells were in the adjacent regions of MT and FST (Fig. 2B–D). A few labeled neurons were also found in ventral V3, ventral V2, and also in V1. Some cells were labeled in ITc (1.7% on average). Only a few labeled neurons were in the rostral portion of area PMD (PMDr). Even a large injection, as in case 04-26, did not label cells in PMDr (Fig. 5A). No labeled neurons were found in PMV, ventral to the anterior frontal sulcus (FSa), in M1, or SMA. Single, isolated labeled neurons were found in the somatosensory areas of the anterior parietal cortex (0.2%) and cortex in the LS (0.2%) in case 04-47, but not in the other 2 cases (Table 4).
Figure 4.
Distributions of labeled neurons after tracer injections in dorsal PPCc in cases (A) 04-39 and (B) 04-47. Core of each injection is marked in black and small diffusion zone of FR in (B) is marked in white. Black dots indicate the locations of labeled cells and the regions with labeled axon terminals are filled with dark gray. In case 04-47 (B) without ICMS mapping PPCr/PPCc border is estimated (line of opened circles). Both injections were placed close to the posterior tip of the IPS. Note that the patterns of connections are similar, and in both cases almost all labeled neurons (and axon terminals in case 04-39) are concentrated in the posterior half of the hemisphere. Both injections into the dorsal PPCc resulted in major label in PPCc, dorsal parts of visual area V2, and V3, as well as in DM, DL, and areas MST, FST, and MT. There were also connections with CgC, retrosplenial cortex (case 04-39), and less dense connections with PMDr and ITc. Conventions are the same as in Figures 1 and 3.
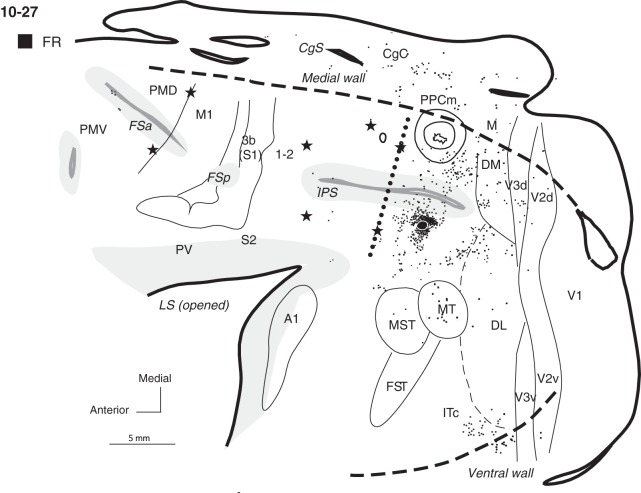
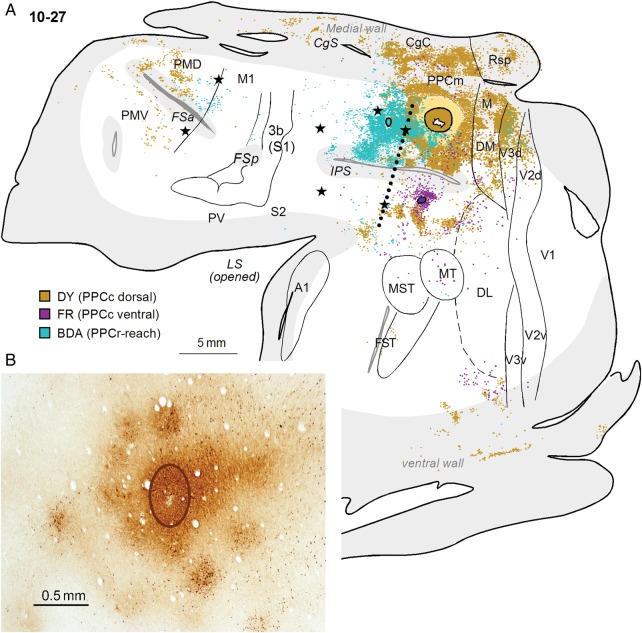
A large DY injection in case 10-27 (Fig. 5B) was placed more medially than other dorsal PPCc injections, and the dense halo surrounding the injection site involved some cortex on the medial surface. Thus, this injection labeled many more neurons on the medial wall than in the other cases with more lateral injections. Large, dense patches of DY-labeled neurons were located in PPCm (12.6%) and in mostly caudal CgC (25.8%). DY-labeled neurons were also located in area M (6.6%), Rsp (4.6%) and possibly in area prostriata, Pst, a poorly understood cortical area located in the anterior portion of the calcarine sulcus. Similar to the other cases (e.g., case 04-07, Fig. 3A) with more rostral injections in dorsal PPCc, the dense distribution of labeled neurons around the injection site spread anteriorly into the adjacent PPCr region (16.4%). Our ICMS mapping revealed that this labeled part of PPCr, marked by 2 caudal microlesions, represents reaching behavior. The major distribution of labeled neurons from this injection included the dorsal bank of IPS, while the ventral bank was almost free of label. More laterally, some foci of label were also found in the ventral PPCc, half way between the IPS and MT, as well as in the dorsal DL. Caudally, the areas DM, dorsal V3, and dorsal V2 contained very dense patches of label (8.8, 8.9, and 2%, respectively). No labeled cells were found in V1. Ventrally, there were few patches of labeled neurons in the caudal IT (ITc, 5.4%). As in case 04-07 with a large injection, many labeled cells were in PMD (3.8%), mostly its rostral part, and in the FEF/PFC (2.9%). The distribution of labeled neurons in this case spread somewhat more medially, possibly involving SMA, and laterally into PMV, where label was sparse (1.2%).
In summary (as shown in Fig. 6C), areas in dorsal PPCc were densely connected with dorsal and ventral PPCc, and with adjacent parts of PPCr representing reaching and defensive behaviors (15.7%), as well as with PPCm (11.4%). Outside the PPC, very dense connections were mainly with dorsal V2 (12.8%), V3 (10.3%), and DL (8.2%), as well as with DM (7.8%). The predominance of labeled neurons in the dorsal portions of these visual areas that represent the lower visual quadrant (Allman et al. 1973; Rosa et al. 1997; Fan et al. 2012) suggests that the dorsal PPCc largely represents the lower visual quadrant. More anterior parts of PPCc (e.g., case 04-07) seem to be related to parts of these visual areas representing more peripheral vision, than more posterior PPCc (case 04-04). Cortex dorsal and anterior to MT, including the expected location of MST, also projected to dorsal PPCc (2.8%). MT, however, appeared to be only weakly connected to dorsal PPCc (1%). Some other connections of dorsal PPCc were with CgC (11%) and they were especially dense in its caudal region, whereas connections with rostral CgC were less dense or they were not present. Within the frontal cortex, 5.2% of all labeled neurons were found, and most of such neurons were in rostral PMD and FEF, areas which both represent eye movements (Wu et al. 2000). Sparse connections were found with caudal PMD and PMV. Large regions of cortex, including M1, SMA, and somatosensory areas, were commonly devoid of label. The total number of neurons labeled in each cortical area after dorsal PPCc injections is shown in Table 3, and the proportion of such neurons in each of these areas is summarized in Figure 6C.
Connections of Ventral PPCc
Five galagos received tracer injections into ventral PPCc below the IPS (Table 1). Three of these galagos (04-47, 10-27, 04-39) received injections of different tracers in ventral and dorsal PPCc (described above), and these cases have been used to directly compare connection patterns to dorsal and ventral regions of PPCc. Injections to ventral PPCc were placed within area 7a of Preuss and Goldman-Rakic (1991a). Injection sites were limited to the area 7a-l on the lateral surface of the brain (e.g., 10-27 or 04-43), area 7a-m on the lip and the lower bank of the IPS (e.g., case 04-39), or involved both 7a-l and 7a-m areas (e.g., 04-47).
Cases 04-47, 04-43, and 10-27 (Figs 7 and 8) received tracer injections in the middle portion of ventral PPCc below the IPS, and cortical connections in each of these cases were distributed in similar manners. As expected, a large number of labeled neurons surrounded the injection site, including both banks of the IPS and dorsal PPCc. In case 04-47 (Fig. 7A), a dense distribution of anterogradely labeled axon terminals was concentrated in the ventral PPCc, but some patches of labeled terminals were also in dorsal PPCc. In case 04-43 (Fig. 7B) some of these short intrinsic connections were not depicted, since a portion of cortex around the injection site was damaged during brain processing. In all 3 cases some labeled neurons were also present in PPCr (∼16% on average), especially in the ventral portion, which represents defensive face (and forelimb) movements, but medial PPC was almost free of labeled neurons (∼3.5% on average). Thus, the middle portion of PPCc was widely connected to the other portions of PPC, with PPCm connected only weakly. Outside PPC, labeled neurons were in visual areas MST, MT, and FST, and cortex caudal and dorsal to MT (areas DL and DM). In case 04-47, BDA-labeled neurons in these areas were mixed with patches of labeled terminals. Isolated patches of such terminals were also present in V3d, MST, and the cortex anterior to MST and FST. Some patches of sparsely distributed labeled terminals and neurons were also found in ITc cortex. The labeled neurons were extremely dense in ITc in case 04-43 (15.9%, Fig. 7B). Neurons were also labeled in ventral V2 (∼5.8% on average) and V3 (2% on average), and in cases 04-47 and 10-27, some of such neurons were also found in the dorsal portions of these areas. Few labeled neurons were found in V1 (∼0.5% on average). CgC contained sparsely distributed labeled neurons (6.8% on average). In case 04-47, neurons were labeled in PMD, PMV, PFC, and FEF (4% total) and occasionally in the cortex spanning areas M1, 3a, 3b, and 1-2 (1.5% total); but in 2 other cases only isolated labeled neurons were found in these areas. Very sparse anterograde label was limited to a small PMD region in case 04-47.
Figure 8.
The distribution of labeled neurons in case 10-27 with a very small injection of FR in ventral PPCc. Labeled neurons are very sparsely distributed but in the same cortical areas (mostly the caudal half of the hemisphere) as in other cases with ventral tracer injections (see Figs 7 and 9). Conventions are the same as in Figures 1 and 3.
In 2 other cases, 04-39 and 10-41 (Fig. 9A,B), injections were placed in the caudal extreme of ventral PPCc, close to the caudal tip of the IPS. In case 04-39 (Fig. 9A), the injection site was mostly in the ventral bank of the IPS, and in case 10-41 (Fig. 9B) the injection site was situated more lateral on the cortical surface, just below the IPS. The patterns of connections were similar in both cases. Most labeled neurons were located in the immediate surround of the injection core, involving both banks of the IPS. The distribution of labeled neurons spread anteriorly across the caudal half of PPC. But in contrast to cases with more anteriorly placed injections, the zone of labeled neurons did not cross the PPCc/PPCr border, suggesting that the most caudal PPC is not significantly connected to rostral PPC. Some patches of neurons were labeled in the dorsal PPCc, but the label did not invade PPCm. Caudally labeled neurons were found in the location of DM (Lyon and Kaas 2002a) (2.3% in 04-39 and 13.5% in 10-41) and ventral to DM. Multiple large clusters of densely distributed labeled neurons were in the cortex below and caudal to MT (e.g., in DL 31.5 and 32.2%, and in ITc 54.2% and 17.4%). Only scattered labeled neurons were found in areas V2 (1.8 and 0.4%) and V3 (3.1 and 0.4%), mostly in their ventral halves. In case 04-39, a few labeled neurons were found in ventral V1 (1.1%) near the border with V2. Neurons in areas MST, MT, and FST were occasionally labeled. In case 10-41, the labeling of neurons was more effective, and there were additional dense patches of labeled neurons in the caudal CgC (7.6%) and in the premotor cortex (mostly rostral PMD), above and within FSa as well as in the FEF (total 4.6%). There were no connections of the ventral PPCc with primary motor or somatosensory cortex.
In summary, cortex ventral to the IPS, has major connections with the cortex of the caudal half of a hemisphere, and less dense connections with frontal cortex. Thus, the ventral portion of PPCc receives predominantly visual inputs, and the intrinsic connection patterns suggest that this visual information spreads within cortex in and around the IPS, but not into PPCm where only a small proportion of neurons was labeled. As shown in Figure 6, ventral PPCc connects strongly with areas DL (24.4%) and ITc (24.2%), and less strongly with areas DM (6.6%), V2 (5.6%), and V3 (3.3%). The ventral distribution of labeled neurons in V2 and V3 suggests that the ventral PPCc mainly represents the upper visual quadrant, as the ventral portions of these visual areas represent the upper visual quadrant (Allman et al. 1973; Rosa et al. 1997). Connections with other visual areas (MT 2.1%, MST 5.4%, and FST 1.6%) and visuomotor cortex (FEF 1%; Fig. 9B) are sparser as are connections with CgC (3.4%). Less significant are connections of ventral PPCc with motor and premotor cortex (1.3%) and especially with somatosensory cortex (0.2%). The total number of neurons labeled in each cortical area after ventral PPCc injections is shown in Table 3, and the densities of such neurons in these areas are summarized in Figure 6D.
One case (05-15, not illustrated) with PPCc injections was cut coronally to reveal the laminar distribution of labeled cells. Small injections of FR and BDA in this case were in the most caudal PPC close to the tip of IPS (Fig. 1). The majority of labeled neurons were found in the supragranular layers 3 and 2 of extrastriate areas, demonstrating that the projections to PPC from visual cortex were of the feedforward type (Rockland and Pandya 1979; Maunsell and Van Essen 1983). As in other cases with caudal PPC injections, these feedforward projections primarily originated from areas V2-V3, DM, DL, and ITc. Labeled neurons were also distributed throughout the caudal portion of PPC, where they were located mainly in layer 3. There was also some label in the supragranular (mostly layer 3) and infragranular (mostly layer 5) layers in the middle temporal cortex (areas MST and MT), as well as cortex on the medial wall (areas M and CgC). PPCm did not contain many labeled neurons in this case, but they were mostly in infragranular layer 5, as were neurons labeled in Rsp. As areal boundaries can be more difficult to estimate in surface views reconstructed from coronal sections, numbers of labeled cells in case 05-15 were not quantified; and results from this case were not included in Figure 6 or Tables 3 and 4.
Comparison of PPCc and PPCr Connections
Most cases described in this article received tracer injections into PPCc and PPCr (Table 1), making possible the direct comparison of connections to both halves of PPC. In this section, we compare the patterns of these connections in 3 cases, 04-07, 04-26, and 10-27. The PPCr connections in cases 04-07 and 04-26 have been published previously (Stepniewska, Cerkevich et al. 2009). Here we include these connection patterns for comparison with the patterns of PPCc connections. Results from PPCr and PPCc injections in case 10-27 have not been published previously.
Case 04-07 (Fig. 10A) with a dorsal PPCc injection also received concurrent injections of 2 tracers into dorsal PPCr, in hand-to-mouth and forelimb defensive domains (for the supporting physiological data see Stepniewska, Fang et al. 2009). The results in this case reveal that projections to the anterior and posterior halves of PPC are mostly segregated. Thus, PPCc connections largely originate from neurons in the visual cortex of the posterior half of the cerebral hemisphere. In contrast, PPCr connections, especially those of the anterior hand-to-mouth domain, originate mainly from neurons in motor and somatosensory cortex of the anterior half of the cerebral hemisphere. Some overlap of neurons connecting to PPCc and PPCr occurred in the middle of PPC around the PPCr/PPCc border and in dorsal portions of visual areas V3 and V2 (Fig. 10A; see also Fig. 3A). Remarkably, patches of labeled cells in V3d contained mixtures of neurons that were labeled by each of the 3 injections, 2 in PPCr and one in PPCc. This patch-like pattern is consistent with other evidence for modular organization in V3d (Fan et al. 2012). An overlap between the distribution of neurons labeled by the more caudal PPCr injection (in the forelimb defensive domain) and the PPCc injection was also observed in PMD, where both populations of labeled neurons were grouped in rostral PMD (PMDr) and FEF. In contrast, neurons labeled after rostral PPCr injection (in the hand-to-mouth domain) grouped in the caudal PMD (PMDc) extending into M1. All injections labeled neurons in PPCm, and zones of labeled neurons were organized topographically in a rostro-caudal manner complementary to the injection sites. The same arrangement of label (although less dense) was observed in the CgC, where PPCr injections labeled neurons of the anterior cingulate area while PPCc injections labeled more caudal CgC.
Figure 10.
Comparison of PPCr and PPCc connections. (A) Case 04-07 with large CTB injection in dorsal PPCc and 2 small injections in dorsal PPCr. CTB injected in PPCc labeled mostly extrastriate visual areas in the caudal half of the hemisphere and less densely in PMDr and FEF in the frontal cortex. A similar pattern of connections was observed after the FE injection in the defensive face zone of PPCr, although the label was much less dense. CTB and FE labeled neurons in V2 and V3 were arranged in bands and both populations of neurons intermingled. In contrast, the FR injection in the hand-to-mouth zone in PPCr heavily labeled somatosensory and motor areas in the anterior half of the hemisphere and only few cells in the visual area V3d. All 3 injections sparsely labeled CgC on the medial wall and ventral PFC. For the detailed microstimulation map of this case see Figure 2B in Stepniewska, Fang et al. (2009). (B) Case 04-26 with large FB injection in dorsal PPCc and 2 small injections in dorsal and ventral PPCr. As in case 04-07, FR injection in the face defensive zone in ventral PPCr labeled neurons in somatosensory and motor cortex, but also in extrastriate visual areas V3 and MST-MT, as well as in nonprimary auditory areas. Neurons in V3d were arranged in bands that were complementary to the bands with neurons labeled after PPCc injections. Conventions are the same as in Figures 1, 3, and 4.
Injections of PPCr and PPCc in 2 other cases provide similar results. In case 04-26, PPCr received 2 small injections, one in anterodorsal PPCr (hand-to-mouth domain) and another one in ventral PPCr (face defensive domain) (Fig. 10B); and their connection patterns were compared with the pattern that resulted from a large injection in dorsal PPCc. As in case 04-07, connections of PPCr were located mostly in the sensorimotor areas, and connections of dorsal PPCc were exclusively in visual areas DM, V3, V2, MST-MT, and between the STS and IPS (for details see above). The distribution of labeled neurons from the ventral PPCr injection in the face defensive domain, but not the dorsal PPCr injection in the hand-to-mouth domain, and the distribution of labeled neurons after PPCc injection overlapped in dorsal parts of visual areas V3 and V2. These 2 neuronal populations were mostly segregated and formed alternating stripes, suggesting that functional modules in V3 differentially project to rostrolateral or caudal PPC. A similar alternation of patches of connections in V3 was seen in case 05-15, not illustrated here. Other visual areas (e.g., MST and ITc) also connected to both ventral PPCr and PPCc. On the medial wall, in PPCm and in CgC neurons labeled after PPCr injections were less numerous and they were located more anterior than neurons labeled after injections into PPCc.
In addition to injections in dorsal and ventral PPCc, (described above), case 10-27 received a BDA injection into the PPCr reach domain (Fig. 11A). Dorsal injections in PPCc and PPCr resulted in more overlap of labeled neurons than in other cases. Such overlap was observed in dorsomedial PPC, V3d, and to lesser degree in the cortex between the LS and IPS (see Fig 11A). As in case 04-07 (Fig. 10A), neurons labeled by the dorsal PPCc injection were distributed throughout the dorsomedial V3d and V2d, whereas the PPCr injection only labeled V3d and V2d neurons on the medial wall in the region representing more peripheral vision. In the frontal cortex, neurons connected to the PPCr reach domain or PPCc were mostly segregated, with neurons projecting to PPCc located mostly in PMDr and PMV, and neurons projecting to PPCr in M1 and PMDc. There was no overlap of neurons connected to PPCr reach domain and to ventral PPCc. Although the tracer injected in the PPCr reach zone in case 10-27 did not label the neurons in somatosensory cortical areas, other cases have shown quite strong connections between areas S2/PV in the LS and PPCr reach domain (see Fig. 7B in Stepniewska, Cerkevich et al. 2009). Neither dorsal nor ventral injections to PPCc in this case revealed connections with somatosensory cortex. Finally, the intrinsic connections revealed by the BDA injections in dorsal PPCr were quite patchy (Fig. 11B), suggesting the existence of a modular organization of functional neuron types within this cortex. In line with this finding, our optical imaging results (Stepniewska et al. 2011) indicated that the local excitatory activation resulting from electrically stimulating PPCr domains does not spread widely or randomly. Instead, increases in overall activity are confined to the stimulated domain, and the immediate surround. The patchy activation of cortex in the immediate surround of the stimulated domain suggests that this cortex is functionally tied to the domain, and we refer to it as the satellite cortex subserving each domain.
Figure 11.
(A) A comparison of the connections of PPCr and PPCc in case10-27. The patterns of label after large DY injection in dorsal PPCc and small injection of FR in ventral PPC are compared with the pattern of label after a BDA injection in the reach zone of PPCr. Note the overlap of neurons labeled by 2 dorsal injections, especially in middle PPC, but also in area V3d. Two populations of labeled neurons are mostly separated in PMD. Conventions are the same as in Figures 1 and 3. (B) Patchy distribution of BDA-labeled neurons and axon terminals after small injection in the PPCr reaching zone in case 10-27. The oval outlines the core of injection.
Discussion
Our present study is part of an extended effort to evaluate and compare patterns of cortical organization across members of the major branches of primate evolution. In rostral PPC (PPCr) of galagos and New World monkeys, we have defined several functional domains, including those related to grasping, defensive behaviors, and reaching; and they appear to be spatially and functionally related to AIP, VIP, and MIP subdivisions of intraparietal region within PPC in macaques (Kaas et al. 2011, 2013). This assumption is supported by the evidence from our studies that a grasping domain, identified by microstimulation, exists in part of the grasp-related cortex of anterior PPC in macaques (Gharbawie et al. 2011b); that microstimulation evokes defensive movements in the region of VIP (Cooke et al. 2003); and that MIP is part of a larger region that microelectrode recording identifies as PRR (Andersen and Buneo 2002). While the location of LIP, an eye movement region in the IPS, has been identified by microstimulation in macaques (e.g., Thier and Andersen 1998), such an eye movement domain has not been fully studied with microstimulation in galagos (Stepniewska, Fang et al. 2009) or New World owl and squirrel monkeys (Friedman et al. 2014). Overall, such functionally related information seems more likely to identify homologous subdivisions of PPC across primate taxa than cortical architecture. In our experiments, these movement regions occupy much of PPCr, but not PPCc. Thus, a study of connection patterns may be best suited in a comparative effort to reveal the organization of PPCc, although it is not yet clear how variable connections of homologous cortical areas in primates might be.
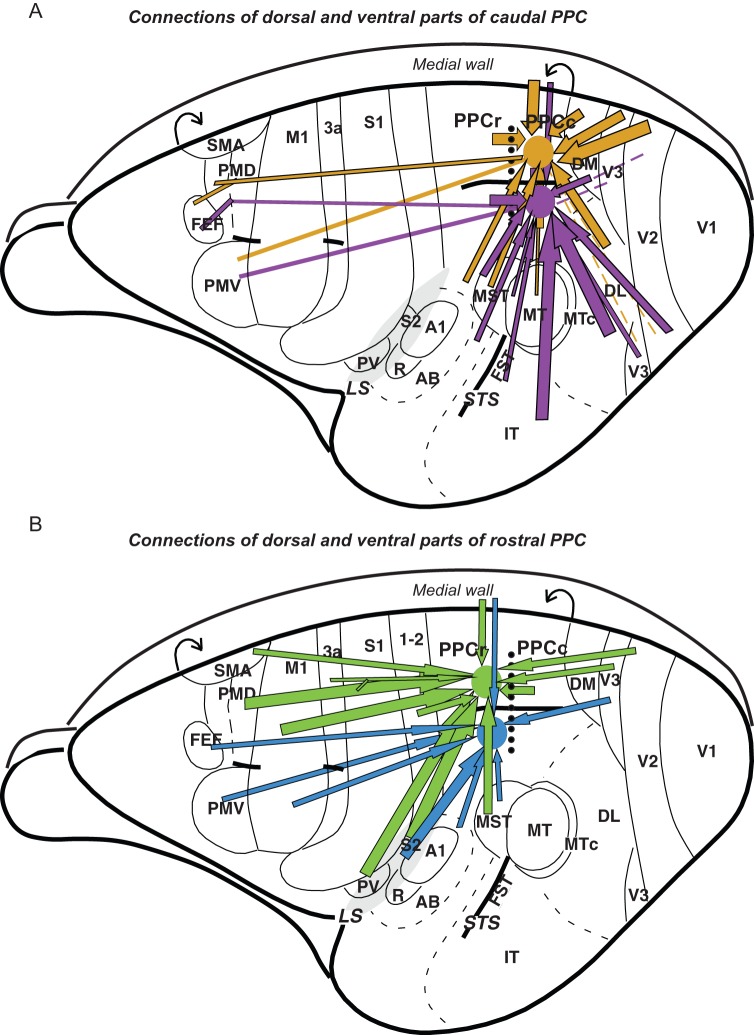
The major ipsilateral cortical connections of PPCc in galagos are summarized and compared with PPCr connections in Figure 12. These connectional patterns as well as results of our quantitative analysis of labeled cells distribution (Fig. 6A–D) suggest the following conclusions. 1) PPCc is much more densely connected with caudal visual areas than PPCr, which gets major inputs from somatosensory areas. Direct visual inputs to PPCc come from areas V2, V3, DM (V3a), DL (V4), MST, FST, and ITc. Injections in PPCc labeled neurons in MT, but not many. 2) Dorsal parts of visuotopic areas V2, V3, and DL representing the lower visual field, project to more dorsal PPCc, while ventral parts of these fields representing the upper visual field, project to more ventral PPCc. Thus, much of PPCc appears to correspond to a single crude representation of the contralateral visual hemifield. 3) The most dorsal part of PPCc is densely connected with adjoining cortex of the medial wall, medial PPC, and ventrally adjoining CgC. 4) Injections in either PPCc or PPCr indicate that these 2 regions are densely connected, but mainly in the caudal half of PPCr. This indicates that the more rostral functional domains in PPCr are less directly driven by visual inputs. (5) PPCc has long connections with the rostral half of PMD, FEF, and adjoining PFC. (6) Intrinsic connections within PPCc are widespread, allowing for widespread functional interactions. Below we discuss these connection patterns further, while comparing them to related findings in galagos and other primates.
Figure 12.
A summary of the PPC connections in galago. Major connections of the caudal (A) and rostral (B) PPC marked with arrows are shown on the schematic dorsolateral views of the left hemisphere. The border between responsive to electrical stimulation PPCr and unresponsive PPCc is marked with dotted line. Cortical connections to dorsal and ventral regions of caudal PPC are marked with gold and violet arrows, respectively, and connections to dorsal and ventral PPCr are marked with green and blue arrows. Thick arrows represent strong connections and thin arrows represent weak connections. Note that major input to PPCc comes from visual areas and to PPCr from somatosensory and motor areas. Rough topography of PPCc connections indicates that dorsal PPCc is connected strongly to dorsal parts of extrastriate cortex and ventral PPCc is connected to ventral parts of extrastriate cortex. Although visual input, especially from V3d, reaches PPCr directly, it is limited to the caudal PPC areas representing reaching and defensive behavior. Major visual information is sent to PPCr indirectly, via PPCc, as PPCc and PPCr are interconnected. Both PPCc and PPCr have connections with motor areas of frontal cortex, although these connections are denser for PPCr.
Connections of PPCc with Visual Areas in Galagos and Other Primates
The primary visual area, V1, appears to project only sparsely to PPCc. Our injections in PPCc either did not label neurons in V1, or labeled a sparse scattering of neurons. When neurons were labeled in V1, they followed the general pattern of dorsal PPCc injections labeling the (dorsal) lower visual quadrant portions of visual areas, and ventral PPCc injections labeling the (ventral) upper field representation of visual areas. The reasons for the variable labeling of neurons in V1 are not apparent, but injections that labeled more neurons were more likely to label some in V1. The existence of a sparse V1 projection to PPCc would not be surprising, as injections in V1 of galagos sometimes labeled a few neurons in PPCc (Lyon and Kaas 2002a) as do injections in V1 of marmosets (Lyon and Kaas 2001), owl monkeys, titi monkeys, and squirrel monkeys (∼1% of labeled neurons, Lyon and Kaas 2002c), and macaque monkeys (∼1%, Lyon and Kaas 2002b; also see Barone et al. 2000; Borra and Rockland 2011; Markov et al. 2013).
The second visual area, V2, had much denser projections to PPCc. Injections in dorsal PPCc labeled neurons most strongly in dorsal V2 representing lower vision. Projecting neurons were sometimes concentrated in several nearby bands crossing much of V2, and suggesting that some of the 4 types of functionally distinct band-like modules of V2 exist in galagos (Federer et al. 2013; Jeffs et al. 2013; see also Xu et al. 2005; Fan et al. 2012), but clear histological evidence for bands is lacking (Collins et al. 2001). Our injections in PPCc did not label neurons in the central parts of V2 representing central vision. Injections in V2 of galagos labeled neurons in the caudal parts of the PPCc region (Collins et al. 2001). Connections of V2 with the caudal PPC region have been revealed in New World monkeys (Cusick and Kaas 1988) and macaques (Stepniewska and Kaas 1996; Gattass et al. 1997).
The third visual area, V3, and especially ventral V3 has been inconsistently identified in primates (Kaas and Lyon 2001), but more recent studies of V1 projection patterns as well as optical imaging studies of the visuotopic organization of dorsal V3 have provided compelling evidence for both a dorsal V3 and ventral V3 in galagos (Lyon and Kaas 2002a, Fan et al. 2012) and other primates (Lyon and Kaas 2001; 2002b; 2002c). Here, we presented evidence that V3d projects densely to dorsal PPCc in galagos, and V3v projects to ventral PPCc, although only one of our injections (04-43) labeled V3v quite densely (Fig. 7B). Evidence for connections between posterior PPC and V3 also comes from our previous study of connections in galagos (Fang et al. 2005) and from studies of PPC connections in macaque monkeys (Neal et al. 1990; Lewis and Van Essen 2000; Nakamura et al. 2001).
The dorsomedial visual area (DM) was consistently labeled by our PPCc injections. Although in some cases dorsal injections labeled more neurons in posterior parts of DM representing the lower visual field (10-27, 04-47) and ventral injections labeled more neurons in anterior DM representing the upper visual field (10-41, 04-39), in most cases the labeled neurons were distributed quite evenly throughout the antero-posterior DM dimension. However, none of the injections in PPCc labeled cells in the most lateral portion of architectonically defined DM, which may correspond to the representation of central vision (Rosa et al. 1997). Similar connections between PPC and DM were reported in owl and squirrel monkeys (Kaas et al. 1977; Krubitzer and Kaas 1993; Beck and Kaas 1998a) and macaque monkeys (Felleman et al. 1997; Beck and Kaas 1999).
The dorsolateral visual area (DL) is another region consistently labeled after PPCc injections. As for other visual areas, label resulting from injections into the dorsal PPCc was mostly located in the dorsal half of DL representing lower vision; while injections placed in ventral portions of PPCc, below or in the lower bank of the IPS, labeled cells in more ventral portions of DL representing upper vision (Allman and Kaas 1974; Rosa et al. 1997). Although this distinction in some cases was not obvious, our unpublished observations from the DL injections in galagos are in agreement with a dorsal-to-dorsal and ventral-to-ventral topography of DL-PPCc connections. Evidence for this retinotopy within DL in galagos also comes from a study of V1 connections (Lyon and Kaas 2002a).
The pattern of DL connections with PPCc in galagos, like the pattern of DL connections with V1 (Lyon and Kaas 2002a) and V2 (Collins et al. 2001), did not clearly identify rostral and caudal subdivisions of DL, which have been proposed for monkeys (Cusick and Kaas 1988; Steele et al. 1991; Weller et al. 1991; Stepniewska and Kaas 1996). Such subdivisions were also not architectonically apparent in galagos (present study; Kaskan and Kaas 2007). The rostral division of DL in monkeys is thought to be more involved in dorsal stream processing, due to its connections with MT and DM areas. Thus, it is possible that DL is not subdivided in galagos as it appears to be in monkeys.
In macaques, dense connections have been revealed between V4 (DL) and PPC areas within the IPS (e.g., LIP, VIP), as well as areas PO and 7a in caudal PPC (Seltzer and Pandya 1980; Rockland and Pandya 1981; Cavada and Goldman-Rakic 1989; Andersen et al. 1990; Ungerleider et al. 2008). These projections were predominantly from the peripheral field representation of V4 (Colby et al. 1988; Ungerleider et al. 2008). The portion of V4 representing the central visual field was connected to the deep part of the posterior wall of IPS and IT cortex (Neal et al. 1987; Ungerleider et al. 2008). In most of our galagos with PPCc injections, the DL part representing central vision was not labeled; however, injections that involved the banks of IPS (see 04-39) resulted in some DL label.
DL is joined ventrally by the inferior temporal cortex (IT), which in monkeys is the major target of DL output (e.g., Weller and Kaas 1987; Ungerleider et al. 2008). Difficulties in distinguishing an architectonic border between the 2 areas make interpretations and assignments of label in this region difficult. Previous architectonic studies have identified 2 to 3 areas within the inferior temporal cortex of galagos (Zilles et al. 1979; Preuss and Goldman-Rakic 1991a; Wong and Kaas 2010). In the present study, cortex in the presumptive location of caudal IT, ITc, was specifically connected to the ventral region of PPCc, and contained moderate (as in case 04-47) to dense (as in case 04-39; 10-41) distributions of labeled neurons. This result is interesting, since parietal cortical areas have generally been considered part of the dorsal stream and, as such, only indirectly connected with IT belonging to the ventral stream. Direct connections of the posterior part of PPC with rostral (TE) and caudal (TEO) inferotemporal areas, have been also demonstrated in macaques (Cavada and Goldman-Rakic 1989; Martin-Elkins and Horel 1992; Distler et al. 1993; Webster et al. 1994; Zhong and Rockland 2003; Borra et al. 2010). The moderate-to-sparse label after injections of anterograde tracers into IT (TEO and TE, respectively) was confined to the lateral bank of IPS (LIP), including a small part of area 7a (Webster et al. 1994). The patterns of V1 connections with IT in macaques suggest that little retinotopy exists in IT, yet provide evidence for retinotopy in V4 (DL) (Tootell and Hadjikhani 2001; Fize et al. 2003; Sundberg et al. 2006).
Neurons in areas MST and MT were inconsistently labeled after our PPCc injections. Area MST was more densely labeled than MT, but the density of labeled neurons varied, ranging from no label (e.g., case 10-41) to a moderately dense or even a dense pattern of label (e.g., cases 04-04 and 04-26; 04-47; 04-43), especially after dorsal injections involving the depth of IPS. Only our injection close to the posterior tip of IPS significantly labeled neurons in MT (case 05-15, not illustrated). Some of the labeled neurons in MST and MT after ventral PPCc injections in cases 04-47 and 04-43 may have been due to the very lateral position of the injection, closer to MST than in other cases, or to some tracer uptake by fibers connecting dorsal PPC with MST-MT. A clear retinotopic pattern of PPCc connections with MST-MT was not apparent in these cases. Tracing studies of MT connections in galagos (Krubitzer and Kaas 1990; Kaskan and Kaas 2007) revealed only sparsely distributed axon terminals around the posterior tip of the IPS and below the sulcus in the ventral PPCc.
In contrast, dense connections between caudal PPC and MT-MST have been described in monkeys. In macaques, the region including areas LIP and VIP within the IPS was identified as a major target of MT (Maunsell and Van Essen 1983). Also, the caudal part of PPC below the IPS (area 7a) connects with visual motion areas in the depth of STS, areas MT-MST, FST, (Seltzer and Pandya 1978, 1994; Ungerleider and Desimone 1986; Cavada and Goldman-Rakic 1989; Andersen et al. 1990; Blatt et al. 1990; Boussaoud et al. 1990; Seltzer et al. 1996; Padberg et al. 2003; Rozzi et al. 2006). In New World monkeys, connections between PPCc and areas in the superior temporal sulcus have been reported, as well (Kaas et al. 1977; Krubitzer and Kaas 1990; Weller et al. 1984; Kaas and Morel 1993).
In the present study, the proposed area FST, as well as cortex anterior to FST, which might be a homolog of superior temporal polysensory area (STP) in macaques, was inconsistently labeled. In macaques middle and caudal STP (in the upper bank of STS) have been shown to connect with the ventral area 7a, areas in the lower bank of the IPS, and area 7 m (Seltzer and Pandya 1978, 1994; Neal et al. 1988; Cavada and Goldman-Rakic 1989; Andersen et al. 1990; Seltzer et al. 1996). Relatively dense connections between STP and area 7b have been demonstrated in macaques (Neal et al. 1987; Cavada and Goldman-Rakic 1989; Zhong and Rockland 2003), but they were not found in galagos (Stepniewska, Cerkevich et al. 2009).
Connections with Cortex of the Medial Wall
Medial PPC (PPCm), posterior CgC, and visual area M were densely labeled after dorsal PPCc injections. In contrast, ventral PPCc injections labeled neurons in areas on the medial wall sparsely or not at all (e.g., case 04-39). Our most dorsal injection in case 10-27 encroached somewhat on the medial wall, revealing strong connections with PPCm, posterior CgC (mostly cortex in the ventral bank of cingulate sulcus, CgS), and also with retrosplenial cortex (Rsp) and visual areas (M, DM, V2, V3, and possibly Pst). CgC and Rsp are limbic areas, whereas Pst is a higher-order visual area closely connected to CgC (Rockland 2012). Thus, dorsal PPCc in galagos seems to have more connections with areas of the limbic system than the ventral PPCc or anterior half of PPC (PPCr) (see above).
Similar connections between PPC and areas on the medial wall have been reported in macaque monkeys (Cavada and Goldman-Rakic 1989; Neal et al. 1990; Leichnetz 2001; Morecraft et al. 2012). In macaques, posterior PPC (area 7a) is heavily interconnected with the ventral posterior CgC, Rsp, parahippocampal gyrus and presubiculum, which are parts of limbic system. In contrast, anterior PPC (area 7b) and cortex in the IPS have few limbic associations.
In summary, considering the similarities in sensory and limbic connections (and functions) between different PPC parts in galagos and macaques, we can suggest some general homologies. In both species, moving from posterior to anterior PPC areas, visual connectivity progressively decreases and somatosensory connectivity progressively increases, in agreement with the fact that we are moving from visual to somatic regions of the brain. Thus, galago PPCr with movement domains (for grasp, hand-to-mouth, defense, reach) and strong connections with somatosensory areas (e.g., grasp and hand-to-mouth domains) and some connections with visual areas (e.g., defense and reach domains) could be a homolog of macaque areas 7b and 7ip, with areas within the IPS: AIP for grasp, VIP for defense, MIP for reach, and LIP for looking (Cavada 2001). On the other hand, galago PPCc with strong connections with extrastriate visual areas, and limbic areas could be a homolog of area 7a of macaques.
Connections with Frontal Motor Cortex
In the present study, injections of tracers into the dorsal and ventral PPCc labeled neurons in premotor cortex, mostly in PMD, FEF, and adjacent regions of PFC. Connections of PPCc with PMD, FEF, and PFC were also revealed in our previous study on galagos, which focused on the frontal motor cortex connections (Fang et al. 2005; see also Preuss and Goldman-Rakic 1991b). In agreement with the present results, connections of PMDr were concentrated in a more caudal sector of PPCc and banks of the IPS, whereas PMDc and PMV projected to PPCr (see also Stepniewska, Cerkevich et al. 2009). The evidence that PMDc and PMV relate mainly to PPCr is consistent with the lack of, or very sparse distribution of, labeled neurons in these areas after our PPCc injections. In general, projections from premotor cortex to both PPCr and PPCc were more sparse than projections from these PPC regions to premotor cortex (see Fang et al. 2005), suggesting that feedback projections to PPC are weaker than PPC feedforward projections. It might also suggest that feedback connections are generally more broadly distributed (Rockland et al. 1994; Salin and Bullier 1995), and that our injections did not include the full extent of terminal fields of axons of PM neurons projecting to PPC. Also in agreement with present findings, injections in the location of FEF in galagos-labeled foci of neurons in the caudal half of PPC, in the IPS (Fang et al. 2005), as well as in PPCc on the dorsomedial surface (Stepniewska, Pouget et al. 2009).
Similar patterns of connections between posterior parietal and frontal cortex were described in New World monkeys (Kaas et al. 1977; Dum and Strick 2005; Stepniewska et al. 2006) and Old World macaques, where these connections were studied more fully (Cavada and Goldman-Rakic 1989; Caminiti et al. 1996). In macaques, injections into posterior regions of PPC and frontal cortex revealed close reciprocal connections of area 7a and the PRR (MIP, PO) with the FEF, PFC, and PMD, although connections with PMD were much stronger for PRR than area 7a (Petrides and Pandya 1984; Caminiti et al. 1999; Cohen and Andersen 2002). Areas within the IPS (LIP, VIP) also have prominent connections with the FEF, restricted portions of the PMV, and with PFC (Andersen et al. 1985; Schall et al. 1993; Petrides and Pandya 1999).
The Retinotopic Organization of PPCc
While there have been no microelectrode receptive field mapping studies of PPC in galagos or other prosimian primates, the global connection pattern of PPCc described here suggests that much of PPCc contains an overall single representation of the contralateral visual hemifield with the lower visual quadrant represented dorsally and the upper visual quadrant represented ventrally. Thus, much of PPCc may function as a single large area. However, regional differences in other connections provide evidence for a dorsal division of PPCc with dense connections with CgC, and ventral regions with connections with temporal visual cortex. In contrast, relatively small retinotopic maps have been reported in PPC of macaques and humans. In particular, LIP of macaques has been described as having a retinotopic organization (Blatt et al. 1990; Colby et al. 1996; Ben Hamed et al. 2001; Patel et al. 2010), and the MT projections to VIP suggest a map in that cortex as well (Seltzer and Pandya 1978; Maunsell and Van Essen 1983; Ungerleider and Desimone 1986). In humans, functional magnetic resonance imaging (fMRI) studies have provided evidence for 2 or more retinotopic maps in PPC; some perhaps not found in other primates, but others possibly being homologs of LIP and VIP (Sereno et al. 2001; Grefkes and Fink 2005; Sereno and Tootell 2005; Silver et al. 2005; Swisher et al. 2007; Mars et al. 2011; Sereno and Huang 2014). These findings do not necessarily relate to the proposed retinotopy of PPCc in galagos, where the previously described motor domains are located in PPCr (Stepniewska et al. 2005; Stepniewska, Fang et al. 2009), as they are in New World monkeys (Gharbawie et al. 2011a). Thus, a retinotopic map may exist in the eye movement module in PPCr of galagos (Stepniewska, Fang et al. 2009) and New World monkeys (Friedman et al. 2014), the presumed homolog of LIP. The arrangement of AIP, LIP, VIP, and PRR in macaques suggests that some rotation or displacement has occurred, and consequently, the medial reaching domain is much more caudal in macaques compared with galagos and New World monkeys. This rotation likely reflects the greater expansion of rostromedial parts of PPC in macaques. Thus, PPCc of galagos may correspond to a proportionally smaller (caudal) portion of PPC in macaques and humans. In support of this possibility, parts of caudal PPC in macaques have inputs from early visual areas (Ungerleider and Desimone 1986; Neal et al. 1990; Ungerleider et al. 2008; Borra and Rockland 2011) and neurons in caudal PPC (area 7a) of macaques have neurons that respond to visual stimuli, while having large receptive fields (Blatt et al. 1990; Gattass et al. 2005; Serences and Yantis 2007). Thus, a region with a crude retinotopy could exist in PPCc of other primates.
Interestingly, our results, in line with data from macaques (Seltzer and Pandya 1980; Lewis and Van Essen 2000) and humans (Uddin et al. 2010; Greenberg et al. 2012), show that PPC (as an entity) has stronger connections with dorsal parts of extrastriate visual areas representing the lower visual field than with the ventral parts of these areas representing the upper visual field. Previously, we have also demonstrated that injections into the lower peripheral field in V2, but not injections in the upper field or centrally located injections, labeled cells in PPC in galagos (Collins et al. 2001). The explanation for these findings might be in the different functions that the lower and upper visual fields play in vision. The lower visual quadrant has been linked with global vision in peripersonal space, in contrast to the upper field that is associated with far visual space (Previc 1990; Christman and Niebauer 1997). Thus, the PPC areas concerned with sensorimotor integration and motor planning in near peripersonal space (Fogassi and Luppino 2005; Rozzi et al. 2008) might depend more on inputs from the dorsal extrastriate cortex (see Borra and Rockland 2011).
Dorsal and Ventral Stream Inputs to PPCc
According to the widely accepted and influential hypothesis of 2 functionally distinct streams of visual processing (Ungerleider and Mishkin 1982), PPC is known as the target of the dorsal stream visual projections, which mediate the visual control of skilled actions (Ungerleider and Mishkin 1982; Goodale and Milner 1992; Goodale and Westwood 2004). However, our findings in galagos show that PPCc receives visual input from visual areas of both dorsal and ventral streams. The connections of PPCc with areas such as DM, MT, MST, and FST provide clear evidence for dorsal stream inputs to PPC. For the ventral stream involvement, there are connections with ITc cortex, which provides a substantial source of visual input to ventral PPC. V2, V3, and DL areas are associated with both dorsal and ventral visual streams (Kaas 1997; Kaas and Lyon 2001; Rosa et al. 2009) and they may send mixed visual information to PPC. Consistent with our findings, area 7a in macaques has been shown to get inputs from the visual areas of the dorsal pathway, as well as from higher-order visual areas of the temporal cortex (Blatt et al. 1990; Milner and Goodale 2006; Rozzi et al. 2006) involved in the ventral visual pathway. Thus, the independence of the 2 streams is not complete. Recent functional and neuroanatomical data (including ours) provide evidence for many connections and points of convergence between dorsal and ventral pathways that might contribute to many different forms of visual processing (for review, see Merigan and Maunsell 1993; McIntosh and Schenk 2009; Kravitz et al. 2011, 2013). Information from the 2 pathways can also be integrated in early visual areas by virtue of extensive feedback connections (Deco and Lee 2004). Moreover, physiological properties of neurons in some cortical areas (e.g., Pst) exhibit some of the defining characteristics of either dorsal or ventral pathways of visual areas, and it is possible that they operate in parallel with these pathways (Yu et al. 2012).
Conclusions
The overall pattern of PPC connections in galagos reported here provides suggestions for how specific motor behaviors might be guided by visual information. Thus, the caudal part of PPC receives a combination of inputs from visual areas, and projects in turn to subdivisions of rostral PPC, which get somatosensory and auditory information and provide major outputs to motor and premotor areas of frontal cortex. In addition, some of the visual inputs from PPCc to M1-PM are direct. While our present evidence supports the hypothesis that most or all of PPCc forms a single visual area with a crude retinotopic organization, there might be several distinct visuomotor networks supporting specific actions (e.g., reach; see Caminiti et al. 1996; grasping, see Gharbawie et al. 2011b) that are dependent on visually dominated subregions in the caudal half of PPC.
Funding
This work was supported by the National Institutes of Health (grant number EY 002686 to J.H.K).
Notes
The authors thank Drs Omar Gharbawie and Nicole Young for assistance with the microstimulation mapping, Kurt Schilling for help with cells quantification, Dan Miller and Dr Jamie Reed for helpful comments on the article, Mary Feurtado for surgical assistance and Laura Trice and Mary Varghese for technical assistance. Conflict of Interest: None declared.
References
- Allman JM, Kaas JH. 1974. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgatus). Brain Res. 81:199–213. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH, Lane RH. 1973. The middle temporal visual area (MT) in the bushbaby, Galago senegalensis. Brain Res. 57:197–202. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. 1990. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 296:65–113. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. 2002. Intentional maps in posterior parietal cortex. Ann Rev Neurosci. 25:189–220. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. 1985. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: a study using retrogradely transported fluorescent dyes. J Comp Neurol. 232:443–455. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. 1997. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 20:303–330. [DOI] [PubMed] [Google Scholar]
- Atherton TJ, Kerbyson DJ. 1999. Size invariant circle detection. Image Vis Comput. 17:795–803. [Google Scholar]
- Baker JF, Petersen SE, Newsome WT, Allman JM. 1981. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol. 45:397–416. [DOI] [PubMed] [Google Scholar]
- Barone P, Batardiere A, Knoblauch K, Kennedy H. 2000. Laminar distribution of neurons in extrastriate areas projecting to visual areas V1 and V4 correlates with the hierarchical rank and indicates the operation of a distance rule. J Neurosci. 20:3263–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. 1999. Reach plans in eye-centered coordinates. Science. 285:257–260. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. 1998a. Cortical connections of the dorsomedial visual area in new world owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus). J Comp Neurol. 400:18–34. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. 1999. Cortical connections of the dorsomedial visual area in old world macaque monkeys. J Comp Neurol. 406:487–502. [PubMed] [Google Scholar]
- Beck PD, Kaas JH. 1998b. Cortical connections of the dorsomedial visual area in prosimian primates. J Comp Neurol. 398:162–178. [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. 2001. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res. 140:127–144. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. 1990. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 299:421–445. [DOI] [PubMed] [Google Scholar]
- Borra E, Ichinohe N, Sato T, Tanifuji M, Rockland KS. 2010. Cortical connections to area TE in monkey: hybrid modular and distributed organization. Cereb Cortex. 20:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E, Rockland KS. 2011. Projections to early visual areas v1 and v2 in the calcarine fissure from parietal association areas in the macaque. Front Neuroanat. 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D1, Ungerleider LG, Desimone R. 1990. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 296:462–495. [DOI] [PubMed] [Google Scholar]
- Bruce K, Grofova I. 1992. Notes on a light and electron microscopic double-labeling method combining anterograde tracing with Phaseolus vulgaris leucoagglutinin and retrograde tracing with cholera toxin subunit B. J Neurosci Meth. 45:23–33. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB. 1996. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex. 6:319–328. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P, Ferraina S, Mitsuda T, Giannetti S, Squatrito S, Maioli MG et al. 1999. Early coding of reaching: frontal and parietal association connections of parieto-occipital cortex. Eur J Neurosci. 11:3339–3345. [DOI] [PubMed] [Google Scholar]
- Cavada C. 2001. The visual parietal areas in the macaque monkey: current structural knowledge and ignorance. NeuroImage. 14:S21–S26. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. 1989. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 287:422–445. [DOI] [PubMed] [Google Scholar]
- Christman SD, Niebauer CL. 1997. The relation between left–right and upper–lower visual field differences. In: Christman S, editors. Cerebral asymmetries in sensory and perceptual processing. Amsterdam: Elsevier; p. 263–296. [Google Scholar]
- Cohen YE, Andersen RA. 2002. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 3:553–562. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. 1996. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 76:2841–2852. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. 1988. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 269:392–413. [DOI] [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. 2001. Topographic patterns of v2 cortical connections in a prosimian primate (Galago garnetti). J Comp Neurol. 431:155–167. [PubMed] [Google Scholar]
- Cooke DF, Taylor CR, Moore T, Graziano MSA. 2003. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA. 100:6163–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG, Kaas JH. 1988. Cortical connections of area 18 and dorsolateral visual cortex in squirrel monkeys. Vis Neurosci. 1:211–237. [DOI] [PubMed] [Google Scholar]
- Deco G, Lee TS. 2004. The role of early visual cortex in visual integration: a neural model of recurrent interaction. Eur J Neurosci. 20:1089–1100. [DOI] [PubMed] [Google Scholar]
- Distler C, Boussaoud D, Desimone R, Ungerleider LG. 1993. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 334:125–150. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 2005. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 25:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan RH, Baldwin MK, Jermakowicz WJ, Casagrande VA, Kaas JH, Roe AW. 2012. Intrinsic signal optical imaging evidence for dorsal V3 in the prosimian galago (Otolemur garnettii). J Comp Neurol. 520:4254–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. 2005. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 490:305–333. [DOI] [PubMed] [Google Scholar]
- Federer F, Williams D, Ichida JM, Merlin S, Angelucci A. 2013. Two projection streams from macaque V1 to the pale cytochrome oxidase stripes of V2. J Neurosci. 33:11530–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Burkhalter A, Van Essen DC. 1997. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J Comp Neurol. 379:21–47. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Kaas JH. 1984. Receptive-field properties of neurons in middle temporal visual area (MT) of owl monkeys. J Neurophysiol. 52:488–513. [DOI] [PubMed] [Google Scholar]
- Fize D, Vanduffel W, Nelissen K, Denys K, Chef d'Hotel C, Faugeras O, Orban GA. 2003. The retinotopic organization of primate dorsal V4 and surrounding areas: a functional magnetic resonance imaging study in awake monkeys. J Neurosci. 23:7395–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. 2005. Motor functions of the parietal lobe. Curr Opin Neurobiol. 15:626–631. [DOI] [PubMed] [Google Scholar]
- Friedman RM, Stepniewska I, Roe AW, Kaas JH. 2014. Intracortical microstimulation reveals movement specific domains in awake squirrel monkeys. Program No. 735.08. Neuroscience Meeting Planner Washington, DC: Soc. Neurosci. Online. [Google Scholar]
- Gallyas F. 1979. Silver staining of myelin by means of physical development. Neurol Res. 1:203–209. [DOI] [PubMed] [Google Scholar]
- Gattass R, Nascimento-Silva S, Soares JG, Lima B, Jansen AK, Diogo AC, Farias MF, Botelho MM, Mariani OS, Azzi J et al. 2005. Cortical visual areas in monkeys: location, topography, connections, columns, plasticity and cortical dynamics. Philos Trans R Soc Lond B Biol Sci. 360:709–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. 1997. Cortical projections of area V2 in the macaque. Cereb Cortex. 7:110–129. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. 2011a. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 21:1981–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. 2011b. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 31:11660–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AR, Hansma DI, Houk JC, Robinson FR. 1984. A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res. 298:235–241. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15:20–25. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. 2004. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 14:203–211. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Verstyn T, Chiu YC, Yantis S, Schneider W, Behrmann M. 2012. Visuotopic cortical connectivity underlying attention revealed with white-matter tractography. J Neurosci. 32:2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. 2005. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 207:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley L, Krubitzer LA, Padberg J, Disbrow EA. 2009. Visual-manual exploration and posterior parietal cortex in humans. J Neurophysiol. 102:3433–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J. 1982. Posterior parietal lobe of the primate brain. Physiol Rev. 62:1060–1129. [DOI] [PubMed] [Google Scholar]
- Jeffs L, Beswick S, Martin K, Campbell H, Rose DN, Ferris E. 2013. High- resolution mapping of anatomical connections in marmoset extrastriate cortex reveals complete representation of the visual field bordering dorsal V2. Cereb Cortex. 23:1126–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerison HJ. 1973. Evolution of the brain and intelligence. New York: Academic Press. [Google Scholar]
- Kaas JH. 1997. Topographic maps are fundamental to sensory processing. Brain Res Bull. 44:107–112. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. 2013. Cortical networks for ethologically relevant behaviors in primates. Am J Primatol. 75:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. 2011. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. 2000. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 97:11793–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Lin CS, Wagor E. 1977. Cortical projections of posterior parietal cortex in owl monkeys. J Comp Neurol. 72:387–408. [DOI] [PubMed] [Google Scholar]
- Kaas LH, Lyon DC. 2001. Visual cortex organization in primates: theories of V3 and adjoining visual areas. Prog Brain Res. 134:285–295. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Morel A. 1993. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J Neurosci. 13:534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan PM, Dillenburger BC, Lu HD, Roe AW, Kaas JH. 2010. Orientation and direction-of-motion response in the middle temporal visual area (MT) of New World owl monkeys as revealed by intrinsic-signal optical imaging. Front Neuroanat. 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan PM, Kaas JH. 2007. Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti). Anat Rec (Hoboken). 290:349–366. [DOI] [PubMed] [Google Scholar]
- Kaskan PM1, Lu HD, Dillenburger BC, Kaas JH, Roe AW. 2009. The organization of orientation-selective, luminance-change and binocular-preference domains in the second (V2) and third (V3) visual areas of New World owl monkeys as revealed by intrinsic signal optical imaging. Cereb Cortex. 19:1394–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing. Nat Rev Neurosci. 12:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. 2013. The ventral visual pathway: an expended neural framework for the processing of object quality. Trends Cogn Sci. 17:26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. 1990. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 5:165–204. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. 1993. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture, and homologies in other primates. J Comp Neurol. 334:497–528. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. 2001. Connections of the medial posterior parietal cortex (area 7 m) in the monkey. Anat Rec. 263:215–236. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. 2000. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 428:112–137. [DOI] [PubMed] [Google Scholar]
- Lui LL, Bourne JA, Rosa MGP. 2006. Functional response properties of neurons in the dorsomedial visual area of New World monkeys (Callithrix jacchus). Cereb Cortex. 16:162–177. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. 2001. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J Neurosci. 21:249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. 2002a. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 59:114–129. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. 2002b. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 33:453–461. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. 2002c. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J Comp Neurol. 449:281–297. [DOI] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz M, Lamy C, Ribeiro Gomes AR, Magrou L, Misery P, Giroud P, Barone P, Dehay C, Toroczkai Z et al. 2013. The role of long-range connections on the specificity of the macaque interareal cortical network. Proc Natl Acad Sci USA. 110:5187–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O'Reilley JX, Croxon PL, Olivier E, Noonan MP, Bergmann C, Mitchell AS, Baxter MG et al. 2011. Diffusion-weighted imaging tractography based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 31:4087–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Elkins CL, Horel JA. 1992. Cortical afferents to behaviorally defined regions of the inferior temporal and parahippocampal gyri as demonstrated by WGA-HRP. J Comp Neurol. 321:177–192. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. 1983. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 3:2563–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RD, Schenk T. 2009. Two visual streams for perception and action: current trends. Neuropsychologia. 47:1391–1396. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. 1993. How parallel are the primate visual pathways? Ann Rev Neurosci. 16:369–402. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. 2006. The visual brain in action. 2nd ed Oxford: Oxford University Press. [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. 2012. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 87:457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H1, Kuroda T, Wakita M, Kusunoki M, Kato A, Mikami A, Sakata H, Itoh K. 2001. From three-dimensional space vision to prehensile hand movements: the lateral intraparietal area links the area V3A and the anterior intraparietal area in macaques. J Neurosci. 21:8174–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. 1990. The connections of area PG, 7a, with cortex in the parietal, occipital and temporal lobes of the monkey. Brain Res. 532:249–264. [DOI] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. 1987. The cortico-cortical connections of area 7b, PF, in the parietal lobe of the monkey. Brain Res. 419:341–346. [DOI] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. 1988. The organization of the cortico-cortical connections between the walls of the lower part of the superior temporal sulcus and the inferior parietal lobule in the monkey. Brain Res. 438:351–356. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand J-B, Vanduffel W. 2006. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 44:2647–2667. [DOI] [PubMed] [Google Scholar]
- Padberg J, Seltzer B, Cusick CG. 2003. Architectonics and cortical connections of the upper bank of the superior temporal sulcus in the rhesus monkey: an analysis in the tangential plane. J Comp Neurol. 467:418–434. [DOI] [PubMed] [Google Scholar]
- Patel GH, Shulman GI, Baker JT, Akbudak E, Snyder AZ, Snyder LH, Corbetta M. 2010. Topographic organization of macaque area LIP. Proc Natl Acad Sci USA. 107:4728–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 1999. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 11:1011–1036. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 1984. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 228:105–116. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. 1991a. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 310:475–506. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. 1991b. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate Galago, with comparative comments on anthropoid primates. J Comp Neurol. 310:507–549. [DOI] [PubMed] [Google Scholar]
- Previc FH. 1990. Functional specialization in the lower and upper visual fields in humans: its ecological origins and neurophysiological implications. Behav Brain Sci. 13:519–575. [Google Scholar]
- Rockland KS. 2012. Visual system: prostriata – a visual area off the beaten path. Curr Biol. 22:R571–R573. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1981. Cortical connections of the occipital lobe in the rhesus monkey: interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Res. 212:249–270. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1979. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179:3–20. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Saleem KS, Tanaka K. 1994. Divergent feedback connections from areas V4 and TEO in the macaque. Vis Neurosci. 11:579–600. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Casagrande VA, Preuss T, Kaas JH. 1997. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti). J Neurophysiol. 77:3193–3217. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Palmer SM, Gamberini M, Burman KJ, Yu HH, Reser DH, Bourne JA, Tweedale R, Galletti C. 2009. Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J Neurosci. 29:4548–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. 2006. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 16:1389–1417. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. 2008. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci. 28:1569–1588. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. 1995. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 5:429–438. [DOI] [PubMed] [Google Scholar]
- Salin PA, Bullier J. 1995. Corticocortical connections in the visual system: structure and function. Physiol Rev. 75:107–154. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. 1993. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 15:4464–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Cola MG, Gutierrez C, Massee M, Weldon C, Cusick CG. 1996. Overlapping and nonoverlapping cortical projections to cortex of the superior temporal sulcus in the rhesus monkey: double anterograde tracer studies. J Comp Neurol. 370:173–190. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1978. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 149:1–24. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1980. Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Brain Res. 192:339–351. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1994. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 343:445–463. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. 2007. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 17:284–293. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. 2014. Multisensory maps in parietal cortex. Curr Opin Neurobiol. 24:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. 2001. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 294:1350–1354. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Tootell RB. 2005. From monkeys to humans: what do we now know about brain homologies? Curr Opin Neurobiol 15:135–144. [DOI] [PubMed] [Google Scholar]
- Shikata E, Mc Namara A, Sprenger A, Hamzei F, Glauche V, Buchel C, Binkofski F. 2008. Localization of human intraparietal areas AIP, CIP, and LIP using surface orientation and saccadic eye movement tasks. Hum Brain Mapp. 29:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. 2005. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 94:1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele GE, Weller RE, Cusick CG. 1991. Cortical connections of the caudal subdivision of the dorsolateral area (V4) in monkeys. J Comp Neurol. 306:495–520. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PY, Kaas JH. 2009. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 517:783–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Kaas JH. 2010. Cortical connections of physiologically identified subdivisions of posterior parietal cortex in prosimian primates. FENS Abstr. 5: 051.20. [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. 2005. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA 102:4878–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang P-C, Kaas JH. 2009. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 517:765–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, Kaas JH. 2011. Optical imaging reveals parietal-frontal circuits underlying motor behavior. Proc Natl Acad Sci USA. 108:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. 1996. Topographic patterns of V2 cortical connections in macaque monkeys. J Comp Neurol. 371:129–152. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Pouget P, Baldwin MK, Kaas JH. 2009. Connections of the frontal eye field in prosimian galagos. Program No. 263.10. Neuroscience Meeting Planner: Chicago, IL: Soc Neurosci. Online. [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. 2006. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol. 495:691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg KA, Fallah M, Reynolds JH. 2006. A motion-dependent distortion of retinotopy in area V4. Neuron. 49:447–457. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. 2007. Visual topography of human intraparietal sulcus. J Neurosci. 27:5326–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier P, Andersen RA. 1998. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 80:1713–1735. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N. 2001. Where is ‘dorsal V4’ in human visual cortex? Retinotopic, topographic and functional evidence. Cereb Cortex. 11:298–311. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. 2010. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex 20:2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. 1986. Cortical connections of visual area MT in the macaque. J Comp Neurol. 248:190–222. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. 2008. Cortical connections of area V4 in the macaque. Cereb Cortex. 18:477–499. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. 1982. Two cortical visual systems. In: Ingje DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; p. 549–586. [Google Scholar]
- Veenman CL, Reiner A, Honig MG. 1992. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 41:239–254. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. 1994. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 4:470–483. [DOI] [PubMed] [Google Scholar]
- Weller RE, Kaas JH. 1987. Subdivisions and connections of inferior temporal cortex in owl monkeys. J Comp Neurol. 256:137–172. [DOI] [PubMed] [Google Scholar]
- Weller RE, Steele GE, Cusick CG. 1991. Cortical connections of dorsal cortex rostral to V II in squirrel monkeys. J Comp Neurol. 306:521–537. [DOI] [PubMed] [Google Scholar]
- Weller RE, Wall JT, Kaas JH. 1984. Cortical connections of the middle temporal visual area (MT) and the superior temporal cortex in owl monkeys. J Comp Neurol. 228:81–104. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. 2010. Architectonic subdivisions of neocortex in the Galago (Otolemur garnetti). Anat Rec (Hoboken). 293:1033–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. 1979. Changes in the visual system of monocularly sutured as enucleated cats demonstrable with cytochrome-oxidase histochemistry. Brain Res. 171:11–28. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. 2000. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 423:140–177. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. 2003. Somatosensory cortex of prosimian Galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 457:263–292. [DOI] [PubMed] [Google Scholar]
- Xu X, Bosking WH, White LE, Fitzpatrick D, Casagrande VA. 2005. Functional organization of visual cortex in the prosimian bush baby revealed by optical imaging of intrinsic signals. J Neurophysiol. 94:2748–2762. [DOI] [PubMed] [Google Scholar]
- Yu HH, Chaplin TA, Davies AJ, Verma R, Rosa MG. 2012. A specialized area in limbic cortex for fast analysis of peripheral vision. Curr Biol. 22:1351–1357. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Rockland KS. 2003. Inferior parietal lobule projections to anterior inferotemporal cortex (area TE) in macaque monkey. Cereb Cortex. 13:527–540. [DOI] [PubMed] [Google Scholar]
- Zilles K, Rehkämper G, Stephan H, Schleicher A. 1979. A quantitative approach to cytoarchitectonics. IV. The areal pattern of the cortex of Galago demidovii (e. Geoffroy, 1796), (lorisidae, primates). Anat Embryol (Berl). 157:81–103. [DOI] [PubMed] [Google Scholar]