Abstract
Neural plasticity is a major factor driving cortical reorganization after stroke. We here tested whether repetitively enhancing motor cortex plasticity by means of intermittent theta-burst stimulation (iTBS) prior to physiotherapy might promote recovery of function early after stroke. Functional magnetic resonance imaging (fMRI) was used to elucidate underlying neural mechanisms. Twenty-six hospitalized, first-ever stroke patients (time since stroke: 1–16 days) with hand motor deficits were enrolled in a sham-controlled design and pseudo-randomized into 2 groups. iTBS was administered prior to physiotherapy on 5 consecutive days either over ipsilesional primary motor cortex (M1-stimulation group) or parieto-occipital vertex (control-stimulation group). Hand motor function, cortical excitability, and resting-state fMRI were assessed 1 day prior to the first stimulation and 1 day after the last stimulation. Recovery of grip strength was significantly stronger in the M1-stimulation compared to the control-stimulation group. Higher levels of motor network connectivity were associated with better motor outcome. Consistently, control-stimulated patients featured a decrease in intra- and interhemispheric connectivity of the motor network, which was absent in the M1-stimulation group. Hence, adding iTBS to prime physiotherapy in recovering stroke patients seems to interfere with motor network degradation, possibly reflecting alleviation of post-stroke diaschisis.
Keywords: diaschisis, fMRI, motor network connectivity, motor recovery, stroke, rTMS
Introduction
Recovery from motor stroke is enabled by reorganization of neural circuits at various levels ranging from single cells to large-scale networks (Nudo 2006; Cramer and Riley 2008). Changes in growth factors, transmitter systems, and axonal sprouting have been observed within the first hours after ischemia, promoting the “re-wiring” of the lesioned brain (Langhorne et al. 2011). At the systems level, neuroimaging experiments revealed that connectivity of the primary motor cortex (M1) with remote areas is reduced in the first weeks after stroke, but subsequently increases alongside motor recovery (Park et al. 2011; Grefkes and Fink 2014). The observation that alterations in motor network connectivity relate to neurological impairment has stimulated the idea of using noninvasive brain stimulation techniques to “correct” pathological network configuration, for example, by using repetitive transcranial magnetic stimulation (rTMS) (Hoyer and Celnik 2011; Bates and Rodger 2015). A common strategy is to combine cortical excitability-enhancing rTMS with motor training (Ackerley et al. 2010; Talelli et al. 2012). The rational underlying this “priming” of training-induced plasticity is that rTMS transiently increases neural excitability which in turn facilitates the efficacy of ensuing motor training (Reis et al. 2008). Indeed, in healthy subjects, rTMS applied over M1 has already been shown to increase both cortical excitability at the stimulation site and connectivity with remote sensorimotor areas (Nettekoven et al. 2014). Therefore, rTMS might not only be useful to modulate local plasticity but also to increase connectivity of the lesioned motor system which has been frequently found to be decreased after stroke (van Meer et al. 2010; Park et al. 2011).
However, despite considerable efforts undertaken in the last decade, to date no study has reported lasting rTMS-induced improvements of motor function that might qualify rTMS to support standard treatment in stroke rehabilitation (Hao et al. 2013). One reason for this disappointing development might lie in the fact that most studies were conducted with chronic stroke patients (Bates and Rodger 2015). However, at this stage of the disease, the mechanisms underlying cerebral reorganization may have returned to a stable albeit low level, possibly constraining the potential of rTMS to induce neural plasticity that subsequent motor training could capitalize on (Grefkes and Fink 2012). This leads to the question whether plasticity-inducing brain stimulation is more effective early after stroke when perilesional neural plasticity is generally enhanced (Cramer and Riley 2008), possibly facilitating the interaction with neural processes underlying neurological recovery. In addition, higher levels of motor network connectivity early after stroke have been related to more favorable motor outcome (Carter et al. 2010; Park et al. 2011). Therefore, increasing motor network connectivity by rTMS in this phase might be particularly effective to enhance motor recovery.
We, therefore, tested whether daily application of cortical excitability-enhancing intermittent theta-burst stimulation (iTBS) applied over ipsilesional M1 priming physiotherapy can be used to promote motor recovery compared with control stimulation in early subacute stroke patients. A number of studies have shown that iTBS is a safe and effective protocol to increase cortical excitability in both healthy subjects (Huang et al. 2005; Nettekoven et al. 2014) and acute stroke patients (Di Lazzaro et al. 2008). Advantages of iTBS over other facilitatory rTMS protocols lie 1) in its short duration (3.5 min) enabling a good integration of this stimulation protocol into clinical training sessions and 2) in its low stimulation intensity, helping to reduce the risk of seizure induction (Rossi et al. 2009).
To this end, hand motor function, cortical excitability and resting-state functional magnetic resonance imaging (fMRI) were assessed 1 day prior to a 5-day intervention (baseline) and 1 day after completion of the intervention (post-intervention). In addition, motor performance was assessed >3 months post-stroke (follow-up). We hypothesized that repeated applications of iTBS over ipsilesional M1 prior to physiotherapy enhance motor recovery of the paretic hand. We, furthermore, expected that stimulation of ipsilesional M1 ameliorates stroke-induced motor network dysconnectivity, thereby representing a system-level mechanism underlying the beneficial impact of iTBS on motor outcome (Grefkes and Fink 2012, 2014).
Materials and Methods
Patients
Twenty-six stroke patients (mean age: 67.2 years ± 13.1 standard deviation; 9 female; 22 right-handed) suffering from a first-ever ischemic stroke causing a unilateral hand motor deficit were recruited from the University Hospital of Cologne, Department of Neurology. Inclusion criteria were: 1) age 40–90 years; 2) ischemic stroke as verified by diffusion-weighted magnetic resonance imaging (DWI); 3) within 2 weeks from symptom onset (average: 7.3 days ± 3.6, 1 patient was included 16 days after stroke); 4) unilateral hand motor deficit; 5) lesion does not extend to the precentral gyrus (M1) as verified by MRI; 6) absence of severe aphasia, apraxia, and neglect; 7) no visual field deficit; and 8) no other neurological disease.
Exclusion criteria were: 1) any contraindication to TMS (e.g., epilepsy); 2) any contraindication to MRI (e.g., cardiac pacemaker); 3) infarcts in multiple territories; and 4) hemorrhagic stroke. Patient details are given in Table 1. This proof-of-principle study was approved by the local ethics committee (File-No. 09-108), and all subjects provided informed written consent.
Table 1.
Demographical, clinical, and behavioral data of stroke patients
| Patient | Age | Sex | Handedness | Lesion side | Lesion location | Days post stroke | Relative grip strength (strength affected/unaffected hand, rounded in [kP/cm2]) |
Follow-up | Stimulation | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ses1 | Ses2 | Ses3 | |||||||||
| 1 | 78 | M | R | L | Cortical (frontal) | 6 | 0.83 (47/57) | 1.02 (51/50) | No | M1 | |
| 2 | 45 | F | R | L | Cortical (frontal) and WM | 5 | 0.72 (58/81) | 1.13 (90/80) | 1.28 (100/78) | Yes | M1 |
| 3 | 64 | M | R | L | Internal capsule | 9 | 0.55 (30/55) | 0.88 (49/55) | 0.71 (50/70) | Yes | M1 |
| 4 | 59 | M | R | R | Internal capsule and subcortical WM | 5 | 0.47 (26/55) | 0.77 (48/63) | 0.86 (50/58) | Yes | M1 |
| 5 | 76 | F | R | L | Internal capsule and subcortical WM | 9 | 0.03 (1/37) | 0.16 (6/37) | No | M1 | |
| 6 | 59 | M | R | L | Internal capsule and subcortical WM | 7 | 0.86 (77/89) | 1.13 (86/76) | 1.04 (88/85) | Yes | M1 |
| 7 | 72 | F | L | L | Cortical (frontoparietal) and subcortical WM | 1 | 0.00 (0/33) | 0.44 (15/35) | 0.69 (22/32) | Yes | M1 |
| 8 | 73 | M | R | R | Internal capsule | 10 | 0.61 (60/98) | 0.66 (63/95) | 0.62 (61/98) | Yes | M1 |
| 9 | 53 | M | R | L | Internal capsule and subcortical WM | 13 | 0.10 (7/70) | 0.22 (6/74) | 0.95 (53/56) | Yes | M1 |
| 10 | 80 | F | L | L | Internal capsule and BG | 7 | 0.09 (3/34) | 0.24 (8/33) | No | M1 | |
| 11 | 89 | F | R | R | Internal capsule and subcortical WM | 7 | 0.36 (17/48) | 0.74 (35/48) | No | M1 | |
| 12 | 86 | M | R | L | Internal capsule and subcortical WM | 7 | 0.77 (29/37) | 0.78 (30/39) | No | M1 | |
| 13 | 72 | F | L | L | Internal capsule | 6 | 0.00 (0/43) | 0.00 (0/47) | No | M1 | |
| 1 | 65 | M | R | L | Internal capsule | 2 | 0.00 (0/108) | 0.00 (0/113.3) | 0.17 (19/112) | Yes | Control |
| 2 | 59 | M | R | R | Cortical (frontoparietal) and subcortical WM | 11 | 0.00 (0/105) | 0.00 (0/121.6) | 0.17 (21/119) | Yes | Control |
| 3 | 73 | M | R | L | Internal capsule and subcortical WM | 4 | 0.89 (45/50) | 0.95 (70/73) | 1.11 (76/69) | Yes | Control |
| 4 | 62 | M | R | R | Internal capsule | 16 | 0.24 (21/88) | 0.43 (33/77) | No | Control | |
| 5 | 42 | M | R | R | Cortical (frontoparietal) and subcortical WM | 5 | 0.44 (52/118) | 0.65 (74/113) | 0.79 (87/110) | Yes | Control |
| 6 | 53 | F | L | L | Pons | 1 | 0.39 (19/48) | 0.64 (31/48) | No | Control | |
| 7 | 89 | M | R | L | Internal capsule | 4 | 0.44 (23/53) | 0.47 (23/49) | 0.56 (29/51) | Yes | Control |
| 8 | 75 | F | R | L | Internal capsule and BG | 8 | 0.63 (22/35) | 0.76 (29/39) | 1.04 (34/33) | Yes | Control |
| 9 | 72 | M | R | L | Internal capsule | 10 | 0.33 (25/75) | 0.40 (29/74) | 0.64 (45/71) | Yes | Control |
| 10 | 58 | M | R | L | Internal capsule | 11 | 0.47 (39/83) | 0.48 (49/103) | 0.61 (60/99) | Yes | Control |
| 11 | 51 | M | R | L | Internal capsule and subcortical WM | 11 | 0.25 (10/40) | 0.47 (18/38) | 0.59 (27/47) | Yes | Control |
| 12 | 83 | F | R | L | Internal capsule and BG | 8 | 0.38 (12/31) | 0.47 (18/39) | No | Control | |
| 13 | 59 | M | R | L | Pons | 7 | 0.18 (20/112) | 0.29 (36/125) | 0.50 (50/99) | Yes | Control |
| Mean | 67.2 | 7.3 | 0.39 (25/65) | 0.55 (35/67) | 0.72 (51/76) | 17/26 | |||||
| SD | 13.1 | 3.6 | 0.29 (21/28) | 0.33 (26/29) | 0.30 (25/27) | ||||||
F, female; M, male; R, right; L, left; WM, white matter; BG, basal ganglia; Ses, session; SD, standard deviation.
Study Design
We used a sham-controlled, pseudo-randomized, single-blinded between-subject design. Pseudo-randomization ensured that both groups were balanced for factors known to influence motor recovery and outcome, that is, 1) age, 2) initial severity of hand motor deficit (as assessed by relative grip strength), and 3) number of days after symptom onset (Coupar et al. 2012). The first 10 patients included in the study were randomly assigned to a treatment group, which resulted in 4 M1-stimulation and 6 control-stimulation patients. All subsequent patients were matched to the most similar patient regarding the randomization factors (1–3) and accordingly assigned to the other treatment group. Of note, matching of patients was performed by an experimenter (C.G.) not involved in the behavioral, electrophysiological, or neuroimaging assessment of patients to exclude any selection bias.
Hand motor function, cortical excitability, and resting-state fMRI were assessed 1 day prior to the first stimulation (baseline) and 1 day after the last stimulation (post-intervention). Thus, the assessment of cortical excitability and resting-state fMRI did not reflect short-term stimulation aftereffects but changes that persisted for at least 24 h after the last stimulation. In addition, 17 patients participated in a third motor assessment 3–6 months post-stroke (follow-up). The follow-up period was chosen based on previous studies showing that >90% of spontaneous motor improvements usually occur during the first 3 months post-stroke (Nakayama et al. 1994).
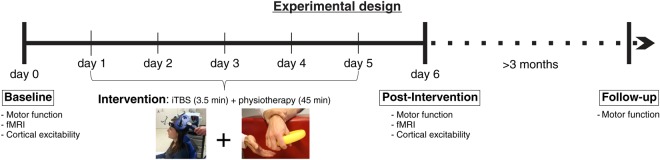
All patients underwent the same experimental protocol with iTBS directly preceding physiotherapy on days 1–5 (Fig. 1). The number of days on which iTBS was applied was determined by practical considerations since the stimulation had to be integrated into the clinical routine of the “early rehabilitation” program provided by the Department of Neurology, University Hospital Cologne, comprising daily physiotherapy, occupational, and speech therapy. All patients were enrolled into this program for 2 weeks, which allowed iTBS to be applied on 5 consecutive days considering the additional time needed for screening and enrolling patients after providing informed consent.
Figure 1.
Experimental design: After recruitment within their first week after stroke, all patients initially participated in motor hand function testing, assessment of cortical excitability via TMS and resting-state fMRI recording (baseline). Then, iTBS was administered preceding physiotherapy (priming) on 5 consecutive working days. On the subsequent day, patients again completed motor tests, assessment of cortical excitability, and fMRI recording (post-intervention). Of note, postinterventional testing was performed without stimulation on the same days, therefore testing rather persisting effects than short-term stimulation aftereffects. Finally, hand motor function was reassessed >3 months after stroke.
Only the stimulation location differed between groups: ipsilesional M1 in the M1-stimulation group and the parieto-occipital vertex in the control-stimulation group.
The experimenter who tested grip strength and cortical excitability was not blinded to the intervention. Then again, we deliberately chose to assess relative grip strength and the Jebsen–Taylor Hand Function Test (JTT), which both involve a device-dependent quantification (i.e., readout of the dynamometer and stop clock) rather than being rating-dependend to minimize any potential bias introduced by the investigator. Importantly, both the patient and the physiotherapist were blinded with respect to the stimulation, that is, whether the patient received M1- or control-stimulation.
Motor Hand Function
We assessed maximum grip strength as primary outcome parameter. The rationales for this were: 1) grip force represents a fundamental feature of recovered hand motor function, 2) it can be measured in an easy and quick but highly standardized fashion, even in severely affected patients still unable to perform more complex grasping movements, and 3) it is rather robust against compensatory movement strategies as the primary movement direction (finger flexion) cannot be significantly modified by recruiting other (e.g., more proximal) muscle groups. Therefore, increases in grip strength predominantly reflect restitution of neurological function rather than compensation achieved via learning alternative movement patterns (Buma et al. 2013). Furthermore, variation in grip strength has been directly related to M1 activity (Dettmers et al. 1995). Therefore, as the intervention aimed at enhancing M1 activity and connectivity, we assumed that grip strength represents a sensitive behavioral marker.
Maximum grip strength was measured via a vigorimeter (KLS Martin Group, Germany), which assesses the grip force exerted on a rubber ball enclosed by the whole hand of the participant. Of note, grip strength assessment derived from a flexible ball vigorimeter rather than a static dynamometer has been shown to be less dependent on individual hand anthropometry (Desrosiers et al. 1995). Patients performed 3 presses with each hand with breaks of 5 s in between to prevent fatigue. A comparison of the first and the last assessments within a session yielded no significant difference (baseline: P = 0.486; post-intervention: P = 0.330; paired t-test). Thus, we found no evidence that grip force assessments were influenced by muscle fatigue. For further analysis, the relative grip strength of the affected hand was computed based on the ratio between the average grip strength of the affected and unaffected hand. This procedure controlled for interindividual differences in absolute grip force, which may considerably vary between patients (e.g., grip force of a well recovered patient might still be lower than grip force of a patient with only little recovery but significantly higher force-levels per se). Furthermore, normalization of grip strength to the unaffected hand also allows controlling for unspecific intra-individual effects such as arousal or fatigue.
To test for more general effects of the intervention on motor function, we assessed performance in the JTT. The JTT is a speed-based test battery probing upper limb function by simulated daily living situations like eating or drinking. In accordance to earlier studies with stroke patients, each subtest of the JTT was limited to 120 s, and this maximum time was assigned if a patient could not perform a given subtest (Duncan et al. 1998). Additionally, statistical analyses were also computed using subtest time limits of 45 and 60 s.
Statistical analysis of the behavioral data was performed using SPSS version 22 (Statistical Package for the Social Sciences, IBM). Treatment effects were evaluated using repeated measures analyses of variance (rm-ANOVAs) comparing the between-subject factor GROUP (2 levels: M1 stimulation and control stimulation) and the within-subject factor SESSION (2 levels: baseline and post-intervention). To account for residual differences between treatment groups, we computed additional rm-ANOVAs including 1) age, 2) time since stroke, and 3) initial motor impairment (relative grip strength at baseline) as covariates of no interest. Post hoc two-sided t-tests were used to elucidate significant interaction effects (P < 0.05). Finally, we tested whether treatment effects observed after the first week of stimulation extended into the follow-up session (one-sided t-test given the clear directional hypothesis).
Cortical Excitability
Cortical excitability was assessed by measuring amplitudes of motor evoked potentials (MEP) which were elicited via neuronavigated single-pulse TMS. Electromyogram (EMG) activity was recorded from the abductor pollicis brevis muscle of the paretic hand. EMG activity was assessed using Ag/AgCl surface electrodes (Tyco Healthcare, Neustadt, Germany) placed in a belly-to-tendon montage, and electrode positions were kept constant between sessions. The EMG signal was amplified, filtered (0.5 Hz high-pass and 30–300 Hz band-pass) and digitized using a Power-Lab 26T device and LabChart software package version 6.0 (ADInstruments Ltd, Dunedin, New Zealand). TMS was performed using a Magstim 2002 stimulator (The Magstim Co. Ltd, Whitland, UK) equipped with a 70-mm figure-of-eight coil. Coil positions were recorded and maintained with a Brainsight2 computerized frameless stereotaxic system (Rogue Research, Inc., Montreal, Canada). The motor hotspot was defined as the position eliciting the MEP of the highest amplitude in response to the TMS pulse applied tangentially to the skull with posterior-anterior current direction. MEP amplitudes were assessed with monophasic pulses using the Magstim 2002 stimulator. In contrast, resting motor thresholds (RMT) were determined with biphasic pulses using the Magstim SuperRapid2, as those were utilized to individualize iTBS intensities. Of note, both motor hotspot and RMT were independently defined for both stimulators. The neuronavigation software confirmed a very high degree of spatial overlap of hotspots defined with biphasic and monophasic pulses.
The RMT was defined using an algorithm provided by the TMS motor threshold assessment tool (MTAT) 2.0 (http://www.clinicalresearcher.org/software.htm) (Awiszus 2003) which performs parameter estimation by sequential testing. The algorithm proposes stimulation intensities that are subsequently tested regarding their ability to induce an EMG response >50 µV, which is accordingly entered by the experimenter. The MTAT has been shown to accurately estimate motor thresholds using less stimuli than the standard 5-out-of-10 rule (Awiszus 2003).
Of note, RMT assessment was defined using the same hotspot (i.e., defined at baseline) for both the assessment before and after the intervention. Although motor representations may change during recovery post-stroke, individual hotspots separately identified for both sessions (baseline and post-intervention) were located in the range of <10 mm according to the neuronavigation software thus rendering a significant bias induced by the use of the same hotspot across sessions highly unlikely given the suspected TMS resolution within a very similar range (Thielscher and Wichmann 2009). At baseline and post-intervention, 36 MEPs were recorded at an intensity of 120% RMT at a frequency of ∼0.1–0.2 Hz.
Intervention
M1-stimulation patients received iTBS over ipsilesional M1. Control stimulation was delivered over the parieto-occipital vertex using a stimulation intensity that was individualized using the same procedure utilized for M1-stimulation. To reduce possible cortical stimulation effects in the control condition, the coil was angled at 45°, touching the skull not with the center but with the rim opposite the handle. In this position, the coil–cortex distance is essentially larger such that the electromagnetic field, if at all reaching the cortex, is substantially weaker and far outside the target range while the typical TMS skin sensation is preserved (Herwig et al. 2010). Using this procedure, a recent study reported no difference in the perception of real and sham stimulation (Herwig et al. 2010). Of note, we here used a between-subject design of TMS-naïve patients, so there was no prior knowledge that allowed the patients to differentiate between M1- and control-stimulation.
iTBS was applied according to the original description of Huang et al. (2005) using a Magstim SuperRapid2 with a figure-of-eight coil (70-mm standard coil, The Magstim Co. Ltd). iTBS consisted of 3 pulses delivered at a frequency of 50 Hz every 200 ms during 2 s (10 bursts) and repeated every 10 s for a total duration of ∼3.5 min (600 pulses) (Huang et al. 2005). Individual stimulation intensity was set to 70% RMT, representing a slight modification of the original protocol. We decided to individualize iTBS intensity using 70% RMT instead of 80% active motor threshold (AMT), because the latter would have required the patients to perform constant and well-controlled contractions of the TMS target muscle which is usually not possible for the paretic hand, especially in severely impaired patients. Importantly, we and other laboratories have repeatedly demonstrated that iTBS administered at 70% RMT has aftereffects on cortical excitability similar to those achieved with 80% AMT and can hence be regarded to be an effective variant for increasing cortical excitability (Gentner et al. 2008; Cardenas-Morales et al. 2014; Nettekoven et al. 2014).
In 7 patients of the M1-stimulation group and 6 patients of the sham group, stimulation thresholds exceeded the maximum stimulator output (MSO) that could be used for administering iTBS. In these patients, stimulation intensity was set to 50% MSO which represents the upper limit for 50-Hz stimulation with a Magstim SuperRapid2. The proportion of patients stimulated at 50% MSO was balanced between stimulation groups.
About 3 min after administration of iTBS, patients started standard physiotherapy under the guidance of a professional physiotherapist. Physiotherapy focused on hand motor function using a standardized training repertoire adjusted to the individual level of impairment (duration: 45 min). Passive and active grasping and object manipulation movements performed as part of the training repertoire likely impacted on the grip strength level of patients, explicit grip strength training was however not part of the daily training. All patients received an equivalent amount of rehabilitative training between baseline and post-intervention assessment.
Functional Magnetic Resonance Imaging
Resting-state blood oxygenation level dependent activity was acquired on a Siemens Trio 3.0T scanner (Siemens Medical Solutions, Erlangen, Germany). Subjects were instructed to remain motionless and to fixate a red cross on a black screen during scanning. We scanned patients with open eyes in order to prevent fatigue during the ∼7 min session. Patients were monitored by means of an infrared camera attached to the end of the scanner. Data were acquired using gradient echo-planar imaging (EPI) with the following parameters: repetition time (TR) = 2200 ms, echo time (TE) = 30 ms, field of view (FOV) = 200 mm, 33 slices, voxel size: 3.1 × 3.1 × 3.1 mm3, 20% distance factor, flip angle = 90°, 184 volumes. The slices covered the whole brain extending from the vertex to lower parts of the cerebellum.
After completion of the resting-state scans, patients performed a motor task, which served as functional localizer task. This task consisted of visually cued rhythmic fist closures performed in blocks of unimanual movements. Written instructions displayed for 2 s indicated whether the left or the right hand had to be moved in the upcoming block of trials. Subjects were asked to perform fist closures at the frequency of a blinking circle for 15 s until a black screen indicated to rest for 15 s (plus a temporal jitter of 1–2.5 s). The following parameters were used: TR = 2200 ms, TE = 30 ms, FOV = 200 mm, 33 slices, voxel size: 3.1 × 3.1 × 3.1 mm3, 20% distance factor, flip angle = 90°, 283 volumes.
In addition to the fMRI measurements, DWI images were acquired to identify the location and extent of the stroke lesion (TR = 5100 ms, TE = 104 ms, FOV = 230 mm, 30 slices, voxel size =1.8 × 1.8 × 3.0 mm3).
Resting-state Functional Connectivity
FMRI data were analyzed using the Statistical Parametric Mapping software package (SPM8, http://www.fil.ion.ucl.ac.uk/spm/). Patients with right-hemispheric lesions were flipped along the midsagittal plane, so that all lesions were in the left hemisphere. Notably, the number of patients with right-sided lesions was balanced across groups (3 per group, see Table 1). The first 4 volumes (“dummy” images) of each fMRI session were discarded from further analyses to allow for magnetic field saturation. Remaining EPI volumes were realigned to the mean image of each time series and co-registered with the DWI-weighted image. Lesion masks were constructed from the DWI volume showing the largest lesion extent using MRIcron (www.sph.sc.edu/comd/rorden/MRicron). All images were spatially normalized to the standard MNI template using the unified segmentation approach with masked lesions (Ashburner and Friston 2005) and smoothed using an isotropic Gaussian kernel of 8 mm full-width at half-maximum. Lesion maps were used to mask functional images for further analysis.
Prior to statistical analysis, variance that could be explained by known confounds was removed from the smoothed resting-state time series. Confound regressors included the mean-centered global, gray matter, white matter, and cerebrospinal fluid signal intensities per time point as obtained by averaging across tissue-class-specific voxels identified via the respective SPM8 segmentation and their squared values, the 6 head motion parameters, their squared values as well as their first-order derivatives. Furthermore, signal changes that were coherent across the whole brain as reflected by the first 5 components of a principal component analysis (PCA) decomposition of the whole-brain time series were removed (zu Eulenburg et al. 2012; Satterthwaite et al. 2013). The use of global time-series regression has been a controversial issue in the recent literature, as it has been suggested to bias subsequent correlation analyses, possibly resulting in the miss-localization of correlations (for further details see Saad et al. 2012). Then again, it has been shown that global signal regression reduces head motion based artifacts in a highly efficient fashion and therefore represents an appropriate and helpful step in preprocessing resting-state fMRI data (Power et al. 2014). To exclude a considerable impact of our preprocessing steps on connectivity findings, we re-calculated all neuroimaging analyses without global signal regression or PCA. Apart from the expected decrease in signal-to-noise ratio (Power et al. 2014), we observed comparable group differences in a very similar set of regions. This renders a considerable impact of the global signal regression and PCA on the spatial location of significant correlations with the M1 time course highly unlikely (see Supplementary Fig. 1).
Between-group differences and between-session differences (within-group) in head movement parameters from image realignment were analyzed via comparison of frame-wise displacement and the root mean squared movement (Satterthwaite et al. 2013). These analyses did not show differences in head movement parameters, neither between groups nor between sessions (all t-tests, P > 0.2).
Data were band-pass filtered preserving frequencies between 0.01 and 0.08 Hz for subsequent analysis (Fox and Raichle 2007). Seed-based whole-brain functional connectivity was computed for the ipsilesional M1 at subject-specific coordinates derived from the functional localizer task. The first eigenvariate (representing the main component of activity) of the adjusted time series from all voxels within a 10-mm sphere centered on the seed-voxel was correlated with the time course of every other voxel in the brain by means of linear Pearson's correlation coefficients (zu Eulenburg et al. 2012). Correlation coefficients were converted to Fisher's Z-scores to yield approximately normally distributed data (Biswal et al. 1995). Finally, resting-state maps were masked by cytoarchitectonic probability maps of frontoparietal sensorimotor areas (Brodmann areas 6, 4 a/p, 3 a/b, 2, and 1) to focus inference on functional connectivity within the cortical sensorimotor network as provided by the SPM Anatomy Toolbox (Eickhoff et al. 2005; see Rehme et al. 2015 for further details). We limited the analysis to cortical areas given their pivotal role in functional recovery after stroke (Carter et al. 2010; van Meer et al. 2010; Park et al. 2011).
To assess stimulation-induced within-group differences between baseline and the post-intervention session, changes in functional connectivity were determined by subtracting individual baseline functional connectivity maps from the respective maps after treatment for each subject. For between-group-level analysis, the individual subtraction maps were entered into a random-effects, flexible factorial analysis of variance testing the factor GROUP (2 levels: M1-stimulation and control-stimulation). The statistical threshold was set to P < 0.05, family-wise error (FWE)-corrected at the cluster level (cluster-forming threshold: P < 0.05 uncorrected).
Correlation Analyses
We finally tested whether individual differences in motor outcome as indicated by relative maximum grip strength were related to changes in connectivity. We decided to use “motor outcome” and not “change in motor performance” for 2 reasons. First, motor outcome may be of higher relevance from a patient's point of view, as even a strong relative change (e.g., from 2% to 10% relative grip strength) may be of small significance if the outcome level remains poor. More importantly, surrogates of cortical reorganization (changes in functional connectivity) do not only reflect recovery of function but are also strongly related to the level of initial impairment (Rehme et al. 2011). For example, an increase in relative grip strength from 10% to 20% is likely accompanied by different neural changes compared with a similar 10% increase from 80% to 90% relative grip strength. Therefore, to account for these effects, we considered “motor outcome” as the variable of interest for the correlation analyses.
Analysis of Structural Damage
Individual lesion volumes were calculated from MNI normalized lesion maps (based on DWI images) using FSLv5.0 (http://fsl.fmrib.ox.ac.uk). Furthermore, anatomical lesion distribution was assessed using voxel-based lesion symptom mapping (VLSM) as implemented in MRIcron (www.sph.sc.edu/comd/rorden/MRicron). Finally, damage to the corticospinal tract (CST) is known to critically impact on motor function and recovery thereof (Stinear et al. 2007). The degree of CST damage was assessed by superimposing the individual normalized lesion maps upon probabilistic myeloarchitectonic maps of the CST as implemented in the SPM Anatomy Toolbox (Eickhoff et al. 2005). CST damage was defined as intersection volumes of individual lesions relative to the total CST volume (for further details see Rehme et al. 2011; Volz et al. 2015).
Results
All patients completed the intervention protocol without adverse events. Of the 26 patients included in the study, 17 were available for the follow-up assessment >3 months later (M1-stimulation: n = 7, control-stimulation: n = 10, see Table 1). Drop-outs were due to various reasons like relocation of residence to a distant city, new diseases interfering with mobility (e.g., orthopedic disease) or incompliance. Please note that this study was not designed as a clinical trial to assess long-term effects but rather immediate impact of iTBS on motor recovery and cortical reorganization. Importantly, no difference in mortality or re-infarction rate was evident between groups.
The pseudo-randomization procedure resulted in 2 equally sized groups, which did not differ in baseline levels of initial motor impairment (relative grip strength: M1-stimulation group: 0.41 ± 0.34%, control-stimulation group: 0.36 ± 0.24%, P = 0.620; JTT: M1-stimulation group: 405.4 ± 362.6 s, control-stimulation group: 381.7 ± 317.9 s, P = 0.861). Furthermore, age (M1-stimulation group: 69.7 ± 12.0 years, control-stimulation group: 64.7 ± 13.3 years, P = 0.341) and time since stroke (M1-stimulation group: 7.1 ± 2.9 days, control-stimulation group: 7.5 ± 4.3 days, P = 0.750) did not differ between groups. Likewise, for the subsample that completed the follow-up measurement, none of these baseline parameters differed between groups (all P > 0.4).
Structural Damage
Lesion volumes did not differ significantly between groups (M1-stimulated group: 30 674 ± 23 576 mm3, control-stimulated group: 36 574 ± 46 739 mm3, P = 0.689). Likewise, when comparing lesion locations between groups using VLSM, we found no significant difference (P > 0.1). Furthermore, the percentage of damaged CST fibers was highly similar between groups (M1-stimulated group: 11.4 ± 9.7%, control-stimulated group: 11.4 ± 15.2%, P = 0.999). In summary, there was no group bias with respect to lesion volume, localization, or CST damage.
Motor Hand Function
All patients significantly improved in both motor tests in the post-intervention compared to the baseline assessment (ANOVA, main effect of SESSION, P < 0.05). However, we found a significant interaction between GROUP × SESSION (F1,24 = 4.970, P = 0.035; Fig. 2) for relative grip strength. While the control-stimulated patients gained 10.54 ± 8.82% SD in relative grip strength, M1-stimulated patients improved by 21.38 ± 15.16% between sessions (Cohen's d = 0.904). Importantly, this interaction remained significant after introducing covariates of no-interest accounting for residual group differences in initial motor impairment, age and time since stroke (F1,21 = 6.637, P = 0.018). Since more control-stimulated patients were recruited 10 days or later post-stroke, that is, at a time point when spontaneous resolution of perilesional edema has probably taken place (which might interfere with recovery rates), we recalculated the analyses removing all patients recruited >10 days after stroke (GROUP × SESSION interaction F1,14 = 7.008, P = 0.019) and also compared the resulting subgroup of 8 control-stimulated patients with 8 matched M1-stimulated patients (F1,11 = 8.398, P = 0.014). Likewise, when excluding subjects in whom no MEP could be elicited at baseline (n = 7), this interaction effect also remained significant (F1,17 = 4.984, P = 0.039). Finally, to control for differences in which hemisphere was lesioned, we included this as additional covariate into the model and still observed a significant GROUP × SESSION interaction (F1,20 = 6.351, P = 0.020).
Figure 2.
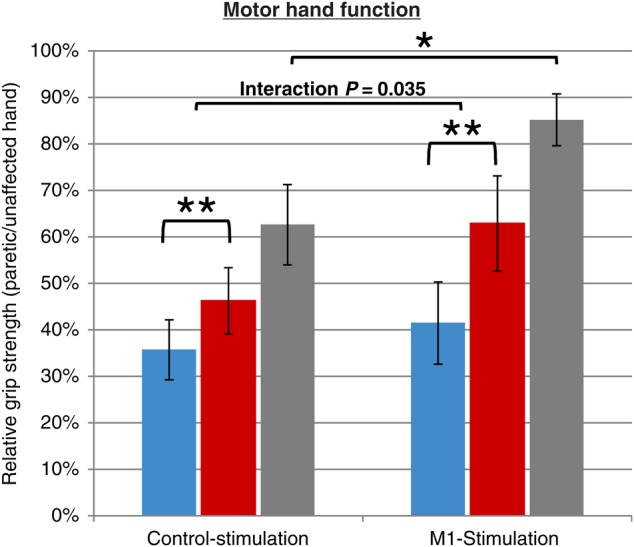
Motor hand function: Relative grip strength (paretic/unaffected hand), at 3 time-points: baseline (blue), post-intervention (red), and follow-up (gray), for the control-stimulation (left) and M1-stimulation group. Patients of both groups significantly improved between baseline and post-intervention (**P < 0.001). Furthermore, comparing the improvement between groups, the M1-stimulated patients recovered significantly stronger between baseline and post-intervention assessment (ANOVA interaction [SESSION × GROUP]: P = 0.035). Finally, >3 months post-stroke (n = 17), motor function was still significantly different between groups (P = 0.031), indicating repeated application of iTBS to improve hand motor function in acute stroke beyond the intervention period.
Post hoc t-tests revealed that relative grip strength was significantly higher in the M1-stimulation compared with the control-stimulation group post-intervention (P = 0.015). Of note, we observed no significant difference between the change in grip strength of the unaffected hand between groups (t-test, P > 0.1), indicating that the effect observed for the relative grip strength was in fact driven by improvements of the paretic hand.
Furthermore, improvements in relative grip strength over time were higher in M1-stimulated compared with control-stimulated patients (P = 0.035). In the subsample of patients that could be recruited for follow-up measurements more than 3 months later, relative grip strength was significantly higher in M1-stimulated compared with control-stimulated patients (P = 0.031, Fig. 2). However, the latter finding has to be treated with caution regarding the reduced sample size and post-intervention treatments that were heterogeneous across patients. In contrast, we found no interaction effects (GROUP × SESSION) for the JTT (F1,24 = 0.860, P = 0.363). Likewise, no significant interaction effects were observed when recalculating the analysis of the JTT data using 45 or 60 s subtest time limits (all P > 0.1).
In summary, these data suggest a positive effect of iTBS applied over M1 on grip strength.
Cortical Excitability
No significant difference was evident between MEP amplitudes recorded at baseline (M1-stimulation group: 0.3 ± 0.2 mV, control-stimulation group: 0.2 ± 0.2 mV, P = 0.210). Likewise, TMS motor thresholds were highly similar across groups at baseline (M1-stimulation group: 67.0 ± 22.8% MSO, control-stimulation group: 66.4 ± 27.1% MSO, P = 0.951). Therefore, stimulation intensities used for iTBS application were also very similar across treatment groups. Furthermore, subjects in whom RMT exceeded the MSO of the TMS machine were balanced across groups (M1-stimulation: n = 3, control-stimulation: n = 4) which may be critical regarding the individual capacity for motor recovery (Stinear et al. 2012). No significant change in cortical excitability (MEP amplitudes in mV) was observed between baseline and post-intervention measurements across all subjects (main effect of SESSION: F1,24 = 2.173, P = 0.153). Similarly, no significant change in RMTs was observed (main effect of SESSION: F1,24 = 2.212, P = 0.150). Importantly, the, assessment of cortical excitability was performed 1 day prior to the first and again 1 day after the last administration of iTBS. This, however, does not exclude that iTBS led to effective modulation of cortical excitability immediately after stimulation since iTBS only transiently enhances cortical excitability for about 20–60 min (Huang et al. 2005; Cardenas-Morales et al. 2014).
Resting-State Functional Connectivity
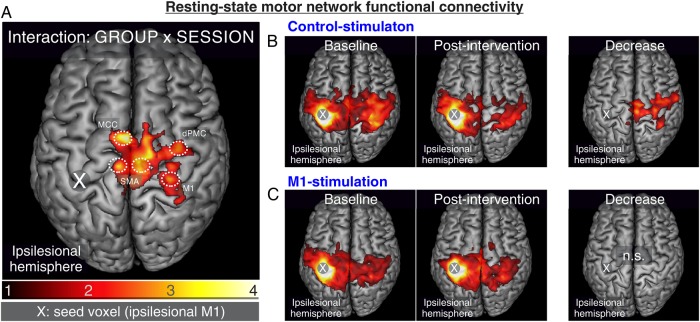
We observed significant functional connectivity between ipsilesional M1 (seed region) and a bilateral cortical motor network (Fig. 3). Differential contrasts between connectivity maps at baseline did not show differences between the M1-stimulation group and control-stimulation group (P > 0.1, FWE-corrected at the cluster level).
Figure 3.
Resting-state motor network functional connectivity: Seed-based resting-state connectivity of ipsilesional M1 (P < 0.05, FWE-corrected at the cluster level). iTBS resulted in higher functional connectivity of the stimulated M1 with a bihemispheric network comprising ipsilateral MCC, bilateral SMA, contralesional dPMC, and contralesional M1 (A, ANOVA: interaction [GROUP × SESSION]). In accordance with the literature, interhemispheric resting-state connectivity of ipsilesional M1 decreased in the early subacute phase in control-stimulated patients (B). Interestingly, no significant decrease was evident in the M1-stimulation group (C), which instead featured connectivity increases at an uncorrected level.
Computing an interaction contrast between GROUP and SESSION demonstrated significantly higher connectivity in the M1-stimulation group of the stimulated region with ipsilesional supplementary motor area (SMA) (MNI-coordinates: −9/−18/54), ipsilesional midcingulate cortex (MCC, −2/0/44), contralesional SMA (6/−15/54), contralesional dorsal premotor cortex (dPMC) (34/−6/51), and contralesional M1 (27/−27/56) after treatment compared to baseline (Fig. 3A, P < 0.05, FWE-corrected at the cluster level). Of note, recalculating changes in connectivity on a whole-brain level, that is, without masking for cortical motor areas, showed a significant interaction for exactly the same cluster (P < 0.05, FWE-corrected at the cluster level), without any additional significant connectivity changes in non-motor regions. Likewise, when recalculating the analysis for the subgroup of 8 control-stimulated patients included <10 days post stroke and 8 matched M1-stimulated patients, we found highly similar results (see Supplementary Fig. 2).
Comparing connectivity maps pre- and post-stimulation revealed that M1-connectivity with motor areas of the contralesional hemisphere decreased over time in control-stimulated patients (Fig. 3B, P < 0.05, FWE-corrected at the cluster level). In contrast, no significant decrease in connectivity was observed in the M1-stimulation group (Fig. 3C). Furthermore, no significant increases in M1 connectivity were observed at a corrected level for neither group. However, at an uncorrected level (P < 0.05), functional connectivity between ipsilesional M1 and ipsilesional areas (i.e., MCC and SMA) featured significant increases for the M1-stimulation group.
In summary, a decrease in functional connectivity between ipsilesional M1 and a bilateral motor network was evident in the control-stimulation group, whereas no significant decreases in motor network connectivity were observed after M1-stimulation.
Correlation Analyses: Reorganization and Motor Function
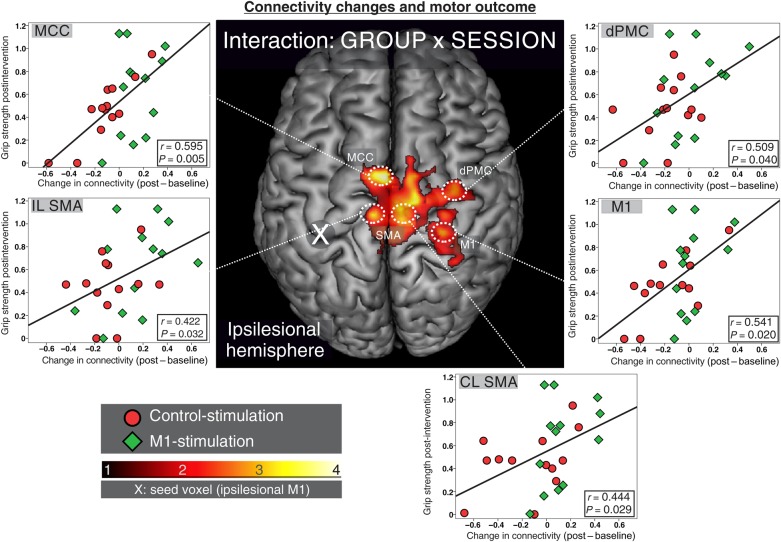
We next tested, whether changes in functional connectivity were correlated with relative grip strength.
Therefore, changes in connectivity estimates were extracted from the local maxima (including all voxels within spheres of 4-mm radius) of the interaction contrast between GROUP and SESSION (see above, Fig. 3A), and subsequently correlated with post-interventional relative grip strength (Fig. 4). We observed significant positive correlations between post-interventional relative grip strength and connectivity changes in ipsilesional MCC (r = 0.595, P = 0.005), ipsilesional SMA (r = 0.422, P = 0.032), contralesional SMA (r = 0.444, P = 0.029), contralesional dPMC (r = 0.509, P = 0.040), and contralesional M1 (r = 0.541, P = 0.020, all false discovery rate--corrected for multiple comparisons). Thus, patients with better motor outcome featured increased (or at least less decreased) functional connectivity between M1 and bilateral motor areas. Of note, functional connectivity between areas and ipsilesional M1 was significantly enhanced following M1-stimulation compared to control-stimulation.
Figure 4.
Connectivity changes and motor outcome: Changes in connectivity in the local maxima of the interaction contrast (GROUP × SESSION) significantly correlated with motor outcome post-intervention for ipsilesional MCC, bilateral SMA, contralesional dPMC, and contralesional M1 (M1-stimulated patients are indicated by green diamonds, control by red circles, P < 0.05 false discovery rate-corrected). Increases in connectivity between M1 and all of these regions were observed in patients featuring good motor outcome. Thus, M1-stimulation-induced increases in connectivity seem to promote recovery of motor function (IL, ipsilesional; CL, contralesional).
Discussion
Priming physiotherapy with iTBS applied to ipsilesional M1 significantly enhanced recovery of grip strength compared with control stimulation in subacute stroke. At the network level, M1-stimulation seems to have prevented a decrease of motor system connectivity assessed at rest, which was observed in control-stimulated patients. Less decline of functional connectivity was associated with better outcome, suggesting stimulation-induced increases in connectivity to represent a neural mechanism contributing to recovery of grip strength in subacute stroke.
rTMS in Motor Rehabilitation After Stroke
Behavioral aftereffects resulting from rTMS of ipsilesional M1 in stroke patients are thought to derive from the induction of cortical plasticity (Bates and Rodger 2015). At the cellular level, the induction of long-term potentiation (LTP)-like plasticity by iTBS might amplify the impact of subsequent motor training (Reis et al. 2008; Ackerley et al. 2010). However, while a single application of iTBS priming subsequent motor training has been shown to transiently improve motor function (Talelli et al. 2007; Ackerley et al. 2010), lasting effects could thus far not be achieved in chronic stroke, even when repeating the stimulation over 10 consecutive days (Talelli et al. 2012). One explanation for this negative finding might be that spontaneous post-stroke reorganization is already close to completion, putatively limiting the induction of neural plasticity by iTBS in the chronic post-stroke phase. This may ultimately preclude an effective promotion of training effects achieved via subsequent physiotherapy (Grefkes and Fink 2012). Indeed, animal studies showed that cellular processes enhancing neuronal plasticity like, for example, neuronal sprouting, changes in genetic transcription/translation as well as secretion of growth factors are especially active in the acute phase after a lesion, thereby opening a critical time window for brain reorganization (Hermann and Chopp 2012). For example, Carmichael et al. (2005) reported that changes in gene expression promoting axonal growth last for 2–3 weeks after stroke. Assuming a similar time window in humans, we stimulated our patients in this early period, hypothesizing that enhancing neural plasticity by iTBS might be especially effective when plasticity of the brain is generally enhanced. Our data support the hypothesis that plasticity induction via iTBS increases the efficacy of subsequent training as patients who received M1-stimulation before physiotherapy featured better motor outcome in terms of relative grip strength, not only immediately after the intervention period but also in the chronic phase (Fig. 2).
From a mechanistic perspective, the connectivity findings of the present study imply that M1-stimulation induced a network-level effect beyond the induction of synaptic plasticity, which probably contributed to the recovery of motor function. In principle, improvements in grip strength could also result from reorganizational changes at the spinal level induced by propagation of iTBS effects through the corticospinal tract (Mori et al. 2010). While we cannot exclude such effects, the fMRI finding of significant iTBS effects on motor network connectivity in areas whose connectivity co-vary with hand motor performance strongly imply cortical changes to be engaged in iTBS-associated improvements. Consistent with the latter, a number of studies demonstrated that iTBS applied over M1 primarily interferes with properties of the motor cortex (Funke and Benali 2011; Murakami et al. 2012; Nettekoven et al. 2014).
Network Disturbance After Stroke
Pathophysiological alterations and reorganization after stroke are not limited to perilesional tissue but also extend to large-scale neural networks. Such system-level effects can be analyzed by assessing connectivity between brain regions using neuroimaging data (Grefkes and Fink 2011). A number of studies have used resting-state functional connectivity to demonstrate characteristic network alterations over time in association with motor recovery of stroke patients (Grefkes and Ward 2014; Silasi and Murphy 2014). Both animal and human studies reported gradual decreases in motor network resting-state connectivity early after stroke with declining connectivity observed within the first 2–4 weeks after stroke (van Meer et al. 2010; Park et al. 2011). Using an animal stroke model, van Meer et al. (2010) demonstrated a characteristic time-course of network changes post-stroke: interhemispheric resting-state connectivity between sensorimotor areas progressively decreases over the first 2 weeks, with a subsequent re-increase alongside recovery of sensorimotor functions. Park et al. (2011) reported highly similar findings in humans, and found the lowest level of interhemispheric motor network connectivity to occur 1 month after stroke, followed by a subsequent re-increase. Our data converge with these findings, showing a significant decrease in functional connectivity between ipsilesional M1 and a network of contralesional motor areas including contralesional M1 in control-stimulated patients (Fig. 3). Several mechanisms have been discussed to contribute to this gradual, early decrease in functional connectivity, such as diminished gamma-aminobutyric acid-mediated interhemispheric inhibition (Witte et al. 2000) or depressed perilesional glutamatergic neurotransmitter metabolism (van der Zijden et al. 2008).
From a functional perspective, decreases in functional motor network connectivity after stroke have been frequently associated with the concept of diaschisis (for a review see Carrera and Tononi 2014), originally proposed by von Monakow (1914). This concept postulates that an acute lesion to one part of the brain consecutively leads to a reduction of excitatory input into regions remote of but connected to the lesion. Accordingly, recovery of function is thought to reflect at least in part a re-activation of initially functionally deafferented brain regions (von Monakow 1914).
Seminal work by Bestmann et al. (2003, 2005) demonstrated that rTMS not only influences neuronal properties of the stimulated region but also modulates activity levels of remote but interconnected brain areas. Likewise, we recently demonstrated that a single application of iTBS to M1 in healthy subjects increases resting-state connectivity of the stimulation site with remote motor areas (ipsi- and contralateral premotor cortex) (Nettekoven et al. 2014). In fact, in the present study, we observed significantly higher motor network connectivity for the M1-stimulated compared with control-stimulation group as highlighted by the significant interaction contrast (Fig. 3). Hence, iTBS applied over ipsilesional M1 seems to prevent a decrease of connectivity in the motor network.
A potential mechanism underlying this observation lies in the induction of cortical plasticity, which might concurrently increase functional connectivity between the stimulated M1 and remote motor areas. Support for this hypothesis stems from a recent animal study, which reported repetitive stimulation of the ipsilesional M1 to induce the expression of neurotrophic factors in contralesional M1, strongly suggesting the induction of synaptic plasticity not only locally but also in remote motor areas (Cheng et al. 2014). A functional relevance of motor network connectivity is also indicated by our data demonstrating a significant correlation of connectivity changes in these areas and motor outcome (Fig. 4). Here, especially those patients with almost no decrease or even an increase in resting-state connectivity featured the best motor outcome. However, it is important to note that the correlation analysis included all patients and was not exclusive for the M1-stimulation group. Hence, the relationship between increased levels of M1-connectivity and motor outcome is not specific to the iTBS intervention, but rather reflects a more general aspect of motor recovery. In line with this observation, numerous studies have described higher levels of motor network connectivity to be associated with better motor recovery (Carter et al. 2010; van Meer et al. 2010; Park et al. 2011). Therefore, higher levels of motor network connectivity in the M1-stimulation group as found in the present study imply that M1-stimulation may have facilitated network reorganization compared with the control-stimulation group.
We can only speculate whether similar changes in connectivity would have been observed if connectivity was assessed during performance of a motor task. For healthy subjects, we have recently shown that resting-state connectivity within the motor system is only weakly correlated with coupling parameters computed for simple motor tasks, highlighting the state dependency of connectivity estimates (Rehme et al. 2013). However, this does not exclude that lesion-associated changes in resting-state connectivity correlate with task-state connectivity. For example, resting-state and task-based connectivity studies with stroke patients have congruently reported altered interhemispheric connectivity between bilateral M1 to be associated with motor impairment and recovery (Carter et al. 2010; van Meer et al. 2010; Grefkes and Fink 2011; Park et al. 2011; Rehme et al. 2011; Volz et al. 2015). Therefore, it may well be that stroke-induced changes in interhemispheric motor network connectivity during rest may relate to task-state connectivity which needs to be assessed in future studies.
Limitations
One limitation of the study design is that we could not assess the immediate aftereffects of iTBS on M1 excitability, for example by means of input–output curves, as this would have delayed the start of the training session, which is critical given the decay of the iTBS effect within the first 20 min post-stimulation (Huang et al. 2005; Cardenas-Morales et al. 2014). Therefore, it remains unclear how patients responded to iTBS in terms of changes in cortical excitability. Hamada et al. (2013) reported that only about 50% of healthy subjects feature a canonical response to iTBS, that is, show increased cortical excitability after stimulation, giving rise to the question whether the beneficial impact of iTBS on motor function might be even greater if only “responding” patients were included in our study. Then again, Di Lazzaro et al. have previously shown that iTBS is an effective method to induce cortical plasticity in subacute stroke patients. Thus, we have strong reasons to assume that iTBS actually induced cortical plasticity in our patient cohort (Di Lazzaro et al. 2008). Nevertheless, using electrophysiological and neuroimaging data to identify biomarkers, which may predict the individual response to iTBS and the potential to promote motor recovery by neuromodulatory interventions, represents a critical goal for future research.
Furthermore, stimulation intensities were limited to 50% MSO due to technical reasons. Thus, patients with very high motor thresholds (i.e., above 71%) could not be stimulated with 70% RMT. However, limiting the maximum intensity also prevented the use of excessive stimulation intensities in patients featuring highly increased RMT, which might cause different aftereffects on a cellular level compared with lower intensity stimulation and increase the risk of seizure induction. Arguably, this procedure resulted in 2 different intervention protocols regarding the individual adjustment of stimulation intensities. Furthermore, one might argue that we could have used cortical excitability measures of the contralesional hemisphere to individualize stimulation intensities instead. Then again, cortical excitability in the contralesional hemisphere may also be altered following stroke (Volz et al. 2015), hence also biasing the definition of individual stimulation intensities. To exclude that the difference in intensity individualization substantially impacted on iTBS effects on motor recovery, we compared patients for whom M1-stimulation intensity was individualized to those that received iTBS applied at 50% MSO and found no difference in motor recovery (relative grip strength: P = 0.369; JTT: P = 0.625; two-sided t-test).
Our study is certainly limited by the small sample size and the fact that patients and physiotherapists but not experimenters were blinded to the nature of the intervention. However, the fact that we observed significant group differences in recovery of motor function as well as in changes in motor network connectivity (which were completely independent of the behavioral assessment) corroborates our findings. Before any conclusion can be drawn for potential clinical utilization, prospective double-blinded clinical trials are needed to further evaluate the effect of iTBS on motor recovery in larger cohorts of patients.
An additional problem especially encountered in small samples lies in the heterogeneity of stroke patients regarding factors possibly impacting on individual motor recovery. To account for these differences, we balanced groups for putative confounds such as age, time since stroke, and initial motor impairment. Likewise, lesion volumes and locations as well as cortical excitability were not different between groups, providing further support that observed differences in motor outcome indeed resulted from the iTBS intervention. In particular, one might argue that the slight remaining group difference in relative grip strength (5%) with higher levels observed in the M1-stimulation group indicates the M1-stimulation group to suffer from less motor impairment compared with control-stimulated patients, possibly accounting for motor improvements independent of the intervention. Then again, baseline JTT values suggest the M1-stimulation group to feature stronger initial motor impairment compared to the control-stimulation group, thus rendering a systematic difference in initial motor impairment highly unlikely.
The question arises why the positive effects on recovery of relative grip strength observed in the M1-stimulation group were not paralleled by the JTT. From a clinical perspective, it is well known that basic motor parameters like grip force show faster recovery than motor abilities in more complex tasks which rely not only on grip strength but also on speed and coordination of movements. As discussed above, modulation of grip strength has been shown to directly depend on M1 activity, while reaching and grasping tasks (as tested in the JTT) strongly rely on activity in frontoparietal networks (for a review see Turella and Lingnau 2014). Therefore, it is conceivable that enhancing M1 activity by means of iTBS also shows strongest effects in motor aspects relying on M1 activity and connectivity. Whether or not other stimulation targets (e.g., premotor or posterior parietal cortex) would differentially impact on the recovery of more complex motor tasks needs to be addressed in future studies.
Summary and Conclusion
We show that iTBS can be used to promote recovery of grip strength, when repetitively “priming” physiotherapy in the early subacute phase post-stroke. Importantly, the beneficial impact of the intervention seems to not only result from the induction of local LTP-like processes at the stimulation site priming motor training, but also from the enhancement of motor network connectivity. From a conceptual perspective, the stimulation-induced enhancement of connectivity might well represent alleviation of diaschisis contributing to early recovery of motor function. The promising iTBS effects found in the present study need to be replicated in a randomized clinical trial with a large sample of patients in order to further determine the potential of iTBS-primed physiotherapy for recovery of motor function in early subacute stroke.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
C.G. is supported by the German Research Foundation (DFG GR 3285/2-1) and partly funded by the University of Cologne Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative. S.B.E. acknowledges funding by the Helmholtz Initiative on Systems-Biology ‘The Human Brain Model’ and the NIH (R01-MH074457). G.R.F. gratefully acknowledges additional support from the Marga and Walter Boll Stiftung. Funding to pay the Open Access publication charges for this article was provided by the Jülich Research Center (INM-3).
Supplementary Material
Notes
We thank Dr Lizbeth Cárdenas-Morales for her support with data acquisition. Conflict of Interest: None declared.
References
- Ackerley SJ, Stinear CM, Barber PA, Byblow WD. 2010. Combining theta burst stimulation with training after subcortical stroke. Stroke. 41(7):1568–1572. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26(3):839–851. [DOI] [PubMed] [Google Scholar]
- Awiszus F. 2003. TMS and threshold hunting. Suppl Clin Neurophysiol. 56:13–23. [DOI] [PubMed] [Google Scholar]
- Bates KA, Rodger J. 2015. Repetitive transcranial magnetic stimulation for stroke rehabilitation-potential therapy or misplaced hope? Restor Neurol Neurosci. 334:557–569. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. 2003. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 20(3):1685–1696. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. 2005. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 28(1):22–29. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Buma F, Kwakkel G, Ramsey N. 2013. Understanding upper limb recovery after stroke. Restor Neurol Neurosci. 31(6):707–722. [DOI] [PubMed] [Google Scholar]
- Cardenas-Morales L, Volz LJ, Michely J, Rehme AK, Pool EM, Nettekoven C et al. 2014. Network connectivity and individual responses to brain stimulation in the human motor system. Cereb Cortex. 24(7):1697–1707. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. 2005. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 193(2):291–311. [DOI] [PubMed] [Google Scholar]
- Carrera E, Tononi G. 2014. Diaschisis: past, present, future. Brain. 137(Pt 9):2408–2422. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DLW, Shulman GL, Corbetta M.. 2010. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 67(3):365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK.. 2014. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci USA. 111(35):12913–12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. 2012. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 26(4):291–313. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Riley JD. 2008. Neuroplasticity and brain repair after stroke. Curr Opin Neurol. 21(1):76–82. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hébert R, Bravo G, Dutil É. 1995. Comparison of the Jamar dynamometer and the Martin vigorimeter for grip strength measurements in a healthy elderly population. Scand J Rehabil Med Suppl. 27:137–143. [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS.. 1995. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 74(2):802–815. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, Musumeci G, Cianfoni A, Pasqualetti P, Tonali PA.. 2008. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol. 119(3):715–723. [DOI] [PubMed] [Google Scholar]
- Duncan P, Richards L, Wallis D, Stoker-Yates J, Pohl P, Luchies C, Ogle A, Studenski S.. 1998. A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke. 29:2055–2060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K.. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25(4):1325–1335. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8(9):700–711. [DOI] [PubMed] [Google Scholar]
- Funke K, Benali A. 2011. Modulation of cortical inhibition by rTMS—findings obtained from animal models. J Physiol. 589(Pt 18):4423–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. 2008. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 18(9):2046–2053. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. 2011. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 134(Pt 5):1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. 2012. Disruption of motor network connectivity post-stroke and its noninvasive neuromodulation. Curr Opin Neurol. 25(6):670–675. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. 2014. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 13(2):206–216. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Ward NS. 2014. Cortical reorganization after stroke: how much and how functional? Neuroscientist. 20(1):56–70. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. 2013. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 23:1593–1605. [DOI] [PubMed] [Google Scholar]
- Hao Z, Wang D, Zeng Y, Liu M. 2013. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 5:CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Chopp M. 2012. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 11(4):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Cardenas-Morales L, Connemann BJ, Kammer T, Schonfeldt-Lecuona C. 2010. Sham or real—post hoc estimation of stimulation condition in a randomized transcranial magnetic stimulation trial. Neurosci Lett. 471(1):30–33. [DOI] [PubMed] [Google Scholar]
- Hoyer EH, Celnik PA. 2011. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci. 29(6):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. 2005. Theta burst stimulation of the human motor cortex. Neuron. 45(2):201–206. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J, Kwakkel G. 2011. Stroke rehabilitation. Lancet. 377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, Bernardi G, Koch G, Centonze D. 2010. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. 17:295–300. [DOI] [PubMed] [Google Scholar]
- Murakami T, Muller-Dahlhaus F, Lu MK, Ziemann U. 2012. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol. 590(Pt 22):5765–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. 1994. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 25(4):808–813. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C.. 2014. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 34(20):6849–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. 2006. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 16(6):638–644. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH.. 2011. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 42(5):1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Fink GR, von Cramon DY, Grefkes C. 2011. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 21(4):756–768. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Grefkes C. 2013. State-dependent differences between functional and effective connectivity of the human cortical motor system. Neuroimage. 67:237–246. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Volz LJ, Feis DL, Bomilcar-Focke I, Liebig T, Eickhoff SB, Fink GR, Grefkes C.. 2015. Identifying neuroimaging markers of motor disability in acute stroke by machine learning techniques. Cereb Cortex. 259:3046–3056. [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik PA et al. 2008. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?” Brain Stimul. 1(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group. 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 120(12):2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE et al. 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi G, Murphy TH. 2014. Stroke and the connectome: how connectivity guides therapeutic intervention. Neuron. 83(6):1354–1368. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. 2007. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 130(Pt 1):170–180. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. 2012. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 135(Pt 8):2527–2535. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. 2007. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 118(2):333–342. [DOI] [PubMed] [Google Scholar]
- Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, VandenBos M, Hammerbeck U, Barratt K, Gillini C et al. 2012. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 26(8):976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A, Wichmann FA. 2009. Determining the cortical target of transcranial magnetic stimulation. Neuroimage. 47:1319–1330. [DOI] [PubMed] [Google Scholar]
- Turella L, Lingnau A. 2014. Neural correlates of grasping. Front Hum Neurosci. 8:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zijden JP, van Eijsden P, de Graaf RA, Dijkhuizen RM. 2008. 1H/13C MR spectroscopic imaging of regionally specific metabolic alterations after experimental stroke. Brain. 131(Pt 8):2209–2219. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM.. 2010. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 30(11):3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz LJ, Sarfeld AS, Diekhoff S, Rehme AK, Pool EM, Eickhoff SB, Fink GR, Grefkes C.. 2015. Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Funct. 2202:1093–1107. [DOI] [PubMed] [Google Scholar]
- von Monakow C. 1914. Die Lokalisation im Grosshirn und der Abbau der Funktion durch kortikale Herde. Wiesbaden: Bergmann JF. [Google Scholar]
- Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. 2000. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 20(8):1149–1165. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. 2012. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 60(1):162–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.