Abstract
Processing eye-gaze information is a key step to human social interaction. Neuroimaging studies have shown that superior temporal sulcus (STS) is highly implicated in eye-gaze perception. In autism, a lack of preference for the eyes, as well as anatomo-functional abnormalities within the STS, has been described. To date, there are no experimental data in humans showing whether it is possible to interfere with eye-gaze processing by modulating STS neural activity. Here, we measured eye-gaze perception before and after inhibitory transcranial magnetic stimulation (TMS) applied over the posterior STS (pSTS) in young healthy volunteers. Eye-gaze processing, namely overt orienting toward the eyes, was measured using eye tracking during passive visualization of social movies. Inhibition of the right pSTS led participants to look less to the eyes of characters during visualization of social movies. Such effect was specific for the eyes and was not observed after inhibition of the left pSTS nor after placebo TMS. These results indicate for the first time that interfering with the right pSTS neural activity transitorily disrupts the behavior of orienting toward the eyes and thus indirectly gaze perception, a fundamental process for human social cognition. These results could open up new perspectives in therapeutic interventions in autism.
Keywords: eye-gaze perception, social cognition, STS, TMS
Introduction
The processing of eye-gaze information is a key step to engage in social interactions. This ability is characteristic of humans and nonhuman primates living in complex social environments (Adolphs 2003). Eye contact helps infer the intentions and feelings of the conspecifics, which is crucial for survival and social integration (Klein et al. 2009). Spontaneous perception and acute analysis of the eye movement is essential for maintaining optimal social relationships throughout the primate lifespan. Moreover, the preference for the eyes as a privileged attention target is evident extremely early in the normal development, suggesting that this preference is a core mechanism for the subsequent development of a larger expertise of human social cognition (Frith and Frith 2012). As the most reliable cue to understanding what another person is thinking, feeling or intending, the eyes become, in fact, a “window to the soul.” Interestingly, in developmental disorders, such as autism spectrum disorders (ASD), this behavior seems to be disrupted and deficits in eye contact are a hallmark of autism (Jones and Klin 2013). Indeed, in both adults and children with ASD, a lack of preference for the eyes has been demonstrated (Klin et al. 2002; Jones et al. 2008), which may account for their difficulties in social interactions.
A substantial body of evidence has emphasized the importance of the superior temporal sulcus (STS) in gaze perception. In monkeys, single unit recording studies have consistently indicated the involvement of the posterior STS (pSTS; Perrett et al. 1985). In humans, neuroimaging studies have described STS activation, mainly within the right STS, in the processing of eye gaze (Pelphrey, Morris, Michelich, et al. 2005; Hadjikhani et al. 2008; Sato et al. 2008; Nummenmaa et al. 2010; Carlin and Calder 2013). Indeed, several functional MRI studies have revealed a well-defined anatomical region within the pSTS consistently implicated in gaze perception. Furthermore, a number of brain imaging studies have reported the presence of anatomical and functional abnormalities within the pSTS in children and in adults with ASD (Boddaert et al. 2004; Pelphrey, Morris, McCarthy 2005; Zilbovicius et al. 2006; Duchesnay et al. 2011; Philip et al. 2012).
To date, no experimental data exist for humans that demonstrate whether gaze processing can be altered by an artificial modulation of the STS neural network. We hypothesize that a transitory inhibition of the right pSTS would selectively interfere with eye-gaze processing. To test this hypothesis, we examined eye-tracking recordings from young healthy volunteers during passive visualization of naturalistic social movies, without any specific task performance. To provide an ecological and naturalistic setting, we have used short movie fragments extracted from commercial films. We aimed to measure the changes in gaze perception, namely overt orienting toward the eyes, induced by an inhibitory theta-burst transcranial magnetic stimulation (TMS) applied to the right pSTS.
Materials and Methods
Participants
Thirty healthy volunteers, divided into 2 groups, participated in the first phase of the study. Group 1 was composed of 16 participants who underwent the Sham–Actual Inhibition protocol applied to the right pSTS (5 women; 22.1 ± 2.5 years); Group 2 was composed of 14 participants who underwent the Sham–Sham protocol applied to the right pSTS (3 women; 23.1 ± 3.1 years). After analysis comparing these 2 groups, additional data were collected to form a third group (Group 3). Group 3 was composed of 14 participants who underwent the Sham–Actual Inhibition protocol applied to the left pSTS (3 women; 24.1 ± 3.4 years). There was no significant age difference between the 3 groups (F2,41 = 1.67; P = 0.20). All participants were right-handed, had normal or corrected-to-normal sight, were free of psychiatric, neurological, and general health problems, and presented no contraindication for the TMS. All participants provided written informed consent in accordance with the Ethical Committee at the Saint-Louis Hospital, Paris, France, and the participants were monetarily compensated for their participation in this study.
Experimental Design
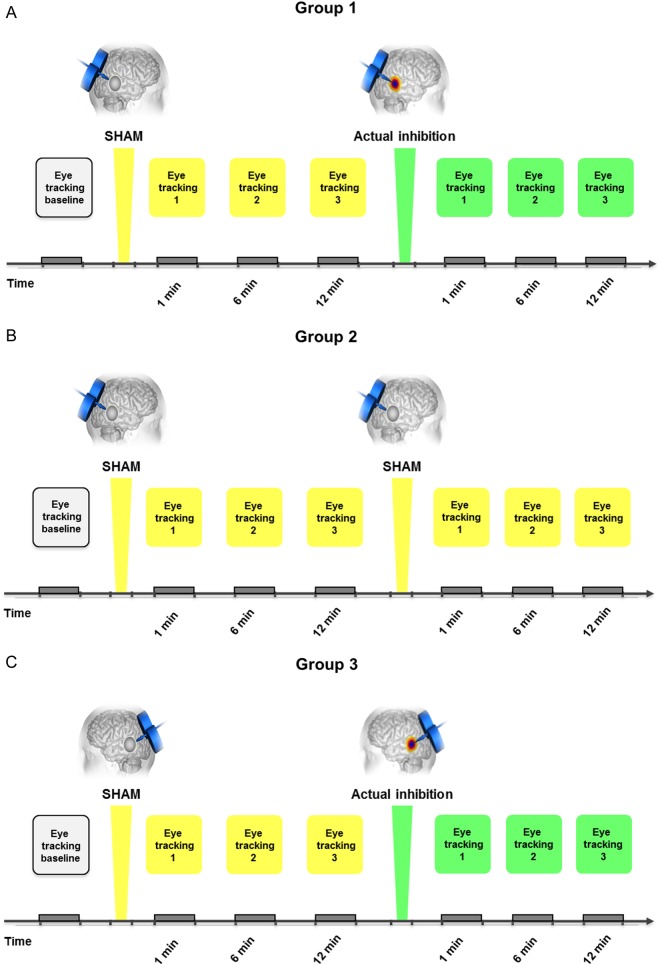
The study was initially based on 2 different experimental sets: 1) Sham–Actual Inhibition protocol applied to right pSTS (Group 1; Fig. 1A); 2) Sham–Sham protocol applied to the right pSTS (Group 2; Fig. 1B). In Group 1, actual inhibition was delivered to the right pSTS with a continuous theta-burst stimulation (cTBS). This type of TMS has effects that largely outlast (by at least 30 min) the stimulation duration (40 s), which allows the experimental measures to be conducted separately from the stimulation itself (Huang et al. 2005). However, this long-lasting effect did not allow for a classical crossover randomized experimental design. Therefore, a Sham–Sham protocol, in which actual STS inhibition was replaced by a second sham, was designed and applied to Group 2. Finally, to verify the specificity of the findings for the target area in the right pSTS, subsequently to analysis comparing Groups 1 and 2, additional data were collected in a new experimental set: Sham–Actual Inhibition protocol applied to left pSTS (Group 3; Fig. 1C) in which actual inhibition was delivered to the left pSTS. All subjects were blinded to the protocol type and to the intervention. In addition, after the experiment subjects were debriefed and did not manifest knowledge regarding sham or active condition.
Figure 1.
Experimental design: (A) Group 1: Sham–Actual Inhibition protocol applied to the right pSTS. The white square on the beginning of the time line indicates the baseline eye-tracking measure. The yellow squares over time indicate each eye-tracking measure performed after the sham (1, 6, and 12 min); the green squares over time indicate each eye-tracking measure performed after the inhibitory TMS administered over the right pSTS (1, 6, and 12 min). (B) Group 2: Sham–Sham protocol applied to the right pSTS. The white square on the beginning of the time line indicates the baseline eye-tracking measure. The yellow squares over time indicate each eye-tracking measure performed after the first sham (1, 6, and 12 min) and after the second sham (1, 6, and 12 min) administered over the right pSTS. (C) Group 3: Sham–Actual Inhibition protocol applied to the left pSTS. The white square on the beginning of the time line indicates the baseline eye-tracking measure. The yellow squares over time indicate each eye-tracking measure performed after the sham (1, 6, and 12 min); the green squares over time indicate each eye-tracking measure performed after the inhibitory TMS administered over the left pSTS (1, 6, and 12 min).
Before the TMS session, an anatomical 3DT1 MRI scan was acquired for all participants. Based on 4 fMRI studies on gaze perception (Pelphrey, Morris, Michelich, et al. 2005; Hadjikhani et al. 2008; Sato et al. 2008; Nummenmaa et al. 2010), the precise target location within the pSTS was identified on the individual scans. A baseline eye-tracking measure was performed prior to the TMS procedures. The participants underwent the first intervention (sham) and eye-tracking measures were recorded at 1, 6, and 12 min after the intervention. The participants receiving the Sham–Actual Inhibition protocol underwent the second intervention in the form of an actual inhibition (Fig. 1A,C). Eye-tracking measures were recorded at 1, 6, and 12 min after the inhibition. The participants receiving the Sham–Sham protocol underwent the second intervention in the form of a sham (Fig. 1B). Eye-tracking measures were recorded at 1, 6, and 12 min after the sham intervention. The entire session lasted approximately 90 min.
Structural MRI
All participants underwent a 3D T1-weighted FSPGR sequence (TR/TE/TI/NEX: 10.5/2.2/600/1, flip angle 10°, and matrix size 256 × 192, yielding 124 axial slices at a thickness of 1.2 mm and a 22 cm field of view) acquired with a 1.5T (Signa General Electric) scanner at the Necker Hospital, Paris, France.
Targeting the pSTS
The cortical 3D representation of each individual was reconstructed from the previously acquired structural MRI using the eXimia software (Nextim Ltd, Helsinki, Finland). The target was visually identified on the 3D reconstruction by an experimented neuro-radiologist based on the mean Talairach coordinates (x = 50, y = −53, z = 15) provided by 4 fMRI studies on the gaze perception, mainly on passive viewing of eye movements (Pelphrey, Morris, Michelich, et al. 2005; Hadjikhani et al. 2008; Sato et al. 2008; Nummenmaa et al. 2010). The frameless stereotaxic neuronavigation system localizes the coil placement and the orientation with an accuracy below 2 mm, which allows a maximum mismatch of 4 mm between the TMS hotspot and the MRI target (Neggers et al. 2004). The spatial resolution of the magnetic pulse cone being of approximately 1–2 cm assures that the induced electrical field encompasses the target region.
Actual Inhibition
The repetitive TMS was delivered with a continuous theta-burst pattern (cTBS) to inhibit the right or left pSTS. Continuous TBS is assumed to activate preferentially an inhibitory cascade of events, as demonstrated on the primary motor cortex. Such a stimulation paradigm would induce a depression in the excitability of pyramidal neurons within the target area, reversibly reducing the cortical excitability for at least 30 min and up to 1 h (Huang et al. 2005). The cTBS inhibition was delivered with a SuperRapid2 (Magstim Co., Whitland, UK) via a figure-eight cooled coil with a wing diameter of 70 mm. The coil was held in such a way as the handle (and the induced field) would be perpendicular to the targeted. The cTBS consisted of trains of 3 magnetic pulse separated by 20 ms (50 Hz); each train was repeated every 200 ms (5 Hz) until the total number of pulses (n = 600) was delivered (total stimulation time: 40 s). In this protocol, the cTBS was delivered with an intensity of 90% of the active motor threshold (AMT) of the first interosseous right hand muscle. The AMT was defined as the lowest intensity of a single magnetic pulse delivered over the primary motor cortex (M1), which produced a motor evoked potential >0.2 mV in at least 5 of the 10 trials when the subject exerted a 10% maximum voluntary target muscle contraction using visual feedback (Rothwell 1997). The subjects were required to wear MRI-grade earplugs during the procedure.
Sham TMS
The sham TMS consisted of 600 pulses delivered in the same cTBS pattern with a special sham coil (double 70 mm air-cooled sham coil; Eng Spc. SP15878, Magstim Co., Whitland, UK), designed to replicate the appearance and operation of the standard double 70 mm coil used in the active condition. This coil provides discharge noise and a slight sensory stimulation without stimulating cortical tissue. The subjects were blinded to the type of intervention performed. The subjects were required to wear MRI-grade earplugs during the procedure.
Eye Tracking
The study was performed using the Tobii™ T120 eye tracker equipment, based on infra-red technology, consisting of a 17-in. TFT monitor with a resolution of 1280 × 1024 pixels, from which the stimuli were presented in full screen, and the gaze behavior was simultaneously recorded. The eye-tracking system was completely noninvasive with little indication that the eye movements were being tracked and with no artificial constraints of the head or body movements. The system tracked both eyes to a rated accuracy of 0.5° with a sampling rate of 60 Hz. The Tobii™ equipment was connected to a HP Pavilion dv6 laptop computer (Windows 7 Professional).
The participants were individually tested and were seated facing the eye-tracker monitor at a distance of approximately 60 cm; the experimenter sat next to the participant to control the computer without interfering with the viewing behavior. A calibration test consisting of 5 registration points was performed before each set of stimuli. The calibration test was repeated if the examiner considered one of the 5 points not valid according to the eye-tracker criteria (recorded gaze extrapolating the limits of the calibration-designed area or the absence of recording for one of the 5 points). All participants matched general recording quality criteria, based on the amount of valid and missing data, as indicated by Tobii Studio™ software. A repeated-measure ANOVA with recoding quality as the dependent variable showed no significant interaction between group and TMS type (F2,41 = 0.62; P = 0.55). The participants were instructed that they would see a sequence of movie fragments and all they had to do was watch them. The stimuli creation, the calibration procedures, and the data acquisition and visualization were performed using the Tobii Studio™ software.
Stimuli
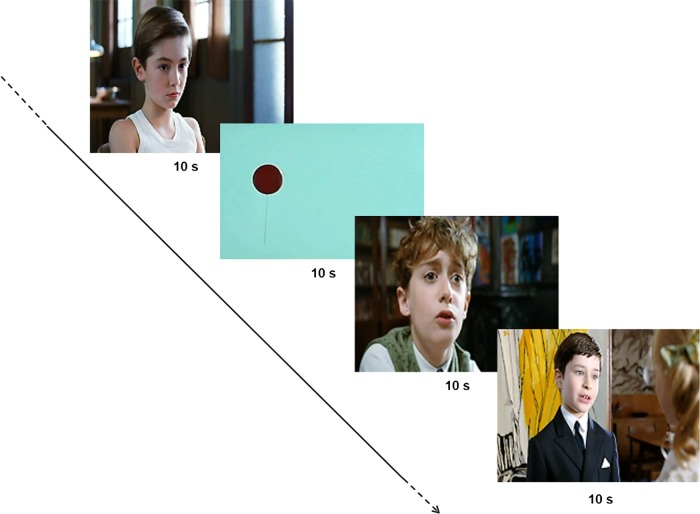
To have the most ecological and naturalistic stimuli set, we have deliberately used movies fragments extracted from French commercial films (25 fps). A total of 8 movie fragments, 10 s each, were selected and assembled together (see Supplementary Material). Six fragments displayed social scenes with 2 characters engaged in peer to peer social interactions (Le Petit Nicolas®), and 2 fragments displayed a simple nonsocial scene with a red balloon flying against a blue sky (Le ballon rouge®), to control for changes linked to the perception of nonbiological movement (Fig. 2). Sounds in the movies were dialogs in the social scenes and soft music in the nonsocial scenes. Factors as scene background, characters' position, balloon size, or speed were not controlled for. Seven finalized movies, 80 s each, were created. Each finalized movie presented all 8 fragments in a randomized order and a different movie was presented in each measure (baseline, 3 time points after the first intervention and 3 time points after the second intervention).
Figure 2.
Example of stimuli set: for each eye-tracking measure, a finalized stimuli set assembling 8 movie fragments of 10 s each (6 displaying social scenes and 2 displaying nonsocial scenes) was presented in a randomized order.
A pilot study was conducted prior to the current experiment to investigate putative habituation effects due to the repetitive visualization of the movie fragments. Fourteen participants successively watched the 7 finalized movies presented in this study. Results showed that repetitive visualization of the stimuli set has no effect on gaze pattern. Indeed, number of fixations to the eyes did not differ over the 7 visualizations (F1,13= 0.85; P = 0.37) (unpublished data).
Data Analyses
In each movie fragment, the following dynamic areas of interest (AOIs) were selected for analysis: the eyes and mouth of the characters in the social movie fragments and the balloon in the nonsocial movie fragments. Eye-tracking software interpolates the shape and position of the AOI, so that it moves smoothly from one frame to the next. More importantly, AOIs sizes and shapes remained stable across measures. The number of fixations in each AOI was recorded using the Tobii Studio™ software. A fixation event was defined as such by the Tobii fixation filter based on 0.42 pixels/ms threshold. Number of fixations was selected since it is an absolute variable that informs on exploratory behavior toward a defined region: higher number of fixations indicates that people further explore the region. The number of fixations to the eyes was pooled together from all social fragments for each one of the 7 visualizations. The same procedure was applied for the fixations made to the mouth and to the balloon. To render the behaviors comparable across all subjects, we used adjusted values (i.e., number of fixations to each AOI divided by the baseline data). Finally, for each AOI data from the 3 time points after each intervention were averaged (since measurements remained stable after each intervention), providing 1 final single data after each intervention.
We initially compared Groups 1 and 2, by performing a repeated-measures ANOVA, using Bonferroni corrections for multiple comparisons, for each AOI. The average number of fixations after each intervention served as the repeated factor, and the TMS type (sham or actual inhibition) and group (Group 1 and Group 2) served as the independent factor. Following results from this first analysis, additional data were collected (Group 3) to confirm that the reduction in number of fixations to the eyes was a specific consequence of inhibition of the right pSTS, and further analysis was performed comparing Groups 1, 2, and 3. The average number of fixations after each intervention served as the repeated factor, and the TMS type (sham or actual inhibition) and group (Group 1; Group 2 and Group 3) served as the independent factor. In all analyses, TMS type by group interaction was investigated.
Results
Transitory inhibition of the right pSTS by TMS induced modifications in spontaneous perception of the eyes. Repeated-measures ANOVA comparing Groups 1 and 2 showed an interaction between TMS type (sham or actual inhibition) and group (Group 1 and Group 2) on the number of fixations to the eyes (F1,28= 4.74; P = 0.04). Indeed, a significant reduction in the number of fixations to the eyes during the visualization of social movies was observed only in the group receiving TMS actual inhibition applied to the right pSTS (F1,15=13.84, P = 0.002) and not in the group receiving sham TMS applied to the right pSTS (F1,13= 1.53, P = 0.24). In addition, we did not find any significant interaction between TMS type (sham or actual inhibition) and group (Group 1 and Group 2) regarding the number of fixations made to other AOIs, that is, the mouth in the social scenes (F1,28 = 0.07, P = 0.80) and the balloon in the nonsocial control scenes (F1,28 = 0.05, P = 0.81).
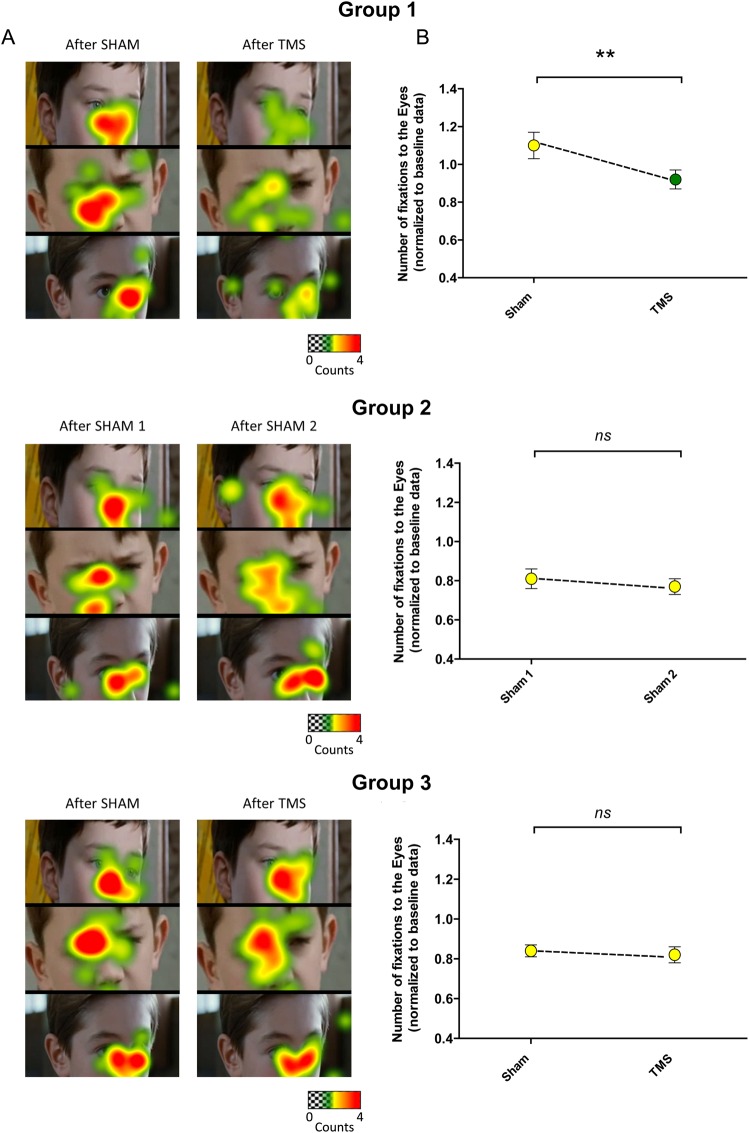
Following results from analysis comparing Groups 1 and 2, additional data on inhibition of the left pSTS were collected (Group 3) to confirm that the reduction in number of fixations to the eyes was a specific consequence of inhibition of the right pSTS. Further analysis comparing the TMS effects on gaze pattern among all 3 groups was performed. Results showed an interaction between TMS type (sham or actual inhibition) and group (Group 1; Group 2 and Group 3) on the number of fixations to the eyes (F2,41= 3.76; P = 0.03). Indeed, a significant reduction in the number of fixations to the eyes during the visualization of social movies was observed only in the group receiving TMS actual inhibition applied to the right pSTS (F1,15= 13.84, P = 0.002) and not in the group receiving sham TMS applied to the right pSTS (F1,13= 1.53, P = 0.24) nor in the group receiving TMS actual inhibition applied to the left pSTS (F1,13= 0.14, P = 0.71) (Fig. 3). In addition, we did not find any significant interaction between TMS type (sham or actual inhibition) and group (Group 1; Group 2 and Group 3) regarding the number of fixations made to other AOIs, that is, the mouth in the social scenes (F2,41= 0.48, P = 0.96) and the balloon in the nonsocial control scenes (F2,41= 0.12, P = 0.89).
Figure 3.
Reduction in the number of fixations to the eyes only after inhibitory TMS applied to the right pSTS. (A) Examples of close-up heatmap from group data before and after each intervention (Sham or inhibitory TMS) in the 3 groups (warm colors denote a greater number of fixations and cold colors denote fewer fixations). Scenes were selected for illustrative purposes. Heatmaps illustrate the reduction in number of fixations to the eye at the group level only in Group 1, while Groups 2 and 3 show no significant changes. (B) The plots illustrate that a significant reduction in the normalized values of number of fixations to the eyes during the visualization of naturalistic social movies was only observed after inhibitory TMS applied over the right pSTS (Group 1) and not after Sham applied to the right pSTS (Group 2) nor after inhibitory TMS applied to the left pSTS (Group 3). The error bars represent the SEM. *P = 0.002.
Discussion
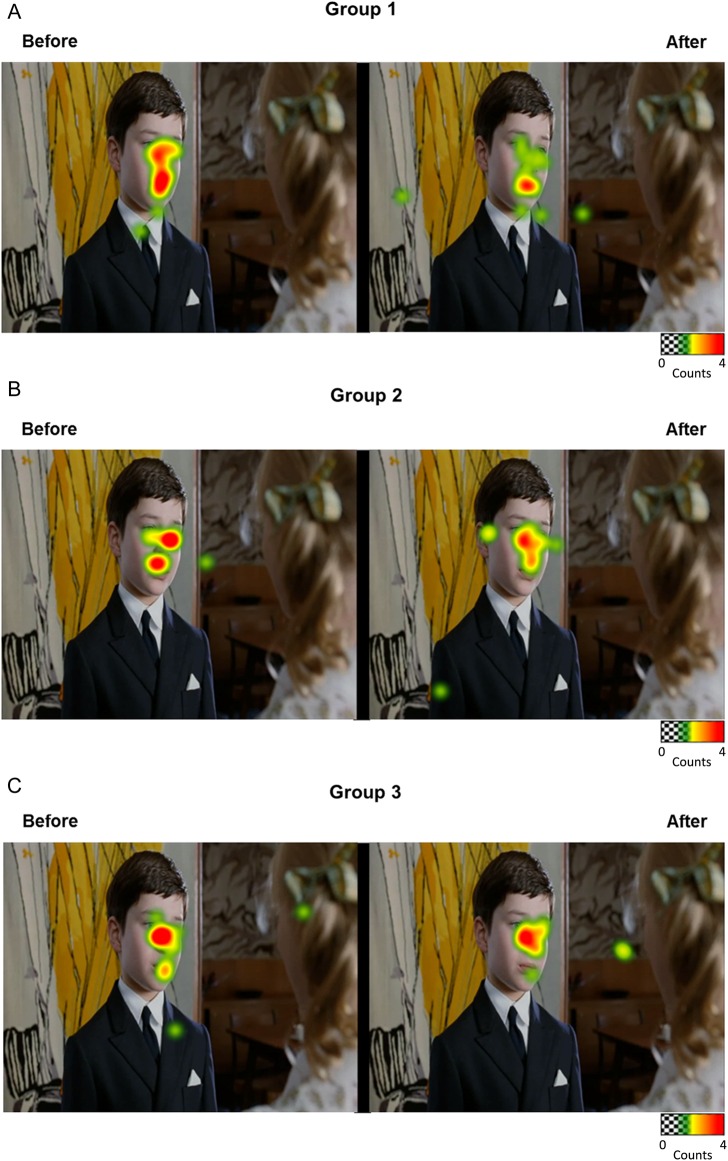
This TMS study demonstrates for the first time that the artificial disruption of the right pSTS neural network interferes with the spontaneous act of looking to the eyes. As predicted, inhibition of the right pSTS induced a selective change in the gaze pattern of healthy volunteers, that is, fewer fixations to the eyes during the visualization of naturalistic social movies (Fig. 3). This result does not seem to be associated with a disruption of global visual perception, since the effect was observed specifically for perception of eyes of characters, while no significant changes in gaze pattern were observed for the perception of the mouth of characters in the social movies nor for the perception of the moving object (balloon) in the nonsocial movies, even though total visualization time was shorter for the nonsocial scenes. Importantly, no significant changes in gaze pattern were observed in participants receiving the Sham–Sham protocol, strongly suggesting that the observed changes were indeed due to the inhibition of the right pSTS neural activity and were not related to a placebo effect or to the repetitive visualization of the movies. Furthermore, the absence of changes in gaze pattern following all placebo stimulations points to an extreme intraindividual stability of spontaneous gaze behavior, which could be considered an individual signature in social behavior (Fig. 4).
Figure 4.
Whole-frame heatmaps representing group gaze behaviors before and after each intervention in (A) Group 1 (inhibitory TMS applied over the right pSTS), (B) Group 2 (Sham applied over the right pSTS), and (C) Group 3 (inhibitory TMS applied over the left pSTS) (selected for illustrative purposes).
Interestingly, the inhibition of the left pSTS had no impact on the spontaneous perception of the eyes. This result suggests an asymmetry in the cortical processing of eye-gaze information, occurring predominantly within the right pSTS, which has been proposed by previous brain imaging studies (Pelphrey and Carter 2008; Greene and Zaidel 2011). For instance, an fMRI study investigating gaze perception showed strongly right lateralized activation within the STS (Pelphrey et al. 2004). In addition, another study investigating the brain network involved in the event-related potential response to direct gaze reported significant clusters in the right STS region (Conty et al. 2007). Moreover, a recent fMRI study showed that, although stimuli presenting gaze shifts elicited significant bold responses in both right and left pSTS, only the right pSTS was sensitive to the social meaning of different gaze directions, with enhanced responses when the eyes shifted toward rather than away from the viewer (Ethofer et al. 2011). Finally, it has been suggested that the left STS would be further implicated in the processing of multimodal information, such as integration of speech and gesture (Willems et al. 2009; Holle et al. 2010).
Few previous studies have used TMS to better characterize the role of the STS in the perception of biological motion. Indeed, it has been shown that inhibitory rTMS applied over the pSTS temporarily impairs the ability to detect and discriminate point-light animations depicting human movement (Grossman et al. 2005). It has also been shown that judgment of unfamiliar faces as being trustworthy or untrustworthy was disrupted when rTMS was delivered over the STS (Dzhelyova et al. 2011). In addition, single-pulse TMS applied over the right temporal cortex has been shown to impair subject's perception of gaze shifts over presentation of 2 consecutive face stimuli (Pourtois et al. 2004). However, the implication of the right pSTS in the very particular and subtle behavior of spontaneous looking to the eyes during passive visualization of ecological social stimuli, verified by an objective non-self-reported measure such as eye tracking, had not yet been demonstrated.
The present results provide then 2 major findings. First, these results help to elucidate some of the basic mechanisms of social cognition, by establishing a direct link between a very specific behavior (looking to the eyes) and a localized anatomical region (the right pSTS). Indeed, recent fMRI studies have indicated that the right pSTS is highly implicated in gaze perception (Hadjikhani et al. 2008; Sato et al. 2008; Nummenmaa et al. 2010; Carlin and Calder 2013). However, due to the lack of lesion models circumscribed to this region, no direct evidence of this association exists. Here, the right pSTS virtual lesion caused a significant and selective decrease in perception of the eyes, providing direct evidence implicating the right pSTS in this precise behavior.
Secondly, these results show that it is possible to interfere with a key behavior of social cognition, opening up interesting perspectives on interventions in psychiatric disorders. By disrupting the right pSTS neural network in healthy volunteers, we artificially induced a gaze pattern that is similar to the gaze pattern observed in persons with autism, who present anatomical and functional abnormalities of the pSTS (Klin et al. 2002; Zilbovicius et al. 2006). Indeed, a core symptom of autism is deficits in social perception, mainly a lack of preference for the eyes, which has been demonstrated in several eye-tracking studies (Klin et al. 2002; Pelphrey et al. 2002; Jones et al. 2008). If stimulation of the STS by excitatory TMS is able to change this pattern and induce an increase in looking to the eyes, new perspectives on therapeutic interventions for ASD could emerge.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
The study was supported by AP-HP PHRC and Fondation de France grants. A.S. received funding from Fondation Orange. T.P. and J-C. L. received funding from the program “Investissements d'avenir” ANR-10-IAIHU-06, Paris Institute of Translational Neuroscience. Eye-Tracking device was financed by “Les Amis d'Arthur” association. Funding to pay the Open Access publication charges for this article was provided by INSERM U1000.
Supplementary Material
Notes
We thank Michel Siksik for assistance with video work. Conflict of Interest: None declared.
References
- Adolphs R. 2003. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 4:165–178. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y et al. 2004. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 23:364–369. [DOI] [PubMed] [Google Scholar]
- Carlin JD, Calder AJ. 2013. The neural basis of eye gaze processing. Curr Opin Neurobiol. 23:450–455. [DOI] [PubMed] [Google Scholar]
- Conty L, N'Diaye K, Tijus C, George N. 2007. When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia. 45:3024–3037. [DOI] [PubMed] [Google Scholar]
- Duchesnay E, Cachia A, Boddaert N, Chabane N, Mangin JF, Martinot JL, Brunelle F, Zilbovicius M. 2011. Feature selection and classification of imbalanced datasets: application to PET images of children with autistic spectrum disorders. Neuroimage. 57:1003–1014. [DOI] [PubMed] [Google Scholar]
- Dzhelyova MP, Ellison A, Atkinson AP. 2011. Event-related repetitive TMS reveals distinct, critical roles for right OFA and bilateral posterior STS in judging the sex and trustworthiness of faces. J Cogn Neurosci. 23:2782–2796. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Gschwind M, Vuilleumier P. 2011. Processing social aspects of human gaze: a combined fMRI-DTI study. Neuroimage. 55:411–419. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. 2012. Mechanisms of social cognition. Annu Rev Psychol. 63:287–313. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Zaidel E. 2011. Hemispheric differences in attentional orienting by social cues. Neuropsychologia. 49:61–68. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. 2005. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res. 45:2847–2853. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Hoge R, Snyder J, de Gelder B. 2008. Pointing with the eyes: the role of gaze in communicating danger. Brain Cogn. 68:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle H, Obleser J, Rueschemeyer SA, Gunter TC. 2010. Integration of iconic gestures and speech in left superior temporal areas boosts speech comprehension under adverse listening conditions. Neuroimage. 49:875–884. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. 2005. Theta burst stimulation of the human motor cortex. Neuron. 45:201–206. [DOI] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. 2008. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 65:946–954. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. 2013. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 504:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML. 2009. Social attention and the brain. Curr Biol. 19:R958–R962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 59:809–816. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Langerak TR, Schutter DJ, Mandl RC, Ramsey NF, Lemmens PJ, Postma A. 2004. A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage. 21:1805–1817. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Passamonti L, Rowe J, Engell AD, Calder AJ. 2010. Connectivity analysis reveals a cortical network for eye gaze perception. Cereb Cortex. 20:1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. 2008. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 1145:283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. 2005. Neural basis of eye gaze processing deficits in autism. Brain. 128:1038–1048. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. 2005. Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cereb Cortex. 15:1866–1876. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. 2002. Visual scanning of faces in autism. J Autism Dev Disord. 32:249–261. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. 2004. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychol Sci. 15:598–603. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. 1985. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B Biol Sci. 223:293–317. [DOI] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. 2012. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 36:901–942. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Sander D, Andres M, Grandjean D, Reveret L, Olivier E, Vuilleumier P. 2004. Dissociable roles of the human somatosensory and superior temporal cortices for processing social face signals. Eur J Neurosci. 20:3507–3515. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. 1997. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 74:113–122. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. 2008. Time course of superior temporal sulcus activity in response to eye gaze: a combined fMRI and MEG study. Soc Cogn Affect Neurosci. 3:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Ozyurek A, Hagoort P. 2009. Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. Neuroimage. 47:1992–2004. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. 2006. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 29:359–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.