Abstract
Ca2+ release from the Golgi apparatus regulates key functions of the organelle, including vesicle trafficking. However, the signaling pathways that control this form of Ca2+ release are poorly understood and evidence of discrete Golgi Ca2+ release events is lacking. Here, we identified the Golgi apparatus as the source of prolonged Ca2+ release events that originate from the nuclear ‘poles’ of primary cardiac cells. Once initiated, Golgi Ca2+ release was unaffected by global depletion of sarcoplasmic reticulum Ca2+, and disruption of the Golgi apparatus abolished Golgi Ca2+ release without affecting sarcoplasmic reticulum function, suggesting functional and anatomical independence of Golgi and sarcoplasmic reticulum Ca2+ stores. Maximal activation of β1-adrenoceptors had only a small stimulating effect on Golgi Ca2+ release. However, inhibition of phosphodiesterase (PDE) 3 or 4, or downregulation of PDE 3 and 4 in heart failure markedly potentiated β1-adrenergic stimulation of Golgi Ca2+ release, consistent with compartmentalization of cAMP signaling within the Golgi apparatus microenvironment. β1-adrenergic stimulation of Golgi Ca2+ release involved activation of both Epac and PKA signaling pathways and CaMKII. Interventions that stimulated Golgi Ca2+ release induced trafficking of vascular growth factor receptor-1 (VEGFR-1) from the Golgi apparatus to the surface membrane. These data establish the Golgi apparatus as a juxtanuclear focal point for Ca2+ and β1-adrenergic signaling, which functions independently from the sarcoplasmic reticulum and the global Ca2+ transients that underlie the primary contractile function of the cell.
Introduction
The Golgi apparatus has an integral role in the modification, sorting and packaging of macromolecules originating from the rough endoplasmic reticulum (ER), vesicular transport of secreted lipids, proteins and carbohydrates, and the formation of lysosomes1. Previous findings have shown that Ca2+ within the Golgi apparatus can regulate both its structure and function. For example, reduced Golgi Ca2+ uptake was associated with changes in structure, protein sorting and vesicle trafficking2,3. It has also been suggested that localized increases in cytosolic Ca2+ concentration due to Golgi Ca2+ release control vesicle fusion and cargo transport3–5. This is supported by work identifying Ca2+ binding proteins associated with the Golgi membrane, which transduce local cytosolic Ca2+ signals into regulatory events6. However, relatively little is known about the role of the Golgi apparatus as a Ca2+ signaling organelle or the pathways that regulate Golgi Ca2+ release.
The Ca2+ concentration gradient across the Golgi apparatus membrane is generated by the secretory pathway Ca2+ ATPase1 (SPCA1)2,7,8 and SERCA9–11. Ca2+ binding proteins within the Golgi lumen, such as calnuc, increase the Ca2+ storage capacity of the organelle in a manner analogous to calsequestrin in the SR/ER12. Most previous studies have concluded that inositol 1,4,5-trisphosphate (InsP3) receptors (InsP3Rs) mediate Golgi Ca2+ efflux3,13–15. However, this is based on data from cell lines, where the InsP3 Ca2+ signaling is dominant. In neonatal cardiac myocytes, trans Golgi apparatus Ca2+ depletion occurs in response to agonists of the ryanodine receptor (RyR), but not the InsP3R2, indicating that Golgi Ca2+ regulation exhibits cell type specialization.
The Golgi apparatus Ca2+ efflux mechanism has been studied indirectly by targeting Ca2+ probes to the lumen of the organelle8,13,14,16, or by using selective disrupting agents to assess its contribution to cytosolic Ca2+ transients8,17,18. However, direct evidence of Ca2+-release events originating from the Golgi apparatus is lacking; it is possible that in cells that exhibit large ER/SR cytosolic Ca2+ transients, Golgi Ca2+ efflux may be obscured. Alternatively, by virtue of its location, the organelle might dictate local Ca2+ signaling despite global cytosolic Ca2+ transients; the Golgi apparatus typically appears as a continuous ribbon of flattened stacks, either encircling or adjacent to the nucleus2,19. Released Ca2+ may, therefore, have privileged access both to the Golgi apparatus microenvironment and the nucleoplasm.
The aim of the present study was to establish whether Golgi Ca2+-release events can be identified in cells that exhibit large cytosolic ER/SR derived Ca2+ transients and if present, to characterize the signaling pathways that control Ca2+-release from the organelle. Our findings establish the Golgi apparatus as a nexus for β1-adrenergic signaling and demonstrate its capacity to dictate the local Ca2+ concentration within the Golgi microenvironment, with consequent effects on protein trafficking in primary cardiac cells.
Results
Prolonged Ca2+-release events occur at the nuclear poles
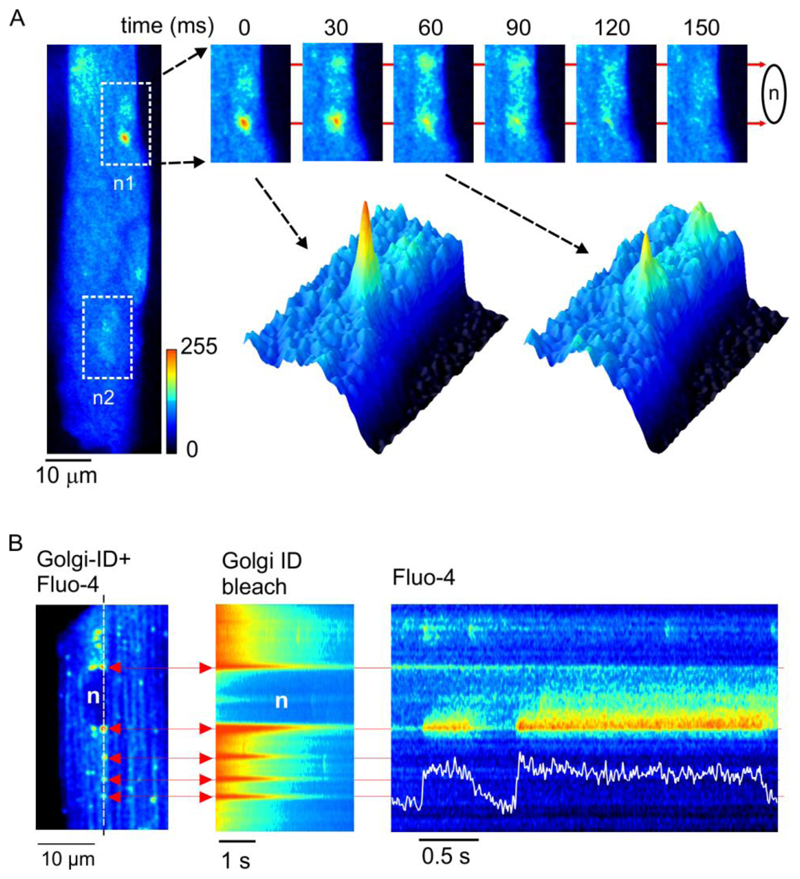
Experiments were carried out to detect changes in local Ca2+-release in the vicinity of the Golgi apparatus and associated nuclei. Sequential x-y images obtained from a fluo-4 loaded adult rat ventricular myocyte (ARVM) with the confocal plane adjusted such that one of the 2 nuclei was in sharp focus is shown (Fig. 1A). A localized Ca2+-release event is apparent at the lower ‘nuclear pole’. Subsequent frames (Fig. 1A, upper right) and corresponding surface plots (Fig. 1A, lower right) reveal a prolonged elevation of nucleoplasmic Ca2+ concentration. In line-scan images, localized Ca2+-release events arising at the nuclear poles were of similar amplitude to Ca2+ sparks, but often exhibited a plateau phase of variable duration (Fig. S1).
Fig. 1. Prolonged Ca2+-release events co-localize with the Golgi apparatus.
(A) x-y confocal images of an intact ARVM. White boxes indicate the location of the 2 nuclei (n1 & n2). (B) x-y image showing dual loading of a myocyte with fluo-4 and Golgi-ID (left). Diffuse fluorescence reflects cytosolic fluo-4, punctuate fluorescence highlights the Golgi apparatus at the ends of the nucleus and also cytosolic vesicles. Similar effects were seen in cells from 4 hearts.
The Golgi apparatus is consistently present at the nuclear poles in ARVMs, where it appears as a series of flattened membranous sacs in electron micrographs (Fig. S2A). In this example, localization of the Golgi at one nuclear pole was highlighted by DAB staining of the Golgi matrix protein GM130. The cis face of the Golgi apparatus was in close apposition to the nuclear envelope, while the trans face was bounded by mitochondria. Using confocal microscopy, expression of GFP targeted to the Golgi apparatus identified the organelle at the nuclear poles and also Golgi transfer vesicles within the cytosol (Fig. S2B, left). Pre-exposure of GFP expressing cells to the selective Golgi disrupting agent ilimaquinone (IQ)20 resulted in complete loss of both the Golgi apparatus at the nuclear poles and the transfer vesicles (Fig. S2C, right). Given the consistent presence of the Golgi apparatus at the nuclear poles in ARVMs, experiments were carried out to establish whether the prolonged Ca2+-release events co-localize with the organelle.
Cells were co-loaded with Golgi-ID green to identify the Golgi apparatus, and fluo-4 to detect changes in cytosolic Ca2+ concentration. An initial x-y confocal image revealed both the location of the Golgi apparatus and the evenly distributed cytosolic fluo-4 signal (Fig. 1B, left). The x-y image was then used to position the scan line through the Golgi apparatus at both ends of the nucleus and several bright cytosolic vesicles (Fig 1B, broken line). On initiation of the line scan, the Golgi apparatus was initially highlighted as a series of bright horizontal lines corresponding to the Golgi-ID green signal (Fig. 1B, middle). However, because Golgi-ID green bleaches rapidly (Fig. S3), cytosolic fluo-4 fluorescence was left as the remaining stable component of the signal (Fig. 1D, right). The prolonged Ca2+-release events at the nuclear poles consistently aligned with the Golgi-ID signal. Co-localization of prolonged Ca2+-release with cytosolic vesicles did not typically occur.
Prolonged Ca2+-release events are abolished by selective Golgi disruption
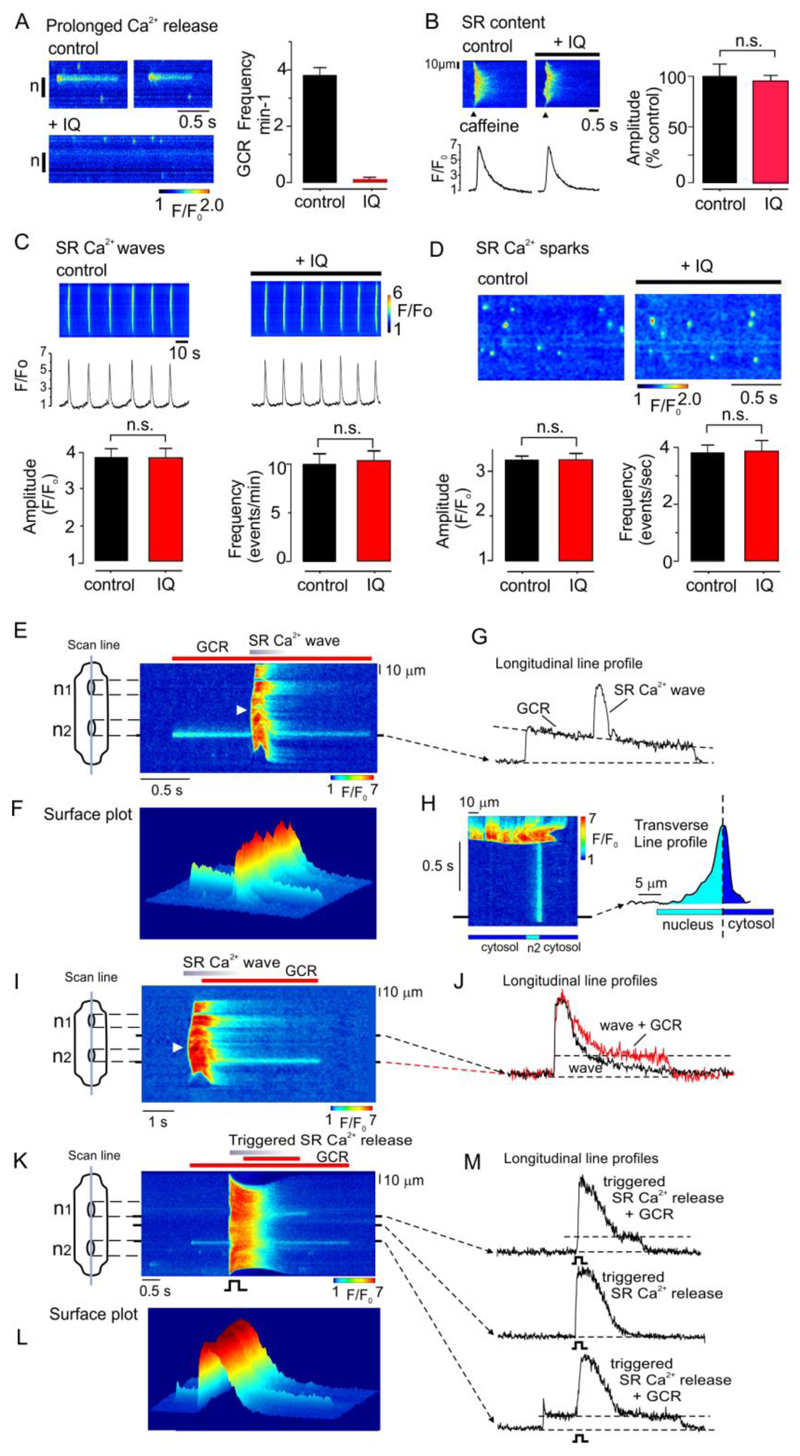
The possibility that the Golgi apparatus is the source of the prolonged Ca2+-release events was investigated further by using IQ to disrupt the organelle. Confocal imaging was carried out in fluo-4 loaded ARVMs, with the scan line positioned longitudinally through the poles of at least one nucleus. In cells initially exhibiting repetitive Ca2+-release events (Fig. 2A), exposure to IQ abolished Ca2+-release. In contrast, IQ had no influence on SR Ca2+ regulation, as indicated by a lack of effect on the amplitude of the caffeine-induced Ca2+ transient (Fig. 2B), the frequency or amplitude of spontaneous SR Ca2+ waves (Fig. 2C), or the properties of localized SR Ca2+ sparks (Fig. 2D). Both the location of the prolonged Ca2+-release events and their selective abolition with IQ indicate that the site of origin is the Golgi apparatus. Therefore, prolonged Ca2+-release events arising at the nuclear poles will now be referred to as Golgi Ca2+-release.
Fig. 2. IQ inhibits Golgi Ca2+ release but not SR Ca2+-release.
(A) Line scan (left) and mean data (right) showing that in cells initially exhibiting repetitive Golgi Ca2+ release, a subsequent 15 minute exposure to IQ (laser off) abolished Golgi Ca2+ release (n=26 cells from 3 hearts). (B) original line scan (left) and mean data (right) showing that IQ had no significant effect on the SR Ca2+ content assessed as assessed by rapid application caffeine (n=6 cells from 3 hearts) (C) Original line scan (upper) and mean data (lower) showing that IQ has no significant effect on spontaneous SR Ca2+ wave amplitude or frequency.(n=8 cells from 3 hearts) (D) Original line scan (upper) and mean data (lower) showing absence of effect of IQ had on the frequency or amplitude of spontaneous Ca2+ sparks (n=8 cells from 3 hearts). * p<0.05. n.s., not significant. (E) A line scan image (upper left) and corresponding surface plots (lower left & right) obtained from a permeabilized ARVM in which a spontaneous SR Ca2+ wave occurred during a prolonged Ca2+-release event. The line profile is positioned through the prolonged Ca2+-release event (upper right). A surface plot orientated from the direction of the arrow (lower right) and the accompanying line profile (right) indicates diffusion of Ca2+ . (F) A line scan image showing an SR Ca2+ wave originating near the center of a permeabilized cell (arrow) triggered a prolonged Ca2+-release event from the upper pole of n2 (left) and associated line profiles (right). (G) A line scan image from an intact myocyte showing a global SR Ca2+-release induced by field stimulation, during a prolonged Ca2+-release event originating from one end of n2. In E-G, similar results were obtained in cells from 2-3 hearts.
Golgi Ca2+ release arises from a Ca2+ pool that is distinct from the SR
Experiments were done to characterize the properties of Golgi Ca2+ release and its relationship to Ca2+-release from the SR. Cell permeabilization allowed the cytosolic conditions to be adjusted, such that regular spontaneous SR Ca2+ waves could be induced. A line-scan image was obtained from a permeabilized cell, with the scan line positioned longitudinally through both nuclei (Fig. 2E, upper left). The image shows a Golgi Ca2+ release event that originated close to one end of the lower nucleus. In this example, an SR Ca2+ wave arose spontaneously in the middle of the cell during the Golgi Ca2+ release (Fig. 2E, arrow), before propagating in both directions to the ends of the myocyte. The SR Ca2+ wave added to the Golgi Ca2+-release event, but did not affect the underlying time course (Fig, 2E, upper right), providing strong evidence that the SR and Golgi Ca2+ stores are not interconnected.
Another consistent feature was that Golgi Ca2+ release diffused further into the nucleoplasm than the cytosol (Fig. 2E, lower right). This might be explained by the close proximity of the trans-Golgi apparatus to mitochondria (Fig S2), which acts as a Ca2+ sink. Inhibition of mitochondrial Ca2+ uptake with RU360 was found to stimulate GCR (Fig. S4). This is likely due to Ca2+ loss from the mitochondria and consequent effects in the Golgi Ca2+ uptake/release.
Further experiments provided evidence of a limited interaction between SR Ca2+-release and CGR. In the line-scan image shown in Fig. 2F, a spontaneous SR Ca2+ wave arose close to the center of a permeabilized cell (arrow) and then propagated in both directions to the ends of the cell. In this example, the SR Ca2+ wave triggered a prolonged Golgi Ca2+ release originating at the pole of one nucleus. Again, this event exhibited a typical plateau phase and a local increase in both cytosolic and nucleoplasmic Ca2+ concentration was apparent.
A similar interplay between SR Ca2+-release and Golgi Ca2+ release was observed in intact myocytes. Fig. 2G shows Golgi Ca2+ release originating close to one end of the lower nucleus. The cell was then field stimulated during the plateau phase and as shown by the line profile (lower right), this form of global SR Ca2+-release also had no detectable influence on the amplitude or time course of Golgi Ca2+ release. However, the global SR Ca2+-release triggered Golgi Ca2+ release at one pole of the other nucleus (upper right).
Golgi apparatus Ca2+ release involves RyRs
A number of interventions were carried out to investigate the mechanisms underlying Ca2+ uptake and-release from the Golgi apparatus (Table 1). In permeabilized cells perfused with a mock intracellular solution containing 120 nM free Ca2+ concentration, 17.6% of myocytes exhibited Golgi Ca2+ release during a standardized 3 minute line scan. Lowering the free Ca2+ concentration decreased, while raising significantly increased the percentage of cells exhibiting Golgi Ca2+ release. In intact ARVMs, pre-incubation with ryanodine at a concentration that blocks RyR2 in its closed state abolished Golgi Ca2+ release, as did pre-incubation with the SERCA inhibitor thapsigargin. These findings suggest that RyR Ca2+ efflux and SERCA mediated Ca2+ uptake have a major role in Golgi Ca2+ regulation in ARVMs.
Table 1. Modulation of Golgi Ca2+ release in permeabilized and intact ARVMs.
Confocal line scan imaging was used to assess the effect of various interventions on Golgi Ca2+ release in permeabilized or intact ARVMs. In permeabilized cells, lowering the Ca2+ concentration from the control level of 120 nM to 70 nM significantly reduced, while raising to 250 nM significantly increased the percentage of cells exhibiting Golgi Ca2+ release (n=31). Adenophostin-A had no significant effect on Golgi Ca2+ release. In intact ARVMs, after treatment with ryanodine or thapsigargin Golgi Ca2+ release was never observed. Neither ET-1 nor 2-APB had a significant effect on Golgi Ca2+ release. Data were obtained from 3-6 hearts per intervention.
| Permeabilized ARVMs | Intact ARVMs | ||||||
|---|---|---|---|---|---|---|---|
| Intervention | [Ca2+] 70 nM | [Ca2+] 250 nM | adenophostin-A | ryanodine | thapsigargn | ET-1 | 2-APB |
| Relative change | ↓ | ↑ | – | abolished | abolished | – | – |
| n | n=31 | n=31 | n=57 | n=10 | n=10 | n=47 | n=60 |
| p value | <0.05 | <0.005 | >0.05 | <0.05 | <0.05 | >0.05 | >0.05 |
InsP3 can induce depletion of Golgi apparatus luminal Ca2+ in various cell types3,13–15. In addition, studies on mouse myocytes have provided evidence that endothelin-1 (ET-1: which increases [InsP3]) or InsP3R agonists, can induce Ca2+ efflux from the closely apposed nuclear envelope21. However, in the present study ET-1 did not stimulate Golgi Ca2+ release in intact ARVMs and the InsP3R blocker 2-APB had no significant effect on the percentage of cells exhibiting Golgi Ca2+ release under basal conditions. Similarly, in permeabilized myocytes, the InsP3R agonist adenophostin-A did not stimulate Ca2+-release at the nuclear poles.
The β1-adrenergic pathway modulates Golgi Ca2+ release
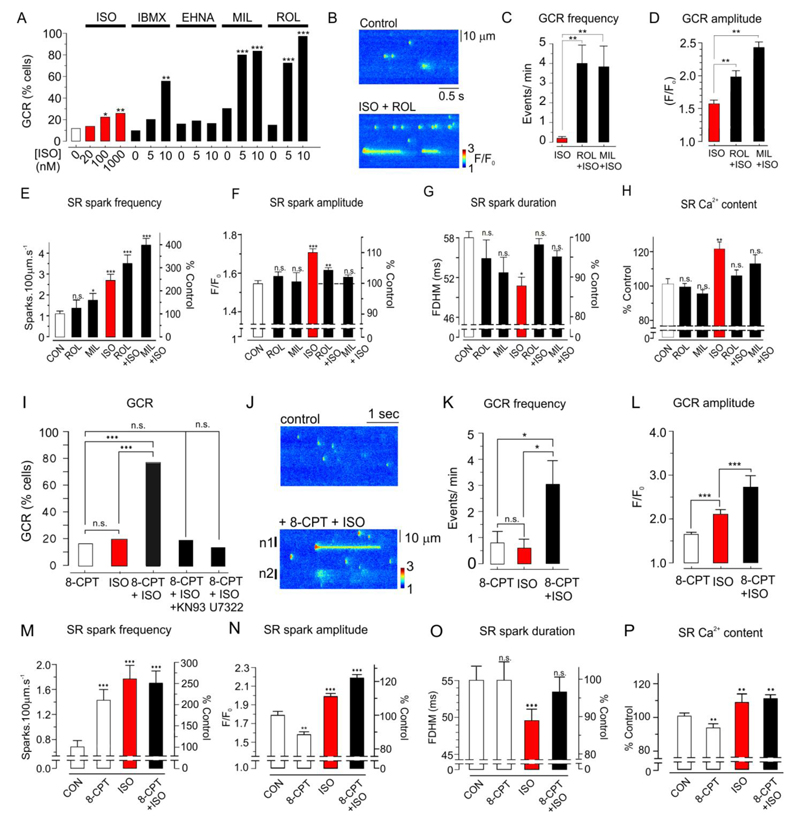
β1-adrenergic signaling has major effects on SR Ca2+ regulation, which involve a rise in cAMP concentration and activation of both PKA and Epac pathways22,23. Therefore, experiments were carried out to establish whether β1-adrenoceptor activation also stimulates Golgi Ca2+ release in intact ARVMs. In this series of experiments, 11.4 % of intact ARVMs under control conditions. In the presence of 20, 100 and 1000 nM isoproterenol, the percentage of cells exhibiting Golgi Ca2+ release increased to 13%, 23.3% and 26.3% respectively (Fig. 3A).
Fig. 3. Stimulation of Golgi Ca2+ release by the β1-adrenergic signaling pathway.
(A) Effects of isoproterenol (ISO) (20-1000 nM) or ISO (0, 5 or 10 nM) plus IBMX, EHNA, milrinone (MIL) or rolipram (ROL) on the percentage of cells exhibiting Golgi Ca2+ release within a 3 minute line-scan (n=22-30 cells). (B) Line-scan of ARVM under control conditions and after introduction of ISO (plus ROL. (C) Effects of ROL or MIL + 10 nM ISO on Golgi Ca2+ release frequency (n=12-21 cells). (D) Effects of PDE 3 or 4 inhibition on Golgi Ca2+ release amplitude relative to 10 nM ISO alone (n=12-30 cells). (E-G) Effects of ROL, MIL, ISO, ROL+ISO and MIL+ISO on SR Ca2+ spark frequency, amplitude (F/F0), and full duration at half maximum width (FDHM, n=13-25 cells). (H) Effects of ROL, MIL, ISO, ROL+ISO on the SR Ca2+ content assessed by application of caffeine (n=8 cells). (I) Effect of 8-CPT, ISO , 8-CPT+ISO, 8-CPT+ISO +KN93 and 8-CPT+ISO +U7322 on the percentage of cells exhibiting Golgi Ca2+ release (n=10-13 cells) (J) line-scan images before and after introduction of 8-CPT+ISO. (K) Effects of 8-CPT, ISO and ISO+8-CPT on Golgi Ca2+ release frequency (n=10-12 cells). (L) Effects of 8-CPT, ISO and ISO+8-CPT on Golgi Ca2+ release amplitude (n=10-12 cells). (M) Effects of 8-CPT, ISO and ISO+8-CPT on SR Ca2+ spark frequency relative to the mean control level (n=15-23 cells). (N) Effects of 8-CPT, ISO and ISO + 8-CPT on spark amplitude. (O) Effects of 8-CPT, ISO and ISO+8-CPT on Ca2+ spark duration (n=22-58 cells). (P) Effects of 8-CPT, ISO and ISO+8-CPT on the SR Ca2+ content, assessed by rapid application of caffeine (n=10-11 cells). In A-H data was obtained from 3-5 hearts per protocol. In I-P, data was obtained from 3-4 hearts per protocol. *p<0.05, ** p<0.01, ***, p<0.005.
This relatively small effect of isoproterenol on Golgi Ca2+ release might be explained if the cAMP concentration is low in the vicinity of the Golgi apparatus, for example, due to local PDE activity. Consistent with this, in the presence of only 10 nM isoproterenol, which did not itself influence Golgi Ca2+ release, the non-specific PDE inhibitor IBMX increased the percentage of cells exhibiting Golgi Ca2+ release to 55% (Fig. 3A). The selective PDE2 inhibitor EHNA had no significant effect with or without isoproterenol. However, in the presence of 5 or 10 nM isoproterenol, PDE3 inhibition with milrinone increased the percentage of cells exhibiting Golgi Ca2+ release to 80% and 83.3% respectively. Inhibition of PDE4 with rolipram increased the percentage of cells exhibiting Golgi Ca2+ release to 72.7% and 100% (Fig. 3A). Inhibition of PDE 3 or 4 typically induced repetitive Golgi Ca2+ release, and on average, rolipram or milrinone increased the frequency of Golgi Ca2+ release >10 fold relative to isoproterenol alone (Fig. 3 B&C). PDE 3 or 4 inhibition also increased Golgi Ca2+ release amplitude relative to isoproterenol alone (Fig. 3D).
The influence of isoproterenol and PDE inhibition on Ca2+ regulation by the SR was also studied to allow comparison with Golgi Ca2+ regulation. In the absence of isoproterenol, rolipram increased spark frequency by 28.6% ± 24.1, although this was not statistically significant (Fig. 3E). Milrinone increased spark frequency by 55 ± 20.8%. isoproterenol (10 nM) increased spark frequency by 144.4 ± 28.8%, while addition of isopproterenol with rolipram or milrinone increased spark frequency by 215.7 ± 38.2% and 297.2 ± 29.63% respectively. Rolipram or milrinone alone had no significant effect on Ca2+ spark amplitude, while isoproterenol significantly increased spark amplitude by 9.8 ± 1.41 % (Fig. 3F). Unexpectedly, however, in the presence of isoproterenol+rolipram or isoproterenol+milrinone, spark amplitude was not significantly different to control values. There was a trend towards reduced spark duration, although this was only significant with isoproterenol alone (Fig. 3G).
Assessment of the SR Ca2+ content with caffeine also revealed differences between the effects of isoproterenol and PDE 3 or 4 inhibition (Fig. 3H). Application of isoproterenol alone increased the SR Ca2+ content to 121.9 ± 2.68%. However, with isoproterenol combined with PDE 3 or 4 inhibition, the SR Ca2+ content was not significantly different to control, consistent with an increased Ca2+ leak from the SR.
Both Epac and PKA activation are required for stimulation of Golgi Ca2+ release
The SR Ca2+ leak that occurs in the presence of isoproterenol is partly due to activation of the Epac pathway and subsequent modification of RyR function by CaMKII22. Therefore, the apparent ability of PDE 3 or 4 inhibition to increase the RyR2 mediated Ca2+ leak (Fig. 3H) might be explained if a local increase in [cAMP] increases Epac activation.
In ARVMs, the selective Epac activator 8-CPT alone failed to increase the proportion of cells exhibiting Golgi Ca2+ release above the basal level (Fig. 3I). However, in the presence of 2 nM, 8-CPT increased the proportion of cells exhibiting Golgi Ca2+ release to 76.9 %. This level of isoproterenol was used to ensure that PKA activation was minimized, while still producing the effect. This effect of 8-CPT was blocked by the CaMKII inhibitor KN93 or by the phospholipase-C (PLC) inhibitor U73122. Line-scan images of a cell under control conditions and immediately after exposure to 8-CPT+isoproterenol, where both sparks and Golgi Ca2+ release are apparent, are shown (Fig. 3J). Relative to 8-CPT alone, addition of 8-CPT+isoproterenol increased Golgi Ca2+ release frequency ~3 fold, while isoproterenol alone had no significant effect (Fig. 3K). However, relative to 8-CPT alone, isoproterenol did significantly increase Golgi Ca2+ release amplitude, and this further increased in the presence of 8-CPT+isoproterenol (Fig. 3L); an effect is consistent with facilitation of Golgi Ca2+ uptake.
The effects of Epac activation on Ca2+ sparks and the SR Ca2+ content were also studied, with 8-CPT added either in the presence or absence of 2 nM isoproterenol. In contrast to the lack of effect on Golgi Ca2+ release, 8-CPT alone increased Ca2+ spark frequency to 210.2 ± 25.4 of the control value (Fig. 3M). Isoproterenol also increased spark frequency to 261.01 ± 32.20% of the control value and this effect was significantly greater than that of 8-CPT alone. However, the combined addition of 8-CPT and isoproterenol had no additional potentiating effect on spark frequency.
The effects on Ca2+ spark amplitude reveal differences between the effects of 8-CPT and isoproterenol on the SR; 8-CPT significantly decreased, while isoproterenol increased Ca2+ spark amplitude (Fig 3N). Furthermore, when 8-CPT and isoproterenol were added in combination, the net effect was an increase in Ca2+ spark amplitude, which was significantly greater than with isoproterenol alone. 8-CPT alone had no effect on duration, while isoproterenol significantly abbreviated Ca2+ sparks (Fig. 3O). When isoproterenol+8-CPT were added, Ca2+ spark duration was not significantly different to control. As previously described22, the differing effects of 8-CPT and isoproterenol on SR Ca2+ spark properties likely reflect changes in SR Ca2+ content; 8-CPT significantly reduced, while isoproterenol significantly increased the SR Ca2+ content (Fig. 3P). However, when isoproterenol was added with 8-CPT, the SR Ca2+ content was higher than control. The effects of 8-CPT on Ca2+ sparks and the SR content are consistent with previous reports24.
If the Golgi is similarly affected, the combined effect of Epac acting on the RyR and a sustained increase in Golgi Ca2+ content may both be necessary for stimulation of Golgi Ca2+ release (Fig. 3I). Consistent with this possibility, 6-Bnz-cAMP, which activates PKA but not Epac, increased SR Ca2+ spark frequency (Fig. S5 A& B) and the SR Ca2+ content (Fig. S5 C) but failed to stimulate Golgi Ca2+ release (Fig. S5D).
It has been suggested that β1-adrenergic stimulation can cause an increase in [NO], which activates an RyR2 mediated Ca2+ leak25. However, experiments involving measurement of NO with DAF-2 failed to detect a significant increase in NO following a 12 minute exposure to a supramaximal dose of isoproterenol in ARVMs, (Fig. S6 A & B). As a positive control, the NO donor GSNO did increase NO. The nitric oxide synthase (NOS) inhibitor L-NAME failed to block the stimulating effect of isoproterenol on Golgi Ca2+ release, or SR Ca2+ spark parameters (Fig. S6, C-G). These data suggest that NO does not have a major role in the stimulating effect of β1-adrenoceptor activation on Ca2+ sparks, or Golgi Ca2+ release in ARVMs.
Heart failure is associated with enhanced β1-adrenergic stimulation of Golgi Ca2+ release
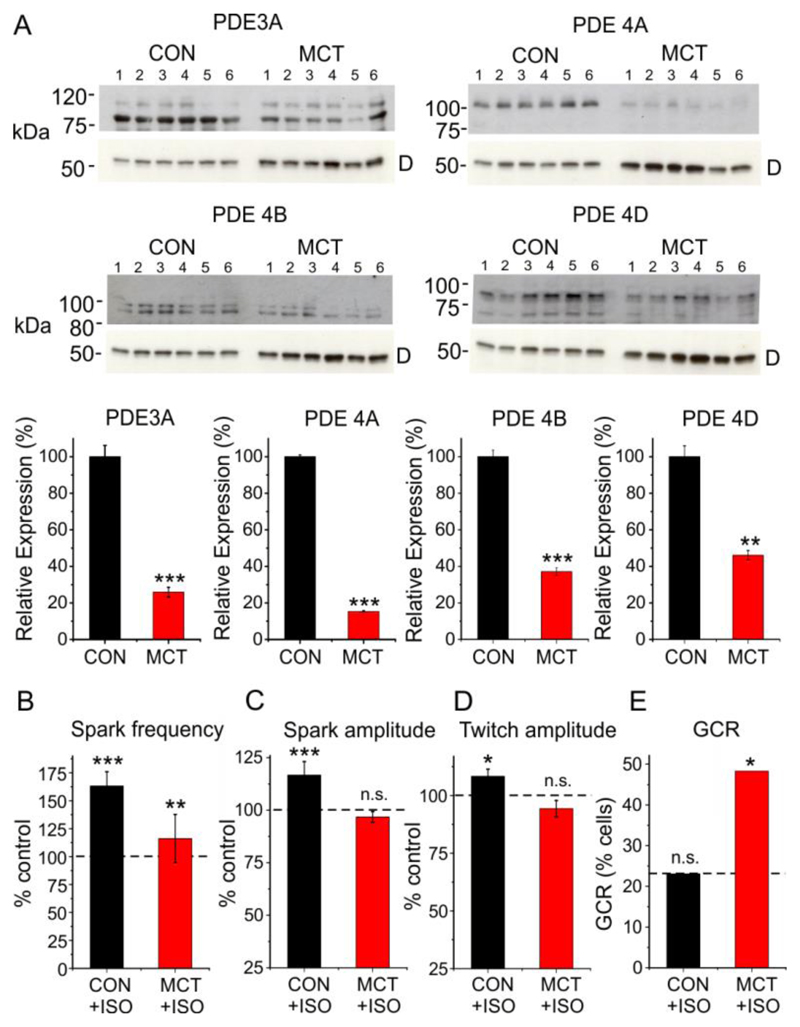
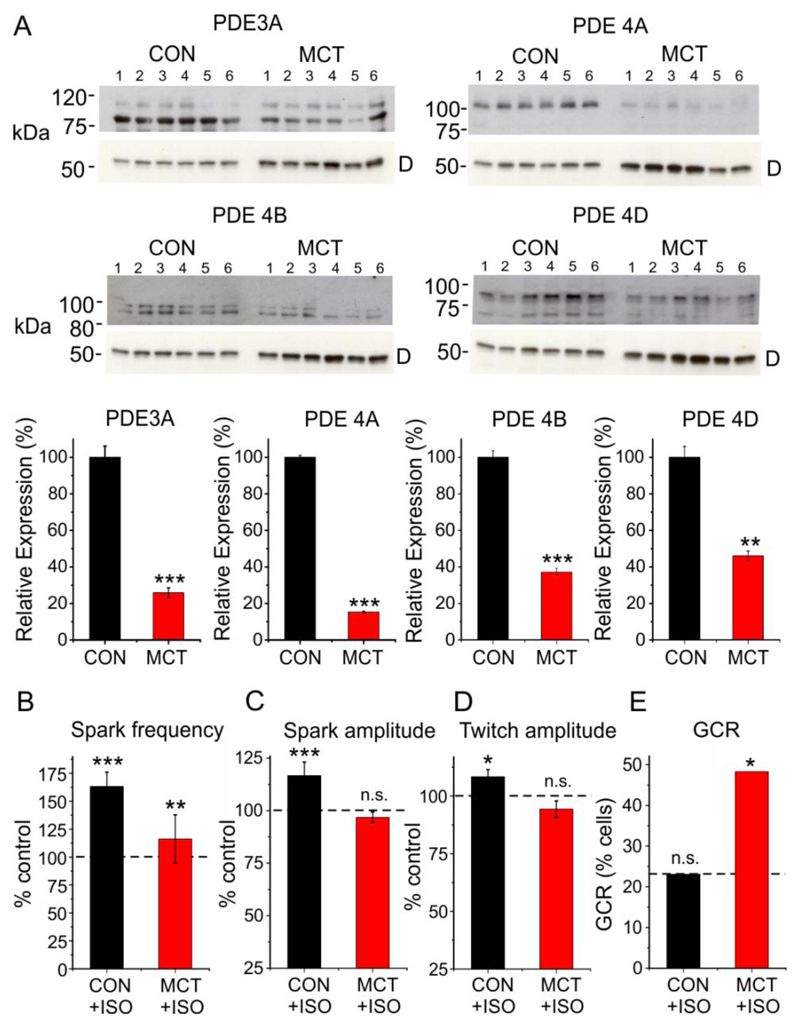
Expression of PDE3 and 4 is reportedly decreased in heart failure, which might, therefore, lead to β1-adrenergic facilitation of Golgi Ca2+ release in the absence of pharmacological agents. Therefore, the response to β1-adrenoceptor stimulation was investigated in the monocrotaline model of right heart failure26. Right heart failure was associated with a marked reduction in expression of PDE3A, PDE4A, PDE4B and PDE4D (Fig 4A). In ARVMs from heart failure animals 20 nM isoproterenol caused only a small increase in SR Ca2+ spark frequency, while spark amplitude and the amplitude of the electrically stimulated Ca2+ transient did not change significantly (Fig. 4B). This contrasts with controls, where spark frequency, spark amplitude and twitch amplitude all increased significantly, consistent with the previously reported blunting of the β1-adrenergic response in MCT induced heart failure27. However, unlike the effects on SR Ca2+ regulation, isoproterenol markedly increased the percentage of heart failure cells exhibiting Golgi Ca2+ release (n=6, p<0.05). This suggests that despite blunting of the β1-adrenergicresponse, downregulation of PDE 3 and 4 facilitates the action of cAMP on the Golgi, as acute PDE inhibition does in normal myocytes.
Fig. 4. Changes in PDE expression and Ca2+ regulation in heart failure.
(A) Western blot data obtained from the hearts of 6 control and 6 MCT-induced heart failure animals (upper) and corresponding mean data (lower) showing PDE 3A, 4A, 4B and 4D expression. ‘D’ indicates band corresponding to desmin at ~ 50 kDa. The data were normalized to desmin expression. (B) Mean data showing changes in spark frequency, spark amplitude, twitch amplitude and the percentage of cells exhibiting Golgi Ca2+ release following exposure to 20 nM ISO in control. For each condition, data were obtained from 22-29 cells from 5 control and 6 MCT hearts. *p<0.05, ** p<0.01, ***, p<0.005.
Conditions that promote Golgi Ca2+ release facilitate trafficking of VEGFR-1 to the surface membrane
Experiments were carried out to investigate whether conditions that induce Golgi Ca2+ release influence trafficking of proteins to the surface membrane, a key role of this organelle. Under control conditions, all ARVMS exhibited a punctate intracellular VEGFR-1 distribution (Fig. 5A), which co-localized with the Golgi apparatus resident protein GM130 (Fig. 5B). When present, the striated VEGFR-1 pattern co-localized with Nav1.1, which is located within the sarcolemma/t-tubules in cardiac cells (Fig 5C). However, only 29.1 ± 9.3% of control cells were striated or partially striated, indicating a sarcolemmal/t-tubule VEGFR-1 distribution (Fig 5D).
Fig. 5. Conditions that promote Golgi Ca2+ release facilitate trafficking of VEGFR-1 to the sarcolemma.
(A) Representative immunofluorescence data showing VEGFR-1 expression under control conditions or following exposure to 10 nM ISO, ISO + 8-CPT or ISO + ROL. Line profiles (inset) indicate the presence or absence of striations, indicating sarcolemmal localization. (B) Representative images showing intracellular VEGFR-1 (green) and GM130 (red) co-localization (yellow). (C) When present, VEGFR-1 striations (green) co-localized with Nav1.1 at the sarcolemma/t-tubules. Line profiles (inset) highlight coincident VEGFR1 and Nav1.1 peak fluorescence intensities. (D) Cumulative data showing percentage of cells exhibiting VEGFR1 striated pattern in the presence of ISO, ISO+ ROL, ISO+MIL and 8-CPT+ISO. Also shown is cumulative data from experiments for similar experiments done during constant field stimulation (stim), field stimulation and ISO+8-CPT or following preincubation in BAPTA-AM. n=60-160 cells from 3-8 hearts. * p<0.05. Bar indicates 4 µm.
These data suggest that the receptor is primarily localized within the Golgi apparatus at the ends of the nuclei and within cytosolic vesicles under control conditions, but not the sarcolemma. Incubation of ARVMs with isoproterenol, which had no effect on Golgi Ca2+ release, failed to influence the cellular distribution of VEGFR-1 (Fig 5A & D). However, all conditions found to stimulate Golgi Ca2+ release (Fig 3) markedly increased the percentage of cells exhibiting a striated VEGFR-1 expression pattern (Fig 5A & D). As shown for the effect of 8-CPT+ isoproterenol, this effect was also present if the cells were subjected to field stimulation throughout the protocol (Fig. 5D). Preincubation of cells with BAPTA-AM to strongly buffer cytosolic [Ca2+] blocked the increase in VEGFR-1 expression at the surface membrane (Fig. 5D). These data suggest that stimulation of Golgi apparatus Ca2+ cycling facilitates trafficking of VEGFR-1 from the Golgi apparatus to the sarcolemma in ARVMs.
Discussion
The present study shows that prolonged Ca2+-release events arise at the nuclear poles in ARVMs and co-localize with the Golgi apparatus (Fig. 1B). Furthermore, IQ-induced disruption of the Golgi apparatus at the nuclear poles (Fig. 2A) abolished Golgi Ca2+ release, without affecting SR Ca2+ regulation (Fig. 2C-D). Together, these findings suggest that the prolonged Ca2+-release events at the nuclear poles are the cytosolic consequence of Golgi apparatus Ca2+ depletion described in earlier studies on a range of other cell types8,13,14,16. The greater diffusion Ca2+ into the nucleus rather than the cytosol (Fig. 2E) is consistent with the close proximity of the trans-Golgi apparatus to mitochondria, which act as a Ca2+ sink28 and also the absence of active Ca2+ uptake mechanisms within the nucleoplasm. Proximity to mitochondria might also explain why the Golgi apparatus at the nuclear poles is more likely to exhibit Ca2+ release than cytosolic vesicles, for example, if mitochondrial Ca2+ fluxes influence Golgi Ca2+ regulation28.
Golgi Ca2+ release involves RyR mediated Ca2+ efflux and SERCA Ca2+ uptake
In ARVMs, Golgi Ca2+ release was abolished by ryanodine. This is consistent with findings in HL-1 cells (which partially retain an adult cardiac phenotype) that functional RyRs are present within the Golgi29. In addition, caffeine-sensitive Ca2+ efflux from both the trans and medial Golgi apparatus has been reported in neonatal cardiac and HL-1 cells respectively2,30. These data suggest that in ARVMs, RyRs are an important component of the Golgi Ca2+ efflux pathway. Basal Golgi Ca2+ release may, therefore, be the equivalent of spontaneous Ca2+ sparks.
The luminal Golgi environment in particular differs from that of the SR in that it has calnuc as the major Ca2+ buffer9 which unlike calsequestrin, is not known to affect RyR gating. The Ca2+ binding protein calumenin is also present within the GA and previous work has shown that it inhibits the direct activating effect of luminal Ca2+ on RyR231. It has been shown that interventions that modify the relationship between RyR2 gating and SR luminal Ca2+ can induce long lasting SR Ca2+ sparks32. The prolonged nature of Golgi Ca2+ release may, therefore, reflect the environment within the Golgi and consequent effects on RyR gating.
In the present study, drugs that increase InsP3 or block InsP3Rs did not affect Golgi Ca2+ release under control conditions. However, PLC and InsP3R receptor activation are part of the signaling cascade that links Epac activation to CaMKII phosphorylation of RyR2, and an increase in SR Ca2+ spark frequency22. As with Ca2+ sparks33, PLC inhibition prevented stimulation of Golgi Ca2+ release by isoproterenol+8-CPT (Fig. 3I). Therefore, InsP3R activation may either directly (via Ca2+ release) or indirectly (via CaMKII) facilitate Golgi Ca2+ release when Epac is activated.
In contrast to neonatal cardiac myocytes where Ca2+ uptake into the trans-Golgi was exclusively dependent on SPCA12, Golgi Ca2+ release was abolished by thapsigargin, implicating SERCA as an important Ca2+ uptake mechanism underlying this form of Ca2+ release in ARVMs. However, this finding does not exclude the possibility that SPCA1 also contributes to Ca2+ uptake in ARVMs.
Golgi apparatus and SR Ca2+-release are functionally independent
One of the most striking findings obtained in the present study was that once initiated, spontaneous or triggered global SR Ca2+-release had no apparent effect on the underlying time course of Golgi Ca2+ release (Fig. 2E-G). As spontaneous SR Ca2+ waves deplete the SR by 80-90%34, it is unlikely that Golgi Ca2+ release and Ca2+ sparks originate from a common Ca2+ pool. Furthermore, previous work on cardiac cells has established (i) that the lumen of the nuclear envelope is contiguous with that of the SR, allowing Ca2+ to diffuse freely between both compartments and (ii) that global SR Ca2+-release is associated with rapid (peak ~170 ms) depletion of nuclear envelope Ca2+ 35. Therefore, the absence of an effect of global SR Ca2+-release on the underlying time course of Golgi Ca2+ release (Fig. 2) excludes the nuclear envelope as a the likely source of Ca2+ and is consistent with anatomical and functional separation of the SR and Golgi Ca2+ stores. Global SR Ca2+-release did occasionally trigger prolonged Golgi Ca2+ release (e.g. Fig. 2F). However, this can be explained by the presence of RyR2 within the Golgi apparatus2, which would be expected to exhibit Ca2+ induced Ca2+-release.
Golgi Ca2+ release is stimulated by activation of the β1-adrenergic signaling pathway
In the present study, introduction of isoproterenol alone had only a small stimulating effect on Golgi Ca2+ release while isoproterenol plus PDE 3 or 4 inhibition markedly stimulated Golgi Ca2+ release. This is consistent with compartmentalization of β1-adrenergic signaling36, such that the cAMP concentration at the Golgi membrane is affected by PDE 3 and 4 activity. In support of this, multiple components of the β1-adrenergic signaling pathway are associated with scaffolding proteins, which are targeted to specific subcellular locations (Fig. S7). For example, muscle-specific A-kinase anchoring proteins (mAKAPs) form signaling complexes comprising PKA, Epac, PDE 4D3 and protein phosphatase 2A37. mAKAPs are targeted to the nuclear envelope, but are also associated with RyRs in cardiac myocytes 38,39. In addition, the scaffold protein myomegalin anchors PDE 4D3 to the Golgi apparatus40 and particulate PDE 3 is membrane-bound and also present in the perinuclear region41.
Golgi Ca2+ regulation has effects on VEGFR-1 trafficking
It has been shown that both the Ca2+ within the Golgi apparatus and local cytosolic Ca2+ exert a regulatory influence on the structure of the organelle and on vesicle trafficking2,3,6. The effects of local cytosolic Ca2+ may involve Golgi-associated calmodulin-like Ca2+ binding proteins (e.g. calneuron1/2, NCS-1), which regulate the activity of phosphatidylinositol 4-OH kinase IIIβ (PI-4Kβ), local synthesis of phospholipids and vesicle trafficking6. β1-adrenergic signaling also has effects on the function of the Golgi apparatus in a variety of cell types42,43.
The present study provides a plausible link between these two sets of observations; as β1-adrenergic stimulation can facilitate Golgi Ca2+ release (Fig 3), the influence of β1-adrenergic stimulation on the Golgi apparatus may involve Ca2+ dependent regulation. This possibility is supported by the effects on VEGFR-1 localization (Fig. 5). As described in endothelial cells44, VEGFR-1 was located primarily within the Golgi apparatus in ARVMs under control conditions (Fig. 5B). However, all factors that facilitated Golgi Ca2+ release increased trafficking of VEGFR-1 to the surface membrane (Fig. 5A & D). This is of is of relevance to work showing that VEGFR-1 is involved in preconditioning, whereby brief periods of intermittent ischemia improve the functional and structural integrity of the heart following subsequent ischemia/reperfusion45. It has been shown that PDE is inhibited during ischemic preconditioning46 and that transient pharmacological PDE inhibition can mimic ischemic preconditioning47. The present work suggests that increased sarcolemmal expression of VEGFR-1, secondary to β1-adrenoceptor mediated effects on Golgi Ca2+, may contribute to these effects. Moreover, our findings suggest that activation of Epac may provide a novel method to increase sarcolemmal VEGFR-1, thereby facilitating the preconditioning response.
The current findings may also be relevant to work implicating local, rather than global, Ca2+ regulation in the control of key cardiac signaling pathways48; β1-adrenoceptor stimulation is known to induce hypertrophy by a pathway that involves activation of Epac and subsequent effects that involve ‘nuclear factor of activated t-cells’ (NFAT) and/or myocyte enhancer factor 2 (MEF2) transcription factors (Fig S5). 33,49. Both of these pathways are Ca2+ regulated, for example, NFAT activation is controlled by the Ca2+/calmodulin-dependent phosphatase calcineurin, which is present at the nuclear poles50. Epac induced activation of InsP3 receptors and consequent effects on nucleoplasmic [Ca2+]/CaMKII are required for the nuclear export of histone deacetylase-5 from the nucleus, which precedes MEF2 activation33. Local Golgi Ca2+ release may influence these signaling pathways, particularly during the development of hypertrophy and heart failure, when sustained β1-adrenergic activation and PDE downregulation occur.
Conclusions
These data establish that the Golgi apparatus is a juxtanuclear focal point for β1-adrenergic signaling in the myocardium, exhibits functional plasticity under pathological conditions and has the capacity to impose local changes in Ca2+ concentration, despite the presence of global cytosolic Ca2+ transients that underlie the primary function of the cell. Furthermore, we show that activation of the β1-adrenergic pathway has distinct effects on Ca2+-release from the Golgi apparatus, which involve local regulation of [cAMP] by PDE 3 and 4, and activation of Epac and PKA, with consequent effects on receptor trafficking.
Materials and Methods
Preparation of cells and solutions
Wistar rats (150-200g) were sacrificed in accordance with the UK Home Office Guidance on the Operation of Animals (Scientific Procedures) Act of 1986. ARVMs were isolated by collagenase digestion as described previously51. Intact myocytes were superfused with solutions containing (mM): 113 NaCl; 5.4 KCl; 1 MgCl2; 1.0 CaCl2; 0.37 Na2HPO4; 5.5 glucose; 5 HEPES, pH 7.1. Changes in cytosolic Ca2+ concentration were detected by loading the myocytes with fluo-4 AM (5 µM) for 15 minutes at room temperature (20-22°C). In some experiments, cells were permeabilized by exposure to saponin (10 µg/ml) in a mock intracellular solution for 10 minutes, before centrifugation and re-suspension. The intracellular solution contained (mM) 100 KCl; 25 HEPES; 0.36 or 0.05 EGTA; 10 phosphocreatine; 5 ATP and fluo-4 (5 µM), pH 7.0. The free Ca2+ and Mg2+ concentrations were adjusted to 120 nM and 1 mM respectively by addition of CaCl2 and MgCl2. Unless otherwise stated, all chemicals were obtained from Sigma Aldrich (Dorset, UK). Isoproterenol was obtained from Martindale Pharmaceuticals, Essex, UK. In some experiments the β2-antagonist ICI 118,551 (100 nM) was included in the solutions, although it was not found to influence the results reported here. The rolipram (10 µM), milrinone (10 µM) and IBMX (10 µM) were added to inhibit PDEs. 8-CPT (10 µM) and 6-Bnz-cAMP (600 µM) used to selectively activate Epac or PKA respectively. Caffeine (20 mM) was rapidly applied to induce a maximal SR Ca2+ release. A high level of ryanodine (100 µM) was use to block RyRs in the closed state. Thapsigargin (2.5 µM) was applied to selectively inhibit SERCA. ET-1 (100 µM) was applied to increase InsP3 in ARVMs. 2-APB (10 µM) was applied as a blocker of IP3Rs. Preincubation with IQ (25 µM) was used to completely disrupt the Golgi apparatus (1 hour) or to disrupt Golgi Ca2+ release (15 mins). KN93 (1 µM) was used to inhibit CaMKII. U7322 (5 µM) was applied to inhibit PLC. RU360 (5 µM) was used to inhibit mitochondrial Ca2+ uptake.
Monocrotaline induced heart failure model
Experiments were approved by the local ethical committee and the United Kingdom Home Office. Male Wistar rats (200 g) received a single intraperitoneal injection of monocrotaline (MCT) to induce right ventricular failure (60 mg/kg in saline) or were injected with an equivalent volume of saline as previously. Animals were weighed weekly for 3 week post-injection and then daily. Ethical approval required that animals were killed upon displaying clinical signs of right heart failure, for example, dyspnea, cold extremities, lethargy, or any weight loss on consecutive days.
Experimental chamber and Ca2+ imaging
The experimental chamber was placed on the stage of a Nikon Diaphot Eclipse TE2000 inverted microscope and the cells were viewed using a 60X water immersion lens (Plan Apo, NA 1.2). In most experiments, cells were imaged in line scan mode using a confocal laser-scanning unit (Bio-Rad, Microradiance 2000, Herts UK) attached to the side port of the microscope. Experiments involving rapid x-y confocal Ca2+ imaging utilized an Andor Revolution confocal unit fitted with a Yokogawa-X1 spinning disk running at 30-60 frames per second. All dyes were excited at 488 nm and emitted fluorescence was measured at >515 nm. Image processing and analysis were done using ImageJ (http://rsb.info.nih.gov/ij/) software with plugins that allow automatic detection of localized Ca2+-release in line-scan (https://sites.google.com/site/sparkmasterhome/) or x-y images 52.
Identification of the Golgi apparatus using electron or confocal microscopy
For electron microscopy, the left ventricle from paraformaldehyde treated rat heart was sectioned at 50 µm on a vibrating microtome and incubated in an antibody to the Golgi protein GM130 (mouse) 1:200 (BD Biosciences, Oxford England). Following washing, sections were incubated in biotinylated donkey anti-mouse 1:200, (Invitrogen, Europe) prior to extravidin peroxidase (1:1500; Sigma) and visualisation with diaminobenzidine.
For confocal microscopy (Zeiss LSM700) of fixed cells, myocytes were cultured overnight in the presence of the CellLight Golgi-GFP baculovirus (Invitrogen, Paisley, UK), which expresses a fusion construct of a Golgi apparatus marker (targeted to N-acetylgalactosaminyl transferase-2) and GFP. The culture medium comprised Medium 199 with HEPES and the following added ingredients (mM) 5 creatine; 5 taurine; 2 Na-pyruvate, 2 L-Carnitine. The solution also contained primocin 2µl/ml and insulin 0.1 µM. 6 well plates were laminin coated before addition of myocytes at ~ 25,000 cells/ml. Following culture, cells were fixed (Dulbecco’s PBS 4% paraformaldehyde, 20 min) and then permeabilized (0.05% Triton-X 100) before DAPI staining to allow both the Golgi apparatus (488 nm excitation, >515 nm emission) and the nucleus (360 nm excitation, 460 nm emission) to be imaged. Colocalization experiments were carried out using Alexa Fluor 488 labeled mouse anti GM130 (1:10, BD Biosciences), a Nav1.1 rabbit primary (1:25, Alomone Labs), followed by donkey anti-rabbit Alexa Fluor 555 (Invitrogen) and a VEGFR-1 antibody (1:25 BD Systems Ltd) followed by donkey anti-goat Alexa Fluor 488 (1:500, Invitrogen).
In experiments involving changes in VEGFR-1 expression at the surface membrane, cells were exposed to a variety of conditions prior to fixing. This included preinculabtion in BAPTA-AM (2.5 µM) for 15 minutes prior to introduction of isoproterenol (10 nM), isoproterenol plus 8-CPT (10 µM) or isoproterenol plus rolipram (10 µM). In some experiments the cells were electrically field stimulated (0.5 Hz) before and during exposure the drugs.
For visualization of the Golgi apparatus in living cells, myocytes were loaded for 15 minutes with Golgi-ID green (0.5 nmoles/ml, Enzo Life Sciences, Exeter, UK), a membrane permeant Golgi apparatus marker (excitation 488 nM, emission > 515 nm). The Golgi ID green signal was found to bleach rapidly (Fig. S2). Consequently, co-loading with fluo-4 and Golgi ID green allowed the Golgi apparatus to be identified in x-y confocal images before bleaching of the dye, which left the stable Ca2+ sensitive fluo-4 signal within the cytosol (see also Fig. 1B).
Immunoblotting
The free right ventricular heart wall was dissected, minced, then homogenised for 4 × 10 s in 2 ml cooled (~10 °C) Laemmli buffer without β-mercaptoethanol [63.5 mM Tris (pH 6.8), 10% glycerol, 2% SDS, protease (Roche Applied Science) and phosphatase (Thermo Scientific Pierce) inhibitor cocktails at maximum power using an Ultra-Turrax T8 hand-held homogeniser. After the final homogenisation step, tubes were centrifuged (2000 g, 10 min at 15 °C) to sediment cell debris and unprocessed material. The supernatant was immediately stored at -20 °C. Protein concentration was determined by bicinchoninic acid assay (Sigma-Aldrich). 5% (v/v) β-mercaptoethanol was added to all samples prior to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred from polyacrylamide gels to polyvinylidene fluoride (PVDF) membranes, after which a standard immunoblotting procedure was employed. The PDE3A antibody was obtained from FabGennix Inc (Frisco, USA) and the PDE4A, 4B and 4D antibodies were a gift from Professor Miles Houslay, University of Glasgow.
Statistics
Results are presented as the mean ± S.E.M. Statistical significance was assessed using a t-test, Chi-squared test or ANOVA as appropriate. Differences between means were considered significant at p<0.05. Statistics and graph plotting were done were using Origin software (Microcal, UK).
Supplementary Material
Fig. S1 Ca2+ sparks and prolonged juxtanuclear Ca2+ release events are of similar amplitude
Fig. S2 The Golgi Apparatus is consistently located at the nuclear poles and can be disrupted by IQ
Fig. S3 Golgi ID green fluorescence bleaches rapidly
Fig. S4 RU360 stimulates Golgi Ca2+ release at the nuclear poles
Fig. S5 6-bnz-cAMP stimulates SR Ca2+ sparks but not GCR
Fig. S6 Effects of isoproterenol are not mediated by an increase in cytosolic NO
Fig. S5 Proposed mechanisms underlying β-adrenergic regulation of SR and GA function in ARVMs
One Sentence Summary.
Golgi apparatus Ca2+regulation and function is controlled by Epac and PKA signaling
Funding
This work was funded by grants from the British Heart Foundation and the Wellcome Trust.
Footnotes
Author contributions: Yang: confocal Ca2+ imaging and DAF-2 experiments; Kirton: Ca2+ imaging and immunofluorescence experiments. MacDougall: Western blot analysis; Boyle: Immunofluorescence measurements; Deuchars: electron microscopy; Frater: electron microscopy; Ponnambalan immunofluorescence experiments and experimental design; Hardy: preparation of heart failure model; White: preparation of heart failure model; Calaghan Western blot analysis and experimental design; Peers: experimental design, immunofluorescence and editing of draft manuscript; Steele: Experimental design, manuscript preparation and overall project management.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Pizzo P, Lissandron V, Capitanio P, Pozzan T. Ca2+ signalling in the Golgi apparatus. Cell Calcium. 2011;50:184–192. doi: 10.1016/j.ceca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci U S A. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micaroni M, Perinetti G, Di Giandomenico D, Bianchi K, Spaar A, Mironov AA. Synchronous intra-Golgi transport induces the release of Ca2+ from the Golgi apparatus. Exp Cell Res. 2010;316:2071–2086. doi: 10.1016/j.yexcr.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Porat A, Elazar Z. Regulation of intra-Golgi membrane transport by calcium. J Biol Chem. 2000;275:29233–29237. doi: 10.1074/jbc.M005316200. [DOI] [PubMed] [Google Scholar]
- 5.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 6.Mikhaylova M, Reddy PP, Munsch T, Landgraf P, Suman SK, Smalla KH, Gundelfinger ED, Sharma Y, Kreutz MR. Calneurons provide a calcium threshold for trans-Golgi network to plasma membrane trafficking. Proc Natl Acad Sci U S A. 2009;106:9093–9098. doi: 10.1073/pnas.0903001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Wuytack F, Vanoevelen J, Van Baelen K, Simoni A, Rizzuto R, Missiaen L. Similar Ca2+-signaling properties in keratinocytes and in COS-1 cells overexpressing the secretory-pathway Ca2+-ATPase SPCA1. Cell Calcium. 2003;34:157–162. doi: 10.1016/s0143-4160(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 8.Van Baelen K, Vanoevelen J, Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Rizzuto R, Missiaen L, Wuytack F. The contribution of the SPCA1 Ca2+ pump to the Ca2+ accumulation in the Golgi apparatus of HeLa cells assessed via RNA-mediated interference. Biochem Biophys Res Commun. 2003;306:430–436. doi: 10.1016/s0006-291x(03)00977-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin P, Yao Y, Hofmeister R, Tsien RY, Farquhar MG. Overexpression of CALNUC (nucleobindin) increases agonist and thapsigargin releasable Ca2+ storage in the Golgi. J Cell Biol. 1999;145:279–289. doi: 10.1083/jcb.145.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas P, Surroca A, Orellana A, Wolff D. Kinetic characterization of calcium uptake by the rat liver Golgi apparatus. Cell Biol Int. 2000;24:229–233. doi: 10.1006/cbir.2000.0496. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RS, Jones SM, Dahl RH, Nordeen MH, Howell KE. Characterization of the Golgi complex cleared of proteins in transit and examination of calcium uptake activities. Mol Biol Cell. 1997;8:1911–1931. doi: 10.1091/mbc.8.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin P, Le Niculescu H, Hofmeister R, McCaffery JM, Jin M, Hennemann H, McQuistan T, De Vries L, Farquhar MG. The mammalian calcium-binding protein, nucleobindin (CALNUC), is a Golgi resident protein. J Cell Biol. 1998;141:1515–1527. doi: 10.1083/jcb.141.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanoevelen J, Raeymaekers L, Parys JB, De Smedt H, Van Baelen K, Callewaert G, Wuytack F, Missiaen L. Inositol trisphosphate producing agonists do not mobilize the thapsigargin-insensitive part of the endoplasmic-reticulum and Golgi Ca2+ store. Cell Calcium. 2004;35:115–121. doi: 10.1016/j.ceca.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Mahieu F, Owsianik G, Verbert L, Janssens A, De Smedt H, Nilius B, Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J Biol Chem. 2007;282:3325–3336. doi: 10.1074/jbc.M605213200. [DOI] [PubMed] [Google Scholar]
- 16.Missiaen L, Van Acker K, Van Baelen K, Raeymaekers L, Wuytack F, Parys JB, De Smedt H, Vanoevelen J, Dode L, Rizzuto R, Callewaert G. Calcium release from the Golgi apparatus and the endoplasmic reticulum in HeLa cells stably expressing targeted aequorin to these compartments. Cell Calcium. 2004;36:479–487. doi: 10.1016/j.ceca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Vanoevelen J, Raeymaekers L, Dode L, Parys JB, De Smedt H, Callewaert G, Wuytack F, Missiaen L. Cytosolic Ca2+ signals depending on the functional state of the Golgi in HeLa cells. Cell Calcium. 2005;38:489–495. doi: 10.1016/j.ceca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Missiaen L, Van Acker K, Parys JB, De Smedt H, Van Baelen K, Weidema AF, Vanoevelen J, Raeymaekers L, Renders J, Callewaert G, Rizzuto R, et al. Baseline cytosolic Ca2+ oscillations derived from a non-endoplasmic reticulum Ca2+ store. J Biol Chem. 2001;276:39161–39170. doi: 10.1074/jbc.M104044200. [DOI] [PubMed] [Google Scholar]
- 19.Rambourg A, Segretain D, Clermont Y. Tridimensional architecture of the Golgi apparatus in the atrial muscle cell of the rat. Am J Anat. 1984;170:163–179. doi: 10.1002/aja.1001700204. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa PA, Yucel JK, Veit B, Faulkner DJ, Deerinck T, Soto G, Ellisman M, Malhotra V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell. 1993;73:1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc'h F, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during beta-adrenergic stimulation of cardiac muscle. Cardiovasc Res. 2013;100:392–401. doi: 10.1093/cvr/cvt201. [DOI] [PubMed] [Google Scholar]
- 26.Benoist D, Stones R, Drinkhill M, Bernus O, White E. Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2011;300:H2230–H2237. doi: 10.1152/ajpheart.01226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leineweber K, Seyfarth T, Abraham G, Gerbershagen HP, Heinroth-Hoffmann I, Ponicke K, Brodde OE. Cardiac beta-adrenoceptor changes in monocrotaline-treated rats: differences between membrane preparations from whole ventricles and isolated ventricular cardiomyocytes. J Cardiovasc Pharmacol. 2003;41:333–342. doi: 10.1097/00005344-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Dolman NJ, Gerasimenko JV, Gerasimenko OV, Voronina SG, Petersen OH, Tepikin AV. Stable Golgi-mitochondria complexes and formation of Golgi Ca2+ gradients in pancreatic acinar cells. J Biol Chem. 2005;280:15794–15799. doi: 10.1074/jbc.M412694200. [DOI] [PubMed] [Google Scholar]
- 29.George CH, Rogers SA, Bertrand BM, Tunwell RE, Thomas NL, Steele DS, Cox EV, Pepper C, Hazeel CJ, Claycomb WC, Lai FA. Alternative splicing of ryanodine receptors modulates cardiomyocyte Ca2+ signaling and susceptibility to apoptosis. Circ Res. 2007;100:874–883. doi: 10.1161/01.RES.0000260804.77807.cf. [DOI] [PubMed] [Google Scholar]
- 30.Wong AK, Capitanio P, Lissandron V, Bortolozzi M, Pozzan T, Pizzo P. Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol. 2013;5:266–276. doi: 10.1093/jmcb/mjt024. [DOI] [PubMed] [Google Scholar]
- 31.Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim dH. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem. 2009;284:31109–31121. doi: 10.1074/jbc.M109.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira L, Ruiz-Hurtado G, Morel E, Laurent AC, Metrich M, Dominguez-Rodriguez A, Lauton-Santos S, Lucas A, Benitah JP, Bers DM, Lezoualc'h F, et al. Epac enhances excitation-transcription coupling in cardiac myocytes. J Mol Cell Cardiol. 2012;52:283–291. doi: 10.1016/j.yjmcc.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacQuaide N, Dempster J, Smith GL. Assessment of sarcoplasmic reticulum Ca2+ depletion during spontaneous Ca2+ waves in isolated permeabilized rabbit ventricular cardiomyocytes. Biophys J. 2009;96:2744–2754. doi: 10.1016/j.bpj.2008.12.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 36.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 37.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 39.Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life. 2007;59:163–169. doi: 10.1080/15216540701358593. [DOI] [PubMed] [Google Scholar]
- 40.Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- 41.Kauffman RF, Crowe VG, Utterback BG, Robertson DW. LY195115: a potent, selective inhibitor of cyclic nucleotide phosphodiesterase located in the sarcoplasmic reticulum. Mol Pharmacol. 1986;30:609–616. [PubMed] [Google Scholar]
- 42.Mayinger P. Signaling at the Golgi. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arakel EC, Brandenburg S, Uchida K, ZHANG H, Lin YW, Kohl T, Schrul B, Sulkin MS, Efimov IR, Nichols CG, Lehnart SE, et al. Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes. J Cell Sci. 2014 doi: 10.1242/jcs.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittar S, Ulyatt C, Howell GJ, Bruns AF, Zachary I, Walker JH, Ponnambalam S. VEGFR1 receptor tyrosine kinase localization to the Golgi apparatus is calcium-dependent. Exp Cell Res. 2009;315:877–889. doi: 10.1016/j.yexcr.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Thirunavukkarasu M, Juhasz B, Zhan L, Menon VP, Tosaki A, Otani H, Maulik N. VEGFR1 (Flt-1+/-) gene knockout leads to the disruption of VEGF-mediated signaling through the nitric oxide/heme oxygenase pathway in ischemic preconditioned myocardium. Free Radic Biol Med. 2007;42:1487–1495. doi: 10.1016/j.freeradbiomed.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lochner A, Genade S, Tromp E, Opie L, Moolman J, Thomas S, Podzuweit T. Role of cyclic nucleotide phosphodiesterases in ischemic preconditioning. Mol Cell Biochem. 1998;186:169–175. [PubMed] [Google Scholar]
- 47.Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, Asakura M, Ogita H, Liao Y, Sakata Y, Ogai A, et al. Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation. 2001;104:705–710. doi: 10.1161/hc3201.092216. [DOI] [PubMed] [Google Scholar]
- 48.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Metrich M, Laurent AC, Breckler M, Duquesnes N, Hmitou I, Courillau D, Blondeau JP, Crozatier B, Lezoualc'h F, Morel E. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell Signal. 2010;22:1459–1468. doi: 10.1016/j.cellsig.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z, Steele DS. Effects of phosphocreatine on SR Ca2+ regulation in isolated saponin-permeabilized rat cardiac myocytes. J Physiol. 2002;539:767–777. doi: 10.1113/jphysiol.2001.012987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steele EM, Steele DS. Automated Detection and Analysis of Ca2+ Sparks in x-y Image Stacks Using a Thresholding Algorithm Implemented within the Open-Source Image Analysis Platform ImageJ. Biophys J. 2014;106:566–576. doi: 10.1016/j.bpj.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.