Summary
Chromium supplementation (Cr) may be useful in the management of diabetes and appears to improve some aspects of glucose handling. However, several studies have used either high doses of Cr supplementation or have placed control animals on a Cr-deficient diet. We therefore wanted to test whether Cr dosages in the ranges that more closely approximate recommended levels of supplementation in humans are efficacious in glycemic control under normal dietary conditions. Euglycemic Wistar or diabetic Goto-Kakizaki (GK) rats (a model of nonobese NIDDM) were assigned to water (control) or chromium picolinate (Cr-P) supplementation (1 or 10 mg/kg/day) groups for up to 32 weeks. Glucose tolerance was tested following an overnight fast by injecting sterile glucose (1.0 g/kg, i.p.) and then measuring blood glucose at select times to determine the sensitivity to glucose by calculation of the area under the curve. Cr-P did not significantly alter the growth of the animals. In the euglycemic Wistar rats, Cr-P supplementation did not alter the response to a glucose tolerance test. In the GK rats, Cr-P supplementation significantly improved glucose tolerance at both levels of Cr-P supplementation (1 mg/kg/day: H20; 100 ± 11%; Cr-P 70 6 8%; 10 mg/kg/day: H20; 100 ± 10%; Cr-P 66 ± 9 %). Cr-P supplementation produced a small improvement in some indices of glycemic control. There were no differences observed for the two levels of Cr-P supplementation suggested that we did not identify a threshold for Cr-P effects, and future studies may use lower doses to find a threshold effect for improving glucose tolerance in diabetics.
Keywords: chromium picolinate, type 2 diabetes, NIDDM, Goto-Kakizaki, dietary supplements, glucose tolerance
INTRODUCTION
Cardiovascular disease is the leading cause of mortality in noninsulin dependent diabetes mellitus patients (NIDDM). Although originally thought to be a metabolic problem, widespread systemic complications are now recognized. Diet modification, exercise, and medications are the current treatment modalities. However, even with strict compliance, tight glycemic control is difficult to achieve for some patients. The use of alternative therapies is rising and despite lacking a solid foundation for its use, chromium is increasingly used as a dietary supplement (1). Some reports have suggested that chromium supplementation (Cr) may be useful in the management of diabetes. However, the efficacy of chromium or safety for long-term use remains unclear. Complications of diabetes place an enormous cost on the health care system and the potential for a low-cost supplement that may aid in the management of diabetes warrants evaluation.
Chromium is an essential element that exists as tri-or hexavalent forms. Although chromium (III) is biologically beneficial, chromium (VI) is cytotoxic (2, 3). Chromium-deficient diets are associated with impaired glucose tolerance, hyperglycemia, and hyperinsulinemia (4–6). More severe forms of Cr deficiency include nerve dysfunction, and Cr is now routinely added to total parenteral nutrition (7). It has been suggested that the average daily dietary intake of Cr of most individuals is below the recommended values for adults (8). Cr levels in diabetics are thought to be depressed as Cr levels in hair samples of type 1 diabetic patients and in the plasma of NIDDM patients were significantly decreased, while urinary excretion was increased in both humans and diabetic animals (5, 9–11). Chromium tissue levels vary by organ and diabetes does not impact equally upon them, although the major depositories in the kidney and muscle have greater depletions as a function of diabetes (11–15).
Chromium is thought to enhance insulin sensitivity or serve as an antioxidant to relieve the increased oxidative stress associated with diabetes (5, 13, 16–18). Some studies have found that chromium supplementation significantly decreased the fasting blood glucose and decreased the HbA1c of diabetic individuals (16, 19). Several reviews including a meta-analysis concluded that Cr was without effect on euglycemic individuals but likely to be somewhat effective on individuals with impaired glucose metabolism (1, 8, 20).
Goto-Kakizaki (GK) rats are a nonobese model of NIDDM, which are hyperinsulinemic and have an elevated fasting glucose (21–25). As a model of type 2 diabetes, the GK rats do not have the confounding factors of hyperlipidemia or hypercholesterolemia observed in obese diabetic rats. GK rats display symptoms associated with diabetic complications, including reduced nerve conduction velocity indicative of peripheral neuropathy (26). Progressive renal involvement in GK rats presents in a manner similar to NIDDM in humans including a thickened glomerular basement membrane (22, 27–31). GK animals also display increases in oxidative stress markers and susceptibility to lipid peroxidation in the hearts of older (12–18 months) but not younger (3–6 months) animals (32–36). The etiology of the GK rats is unknown, but it has been suggested that impaired pancreatic mitochondrial function may partially explain the depressed insulin release (34, 37).
Although there is little indication that Cr supplementation is beneficial in euglycemic individuals, there is evidence for a dose-dependency effect in diabetics (19, 38). There are significant differences between humans and rats in terms of their mass, rates of metabolism, and turnover of metabolites or drugs, and scaling Cr supplementation for the rat is somewhat difficult. Also, problematic with chromium supplementation studies are the high doses of chromium used, the relatively short length of the protocols, or the use of chromium deficient diets as controls (4, 6, 11, 39). We therefore wanted to test under normal dietary conditions whether Cr supplementation is efficacious for glycemic control.
MATERIALS AND METHODS
Animal Model
Female euglycemic Wistar and diabetic Goto-Kakizaki rats were used throughout this study. The GK animals were originally received courtesy of R. Farase (University of South Florida for Health Sciences) (31). The GK rats are a nonobese model of NIDDM that have elevated fasting glucose, impaired response to glucose, and increased HbA1c levels at an early age (21–23). Experimental protocols using animals had institutional approval, and animals were maintained in accordance with institutional polices and the Public Health Service (NIH:PHS) Policy on Humane Care and Use of Laboratory Animals (revised 8/2002).
Chromium Picolinate Supplementation
GK and Wistar rats were assigned to water (distilled) or chromium picolinate (Cr-P) supplementation groups (Table 1). All animals were fed Purina Rat Chow (#5008). Body weights were determined on a biweekly basis. Water consumption was determined three times a week (Monday, Wednesday, Friday) to permit calculation of daily water intake. Chromium picolinate (Nutrition 21, Purchase, NY) was added to the distilled water (7–11 µg/ml) to yield an averaged daily dose for the GK1 and Wistar groups of 1.0 ± 0.02 mg/kg/day. For the high-dose treatment (GK10), Cr-P was added to distilled water to a final concentration of 100 µg/ml to yield a final dose of ~10 mg/kg/day. Treatments lasted 32 weeks for the GK1 and Wistar groups; the GK10 group received chromium supplementation for 16 weeks.
Table 1.
Biometric data
| Body wt (g) |
Kidney wt (g) |
Heart wt (g) |
LV wt (g) |
Kidney/BW (×1000 g/g) |
Heart wt/BW (×1000 g/g) |
LV/BW (×1000 g/g) |
|
|---|---|---|---|---|---|---|---|
| GK 1; water | 237.7 | 0.845 | 0.775 | 0.535 | 3.556 | 3.265 | 2.273 |
| 5.0 | 0.034 | 0.011 | 0.016 | 0.131 | 0.066 | 0.08 | |
| GK 1; Cr-P | 232.9 | 0.801 | 0.740 | 0.505 | 3.437 | 3.175 | 2.167 |
| 2.7 | 0.025 | 0.014 | 0.009 | 0.097 | 0.038 | 0.02 | |
| GK 10; water | 249.9 | 0.873 | 0.830 | 0.565 | 3.5 | 3.33 | 2.267 |
| 7.2 | 0.028 | 0.026 | 0.017 | 0.078 | 0.077 | 0.052 | |
| GK 10; Cr-P | 249.6 | 0.888 | 0.821 | 0.575 | 3.541 | 3.324 | 2.328 |
| 14.2 | 0.064 | 0.037 | 0.027 | 0.083 | 0.135 | 0.095 | |
| Wistar; water | 375.4 | 1.055 | 1.177 | 0.790 | 2.569 | 3.222 | 2.144 |
| 19.6 | 0.034 | 0.062 | 0.040 | 0.052 | 0.306 | 0.17 | |
| Wistar; Cr-P | 379.5 | 1.094 | 1.043 | 0.748 | 2.895* | 2.766 | 1.978 |
| 23.7 | 0.072 | 0.053 | 0.041 | 0.118* | 0.101 | 0.059 |
Values are mean ± SEM.
P < 0.05 compared with respective control.
Glucose Tolerance Test
The tolerance to glucose was tested following an overnight fast; animals were moved to new cages at 4 PM and the glucose tolerance test (GTT) was started between 09:30 and 10:30 the following morning. The animals were weighed and injected with Nembutal (40 mg/kg, i.p.), and then at least 15 min were allotted for the animals to achieve a suitable plane of anesthesia. To perform the GTT, sterile glucose (1.0 g/kg i.p.) was injected into the abdominal cavity being careful to avoid the g-i tract. Tail vein blood (50 µl) was sampled at selected intervals (preinjection, 15, 30, 60, 120 min). Larger aliquots (300 µl) were drawn preinjection and at 60 min for determination of plasma insulin. Blood glucose was determined using an AccuChek monitor (Roche Diagnostics, Indianapolis, IN) calibrated using known standards. Insulin was determined from plasma by ELISA (Crystal Chem, Downers Grove IL). An insulin sensitivity index value was calculated according to the method described by Matsuda and DeFronzo (40).
Statistical Analysis
Values present are mean ± SEM of 6–10 animals per group. Where appropriate, t-test, 1- or 2-way ANOVA analyses were utilized; post-hoc analysis was done using a Fisher’s LSD analysis. Statistical analyses were performed using NCSS 2000 software (NCSS, Kaysville UT). Statistical significance was set at P < 0.05.
RESULTS
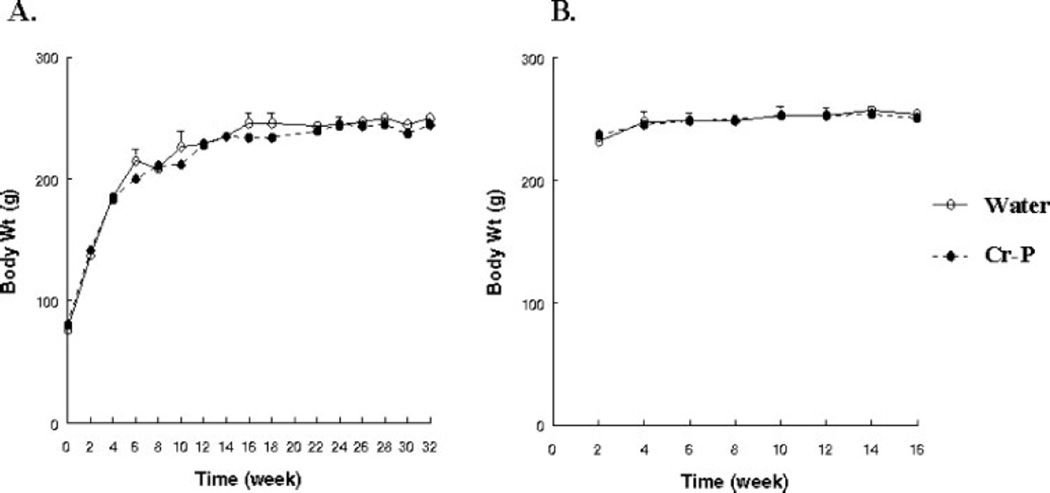
GK and Wistar rats were supplemented with chromium in the form of chromium picolinate (Cr-P). The animals were fed a standard diet (Purina Rat Chow #5008) that contained 2 ppm chromium, and no effort was made to create a chromium deficient diet in the control groups. The addition of Cr-P did not significantly alter water consumption of the GK1 or Wistar groups. Animals were weighed at regular intervals and Cr-P supplementation did not significantly alter the growth of either the GK or Wistar rats (Fig. 1).
Figure 1.
Chromium supplementation did not alter growth. GK rats were assigned to water (control) or Cr-P supplementation and body weights were determined biweekly. (A) 1.0 mg/kg/day. (B) 10.0 mg/kg/day. No significant differences were found between the groups. Cr-P supplementation did not alter the growth of the Wistar rats (data not shown).
At the time of sacrifice, body and organ weights were determined. Both the heart weight:body weight ratio and the kidney weight:body weight ratio were significantly increased in the GK rats when compared with the Wistar rats (Table 1). The GK rats weighed significantly less than the Wistar rats and this may have influenced the organ:body ratio. An analysis of covariance using body weight as the covariant was performed and determined that the GK rats had significant kidney hypertrophy when compared with the age-matched Wistar rats. Covariant analysis using body weight found no strain differences for either heart weight or left ventricle weight. In a separate group of GK and Wistar rats, tibia length was measured and the kidney weight:tibia length was also significantly increased in the GK rats when compared with the Wistar rats (data not shown). Cr-P supplementation did not influence organ weights in the GK rats. Cr-P supplementation did produce a significant increase in the kidney:body weight ratio of the Wistar rats (Table 1).
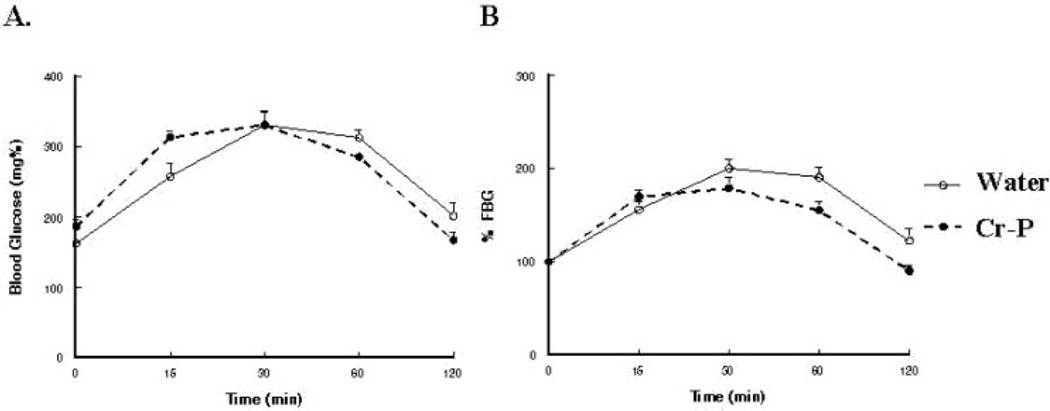
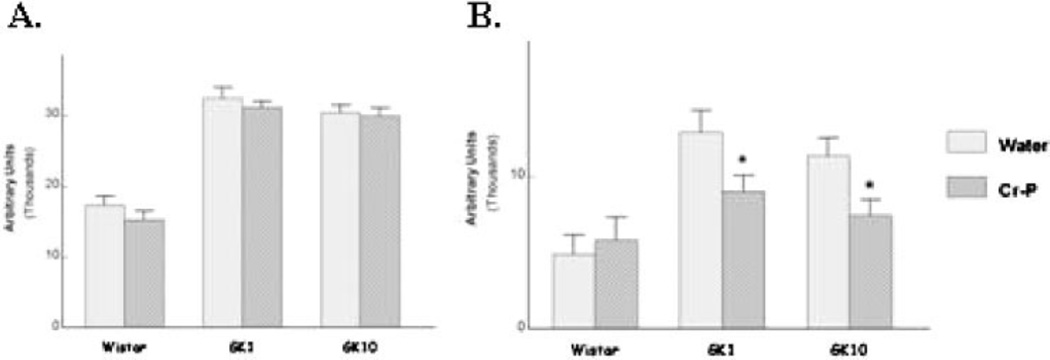
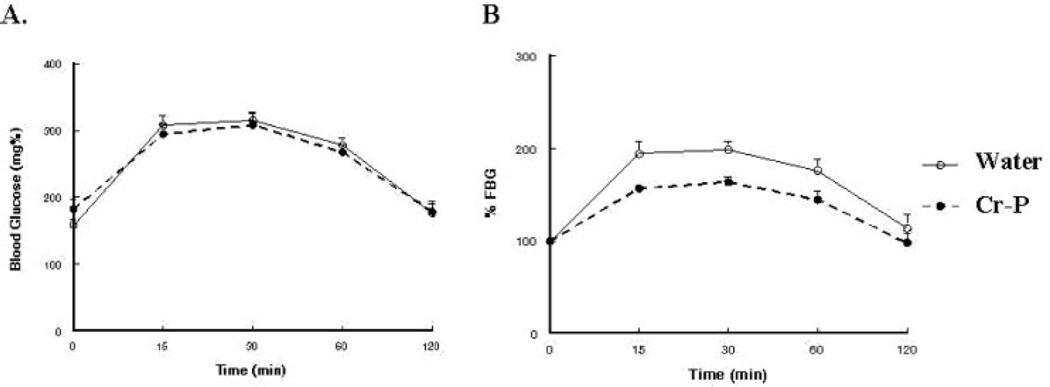
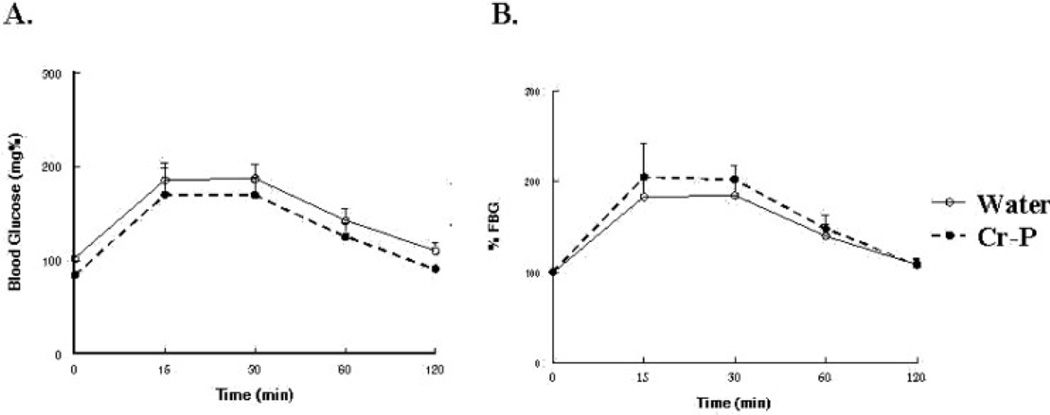
Fasting blood glucose levels and the calculate insulin sensitivity index were not significantly altered by Cr-P supplementation (Table 2). Although the GK rats have reported to be hyper-insulinemic at a young age, we observed that the GK1 group had significantly lower fasting plasma insulin when compared with the age-matched Wistar animals, which chromium supplementation did not influence (Table 2). Glucose tolerance was tested by injection of sterile glucose and determination of blood glucose levels at selected times (Fig. 2). Area under the curve (AUC) was used to analyze for differences in glucose sensitivity. Six weeks of Cr-P supplementation in the GK1 group appeared to lower the relative AUC (data not shown); however, the values did not achieve statistical significance (P = 0.061). When the Cr-P supplementation was extended to 32 weeks for the GK1 group, a significant decrease in the relative AUC was observed (Figs. 2B and 5B). Similarly, 16 weeks of Cr-P supplementation lowered the AUC in the GK10 group (Figs. 3B and 5B). Cr-P supplementation did not alter the AUC blood glucose values of the euglycemic Wistar group (Figs. 4 and 5).
Table 2.
Fasting blood glucose, fasting insulin, and insulin sensitivity index (CISI)
| Fasting blood glucose (mg %) | Fasting insulin (ng/ml) | CISI | ||||
|---|---|---|---|---|---|---|
| Cr-P | − | + | − | + | − | + |
| GK 1 | 163.0 ± 6.4 | 186 ± 9.1 | 0.17 ± 0.02 | 0.22 ± 0.08 | 86.9 ± 5.9 | 108.5 ± 26.5 |
| GK 10 | 159 ± 9.4 | 182 ± 15.3 | 0.37 ± 0.09 | 0.67 ± 0.22 | 83.0 ± 26.8 | 113.6 ± 56.0 |
| Wistar | 93.0 ± 3.3 | 84 ± 2.7* | 1.54 ± 0.48 | 1.44 ± 0.45 | 65.5 ± 16.2 | 85.5 ± 21.3 |
P < 0.05 compared with respective control.
Figure 2.
Glucose tolerance test of GK rats supplemented with 1.0 mg/kg day Cr-P for 32 weeks. Animals were tested after an overnight fast and injected with Nembutal (40 mg/kg, i.p.), and then injected with sterile glucose (1.0 g/kg) as described in Materials and Methods section. (A) Blood glucose values. (B) Blood glucose values normalized to fasting blood glucose.
Figure 5.
Area under the curve analysis of (A) absolute blood glucose values and (B) relative change in blood glucose values. Animals were supplied with water or Cr- P as described in Materials and Methods section. Values are mean ± SEM of 6–10 animals per group. *P < 0.0.05 compared with respective water (control) group.
Figure 3.
Glucose tolerance test of GK rats supplemented with 10.0 mg/kg day Cr-P for 16 weeks. Animals were tested after an overnight fast and injected with Nembutal (40 mg/kg, i.p.), and then injected with sterile glucose (1.0 g/kg) as described in Materials and Methods section. (A) Blood glucose values. (B) Blood glucose values normalized to fasting blood glucose.
Figure 4.
Glucose tolerance test of Wistar rats supplemented with 1.0 mg/kg day Cr-P for 32 weeks. Animals were tested after an overnight fast and injected with Nembutal (40 mg/kg, i.p.), and then injected with sterile glucose (1.0 g/kg) as described in Materials and Methods section. (A) Blood glucose values. (B) Blood glucose values normalized to fasting blood glucose.
DISCUSSION
Chromium deficiency has been associated with diabetic-like symptoms including impaired glucose tolerance. Chromium (Cr) supplementation may be useful in the management of NIDDM (1). Some reports indicate that chromium supplementation will improve some parameters of glycemic control in diabetic individuals (38, 41). Animal studies to date have been insufficient to clearly demonstrate the efficacy of Cr-P on long-term glycemic control. The major findings of this study are that Cr-P supplementation slightly improved glucose tolerance in the GK rat using lower chromium dosages than previously studied.
Direct comparisons of drug dosages between rodents and humans are difficult. Two major approaches have been developed and include an allometric approach and a physiological approach. The allometric approach utilizes modeling based on size or mass to develop an equation to permit scaling. Many studies have utilized the allometric approach because of its ease of use and dose extrapolations are made accordingly based on mg/kg basis. This typically underestimates the relevant dose when going from large to small mammals (42). A second approach has been to base dosing on the body surface area and to express the dose as mg/m2 (43). This approach has been used to calculate the maximum tolerated dose of anticancer drugs in man using the LD10 of different species, with about a sevenfold difference between humans and rats has been determined. Although relatively simple, allometric scaling does not take into account differences in metabolism of a substance or the distribution of a substance to target organs (44). The physiological approach assumes a similarity among species in basic cellular structure and uses a physiologically meaningful parameter such as the period of the heart rate. The resting hearts of rats and humans are typically 400 and 72 beats/min, respectively, about a 5.5-fold difference (45, 46). Using this approach, Mordenti demonstrated that the half-life of ceftizoxime was the same in several species from mouse to human (47). Perhaps a better metabolic parameter may be resting oxygen consumption, since it is directly linked to energy consumption. The resting oxygen consumption of humans (~3.5 ml O2/kg/min) is about eightfold less than that for a rat (~28 ml O2/kg/min) (48). Although imperfect, the six to eightfold differences between humans and rats still places the lower dose used in this study above the levels of Cr-P supplementation typically recommended for human consumption (8, 20).
No significant differences were observed either in the fasting blood glucose or in GK group; and these results are similar to some other studies. Although no significant differences in the absolute changes in the AUC, significant decreases in the relative AUC was similar for the two levels of Cr-P supplementation utilized. This suggests that the response to a glycemic challenge was better handled on the Cr-P supplementation groups. The data also suggests that we did not identify a threshold for Cr-P effects. This is somewhat surprising in light of the lower dosages used. Although a linear relationship between chromium tissue levels and supplementation was reported in one study (15), others have found a plateau effect at higher levels of chromium supplementation (12, 14, 49). This would suggest that the lower doses used here should have uncovered a difference. One study, in which Cr-P was supplemented into the dry feed of obese diabetic mice, observed an improvement in glucose tolerance only at the higher dosage used (11). They also observed that glucose tolerance only improved after 12 weeks, but not 4 weeks, of Cr-P feeding. This is similar to this study in that although there was a tendency toward an improvement in glucose tolerance after 6 weeks of Cr-P treatment (GK1), a significant change in the GTT was not observed until the end of the study period. This would suggest that the impact of Cr-P supplementation may have a time and dose dependency for an effect to be demonstrable. Future studies may use lower doses to determine if they are able to alter glucose tolerance, or whether there is a threshold of Cr-P supplementation that is required to achieve improvements.
Some studies of diabetic patients have reported decreased fasting blood glucose as a result of Cr-P supplementation (1, 8, 20, 38, 41). In this study, fasting blood glucose levels did not decrease as a result of Cr-P supplementation irrespective of dose or group, an observation similar to other animal studies (11, 12, 39, 49, 50). In part, this lack of effect may be methodological. An inherent difficulty in drawing blood from conscious animals is a fear reaction that may cause wide variations in sympathetic activation leading to increased blood glucose. Alternatively, in using anesthetized animals, some forms of anesthetic, particularly ketamine/xylazine, are known to increase blood glucose levels. (51–53). This study utilized Nembutal, which has been shown to have minimal effects on blood glucose levels (52, 53). A second consideration is the impact of the length of the fast and diurnal rhythm of the rats on fasting blood glucose levels. In this study, animals were studied after an overnight fast and anesthesia was initiated between 09:30 and 10:30 h to standardize the test conditions.
Diabetes is a metabolic disease that impacts on growth and it has been suggested that Cr-P may favorably influence growth or weight gains. Studies of Cr-P supplementation in euglycemic humans found that Cr-P supplementation was associated with weight loss and loss of fat (54–56). One study of diabetic patients, found that Cr-P supplementation may have attenuated the weight gains associated with sulfonylurea agents (41). In this study, Cr-P supplementation did not influence growth. This finding is in agreement with Rhodes et al. (57), who also found no change in growth as a result of Cr-P supplementation in F344/N rats or B6C3F mice. Conversely, one study did find enhanced growth following Cr-P supplementation in GK rats (39). That study differs in two ways: (1) the Cr-P dose used was 10 to 100 folds higher than this study, and (2) their study utilized young male GK rats that were still growing. This study used females that had achieved their adult weights at the start or near the start of the treatment period. It is possible that the increases in weight gains of the GK males in that study may have been due to enhanced carbohydrate metabolism of the growing animals.
The GTT is a reflection of both glucose sensing and insulin sensitivity in peripheral tissues. Chromium is thought to improve insulin sensitivity by its interaction at the level of the insulin receptor and there has been no suggestion that it alters pancreatic function (58–61). Although the euglycemic insulin clamp technique is considered the best method to quantitate insulin sensitivity, Matsuda and DeFronzo (40) have developed an insulin sensitivity index from the GTT. Although the values for the index appeared to be increased, our results indicated that chromium supplementation did not significantly increase insulin sensitivity. The GK rats are thought to have some β-cell glucose insensitivity that may be related to impaired mitochondrial function (24, 34, 37, 62). This “central” defect in the pancreas is not something the chromium supplementation will correct. It is possible that improvements in peripheral sensitivity may account for the slight improvements in glucose tolerance observed. This concept is supported by the others, that in response to exogenous insulin, glucose handling was improved in the Cr-P supplementation groups when compared with diabetic controls (39). Future experiments to disassociate glucose sensing from peripheral insulin sensitivity will be necessary to separately determine the site of Cr-P improvement in glucose handling. Also other models such as obesity-induced diabetes, where “peripheral” defects are the underlying cause may be more responsive to chromium supplementation and could be the subject of future studies.
SUMMARY
In the GK rats, Cr-P supplementation did not alter animal growth, individual organ weights, or fasting blood glucose levels compared with the control GK rats group. Both Cr-P supplementation dosages studied improved some indices of glucose handling as reflected by significant decreases in the AUC during a GTT. No significant dose-dependent effect of Cr-P supplementation on the GTT was observed suggesting that the threshold for the impact of Cr-P supplementation was not identified. The GTT is a reflection of both glucose sensing and insulin sensitivity, and it is unclear whether Cr-P supplementation improved either or both of these parameters. Future experiments to more clearly disassociate glucose sensing from peripheral insulin sensitivity will be necessary to separately determine the site of Cr-P improvement in glucose handling. Future works to utilize NIDDM models that are more specific models of insulin resistance to study the efficacy of this supplement are warranted. These results do suggest that Cr-P may improve some indices of glycemic control in GK rats and may be beneficial in the management of diabetes.
Acknowledgments
This work was supported in part by the HL43023 and Castle-Krob Award of the New York Medical College Endowment Fund.
REFERENCES
- 1.Ryan GJ, Wanko NS, Redman AR, Cook CB. Chromium as adjunctive treatment for type 2 diabetes. Ann. Pharmacother. 2003;37:876–885. doi: 10.1345/aph.1C304. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology. 2002;180:5–22. doi: 10.1016/s0300-483x(02)00378-5. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama M. Role of physiological antioxidants in chromium(VI)-induced cellular injury. Free Radic. Biol. Med. 1992;12:397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- 4.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am. J. Clin. Nutr. 1977;30:531–538. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HH, Lai MH, Hou WC, Huang CL. Antioxidant effects of chromium supplementation with type 2 diabetes mellitus and euglycemic subjects. J. Agric. Food Chem. 2004;52:1385–1389. doi: 10.1021/jf035074j. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder HA. Diabetic-like serum glucose levels in chromium deficient rats. Life Sci. 1965;4:2057–2062. doi: 10.1016/0024-3205(65)90322-x. [DOI] [PubMed] [Google Scholar]
- 7.Brown RO, Forloines-Lynn S, Cross RE, Heizer WD. Chromium deficiency after long-term total parenteral nutrition. Dig. Dis. Sci. 1986;31:661–664. doi: 10.1007/BF01318699. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA. Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 1998;17:548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 9.Hambidge KM, Rodgerson DO, O’Brien D. Concentration of chromium in the hair of normal children and children with juvenile diabetes mellitus. Diabetes. 1968;17:517–519. doi: 10.2337/diab.17.8.517. [DOI] [PubMed] [Google Scholar]
- 10.Morris BW, MacNeil S, Hardisty CA, Heller S, Burgin C, Gray TA. Chromium homeostasis in patients with type II (NIDDM) diabetes. J. Trace Elem. Med. Biol. 1999;13:57–61. doi: 10.1016/S0946-672X(99)80024-8. [DOI] [PubMed] [Google Scholar]
- 11.Mita Y, Ishihara K, Fukuchi Y, Fukuya Y, Yasumoto K. Supplementation with chromium picolinate recovers renal Cr concentration and improves carbohydrate metabolism and renal function in type 2 diabetic mice. Biol. Trace Elem. Res. 2005;105:229–248. doi: 10.1385/BTER:105:1-3:229. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Clodfelder BJ, Shute AA, Irvin T, Vincent JB. The biomimetic [Cr(3)O(O(2)CCH(2)CH(3))(6)(H(2)O)(3)](+) decreases plasma insulin, cholesterol, and triglycerides in healthy and type II diabetic rats but not type I diabetic rats. J. Biol. Inorg. Chem. 2002;7:852–862. doi: 10.1007/s00775-002-0366-y. [DOI] [PubMed] [Google Scholar]
- 13.Raz I, Havivi E. Influence of chronic diabetes on tissue and blood cells status of zinc, copper, and chromium in the rat. Diabetes Res. 1988;7:19–23. [PubMed] [Google Scholar]
- 14.Long-Ying Z, Zi-Rong X, Min-Qi M, Liang-Ying G. Effects of chromium nanoparticle dosage on growth, body composition, serum hormones, and tissue chromium in Sprague-Dawley rats. J. Zhejiang Univ. Sci. 2007;8:323–330. doi: 10.1631/jzus.2007.B0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RA, Bryden NA, Polansky MM. Lack of toxicity of chromium chloride and chromium picolinate in rats. J. Am. Coll. Nutr. 1997;16:273–279. doi: 10.1080/07315724.1997.10718685. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RA, Polansky MM, Bryden NA, Bhathena SJ, Canary JJ. Effects of supplemental chromium on patients with symptoms of reactive hypoglycemia. Metabolism. 1987;36:351–355. doi: 10.1016/0026-0495(87)90206-x. [DOI] [PubMed] [Google Scholar]
- 17.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;36:4382–4385. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh D, Bhattacharya B, Mukherjee B, Manna B, Sinha M, Chowdhury J, Chowdhury S. Role of chromium supplementation in Indians with type 2 diabetes mellitus. J. Nutr. Biochem. 2002;13:690–697. doi: 10.1016/s0955-2863(02)00220-6. [DOI] [PubMed] [Google Scholar]
- 19.Bahijiri SM, Mira SA, Mufti AM, Ajabnoor MA. The effects of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med. J. 2000;21:831–837. [PubMed] [Google Scholar]
- 20.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am. J. Clin. Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K, Ishihara A. Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin. Exp. Pharmacol. Physiol. 2002;29:1001–1008. doi: 10.1046/j.1440-1681.2002.03757.x. [DOI] [PubMed] [Google Scholar]
- 22.Yagihashi S, Goto Y, Kakizaki M, Kaseda N. Thickening of glomerular basement membrane in spontaneously diabetic rats. Diabetologia. 1978;15:309–312. doi: 10.1007/BF02573824. [DOI] [PubMed] [Google Scholar]
- 23.Goto Y, Kakizaki M, Masaki N. Production of a spontaneous diabetic rats by repetition of selective breeding. Tohoku J. Exp. Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 24.Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, Giroix MH. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes. 1991;40:486–491. doi: 10.2337/diab.40.4.486. [DOI] [PubMed] [Google Scholar]
- 25.Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, Lemmer B. Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide. 2002;6:85–95. doi: 10.1006/niox.2001.0363. [DOI] [PubMed] [Google Scholar]
- 26.Murakawa Y, Zhang W, Pierson CR, Brismar T, Ostenson CG, Efendic S, Sima AA. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab. Res. Rev. 2002;18:473–483. doi: 10.1002/dmrr.326. [DOI] [PubMed] [Google Scholar]
- 27.Janssen U, Riley SG, Vassiliadou A, Floege J, Phillips AO. Hypertension superimposed on type II diabetes in Goto Kakizaki rats induces progressive nephropathy. Kidney Int. 2003;63:2162–2170. doi: 10.1046/j.1523-1755.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips AO, Baboolal K, Riley S, Grone H, Janssen U, Steadman R, Williams J, Floege J. Association of prolonged hyperglycemia with glomerular hypertrophy and renal basement membrane thickening in the Goto Kakizaki model of non-insulin-dependent diabetes mellitus. Am. J. Kidney Dis. 2001;37:400–410. doi: 10.1053/ajkd.2001.21322. [DOI] [PubMed] [Google Scholar]
- 29.Yagihashi S, Kaseda N, Kakizaki M, Goto Y. Evolution of glomerular lesions in rats with spontaneous diabetes. Tohoku J. Exp. Med. 1979;127:359–367. doi: 10.1620/tjem.127.359. [DOI] [PubMed] [Google Scholar]
- 30.Yagihashi S, Tonosaki A, Yamada K, Kakizaki M, Goto Y. Peripheral neuropathy in selectively-inbred spontaneously diabetic rats: electrophysiological, morphometrical and freeze-replica studies. Tohoku J. Exp. Med. 1982;138:39–48. doi: 10.1620/tjem.138.39. [DOI] [PubMed] [Google Scholar]
- 31.Vesely DL, Gower WR, Jr, Dietz JR, Overton RM, Clark LC, Antwi EK, Farese RV. Elevated atrial natriuretic peptides and early renal failure in type 2 diabetic Goto-Kakizaki rats. Metabolism. 1999;48:771–778. doi: 10.1016/s0026-0495(99)90178-6. [DOI] [PubMed] [Google Scholar]
- 32.Santos DL, Palmeira CM, Seica R, Dias J, Mesquita J, Moreno AJ, Santos MS. Diabetes and mitochondrial oxidative stress: a study using heart mitochondria from the diabetic Goto-Kakizaki rat. Mol. Cell Biochem. 2003;246:163–170. [PubMed] [Google Scholar]
- 33.Santos MS, Santos DL, Palmeira CM, Seica R, Moreno AJ, Oliveira CR. Brain and liver mitochondria isolated from diabetic Goto-Kakizaki rats show different susceptibility to induced oxidative stress. Diabetes Metab. Res. Rev. 2001;17:223–230. doi: 10.1002/dmrr.200. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira PJ, Rolo AP, Seica R, Palmeira CM, Santos MS, Moreno AJ. Decreased susceptibility of heart mitochondria from diabetic GK rats to mitochondrial permeability transition induced by calcium phosphate. Biosci. Rep. 2001;21:45–53. doi: 10.1023/a:1010482017540. [DOI] [PubMed] [Google Scholar]
- 35.Palmeira CM, Ferreira FM, Santos DL, Ceica R, Suzuki K, Santos MS. Higher efficiency of the liver phosphorylative system in diabetic Goto-Kakizaki (GK) rats. FEBS Lett. 1999;458:103–106. doi: 10.1016/s0014-5793(99)01144-8. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira FM, Palmeira CM, Matos MJ, Seica R, Santos MS. Decreased susceptibility to lipid peroxidation of Goto-Kakizaki rats: relationship to mitochondrial antioxidant capacity. Life Sci. 1999;65:1013–1025. doi: 10.1016/s0024-3205(99)00332-x. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira PJ, Rolo AP, Seica R, Sardao V, Monteiro P, Goncalves L, Providencia L, Palmeira CM, Santos MS, Moreno AJ. Impact of diabetes on induction of the mitochondrial permeability transition. Rev. Port Cardiol. 2002;21:759–766. [PubMed] [Google Scholar]
- 38.Anderson RA, Cheng N, Bryden NA, Polansky MM, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 39.Kim DS, Kim TW, Kang JS. Chromium picolinate supplementation improves insulin sensitivity in Goto-Kakizaki diabetic rats. J Trace Elem. Med. Biol. 2004;17:243–247. doi: 10.1016/S0946-672X(04)80025-7. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 41.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 42.Rucker R, Storms D. Interspecies comparisons of micronutrient requirements: metabolic vs. absolute body size. J. Nutr. 2002;132:2999–3000. doi: 10.1093/jn/131.10.2999. [DOI] [PubMed] [Google Scholar]
- 43.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 44.Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J. Pharm. Sci. 1986;75:1028–1040. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- 45.Tipton CM, Barnard RJ, Tcheng TK. Resting heart rate investigations with trained and nontrained hypophysectomized rats. J. Appl. Physiol. 1969;26:585–588. doi: 10.1152/jappl.1969.26.5.585. [DOI] [PubMed] [Google Scholar]
- 46.Barnard RJ, Corre K, Cho H. Effect of training on the resting heart rate of rats. Eur. J. Appl. Physiol. Occup. Physiol. 1976;35:285–289. doi: 10.1007/BF00423288. [DOI] [PubMed] [Google Scholar]
- 47.Mordenti J. Dosage regimen design for pharmaceutical studies conducted in animals. J. Pharm. Sci. 1986;75:852–857. doi: 10.1002/jps.2600750906. [DOI] [PubMed] [Google Scholar]
- 48.Bedford T, Tipton C, Wilson N. Maximum oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. 1979;47:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 49.Clodfelder BJ, Gullick BM, Lukaski HC, Neggers Y, Vincent JB. Oral administration of the biomimetic [Cr3O(O2CCH2CH3)6(H2O)3]+ increases insulin sensitivity and improves blood plasma variables in healthy and type 2 diabetic rats. J. Biol. Inorg. Chem. 2005;10:119–130. doi: 10.1007/s00775-004-0618-0. [DOI] [PubMed] [Google Scholar]
- 50.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J. Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues SF, de Oliveira MA, Martins JO, Sannomiya P, de Cassia Tostes R, Nigro D, Carvalho MH, Fortes ZB. Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route. Life Sci. 2006;79:1630–1637. doi: 10.1016/j.lfs.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Brown ET, Umino Y, Loi T, Solessio E, Barlow R. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis. Neurosci. 2005;22:615–618. doi: 10.1017/S0952523805225105. [DOI] [PubMed] [Google Scholar]
- 53.Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp. Biol. Med. (Maywood) 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- 54.Hallmark MA, Reynolds TH, DeSouza CA, Dotson CO, Anderson RA, Rogers MA. Effects of chromium and resistive training on muscle strength and body composition. Med. Sci. Sports Exerc. 1996;28:139–144. doi: 10.1097/00005768-199601000-00025. [DOI] [PubMed] [Google Scholar]
- 55.Lukaski HC, Bolonchuk WW, Siders WA, Milne DB. Chromium supplementation and resistance training: effects on body composition, strength, and trace element status of men. Am. J Food Chem. Toxicol. Clin. Nutr. 1996;63:954–965. doi: 10.1093/ajcn/63.6.954. [DOI] [PubMed] [Google Scholar]
- 56.Campbell WW, Joseph LJ, Anderson RA, Davey SL, Hinton J, Evans WJ. Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int. J. Sport Nutr. Exerc. Metab. 2002;12:125–135. doi: 10.1123/ijsnem.12.2.125. [DOI] [PubMed] [Google Scholar]
- 57.Rhodes MC, Hebert CD, Herbert RA, Morinello EJ, Roycroft JH, Travlos GS, Abdo KM. Absence of toxic effects in F344/N rats and B6C3F1 mice following subchronic administration of chromium picolinate monohydrate. Food Chem. Toxicol. 2005;43:21–29. doi: 10.1016/j.fct.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Miranda ER, Dey CS. Effect of chromium and zinc on insulin signaling in skeletal muscle cells. Biol. Trace Elem. Res. 2004;101:19–36. doi: 10.1385/BTER:101:1:19. [DOI] [PubMed] [Google Scholar]
- 59.Vincent JB. Mechanisms of chromium action: low-molecular-weight chromium-binding substance. J. Am. Coll. Nutr. 1999;18:6–12. doi: 10.1080/07315724.1999.10718821. [DOI] [PubMed] [Google Scholar]
- 60.Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, Elmendorf JS. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol. Endocrinol. 2006;20:857–870. doi: 10.1210/me.2005-0255. [DOI] [PubMed] [Google Scholar]
- 61.Brautigan DL, Kruszewski A, Wang H. Chromium and vanadate combination increases insulin-induced glucose uptake by 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2006;347:769–773. doi: 10.1016/j.bbrc.2006.06.154. [DOI] [PubMed] [Google Scholar]
- 62.Giroix MH, Vesco L, Portha B. Functional and metabolic perturbations in isolated pancreatic islets from the GK rat, a genetic model of noninsulin-dependent diabetes. Endocrinology. 1993;132:815–822. doi: 10.1210/endo.132.2.8425496. [DOI] [PubMed] [Google Scholar]