Abstract
Purpose
Although the 21-gene recurrence score (RS) assay has been validated to assess the risk of distant recurrence in hormone receptor-positive breast cancer patients, the relationship between RS and the risk of locoregional recurrence (LRR) remains unclear. The purpose of this study was to determine if RS is associated with LRR in breast cancer patients and whether this relationship varies based on the type of local treatment [mastectomy or breast-conserving therapy (BCT)].
Methods
163 consecutive estrogen receptor-positive breast cancer patients at our institution had an RS generated from the primary breast tumor between August 2006 and October 2009. Patients were treated with lumpectomy and radiation (BCT) (n = 110) or mastectomy alone (n = 53). Patients were stratified using a pre-determined RS of 25 and then grouped according to local therapy type.
Results
Median follow-up was 68.2 months. Patients who developed an LRR had stage I or IIA disease, >2 mm surgical margins, and received chemotherapy as directed by RS. While an RS > 25 did not predict for a higher rate of LRR, an RS > 24 was associated with LRR in our subjects. Among mastectomy patients, the 5-year LRR rate was 27.3 % in patients with an RS > 24 versus 10.7 % (p = 0.04) in those whose RS was ≤24. RS was not associated with LRR in patients who received BCT.
Conclusions
Breast cancer patients treated with mastectomy for tumors that have an RS > 24 are at high risk of LRR and may benefit from post-mastectomy radiation.
Over the past decade, genomic profiling has made a significant impact on the treatment of women with breast cancer. The 21-gene recurrence score (RS) assay (Oncotype DX; Genomic Health, Redwood City, CA, USA) has emerged as one of the most commonly used genomic profiling assays to tailor chemotherapy recommendations in patients with estrogen receptor (ER)-positive, lymph node-negative breast cancer.1–3
While the use of the 21-gene RS to determine a patient's risk of developing distant metastatic disease has become increasingly common, the use of this assay to determine the risk of locoregional recurrence (LRR) is only beginning to be explored.4,5 Traditionally, patient, tumor, and treatment characteristics have been associated with LRR risk.6,7 Among patients treated with breast-conserving therapy (BCT) specifically, close or positive surgical margins, higher T stage, and lymphovascular space invasion (LVSI) have been associated with a higher risk of LRR.8 Other studies of breast cancer patients treated with mastectomy alone and no radiation have shown that positive nodes and extranodal extension predict for LRR.9
The impact of breast cancer gene expression on LRR has not been as closely examined. Studies of patients with tumors that are high grade, triple negative, human epidermal growth factor receptor 2 (HER2)-positive, and/or exhibit high Ki67 expression suggest that tumor biology may play a role in the development of LRR.5,10,11 Genomic profiling of breast tumors could therefore potentially improve the ability to predict an LRR in an individual patient and determine whether more aggressive local therapy is warranted (e.g. post-mastectomy radiation or an increased boost dose in patients treated with BCT).
The association between RS and LRR has been examined in two prior studies. Both studies used the original 21-gene RS risk stratification criteria, with RS < 18, RS 18–30, and RS ≥ 31 considered low, intermediate, and high risk, respectively. These risk groups were based on patient score distribution in the developmental training sets rather than clinical outcome data.12 In a retrospective analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and B-20 trials, an association between RS and the risk of LRR was found.13 In these studies, patients were treated with systemic regimens that are not typically first-line therapy (e.g. cyclophosphamide, methotrexate, and fluorouracil and tamoxifen in post-menopausal patients) in the modern era, and therefore the question remains whether a relationship between the RS and LRR exists in patients treated with current systemic agents shown to independently improve local control.14,15 Moreover, in NSABP B-14 and B-20, the ability of the RS to predict LRR appeared to be impacted by patient age and local treatment modality. While a high RS predicted for LRR in mastectomy non-radiation patients, irrespective of age, RS did not predict for LRR in BCT patients over the age of 50 years.13 A retrospective analysis of the Eastern Cooperative Oncology Group (ECOG) E2197 trial also did not find any relationship between LRR and RS among their BCT patients, regardless of age.16 These findings suggest that radiation may obscure the impact of RS to predict LRR, and that the RS could potentially predict which tumors respond to RT.
The present study was performed to explore the relationship between RS and LRR in patients treated with radiation following lumpectomy and in patients treated with mastectomy alone. We hypothesized that RS would predict for LRR in mastectomy patients treated without radiation but would not predict for LRR in BCT patients. We therefore proposed that the RS may be used to identify a subgroup of patients treated with mastectomy alone that may benefit from more aggressive local treatment, including radiation.
Recently, the Trial Assigning Individualized Options for Treatment (TAILORx) used an RS > 25 to define a high-risk group of patients based on clinical outcome data indicating a 20 % risk of distant metastasis at 10 years.1 Additionally, the ongoing RxPonder trial (Rx for Positive Node, Endocrine Responsive breast cancer; clinical trial registry NCT01272037) is using an RS > 25 to define high-risk patients.17 For this study, we therefore chose a pre-defined RS > 25 as a clinically relevant cut-off to determine a group of patients at high enough risk of LRR to perhaps warrant more aggressive local therapy.
Methods and Materials
After obtaining Emory University Institutional Review Board approval, we reviewed the records of 163 consecutive breast cancer patients treated at Emory University between August 2006 and October 2009. All patients had ER-positive tumors with a 21-gene RS generated from the primary breast tumor removed at the time of definitive surgery. Patients who received partial breast irradiation, neoadjuvant chemotherapy, or endocrine therapy were excluded, as were patients who did not receive post-lumpectomy radiation, adjuvant chemotherapy as directed by an RS > 31, or adjuvant endocrine therapy. Patients with HER2 positive disease were also excluded. All tumors were staged according to the 7th edition of the American Joint Committee on Cancer's staging system.18
Patients received BCT (n = 110) or mastectomy alone (n = 53). Lymph nodes were evaluated with either sentinel lymph node biopsy and/or full axillary nodal dissection when indicated. Among BCT patients, the median radiation dose was 45 Gy (45–50.4) with a 14.92 Gy (0–15) boost. All patients with an RS > 31, and 27 of 31 patients (87 %) with an RS > 25, received adjuvant chemotherapy. Twenty patients with an RS ≤ 25 received adjuvant chemotherapy. Taxane-based (n = 33), anthracycline-based (n = 11), and cyclophosphamide, methotrexate, fluorouracil (n = 1) chemotherapy regimens were administered. The specific chemotherapy regimens of two patients were not available in the medical record.
Statistical Analysis
LRR was defined as biopsy-proven tumor recurrence in the ipsilateral breast, chest wall, axilla, supraclavicular or infraclavicular fossae, or internal mammary lymph nodes at any time point (with or without distant metastases). Time to LRR and follow-up was calculated from the date of surgery.
The univariate association of each predictor variable with covariates was examined using the Wilcoxon rank-sum, Chi square or Fisher's exact tests. The RS was dichotomized using an outcome-oriented approach, where an optimal cut-off point is chosen corresponding to the most significant relation with LRR based on the log-rank statistic.19 This cut-point was then verified visually using a Martingale residual plot for risk score.20 Survival functions were estimated using the Kaplan–Meier method, and the log-rank test was used to assess the difference in LRR between patients with high or low risk, classified by RS.21 Univariate survival analysis was carried out with a Cox proportional hazards model, in the entire cohort and in patients divided by treatment (mastectomy or BCT).22 Multivariable survival analyses were further conducted by including all covariates and using a backward variable selection method with an alpha level of removal of 0.1. All analyses were carried out using SAS 9.3 software (SAS Institute, Inc., Cary, NC, USA) and R package version 2.15.3 (The R Foundation for Statistical Computing) with a significance level of 0.05.
Results
Patient and Tumor Characteristics by Local Treatment
Subjects were divided into those who received BCT (n = 110) and those who received mastectomy without radiation (n = 53). Median follow-up time was 5.71 years in both treatment groups. The median age of patients who received BCT was significantly higher than those treated with mastectomy alone [59.5 (range 36–85) vs. 51 (range 39–83) years; p < 0.001]. More BCT patients than mastectomy patients had lymph node-negative (93 vs. 81 %; p = 0.03) disease and pathologic stage I tumors (86 vs. 53 %; p < 0.001). Among the two treatment groups, there were no differences in the proportion of patients with an RS > 25 or those who received adjuvant chemotherapy (Table 1). Additional characteristics are listed in Table 1.
Table 1. Patient and tumor characteristics by local treatment.
| Characteristic | Breast-conserving therapy (N = 110) | Mastectomy alone (N = 53) | p value |
|---|---|---|---|
| Follow-up time [months; median (range)] | 67 (20–109) | 67 (2–88) | 0.46 |
| Age, years | |||
| <50 | 25 (23) | 23 (43) | <0.001 |
| ≥50 | 85 (77) | 30 (57) | |
| pStage | |||
| I | 95 (86) | 28 (53) | <0.001 |
| II | 15 (14) | 25 (47) | |
| T stage | |||
| 1 | 95 (86) | 32 (60) | <0.001 |
| 2 | 15 (14) | 21 (40) | |
| Lymph node | |||
| Negative | 102 (93) | 43 (83) | 0.03 |
| Positive (pN1) | 8 (7) | 10 (19) | |
| Grade | |||
| 1 | 41 (37) | 18 (34) | 0.52 |
| 2 | 59 (54) | 27 (51) | |
| 3 | 10 (9) | 8 (15) | |
| LVSI | |||
| No | 13 (12) | 10 (19) | 0.40 |
| Yes | 94 (88) | 43 (81) | |
| <2 mm surgical margins | |||
| No | 91 (83) | 48 (91) | 0.19 |
| Yes | 8 (7) | 5 (9) | |
| Adjuvant chemotherapy | |||
| No | 78 (71) | 38 (72) | 0.92 |
| Yes | 32 (29) | 15 (28) | |
| RS > 25 | |||
| No | 88 (80) | 43 (81) | 0.87 |
| Yes | 22 (20) | 10 (19) | |
| RS > 24 | |||
| No | 87 (79) | 42 (79) | 0.98 |
| Yes | 23 (21) | 11 (21) |
Data are expressed as n (%) unless otherwise specified
pStage pathological stage, T tumor, LVSI lymphovascular space invasion, RS recurrence score
Locoregional Recurrence, Recurrence Score, and Local Treatment
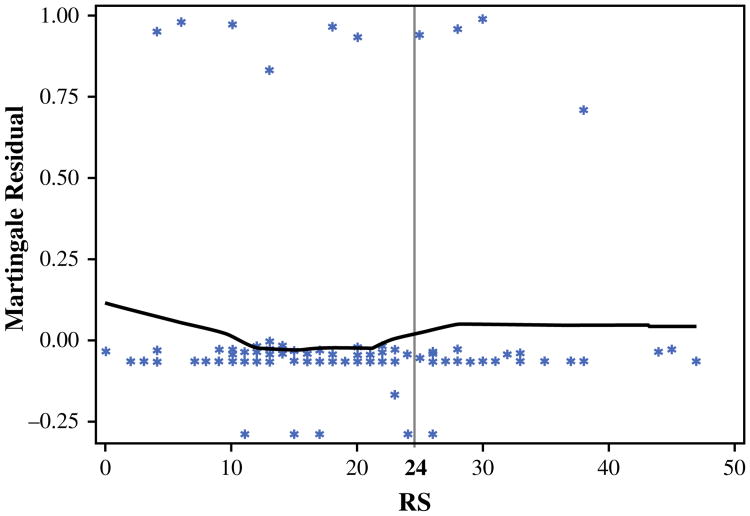
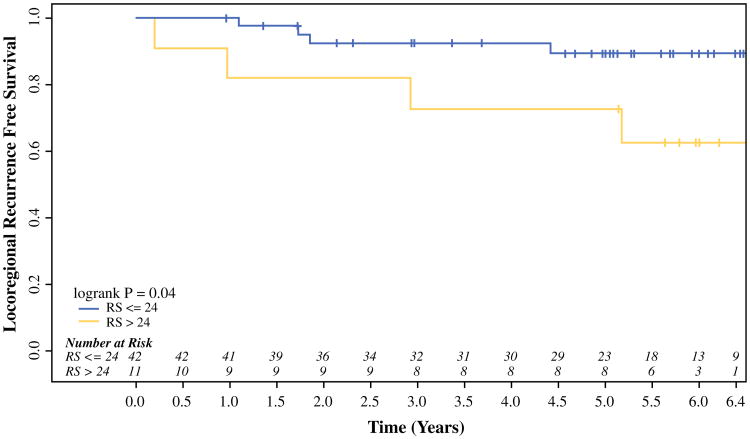
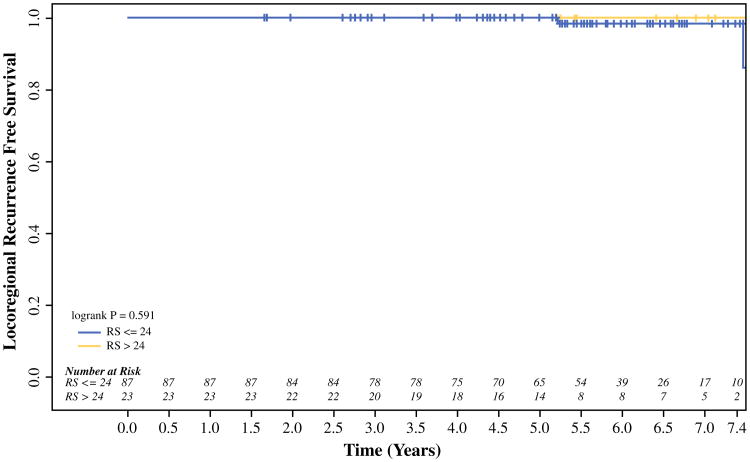
On univariable analysis, an RS > 25 did not predict for LRR in the entire cohort or within patients divided by local treatment type (mastectomy or BCT). Based on the outcome-oriented cut-point approach using the log-rank statistic and Martingale residual plot, an optimal RS cut-off value of 24 was identified, with an RS > 24 predicting for a higher rate of LRR (p = 0.04; Fig. 1). This relationship appeared to be strongest in the mastectomy-alone patients, where the 5-year rate of LRR was significantly higher in patients who had tumors with an RS > 24 than those with an RS ≤ 24 (27.3 vs. 10.7 %, respectively; p = 0.04; Fig. 2). In addition to RS, clinicopathologic and treatment factors (i.e. patient age at diagnosis, race, lymph node status, grade, LVSI, T stage, adjuvant chemotherapy, and surgical margins) previously associated with LRR risk were examined. On univariable analysis, an RS > 24 was the only predictor of LRR in patients treated with mastectomy. Among patients treated with BCT, there was no difference in LRR by RS. No BCT patient developed an LRR at 5 years in either subgroup divided by an RS of 24 (p = 0.59; Fig. 3). None of the above variables predicted for LRR on univariable analysis in the BCT patients.
Fig. 1.
Martingale residual plot of RS and locoregional recurrence. The Martingale residuals represent the difference over time of the observed number of events to the expected number of events under the assumed Cox proportional hazards model. A smoothed curve based on average residual across each covariate value is fit, and if there appears to be a change in slope then the variable can be dichotomized where the change occurs. In the above figure, the average residual decreases as recurrence score increases through scores of 20–25. From that point, the average residual increases slightly before leveling off, indicating an adequate cut-point for recurrence score in that range. This residual plot was used in combination with the method for maximizing the log-rank statistic to identify an optimal cut-point for our covariate (RS) at a value of 24. RS recurrence score
Fig. 2.
RS and locoregional recurrence in breast cancer patients treated with mastectomy. Mastectomy patients treated without radiation were divided by a tumor RS of > 24 or ≤24. Breast cancer patients who had tumors with an RS > 24 had a significantly lower rate of locoregional recurrence-free survival than women with an RS ≤ 24. RS recurrence score
Fig. 3.
RS and locoregional recurrence in breast cancer patients treated with breast-conserving therapy. Breast cancer patients treated with partial mastectomy and whole-breast radiation were divided into two groups by a tumor RS of > 24 or ≤24. There were no significant differences in locoregional recurrence-free survival between these two groups based on RS. RS recurrence score
Limited patient numbers precluded multivariable analysis in either treatment group. Multivariable analysis was therefore performed to identify predictors of LRR in the entire cohort. In addition to RS, the clinicopathologic and treatment factors listed above were examined. None of these additional variables predicted for LRR on univariable analysis. Multivariable analysis revealed that an RS > 24 (p = 0.04) and treatment with mastectomy and no radiation (p = 0.006) were the only significant predictors of LRR.
Of note, all patients who developed an LRR were pStage I or IIA (see Table 2). Eight patients within the mastectomy-alone group and three patients who received BCT developed a recurrence. Among the 11 patients who developed a LRR, two patients had grade 3 disease, two patients had LVSI, one patient had less than a 2 mm margin following mastectomy, and one patient had N1mi disease. The patient with N1mi disease who later developed an LRR was treated with adjuvant chemotherapy for the initial breast tumor.
Table 2. Patient and treatment characteristics of subjects with locoregional recurrence.
| Primary treatment | Age (years) | RS | T stage | N stage | Margin status | LVSI | Chemotherapy | Time to recurrence (months) |
|---|---|---|---|---|---|---|---|---|
| BCS + XRT | 45 | 38 | 1c | 0 | Negative | No | Yes | 48 |
| BCS + XRT | 47 | 13 | 1c | 0 | Negative | No | No | 88 |
| BCS + XRT | 42 | 20 | 1c | 0 | Negative | No | No | 54 |
| Mastectomy | 55 | 18 | 1c | 1mi | Negative | No | Yes | 22 |
| Mastectomy | 65 | 30 | 2 | 0 | Negative | No | Yes | 12 |
| Mastectomy | 46 | 28 | 1c | 0 | Negative | Yes | Yes | 36 |
| Mastectomy | 56 | 30 | 2 | 0 | Negative | Yes | Yes | 14 |
| Mastectomy | 59 | 6 | 2 | 0 | Negative | No | No | 38 |
| Mastectomy | 57 | 4 | 1c | 0 | Negative | No | No | 58 |
| Mastectomy | 40 | 25 | 2 | 0 | Negative | No | No | 63 |
| Mastectomy | 43 | 10 | 1b | 0 | Negative | No | No | 21 |
T tumor, N node, LVSI lymphovascular space invasion, RS recurrence score, BCS breast-conserving surgery, XRT radiation therapy
Discussion
Breast cancer is a biologically heterogeneous disease.23 However, currently, clinicians do not typically make local therapy decisions based on tumor gene expression. The present study indicates that there is a relationship between the 21-gene RS and LRR in breast cancer patients. Although the pre-determined RS of 25 was not associated with LRR, we found that an RS > 24 is predictive of LRR among our patients, which took into account one additional subject with an RS of 25 who later developed an LRR. On univariable analysis, an RS > 24 was predictive of LRR in mastectomy patients but not in BCT patients who received radiation. Mastectomy patients who recurred did not have risk factors that would typically prompt the recommendation for post-mastectomy radiation therapy, and our data suggest the RS may be used to identify a previously unrecognized group of mastectomy patients who could potentially benefit from post-mastectomy radiation.
Various gene expression signatures predictive of LRR in breast cancer have been previously explored. For example, one study found that both a 34-gene expression prediction model and ER negativity predicted for LRR in breast cancer patients treated with mastectomy.24 Studies of patients treated with BCT have yielded mixed results, with most of the positive findings indicating a relationship between genetic signatures and LRR in younger, pre-menopausal patients.4,25 The majority of studies exploring the relationship between gene expression signatures and LRR have been limited by small patient numbers, heterogeneous treatments, and the inclusion of patients with mixed receptor subtypes, lymph node-positive disease, and involved tumor margins after surgery.4,5,24–26 Due to these inconsistent results, none of the above genetic signatures have been adopted into standard practice.
Nevertheless, among genomic profiling assays, the 21-gene RS is attractive due to its widespread use in a relatively homogeneous cohort of breast cancer patients and its ability to predict distant metastasis and potential benefit of chemotherapy. Our findings indicating that the RS may predict LRR in breast cancer patients treated with mastectomy is consistent with previous results.13 Furthermore, our data suggesting that RS does not predict for LRR in patients treated with BCT is also supported by previous research.16 Another study found a relationship between RS and LRR in BCT patients under 50 years of age, but no association was found in women aged 50 years or older.13 Among our BCT patients, 77 % of subjects were aged 50 years or older. Many of these BCT patients were diagnosed with low-risk disease, which may explain why we found no relationship between RS and LRR where the 10-year rate of LRR does not exceed 10 %.
The potential biological mechanism by which the 21-gene RS may be predictive of LRR in mastectomy and not in BCT patients warrants consideration. Previous research has indicated that wound fluids and growth factors that stimulate proliferation, migration and invasion of breast cancer cells are released following surgery in breast cancer patients. Five of the 21 genes used in the RS assay are instrumental in tumor proliferation, and the proliferation group score determined from these five genes are one of the major determinants of the overall RS. It is possible that a high RS is indicative of inherent cellular sensitivity and response to growth factors released at the time of surgery, stimulating residual malignant cells or otherwise dormant cells to de-differentiate and develop into locally recurrent disease. Targeted Intraoperative Radiotherapy (TARGIT) has already been shown to decrease the stimulatory effect of wound response fluids otherwise observed in surgical fluids sampled from BCT patients who do not receive radiation.27 Likewise, post-mastectomy radiation treatment could potentially suppress the release of growth factors following mastectomy and abrogate the LRR risk in a patient with a high RS.
The strengths of our study include a long follow-up period in a relatively large cohort of patients treated with modern systemic therapies shown to impact LRR. In NSABP B-14 and B-20, patients were treated with tamoxifen alone or with older chemotherapy regimens, including methotrexate and fluorouracil or cyclophosphamide, methotrexate, and fluorouracil.28,29 Despite the use of modern systemic chemotherapy in our patients, a relationship between RS and LRR continued to exist. Limitations include a relatively low overall event rate due to the low-risk breast cancer population in which the RS was generated. All patients had stage I and II disease that was ER-positive, and the majority were post-menopausal. Furthermore, only 20 % of patients had an RS > 25. Due to the low event rate, a multivariable analysis for each local treatment group could not be performed. Nevertheless, even within this low-risk breast cancer population, we found a relationship between RS and LRR underscoring the potential of the RS to identify a group of patients thought to otherwise have low risk of LRR based on traditional clinicopathologic factors.
Conclusions
Our study demonstrates that there is a relationship between RS and LRR and that this relationship appears to be most robust in mastectomy patients treated without radiation. The RS may identify patients traditionally treated with mastectomy alone (e.g. pStage I or II) who may benefit from post-mastectomy radiation due to their higher risk of LRR even after treatment with modern systemic agents. Future prospective trials are needed to validate these findings in a larger cohort of patients.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure None.
References
- 1.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28(10):1671–6. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 4.Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8(5):R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreike B, Halfwerk H, Armstrong N, et al. Local recurrence after breast-conserving therapy in relation to gene expression patterns in a large series of patients. Clin Cancer Res. 2009;15(12):4181–90. doi: 10.1158/1078-0432.CCR-08-2644. [DOI] [PubMed] [Google Scholar]
- 6.Connor CS, Touijer AK, Krishnan L, Mayo MS. Local recurrence following breast conservation therapy in African-American women with invasive breast cancer. Am J Surg. 2000;179(1):22–6. doi: 10.1016/s0002-9610(99)00258-5. [DOI] [PubMed] [Google Scholar]
- 7.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22(21):4247–54. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Jones HA, Antonini N, Hart AA, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol. 2009;27(30):4939–47. doi: 10.1200/JCO.2008.21.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21(7):1205–13. doi: 10.1200/JCO.2003.03.130. [DOI] [PubMed] [Google Scholar]
- 10.Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852–8. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selz J, Stevens D, Jouanneau L, Labib A, Le Scodan R. Prognostic value of molecular subtypes, ki67 expression and impact of postmastectomy radiation therapy in breast cancer patients with negative lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2012;84(5):1123–32. doi: 10.1016/j.ijrobp.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721–8. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 13.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–83. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673–82. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 16.Solin LJ, Gray R, Goldstein LJ, et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. 2012;134(2):683–92. doi: 10.1007/s10549-012-2072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Angulo A, Barlow W, Gralow J, et al. OT1-03-01: a randomized phase III clinical trial of standard adjuvant endocrine therapy ± chemotherapy in patients (pts) with 1–3 positive nodes, hormone receptor (HR)-positive and HER2–negative breast cancer with recurrence score (RS) of 25 or less: SWOG S1007. Cancer Res. 2011;71(24 Suppl 3) [Google Scholar]
- 18.Edge SB American Joint Committee on Cancer. AJCC cancer staging manual. 7th. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 19.Mandrekar J, Mandrekar S, Cha S. Cutpoint determination methods in survival analysis using SAS®. Proceedings of the 28th SAS Users Group International Conference (SUGI) 2003 [Google Scholar]
- 20.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990;77(1):147–60. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Vol. 360. New York: Wiley; 2011. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 23.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 24.Cheng SH, Horng CF, West M, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer. J Clin Oncol. 2006;24(28):4594–602. doi: 10.1200/JCO.2005.02.5676. [DOI] [PubMed] [Google Scholar]
- 25.Nimeus-Malmstrom E, Krogh M, Malmstrom P, et al. Gene expression profiling in primary breast cancer distinguishes patients developing local recurrence after breast-conservation surgery, with or without postoperative radiotherapy. Breast Cancer Res. 2008;10(2):R34. doi: 10.1186/bcr1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatier R, Finetti P, Cervera N, et al. Gene expression profiling and its utility in prediction of local relapse after breast-conserving therapy in early breast cancer. Cancer Genomics Proteomics. 2011;8(4):199–209. [PubMed] [Google Scholar]
- 27.Belletti B, Vaidya JS, D'Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. 2008;14(5):1325–32. doi: 10.1158/1078-0432.CCR-07-4453. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor–positive tumors. N Engl J Med. 1989;320(8):479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B, Dignam J, Emir B, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673–82. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]