Summary
In patients with high-risk metastatic neuroblastoma, the benefit of radiation therapy (RT) to metastatic sites as part of primary treatment has not been fully investigated. The purpose of this single-institution study was to evaluate local control of irradiated metastatic sites, and characterize metastatic disease burden and anatomic distribution in patients with high-risk metastatic neuroblastoma. The records of all patients diagnosed with stage 4 neuroblastoma between August 2000 and January 2010 were reviewed. Exclusion criteria included: bone-marrow only metastatic site, total body irradiation, or no imaging follow-up. A total of 37 patients met eligibility criteria. Median follow-up period for patients without relapse was 61 months. Five-year overall survival for all patients was 67%. Thirteen patients (35%) received RT to a metastatic site as part of their primary treatment. Among these patients, in-field recurrence occurred in three patients (23%), including two of three treated calvarial sites. In patients treated with or without RT to a metastatic site, respectively, there was no significant difference in 5-year overall survival (73% vs. 63%, P = 0.84) or relapse-free survival (46% and 55%, P = 0.48). Current metastatic site RT dose may be suboptimal, and certain locations may predict for a poor response. Further studies are necessary to elucidate the optimal role of RT to metastatic sites.
Keywords: neuroblastoma, radiation therapy, metastatic
Neuroblastoma is a neoplasm arising from neural crest cells of the sympathetic nervous system. It stands as the most common cancer diagnosed in the first year of life, and the most common extracranial solid tumor of childhood.1 Of the approximately 650 children diagnosed annually in the United States, 50% present with high risk, stage 4 (International Neuroblastoma Staging System [INSS]) disease.2 Over the past two decades, developments in multimodality treatment regimens have led to better outcomes. A Children's Cancer Group (CCG-3891) randomized study reported improved survival in patients receiving myeloablative chemotherapy, total body irradiation (TBI), and autologous bone marrow transplantation versus intensive chemotherapy alone.3 Furthermore, patients receiving maintenance 13-cis-retinoic acid and anti-GD-2 antibodies have also demonstrated improved survival.4,5 Despite advances in multimodality treatment, 5-year survival rates for this patient population have been generally unsatisfactory (30% to 60%).4–9
Treatment paradigms continue to evolve with the aim of improving upon these outcomes. In recent years, the role of radiation therapy (RT) has shifted from TBI to focal treatment of the primary tumor (following induction chemotherapy, maximal resection, and myeloablative transplant). Multiple reports have demonstrated excellent primary local control with RT.10–18 Notwithstanding progress in systemic therapies, distant failure remains a significant impediment on eventual disease course. The role of RT to metastatic sites as part of primary treatment has not been fully investigated. The purpose of this study is to evaluate local control of irradiated metastatic sites, and to characterize metastatic disease burden and anatomic distribution in relation to outcomes in patients with high-risk metastatic neuroblastoma.
Materials and Methods
Following approval of this retrospective study by the institutional internal review board, we reviewed the records of all patients diagnosed with INSS stage 4 neuroblastoma between August 2000 and January 2010 at our institution. Exclusion criteria consisted of: bone-marrow only meta-static site, TBI, or no imaging follow-up. A total of 37 patients met eligibility criteria. Staging workup included computed tomography (CT) of the chest and abdomen, 123I-metaiodobenzylguanidine (mIBG) scan, technetium-99 bone scan, bilateral bone marrow aspirates, and biopsy for histologic confirmation. Shimada histology and N-myc status were recorded.
The majority of patients were treated per Children's Oncology Group protocols, namely ANBL00P1, ANBL02P1, and ANBL0532, in which patients underwent a regimen of induction chemotherapy that included cyclophosphamide, doxorubicin, vincristine, cisplatin, etoposide, and topotecan. Postinduction chemotherapy response was evaluated by CT, mIBG, bone scan, and bone marrow evaluation. Type of surgery was documented as follows: (1) gross or near-total resection (defined as > 95% surgical resection); (2) subtotal resection; and (3) biopsy alone. Following surgery, patients underwent single or tandem high-dose chemotherapy and autologous stem cell transplant.
RT was subsequently delivered to the primary site using CT-based planning to delineate target volumes. The gross tumor volume of the primary (GTVp) consisted of the postinduction chemotherapy, presurgical disease. For abdominal primaries, the clinical target volume (CTVp) included the para-aortic lymph nodes in addition to the GTVp. To account for set-up uncertainties, the planning target volume (PTVp) was generated by expanding the CTVp approximately 0.5 to 1.0 cm. In cases in which metastatic sites were irradiated, the GTVm consisted of the residual metastatic tumor (following induction chemotherapy) as defined by mIBG, CT, or MRI. An additional 1.0 to 1.5 cm CTVm margin was created to account for microscopic disease, followed by a 0.5 to 1.0 cm PTVm margin to account for set-up uncertainties. RT was delivered by a linear accelerator using 6MV photon energy. The decision to irradiate a metastatic site was made by the treating physician (evaluation based on postinduction response and posttransplant/pre-RT imaging if available) or specified by protocol. Following RT, patients received adjuvant cis-retinoic acid for six cycles as part of their maintenance therapy. A subset of patients also received immunotherapy/antibody treatment.
Metastatic Site Classification
Metastatic sites were classified into four categories based on location: (1) axial skeleton; (2) appendicular skeleton; (3) soft tissue; and (4) calvarium. Image-defined (primarily mIBG scan) involvement of each site was documented for every patient at the following time points: (1) preinduction chemotherapy; (2) postinduction chemotherapy; and (3) post-transplant/pre-RT (if obtained). A mIBG score (reflecting the number of involved sites) at each time point was totaled similarly to the modified Curie score,19,20 which is based on the presence of mIBG uptake in different anatomic regions, including nine skeletal sites (head, chest, T-spine, L-spine, pelvis, upper arms, lower arms, femurs, and lower legs) and a 10th site for soft-tissue lesions.
Definitions and Statistical Analysis
Follow-up visits were performed in the pediatric hematology/oncology and radiation oncology clinics, and included evaluation with clinical examinations, imaging (evaluation of relapse by mIBG scan), and laboratory testing. To compare baseline patient characteristics between the group of patients who received RT to a metastatic site and the group that did not, independent two-sample t-tests were performed for numerical covariates, and the χ2 tests or Fisher exact tests were performed for categorical covariates, with statistical significance defined as a P-value of <0.05. Relapse-free survival (RFS) was calculated for each metastatic and primary site from the start date of induction chemotherapy to the date of relapse or progression or to date of last follow-up if patients remained in remission. In cases in which a patient received RT to a metastatic site, infield recurrence was determined based on mIBG scan correlation with the RT field. It was noted whether the first recurrence was out-of-field and in a de novo site. When a patient was not irradiated to a metastatic site, involvement of each metastatic site location was assessed at first recurrence, and if any metastatic site was involved, it was documented whether it occurred in a de novo or previously involved site. Overall survival (OS) was calculated from the start date of induction chemotherapy to date of last follow-up or date of death. Actuarial rates were determined by the Kaplan-Meier method. A modified Curie score cutoff of 2 (at diagnosis and after induction chemotherapy) has previously demonstrated prognostic significance,21,22 and was therefore examined in our study. Distribution of survival times were compared using the Log-rank tests, with statistical significance defined as a P-value of <0.05. All statistical analyses were carried out using SPSS version 21.0 (IBM, Armonk, NY).
Results
Patient and tumor characteristics are summarized in Table 1. Median age at diagnosis was 3.7 years (range, 8.4 mo to 20.7 y). All patients had INSS stage 4 neuroblastoma. Twenty-six patients had a gross total or near-total resection, nine had a subtotal resection, and two underwent biopsy alone. After resection, all patients underwent high-dose chemotherapy followed by a single transplant in six patients and tandem transplant in 31 patients. There were no significant baseline differences in patient or tumor characteristics between the group of patients who did not receive RT to a metastatic site and the group that did receive RT to a metastatic site.
Table 1. Patient and Tumor Characteristics.
| Characteristics | n (%) | P | ||
|---|---|---|---|---|
|
| ||||
| All Patients (n=37) | No RT to a Metastatic Site (n=24) | RT to a Metastatic Site (n=13) | ||
| Age at diagnosis (years) | ||||
| Median | 3.7 | 3.5 | 3.7 | 0.43 |
| Range | 0.7-20.7 | 0.8-10.7 | 0.7-20.7 | |
| Sex | ||||
| Male | 20 (54) | 13 (54) | 7 (54) | 0.98 |
| Female | 17 (46) | 11 (46) | 6 (46) | |
| Resection extent | ||||
| GTR/NTR | 26 (70) | 18 (75) | 8 (61) | 0.47 |
| STR | 11 (30) | 6 (25) | 5 (39) | |
| Shimada histology | ||||
| Favorable | 4 (11) | 2 (11) | 2 (17) | 0.63 |
| Unfavorable | 27 (73) | 17 (89) | 10 (83) | |
| Unknown | 6 (16) | |||
| N-myc amplification | ||||
| Yes | 12 (32) | 9 (56) | 3 (30) | 0.25 |
| No | 14 (38) | 7 (44) | 7 (70) | |
| Unknown | 11 (27) | |||
| Transplant | ||||
| Single | 6 (16) | 4 (17) | 2 (15) | 1.00 |
| Tandem | 31 (84) | 20 (83) | 11 (85) | |
| Maintenance cis-retinoic acid | ||||
| Completed full course | ||||
| Yes | 33 (89) | 20 (91) | 13 (100) | 0.52 |
| No | 2 (5) | 2 (9) | 0 (0) | |
| Unknown | 2 (5) | |||
| Primary antibody treatment | ||||
| Yes | 5 (14) | 4 (17) | 1 (8) | 1.00 |
| No | 32 (86) | 20 (83) | 12 (92) | |
| No. metastatic sites involved at diagnosis | ||||
| Median | 4 | 4 | 5 | 0.75 |
| Range | 1-12 | 1-12 | 1-12 | |
GTR indicates gross total resection; NTR, near-total resection; RT, radiation therapy; STR, subtotal resection.
All patients received RT to the primary site. The median RT dose to the primary site was 21.6 Gy (range, 21.0 to 30.6 Gy). A total of 13 metastatic sites (in 13 patients) were irradiated concurrently with the primary site. Of these 13 patients, 10 patients had positive mIBG findings following induction chemotherapy. The remaining three patients had negative postinduction mIBG imaging, but were thought to have persistent disease as defined by other postinduction imaging (CT and bone scans), which influenced the decision to irradiate a metastatic site. The median RT dose to metastatic sites was 21.6 Gy (range, 21.0 to 30.6 Gy). Following RT, 33 patients (92%) completed a full course of maintenance cis-retinoic acid. Two patients did not receive maintenance therapy due to early progression of disease, and the status of maintenance therapy was unknown in two patients. Seven patients (19%) were treated with monoclonal antibody therapy, of whom five received as part of upfront therapy and two received at relapse.
Metastatic Site/Imaging Characteristics
Table 2 illustrates the RT details and outcomes of each irradiated metastatic site case (13 patients). Among these patients, the median RFS was 35.1 months. At last follow-up, five patients (38%) were alive with no evidence of disease, three (23%) were alive with disease, and five (38%) died of disease. In-field recurrence occurred in three patients (23%), including two of three calvarial cases and one of ten other metastatic sites. Before therapy, the most commonly involved metastatic sites were the appendicular skeleton (87%), followed by axial skeleton (70%), calvarium (43%), and soft tissue (22%). The median mIBG score at diagnosis and after induction chemotherapy was 4 (range, 1 to 12) and 0 (range, 0 to 7), respectively. Following induction chemotherapy, 14 patients (38%) had involvement of ≥ 1 mIBG + metastatic sites (cumulative mIBG score of 44). Of these patients, ten each underwent RT to one mIBG + site and four did not. Reasons for not receiving RT to a metastatic site(s) included: no residual metastatic disease on posttransplant imaging in one patient; diffuse burden of disease in two patients; and unknown reason in one patient. See Figure 1 for a flow diagram of treatment and disease course of patients with postinduction mIBG + sites.
Table 2. Metastatic Site Radiation Treatment Details (13 Patients).
| Patient No. | Age at Dx (years) | Site | Dose Information (Total Dose (Gy)/No. Fractions) | In-field Recurrence | Relapse-free Survival (months) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 2.7 | Left humerus | 21.6/12 | No | 68.3 | First recurrence out-of-field in de novo site; DOD |
| 2 | 4.6 | Right acetabulum | 21.6/12 | No | 23.9 | First recurrence out-of-field in de novo site; AWD |
| 3 | 2.4 | Right iliac bone | 21.6/12 | No | 75.6 | Alive and NED at last F/U |
| 4 | 4.6 | Left scapula | 21.6/12 | No | 31.2 | First recurrence out-of-field in de novo site; AWD |
| 5 | 3.7 | Lumbar spine* | 23.4/13 | No | 99.8 | Alive and NED at last F/U |
| 6 | 1.2 | Left jaw bone | 21.0/14 | No | 33.7 | First recurrence in de novo and previously involved sites; DOD |
| 7 | 0.7 | Sacrum* | 21.0/14 | No | 63.6 | Alive and NED at last F/U |
| 8 | 20.7 | Left SCV | 30.6/17 | No | 20.8 | First recurrence in de novo site; DOD |
| 9 | 8.2 | Left SCV* | 21.6/12 | No | 55.9 | Alive and NED at last F/U |
| 10 | 6.1 | Left SCV/mediastinum | 21.0/14 | Yes | 22.4 | First recurrence in-field, followed by out-of-field recurrence 18mo later; DOD |
| 11 | 1.7 | Calvarium | 23.4/13 | No | 112.7 | Alive and NED at last F/U |
| 12 | 4.0 | Calvarium | 21.6/12 | Yes | 24.8 | First and only recurrence in-field; AWD |
| 13 | 3.4 | Calvarium | 21.6/12 | Yes | 35.1 | First recurrence occurring both in-field and in de novo and previously involved sites; DOD |
Refers to metastatic site that was postinduction mIBG negative, but was treated with RT due to clinical judgment and other imaging studies.
AWD indicates alive with disease; DOD, died of disease; Dx, diagnosis; NED, no evidence of disease; F/U, follow-up; SCV, supraclavicular nodes.
Figure 1.
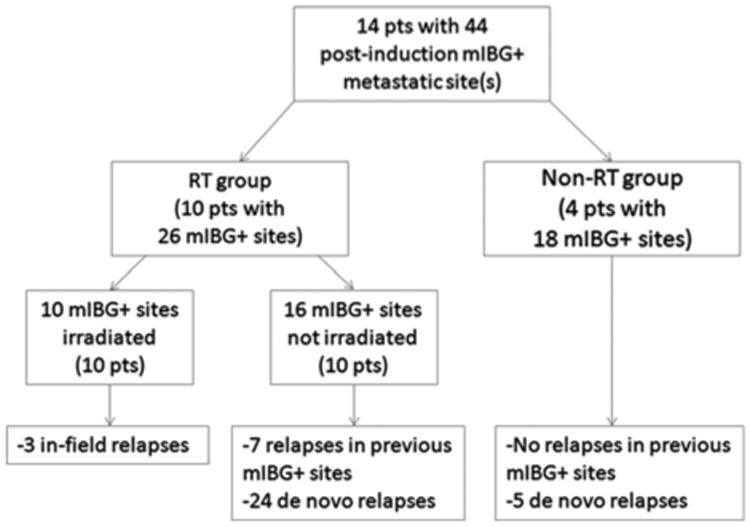
Flow diagram of treatment and disease course of patients with mIBG-positive metastatic sites following induction chemotherapy. RT group patients received RT to the primary site and one metastatic site, whereas non-RT group received RT only to the primary site. mIBG indicates 123I-metaiodobenzylguanidine; RT, radiation therapy.
Survival Outcomes
The median follow-up period for patients without relapse was 61 months (range, 9 to 113 months). The 5-year OS for all patients was 67%. For patients treated with and without RT to a metastatic site, the 5-year OS was 73% and 63%, respectively (P = 0.84), and the 5-year RFS was 46% and 55%, respectively (P = 0.48). Patients with soft-tissue metastases (with or without skeletal metastases) versus skeletal metastases alone at diagnosis had a 5-year RFS of 29% and 58%, respectively (P = 0.18).
In patients who had a mIBG score at diagnosis of ≤2 and > 2, the 5-year RFS was 90% and 40%, respectively (P = 0.09). There was no difference in 5-year RFS for patients with a postinduction mIBG score of ≤2 versus > 2.
Discussion
RT has an integral and evolving role in the management of high-risk neuroblastoma. In our series, the 5-year OS was 67%, highlighting the progress that has been made in this patient population. Furthermore, this study adds to the growing body of the literature demonstrating excellent local control of the primary site with RT (5 year rate of 94%). With such high rates of primary site control, more effective management of metastatic disease sites becomes increasingly important for achieving successful long-term outcomes. Reports of primary site local control with RT have been excellent (84% to 100%)10,11,13–16,18; however, it appears that RT may not be as successful for control of metastatic sites.
Recent Children's Oncology Group high-risk protocols specified irradiation of metastatic sites (to a dose of 21.6 Gy) with persistent active disease demonstrated on the prehematopoietic stem cell transplant (HSCT) evaluation. If a patient had > 5 persistently positive mIBG metastatic sites identified, then a mIBG scan was repeated on day 28 + post-HSCT, with only sites still mIBG + posttransplant requiring radiation. Although these protocol specifications lend guidance, to the best of our knowledge, there have been no prior published studies focusing on primary RT to metastatic sites and describing patterns of recurrence.
We observed that the overall in-field failure rate of irradiated metastatic sites was considerable (23%) while using the currently recommended dose (21.6 Gy). Although this in-field failure rate is based on a small sample size, it does introduce the possibility that current RT dosing to metastatic sites may be insufficient. The lack of surgical resection/debulking before RT in metastatic sites may result in poorer local control compared with primary sites in which there has been a resection. In instances of gross residual disease in primary sites, an additional boost of 14.4 Gy to a total dose of 36 Gy is commonly practiced, which calls into question the standard total dose of 21 Gy for grossly involved metastatic sites. Notably, CCG-3891 specified 20 Gy to extra-abdominal sites followed by 10 Gy TBI dose. A dose-response relationship has only been previously reported in the palliative setting.23,24 Caussa et al23 found improved response rates with higher doses (≥ 20 Gy) to bone metastases, as well as higher doses (≥ 15 Gy) to soft-tissue metastases. However, their classification of a favorable response (reduction in symptomatology or > 25% resolution of the tumor mass) was somewhat more liberal. Therefore, effective palliative doses for response may not necessarily suffice for metastatic disease control in the definitive setting.
Another consideration is whether anatomic location predisposes to poorer RT response. We found calvarial infield failures in two of three cases (67%) compared with one of ten cases (10%) in other metastatic sites. A prior study also found that patients with metastatic neuroblastoma (at diagnosis) involving the dura, epidural space, or bones of the skull had a significantly worse 3-year event-free survival (25%) than patients without involvement (44%).25 These findings suggest that calvarial metastases may be associated with poorer responses to RT as well as systemic therapy; however, this needs to be validated in a larger cohort.
As noted in Figure 1, de novo relapses (metastatic sites never previously involved on diagnostic and postinduction imaging, but later appearing as mIBG + on follow-up imaging) represented the primary pattern of failure in our analysis, suggesting that any potential benefit of local RT to a metastatic site may be diminished if systemic therapy is not entirely effective. Until optimal systemic therapy is achieved, RT dose escalation and targeting of metastatic sites necessitates caution and appreciation of consequential risks of late effects such as secondary malignancies, musculoskeletal deformities, and cognitive and endocrine abnormalities. In the current study, we did not evaluate acute or late toxicity, but this will constitute an area of further study with longer follow-up.
A unique aspect of the study was capturing the volume and distribution of metastatic disease at both pretreatment and postinduction chemotherapy time points. By documenting a total mIBG score similar to the modified Curie score, a validated representation of metastatic disease burden and response to chemotherapy was portrayed. Decarolis et al21 reported a Curie score of ≤2 at diagnosis was significantly associated with improved 5-year event-free survival (70% vs. 27%, P = 0.01) and 5-year OS (90% vs. 42%, P = 0.01). Similarly, we noted a trend to improved 5-year RFS in patients who had a diagnostic mIBG score of ≤2 versus > 2 (90% vs. 40%, P = 0.09). A postinduction Curie score of ≤2 has previously been associated with an improved prognosis22; however, we did not note this.
Important limitations of this study include its retrospective nature, small sample size, and heterogenous tumor characteristics of the patients, all of which preclude the formation of definitive conclusions regarding the impact of RT to metastatic sites. In addition, our study was limited in determining the possible effect of immunotherapy on patients who received RT to a metastatic site due to the small number of patients who received immunotherapy in addition to isotretinoin maintenance. Yu et al26 reported that the addition of immunotherapy with ch14.18 (a monoclonal antibody against the tumor-associated disialoganglioside GD2) to isotretinoin (standard therapy) was associated with significantly improved event-free survival and OS outcomes in high-risk neuroblastoma. In our study, only five patients (14%) received upfront immunotherapy, of whom one received RT to metastatic site. This patient experienced out-of-field recurrence and is alive with disease at last follow-up, whereas the other four patients are alive with no evidence of disease. Additional inquiry is required into whether immunotherapy potentially alters the role and benefit of RT to metastatic sites.
Details pertaining to RT to metastatic sites in stage 4 neuroblastoma remain open to further study. Although intensification of systemic therapy has led to improvement in treatment response and disease outcome, the optimal approach to treating postinduction residual metastatic sites remains a challenge. RT to persistent metastatic sites may contribute to improved outcomes; however, in-field failure may be substantial, especially when compared with the high local control rate of the primary site. RT dose to metastatic sites warrants separate investigation and may need to be tailored to the targeted site; it cannot necessarily be extrapolated from primary site doses. In addition, to justify and attain any practical benefit of local RT to metastatic sites, systemic therapy must minimize failures in other sites. The results from this series require larger studies to fundamentally elucidate the optimal role of definitive RT to metastatic sites.
Acknowledgments
Supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and National Institutes of Health (NIH)/National Cancer Institute (NCI) under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ries L, Smith M, Gurney J, et al. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. NIH Pub No. 99-4649. [Google Scholar]
- 2.Goodman M, Gurney J, Smith M. Sympathetic nervous system tumors, cancer incidence and survival among children and adolescents: United States SEER program 1975-1995. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. SEER Program. Bethesda, MD: National Cancer Institute; 1999. pp. 65–72. [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung NK, Kushner BH, Cheung IY, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 7.De Bernardi B, Nicolas B, Boni L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–1601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko M, Tsuchida Y, Mugishima H, et al. Intensified chemotherapy increases the survival rates in patients with stage 4 neuroblastoma with MYCN amplification. J Pediatr Hematol Oncol. 2002;24:613–621. doi: 10.1097/00043426-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Stram DO, Matthay KK, O'Leary M, et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children's Cancer Group studies. J Clin Oncol. 1996;14:2417–2426. doi: 10.1200/JCO.1996.14.9.2417. [DOI] [PubMed] [Google Scholar]
- 10.Bradfield SM, Douglas JG, Hawkins DS, et al. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuro-blastoma. Cancer. 2004;100:1268–1275. doi: 10.1002/cncr.20091. [DOI] [PubMed] [Google Scholar]
- 11.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–1554. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 12.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 13.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 14.Marcus KJ, Shamberger R, Litman H, et al. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J Pediatr Hematol Oncol. 2003;25:934–940. doi: 10.1097/00043426-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Pai Panandiker AS, Beltran C, Billups CA, et al. Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer. 2013;60:761–765. doi: 10.1002/pbc.24350. [DOI] [PubMed] [Google Scholar]
- 16.Paulino AC, Mayr NA, Simon JH, et al. Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys. 2002;52:1025–1031. doi: 10.1016/s0360-3016(01)02713-4. [DOI] [PubMed] [Google Scholar]
- 17.Robbins JR, Krasin MJ, Pai Panandiker AS, et al. Radiation therapy as part of local control of metastatic neuroblastoma: the St Jude Children's Research Hospital experience. J Pediatr Surg. 2010;45:678–686. doi: 10.1016/j.jpedsurg.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolden SL, Gollamudi SV, Kushner BH, et al. Local control with multimodality therapy for stage 4 neuroblastoma. Int J Radiat Oncol Biol Phys. 2000;46:969–974. doi: 10.1016/s0360-3016(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 19.Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method—application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A:256–261. doi: 10.1016/0959-8049(94)00509-4. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 21.Decarolis B, Schneider C, Hero B, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study. J Clin Oncol. 2013;31:944–951. doi: 10.1200/JCO.2012.45.8794. [DOI] [PubMed] [Google Scholar]
- 22.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. J Nucl Med. 2013;54:541–548. doi: 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caussa L, Hijal T, Michon J, et al. Role of palliative radiotherapy in the management of metastatic pediatric neuroblastoma: a retrospective single-institution study. Int J Radiat Oncol Biol Phys. 2011;79:214–219. doi: 10.1016/j.ijrobp.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Paulino AC. Palliative radiotherapy in children with neuroblastoma. Pediatr Hematol Oncol. 2003;20:111–117. doi: 10.1080/0880010390158702. [DOI] [PubMed] [Google Scholar]
- 25.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]


