Abstract
Objective:
Variations in the genes encoding alcohol dehydrogenase (ADH) enzymes are associated with both alcohol consumption and dependence in multiple populations. Additionally, some environmental factors have been recognized as modifiers of these relationships. This study examined the modifying effect of religious involvement on relationships between ADH gene variants and alcohol consumption–related phenotypes.
Method:
Subjects were African American, European American, and Hispanic American adults with lifetime exposure to alcohol (N = 7,716; 53% female) from the Collaborative Study on the Genetics of Alcoholism. Genetic markers included ADH1B-rs1229984, ADH1B-rs2066702, ADH1C-rs698, ADH4-rs1042364, and ADH4-rs1800759. Phenotypes were maximum drinks consumed in a 24-hour period and total number of alcohol dependence symptoms according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Religious involvement was defined by self-reported religious services attendance.
Results:
Both religious involvement and ADH1B-rs1229984 were negatively associated with the number of maximum drinks consumed and the number of lifetime alcohol dependence symptoms endorsed. The interactions of religious involvement with ADH1B-rs2066702, ADH1C-rs698, and ADH4-rs1042364 were significantly associated with maximum drinks and alcohol dependence symptoms. Risk variants had weaker associations with maximum drinks and alcohol dependence symptoms as a function of increasing religious involvement.
Conclusions:
This study provided initial evidence of a modifying effect for religious involvement on relationships between ADH variants and maximum drinks and alcohol dependence symptoms.
Alcohol dehydrogenase (adh) enzyme genes are consistently associated with both alcohol consumption and dependence (Hurley & Edenberg, 2012). Generally, those variants associated with a higher rate of ethanol metabolism to acetaldehyde are associated with decreased alcohol consumption and lower risk for alcohol dependence. The hypothesized mechanism is that enhanced negative reactions to ethanol (e.g., flushing, higher levels of sedation) are associated with a buildup of acetaldehyde in the body, which tends to limit heavy alcohol consumption (Hurley & Edenberg, 2012; Macgregor et al., 2009; McCarthy et al., 2010). Social factors also influence drinking and risks for alcohol dependence, and are recognized to play a role in varying the genetic susceptibility or protection for this disease. The current study examined the joint associations between religious involvement, as measured by religious services attendance, and ADH gene variants in predicting two alcohol consumption–related phenotypes. Services attendance is negatively associated with alcohol use and is protective against alcoholism (Borders et al., 2010; Edlund et al., 2010). Lower drinking may occur through several recognized mechanisms, including integration into a positive social network, better behavioral self-control, and religious drinking norms (DeWall et al., 2014; Ford, 2006; Ford & Kadushin, 2002). Prior models of gene-by-environment relationships indicate that environments characterized by more social restrictions on alcohol consumption—as with higher religiosity—are associated with reduced genetic influences (Dick & Kendler, 2012; Shanahan & Hofer, 2005). The influence of ADH genes on developing alcohol dependence requires some exposure to alcohol; thus, the genetic effect is susceptible to environmental variation.
Variants in the ADH1B, ADH1C, and ADH4 genes have been studied in association with alcohol consumption–related phenotypes in multiple samples, including those represented in the current sample (African ancestry, European American, and Hispanic populations). There is strong evidence for a negative association between the ADH1B*2 allele (i.e., A allele at rs1229984) and alcohol dependence and alcohol consumption (Ehlers et al., 2012; Konishi et al., 2004; Li et al., 2011; Macgregor et al., 2009), as well as evidence for the ADH1B*3 allele (i.e., T allele at rs2066702) (Edenberg et al., 2006; Ehlers et al., 2007). Bierut et al. (2012) reported reduced risk for alcohol dependence for the A allele at rs1229984 versus the G/G genotype in a combined sample of European Americans and African Americans. Gelernter et al.’s (2014) genome-wide association study reported associations with alcohol dependence at rs1229984 in European Americans and at rs2066702 in African Americans. In Hispanic Americans, the ADH1C*2 allele (i.e., G allele at rs698) was associated with an increased risk for alcohol dependence compared with the ADH1C*1 or A allele (Konishi et al., 2003, 2004). The association for ADH1C*2 may be attributable to its high linkage disequilibrium with ADH1B*1 (Konishi et al., 2004; Osier et al., 1999).
ADH4 gene variants also correlate with the liability for alcohol dependence (Edenberg et al., 2006), including rs1042364 and rs1800759. In individuals of European descent, rs1042364 was associated with a greater risk for alcohol dependence (Luo et al., 2006; Preuss et al., 2011; Turchi et al., 2012). The C/T genotype (corresponding with genotype G/A) at rs1042364 was protective for alcohol dependence in a combined African and European American sample (Luo et al., 2005). Preuss et al. (2011), in their study of Europeans, showed a higher frequency of the rs1042364 A allele in alcohol-dependent cases compared with controls. Edenberg et al. (1999) reported that the ADH4-rs1800759 C allele has lower promoter activity than the A allele, suggesting a greater risk for alcohol dependence for C allele carriers. Rs1800759 has been associated with alcohol dependence in some samples (Guindalini et al., 2005; Luo et al., 2006; Preuss et al., 2011) but not in others (Edenberg et al., 2006; Luo et al., 2005). This could be because of differences in the characteristics for the samples studied (e.g., case–control samples recruited from clinical populations versus family-based samples; Preuss et al., 2011). Taken together, this evidence, across the three populations, provides support for a similar biological mechanism via ADH enzyme genes for alcohol dependence susceptibility or protection, although the specific variants may differ by population.
These genetic influences on alcohol consumption and dependence depend, in part, on the environmental context. Studies of the environment in association with genetic influences and alcohol consumption-related phenotypes have generally included two lines of research, examining the roles of access to alcohol and levels of experienced stress (Dick & Kendler, 2012). Environments characterized by reduced social control and increased access to alcohol or by more severe exposure to stressful events are hypothesized to enable (in the case of access) or induce (in the case of stress) alcohol use in individuals who are genetically vulnerable. For example, an early study by Higuchi et al. (1994) reported decreasing effects for the ALDH2*2 protective flushing allele against alcohol dependence in successive Japanese cohorts. This change over time was hypothesized to reflect increased per capita alcohol use in Japan and less restrictive social norms for drinking in younger cohorts. Hasin et al. (2002) reported weakened effects for the rs1229984 protective allele in immigrants to Israel from heavy drinking cultures. Meyers et al. (2015), also using an Israeli sample, showed stronger effects for the rs1229984 G/G risk genotype in individuals who experienced childhood adversity. These two studies examined environmental contexts, characterized by, respectively, relaxed cultural drinking norms and increased exposure to stressful life events.
For the current study, we evaluated the environmental context of religious involvement in a Gene × Environment interaction analysis. We expected that the associations between ADH variants and alcohol consumption-related phenotypes would be modified by the level of religious involvement (e.g., a weaker effect for risk variants at higher vs. lower levels of religious involvement). Higher levels of religious involvement could act to weaken the effect of risk variants by exerting increased social controls on drinking. This protective relationship may occur because individuals with more frequent services attendance have less access to alcohol by being socially integrated into a low- or non-drinking reference group (Ford & Kadushin, 2002). Religious beliefs and practices could also serve to restrict individual choices and behaviors (Shanahan & Hofer, 2005). DeWall et al. (2014), for example, found that religiousness, which included services attendance, related to lower alcohol use through greater self-control over personal thoughts, emotions, and behaviors. Some religious denominations also have norms that discourage drinking. Ford (2006) showed that lower drinking was associated with prohibitive norms against drinking for Protestants but with greater social integration for Catholics.
Different aspects of religion are associated with alcohol use, including and described above, religious services attendance, denomination, and drinking norms. Using prayer to cope, like services attendance, is a religious practice that is inversely associated with alcoholism (Borders et al., 2010). The combination of having both strong spiritual beliefs and greater religious involvement appears to provide a particularly strong protection against heavy drinking (Brechting et al., 2010; Hodge et al., 2007; Holt et al., 2015). In addition, there are some differences in religious practices by racial/ethnic groups. Most relevant to the current study are the higher rates of religious participation, including services attendance, for African Americans and sometimes Hispanic Americans compared with Whites (Brown et al., 2015; Chatters et al., 2009; Robinson et al., 2012).
Twin studies have examined the joint effects of religiosity and latent genetic factors on alcohol consumption, although these studies were primarily conducted in youth. Koopmans et al. (1999) found that a religious upbringing was associated with smaller effects for genetic influences in predicting risk for alcohol use initiation. Genetic effects on problem drinking were also attenuated with increasing levels of religiosity in adolescents but not for young adults (Button et al., 2010). We are aware of only one study that examined the relationship between a measured alcohol metabolism gene and religious involvement in predicting drinking. Findings from Luczak et al.’s (2003) study in Asian young adults suggested that services attendance is more protective against heavy drinking in individuals who do not carry the protective ALDH2*2 flushing allele. Here we build on the evidence from ALDH and ADH variant and twin studies by examining, in adults, the Gene × Environment interaction between religious involvement and five measured ADH variants (ADH1B [rs1229984, rs2066702], ADH1C [rs698], and ADH4 [rs1042364 and rs1800759]) and their joint influence on maximum drinks consumed in a 24-hour period and number of lifetime endorsed alcohol dependence symptoms.
Method
Sample
Data were from the Collaborative Study on the Genetics of Alcoholism (COGA). Subjects (N = 7,716) were ages 18 years and older, who self-reported non-Hispanic White (i.e., European American), non-Hispanic Black (i.e., African American), or Hispanic race/ethnicity and a lifetime exposure to alcohol (i.e., had consumed at least one drink of alcohol). COGA is a multisite extended family study of probands with alcohol dependence, their relatives, and comparison families. The design of the COGA study is described in more detail elsewhere (Begleiter et al., 1995). Data collection sites included Indiana University; State University of New York at Brooklyn; University of California, San Diego; University of Connecticut; Howard University; University of Iowa; and Washington University in St. Louis. The institutional review boards at all sites reviewed and approved the study protocol. Informed consent was obtained from all subjects by trained COGA research assistants.
Staff at study sites selected probands from consecutive admissions to inpatient and outpatient chemical dependency treatment centers; probands were required to meet lifetime criteria for both alcohol dependence (according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised [DSM-III-R]; American Psychiatric Association, 1987) and Feighner definite alcoholism (Feighner et al., 1972) and to have at least two first-degree relatives available to participate. All available first-degree relatives of probands were invited to join the study. Community-based comparison families, with the same family structure, were recruited at each site through driver’s license registries, dental clinics, university-based studies, and medical clinics. Alcohol dependence, other drug dependence, and psychiatric disorders were not exclusionary criteria for comparison subjects.
Subjects completed the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) interview. The SSAGA is a reliable and valid semi-structured interview designed to assess current and lifetime Axis I disorders, including alcohol use, other drug use, and smoking (Bucholz et al., 1994; Hesselbrock et al., 1999). DNA data were acquired through blood sampling. Single nucleotide polymorphisms (SNPs) in the ADH gene cluster were genotyped using standard Sequenom MassArray technology (Sequenom MassArray system; Sequenom, San Diego, CA). All SNP genotypes were checked for Mendelian inheritance using the program PEDCHECK (O’Connell & Weeks, 1998). Marker allele frequencies and heterozygosities were estimated in the European and African American samples, separately, using founders and computed in Plink (Purcell et al., 2007).
Measures
Two alcohol-related phenotypes were examined: (a) the maximum number of drinks consumed in a 24-hour period and (b) the total number of lifetime alcohol dependence symptoms endorsed according to DSM-IV (American Psychiatric Association, 1994) criteria. Subjects’ responses for maximum drinks in 24 hours were capped at 60 drinks; those reporting greater than 60 drinks comprised 1.57% of the sample. Earlier studies document strong genetic influences for these two traits (Grant et al., 2009; Saccone et al., 2000).
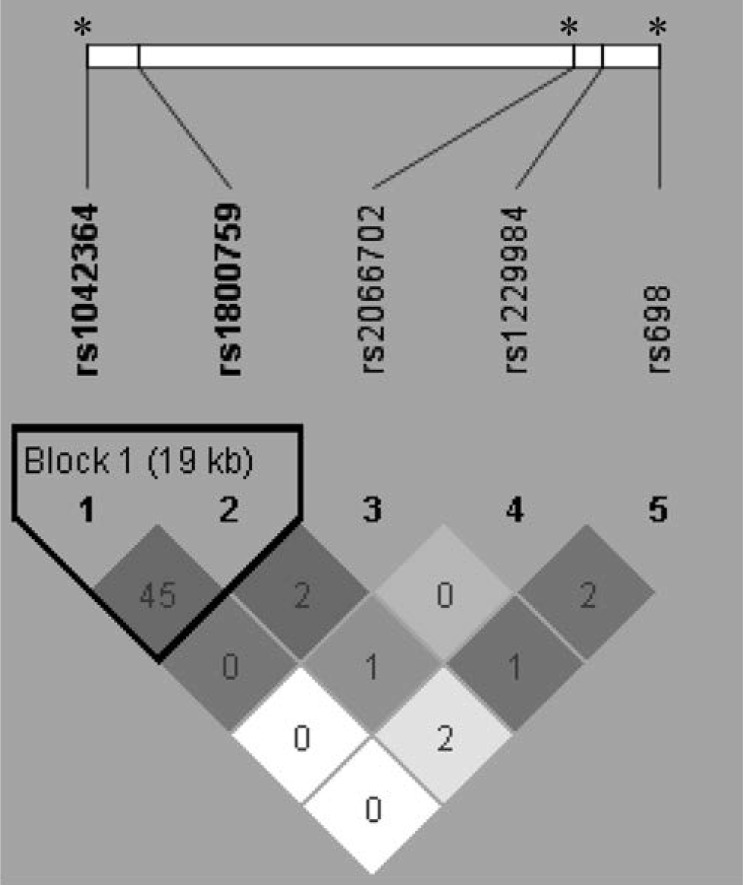
We included SNPs in ADH1B (rs1229984; rs2066702), ADH1C (rs698), and ADH4 (rs1042364; rs1800759). Genetic variants were coded as additive (0, 1, and 2) based on the minor allele count, with the exception of rs1229984 and rs2066702. These ADH1B variants were coded yes/no (carrier of the minor allele; 0, 1) because of the small number of subjects homozygous for the minor allele. Table 1 gives additional details for the ADH markers, including chromosomal position, functional status, alleles, and minor allele and genotype frequencies. Figure 1 shows the linkage disequilibrium between the markers.
Table 1.
Tested alcohol dehydrogenase (ADH) gene variants
| Marker | Chromosomal positiona | Gene | Functionality/placement | Allelesb | Minor allele frequencyc | Genotype frequencyb (n) |
||
| Homozygous for minor allele | Homozygous | Heterozygous for major allele | ||||||
| rs1229984 | 100239319 | AHD1B | missense | G:A | 0.031 | 18 | 449 | 7,204 |
| rs2066702 | 100229017 | ADH1B | missense | C:T | 0.038 | 36 | 457 | 7,183 |
| rs698 | 100260789 | ADH1C | missense | A:G | 0.345 | 978 | 3,313 | 3,360 |
| rs1042364 | 100045574 | ADH4 | 3'-UTR | G:A | 0.247 | 519 | 2,778 | 4,371 |
| rs1800759 | 100065509 | ADH4 | 5' promoter | C:A | 0.487 | 1,928 | 3,452 | 2,272 |
Notes: UTR = Untranslated region.
Positions reference human genome assembly GRCh37.p13;
alleles are listed as major:minor allele with the high-risk allele (based on past studies) in bold;
minor allele and genotype frequencies are for the combined sample. Markers with significant interaction effects (p < .05) are in bold.
Figure 1.
Location (top) and correlations (bottom) between the ADH markers. The numbers in diamonds are r2 × 100. The plot was generated using Haploview in 589 singletons and 385 trios from 1,338 families. Shading is the strength of association between pairs of markers; white represents r2 = 0, shades of gray 0 < r2 < 1, and black r2 = 1. The black triangle groups two ADH4 markers in a highly correlated 19 kilobase (kb) block. The asterisks (*) at the top indicate markers that showed a significant interaction with religious involvement for maximum drinks and alcohol dependence symptoms.
We defined religious involvement by self-reported religious services attendance (i.e., the number of times attended services in the past 12 months before interview). This variable was coded for the analysis at four levels (0 times = never, 1–11 times = less than monthly, 12–51 times = monthly, and 52 or more times = weekly or more).
Control variables included age and gender, genetic ancestry, and religious affiliation. Age was measured in years at the time of interview and gender was male or female. Genetic ancestry was evaluated using a 96 SNP panel developed at the Rutgers University DNA and Cell Repository (RUID™). This panel was enriched with 64 ancestry informative markers, which were used in SNPrelate (Zheng et al., 2012), a library function in R, to estimate principal components. Several HapMap populations were included as reference samples (ASW, CEU, CHB, CHD, JPT, GIH, LWK, MEX, MKK, TSI, YRI) to aid in the assignment of three ancestry groups: European American, African American, and other (primarily Hispanic). This resulted in a 90% concordance with self-reported race/ethnicity. The current study used the first three principal components to capture maximum genetic variability in the European American, African American, and Hispanic American samples. Subjects reported their religious affiliation, categorized as follows: (a) unaffiliated, (b) Catholic affiliation, and (c) other affiliations (e.g., Protestant, fundamentalist Protestant, other Christian, Jewish, and Muslim). Both “having no religious affiliation” or a “Catholic affiliation” have been associated in adults with increased risk for alcoholism and heavier drinking (Heath et al., 1997; Michalak et al., 2007) and with lower levels of religious devotion (e.g., services attendance; Kendler et al., 1997) relative to other religious affiliations. For subjects who identified an affiliation, more than one third of those in three affiliations (i.e., Muslim 85%, fundamentalist Protestant 52%, and Christian, other 46%) reported strict religious rules against drinking alcohol compared with 21% (Protestant) to 0% (Jewish) for other affiliations. The three affiliations were, surprisingly, not different than the other groups in the “other affiliations” category on number of maximum drinks consumed (M [SD] = 14.76 [12.59] vs. 14.33 [14.02], p > .05) and frequency of alcohol dependence symptoms endorsed (1.70 [2.22] vs. 1.77 [2.23], p > .05). Based on these findings, we elected to maintain a combined “other affiliations” category.
Data analysis
Haploview (Barrett et al., 2005) was used to compute the degree of correlation between marker pairs as measured by r2. Other analyses were conducted in SAS Version 9.3 (SAS Institute Inc., Cary, NC). Chi-square statistics tested the gene–environment associations between the ADH variants and religious involvement. Linear regression was performed and accounted for family clustering in the data. Dependent variables (i.e., our phenotypes) were modeled as continuous. Models were tested in two steps for each marker and religious involvement as follows: (a) variant and environment main effects and (b) main effects plus the Gene × Environment interaction term. Interactions were tested for a significant departure from additivity. All models controlled for age, gender, genetic ancestry using principal components, and religious affiliation. Primary analyses used a combined race/ethnicity sample; however, models were run separately in each racial/ethnic group because of population differences on ADH variant genotype frequencies. In additional analyses, genotype frequencies, religious involvement, and alcohol-related phenotypes were examined by racial/ethnic group using the chi-square statistic and analysis of variance with Tukey pairwise comparisons. Statistical significance was assessed at p < .05. Plots of raw data were constructed using IBM SPSS Statistics for Windows, Version 21 (IBM Corp., Armonk, NY) to assist in the interpretation of Gene × Environment interactions.
Results
Sample characteristics are presented in Table 2. Subjects were on average 38 years of age. Slightly more than half (53%) of the sample was female. Most self-reported European American or African American race/ethnicity, with fewer reporting Hispanic American race/ethnicity. In the past 12 months, most subjects attended religious services infrequently or never; however, approximately 40% of the sample attended at least once a month or more. Fifty-two percent of subjects reported a Catholic affiliation or no religious affiliation. On average, subjects reported consuming a maximum number of 15.6 drinks in a 24-hour period. They endorsed an average of 1.8 lifetime alcohol dependence symptoms; approximately 25% met lifetime criteria for DSM-IV alcohol dependence. No significant associations were found between services attendance and the ADH variants (p > .147; individual results not shown).
Table 2.
Sample characteristics (N = 7,716)
| Variable | M (SD) or % |
| Age, in years | 37.94 (14.59) |
| Male, % | 46.55 |
| Race/ethnicity, % | |
| African American | 18.87 |
| European American | 73.38 |
| Hispanic American | 7.75 |
| Services attendance, % | |
| Never | 21.82 |
| <Monthly | 36.07 |
| Monthly | 23.57 |
| Weekly or more | 18.55 |
| Religious affiliation, %a | |
| None | 17.86 |
| Catholic | 34.34 |
| Protestant | 21.67 |
| Fundamentalist Protestant | 10.23 |
| Other Christian | 13.30 |
| Other | 2.60 |
| Maximum drinks in 24 hours | 15.57 (13.04) |
| Alcohol dependence symptoms | 1.81 (2.21) |
| Alcohol dependence diagnosis | 25.49 |
For regression models, religious affiliation was recoded into two dummy variables including no affiliation (0, 1) and Catholic affiliation (0, 1).
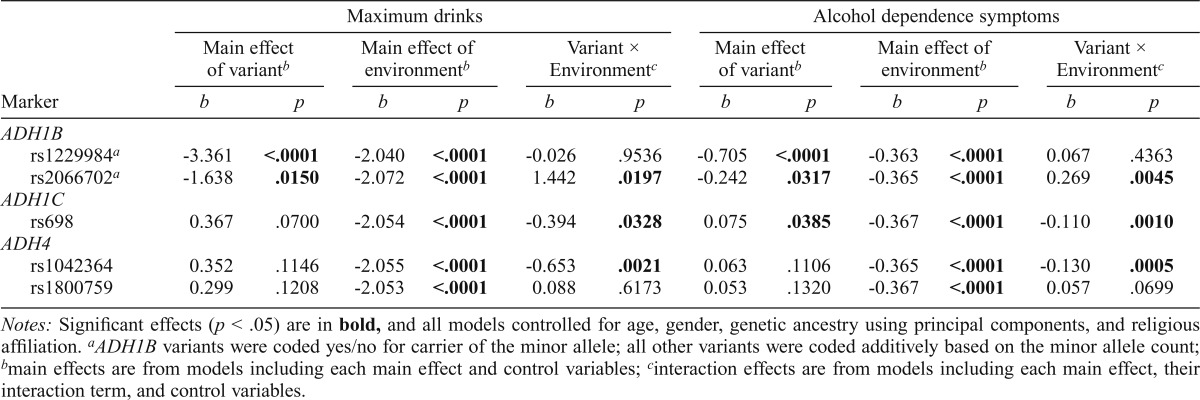
The regression results are provided in Table 3. In main effect models, ADH1B-rs1229984 (0 G/G vs. 1 A/G or A/A) was negatively associated with both the maximum drinks consumed and the number of alcohol dependence symptoms endorsed at p < .0001 (3rd and 9th columns). ADH1B-rs2066702 (0 C/C vs. 1 C/T or T/T) was also negatively associated with maximum drinks (p = .0150) and with alcohol dependence symptoms (p = .0317). The only positive significant association found was between ADH1C-rs698 (0 A/A, 1 A/G, and 2 G/G) and alcohol dependence symptoms (p = .0385). The main effects for ADH4-rs1042364 (0 G/G, 1 G/A, and 2 A/A) and ADH4-rs1800759 (0 C/C, 1 C/A, and 2 A/A) were nonsignificant. Conversely, higher religious involvement was consistently associated (p < .0001) with a lower number of maximum drinks and alcohol dependence symptoms (5th and 11th columns).
Table 3.
ADH gene variants, religious involvement, and associations with maximum drinks and dependence symptoms
| Marker | Maximum drinks |
Alcohol dependence symptoms |
||||||||||
| Main effect of variantb |
Main effect of environmentb |
Variant × Environmentc |
Main effect of variantb |
Main effect of environmentb |
Variant × Environment |
|||||||
| b | p | b | p | b | p | b | p | b | p | b | p | |
| ADH1B | ||||||||||||
| rs1229984a | -3.361 | <.0001 | -2.040 | <.0001 | -0.026 | .9536 | -0.705 | <.0001 | -0.363 | <.0001 | 0.067 | .4363 |
| rs2066702a | -1.638 | .0150 | -2.072 | <.0001 | 1.442 | .0197 | -0.242 | .0317 | -0.365 | <.0001 | 0.269 | .0045 |
| ADH1C | ||||||||||||
| rs698 | 0.367 | .0700 | -2.054 | <.0001 | -0.394 | .0328 | 0.075 | .0385 | -0.367 | <.0001 | -0.110 | .0010 |
| ADH4 | ||||||||||||
| rs1042364 | 0.352 | .1146 | -2.055 | <.0001 | -0.653 | .0021 | 0.063 | .1106 | -0.365 | <.0001 | -0.130 | .0005 |
| rs1800759 | 0.299 | .1208 | -2.053 | <.0001 | 0.088 | .6173 | 0.053 | .1320 | -0.367 | <.0001 | 0.057 | .0699 |
Notes: Significant effects (p < .05) are in bold, and all models controlled for age, gender, genetic ancestry using principal components, and religious affiliation.
ADH1B variants were coded yes/no for carrier of the minor allele; all other variants were coded additively based on the minor allele count;
main effects are from models including each main effect and control variables;
interaction effects are from models including each main effect, their interaction term, and control variables.
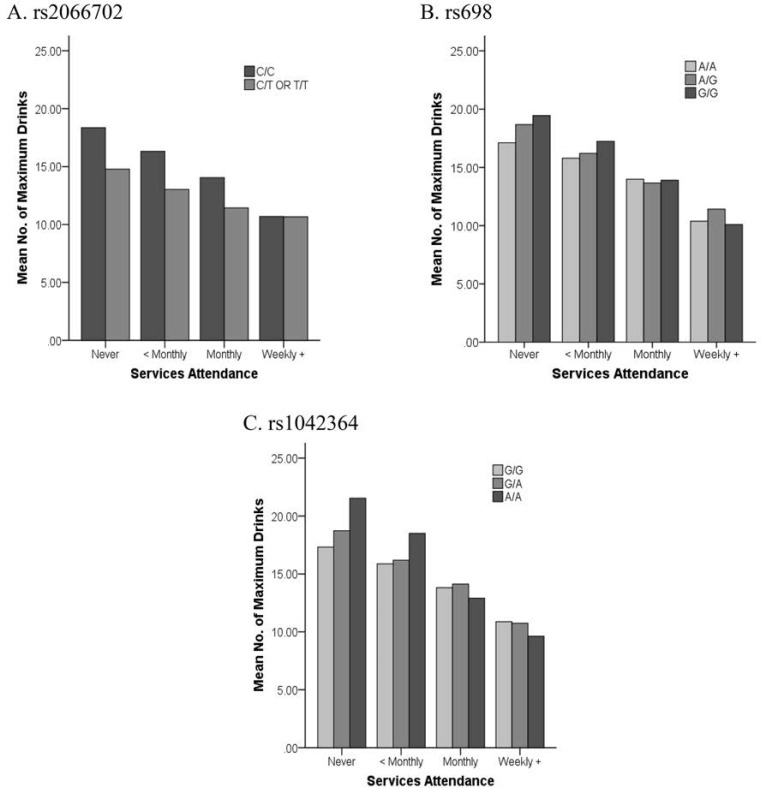
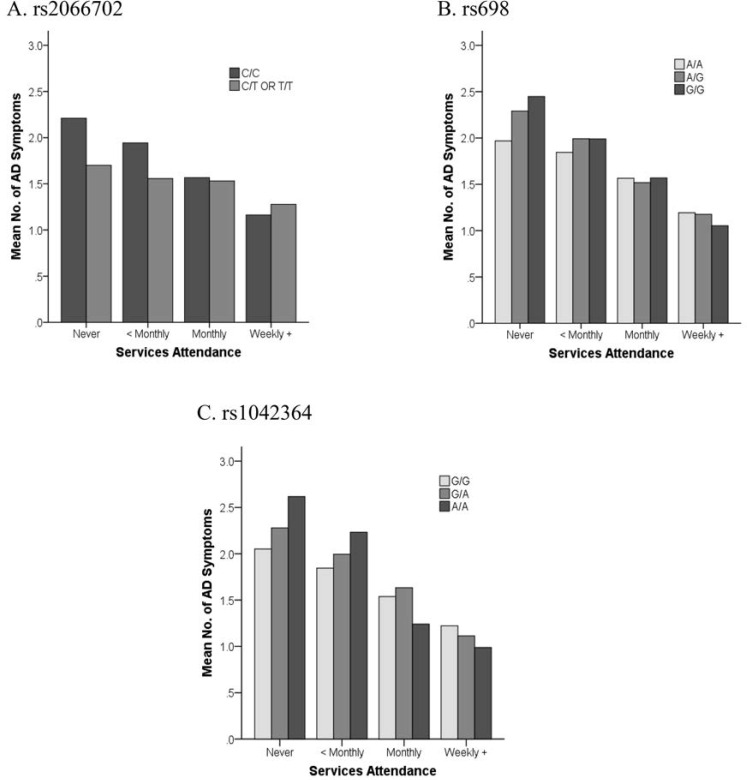
The interaction models (Table 3) showed significant joint effects with religious involvement for three of the five ADH markers (7th and 13th columns). The interaction of religious involvement with rs1042364 was significantly associated (p ≤ .0021) with both maximum drinks and alcohol dependence symptoms. The interaction effects with rs2066702 and rs698 were also associated both with maximum drinks (p < .05) and with alcohol dependence symptoms (p < .005) (Figures 2 and 3). All three (rs2066702, rs698, and rs1042364) risk variants showed weaker associations with maximum drinks and alcohol dependence symptoms in the context of increasing religious involvement. Risk variants were associated with more maximum drinks and alcohol dependence symptoms than protective variants under low or no religious involvement. However, the associations for risk and protective variants with these phenotypes were no longer different under higher levels of religious involvement. The interaction effects for rs1800759 and rs1229984 with religious involvement were nonsignificant.
Figure 2.
Plots of religious involvement with maximum drinks. Interaction effects showed higher maximum drinks consumed for ADH1B-rs2066702, ADH1C-rs698, and ADH4-rs1042364 risk versus protective variants in the context of lower or no religious services attendance. Risk variants are identified by darker shaded bars. No. = number.
Figure 3.
Plots of religious involvement with alcohol dependence symptoms. Interaction effects showed more alcohol dependence symptoms for ADH1B-rs2066702, ADH1C-rs698, and ADH4-rs1042364 risk versus protective variants at lower or no religious services attendance levels. Risk variants are identified by darker shaded bars. No. = number; AD = alcohol dependence.
Additional analyses, by racial/ethnic groups, showed significant differences in genotype frequencies (p < .0001); these allele differences were primarily observed for European Americans and Hispanic Americans compared with African Americans. The ADH1B-rs1229984 A, ADH1C-rs698 G, and ADH4-rs1042364 A alleles were more common in European Americans (respectively, 6.71%, 63.46%, and 48.91%) and Hispanic Americans (8.08%, 51.70%, and 33.45%) than African Americans (2.83%, 29.05%, and 23.88%). Conversely, the ADH1B-rs2006702 T and ADH1B-rs1800759 A alleles were more common in African Americans (respectively, 28.98% and 92.63%) than in European Americans (0.37% and 63.7%) and Hispanic Americans (8.91% and 78.91%). For these groups, Hispanic Americans reported the lowest level of religious involvement. Hispanic Americans had higher rates of attending services never (25.05%) or less than monthly (42.13%) and lower rates of attending monthly (17.08%) or weekly or more (15.75%), compared with African Americans (respectively, for these levels: 22.36%, 37.47%, 21.72%, and 18.94%) and European Americans (21.33%, 35.06%, 24.86%, and 18.75%) (p < .001). Pairwise comparisons (p < .05) showed that European Americans (M [SD] = 16.02 [12.91]) reported more maximum drinks than African Americans (14.18 [13.63]) but not Hispanic Americans (14.73 [13.04]). European Americans (M [SD] = 1.86 [2.22]) also endorsed more alcohol dependence symptoms, this time compared with Hispanic Americans (1.58 [2.11]) but not African Americans (1.72 [2.20]). The rate of DSM-IV alcohol dependence, 23.62%–26.11%, was not different by population (p = .122).
Regression models stratified by racial/ethnic population showed trends that were consistent with those reported for the combined sample, although not always statistically significant. These results are presented here in a brief format. The exceptions included markers with low minor allele frequencies in a racial/ethnic group or the switched direction of an effect that was highly nonsignificant. For example, the associations between rs698 and maximum drinks and alcohol dependence symptoms in Hispanic Americans were negative and highly nonsignificant, and rs2066702 was not associated with either phenotype in Hispanic Americans. Hispanic Americans were the smallest subsample and their results were less stable. In all groups, rs1229984 was negatively associated with maximum drinks and/or alcohol dependence symptoms (p < .05). In African Americans and European Americans, but not in Hispanic Americans, negative main effects for religious involvement with maximum drinks and alcohol dependence symptoms were significant (p < .0001). Few interaction effects were statistically significant except in European Americans, which was the largest group. Consistent with the combined sample, the interaction effect between religious involvement and rs1042364 was significantly associated with maximum drinks and alcohol dependence symptoms (p < .05). Conversely, for alcohol dependence symptoms, the interaction with rs1229984 was positive and statistically significant in Hispanic Americans only; it was not significant in the combined sample. The interaction with rs2066702 in European Americans, a marker with a low minor allele frequency in this population, had a switched direction compared with the combined sample.
Discussion
This study provides initial evidence in adults of a modifying effect for religious involvement on relationships between ADH gene variants and the number of maximum drinks consumed as well as number of lifetime DSM-IV alcohol dependence symptoms endorsed. Under low or no religious involvement, ADH1B-rs2066702, ADH1C-rs698, and ADH4-rs1042364 risk variants were associated with higher maximum drinks and more dependence symptoms relative to protective variants. Conversely, risk and protective variants for these markers had similar associations with the phenotypes at higher religious involvement levels. The modifying effect of religious involvement was observed for three of the ADH gene markers examined (i.e., no significant interaction effects for ADH1B-rs1229984 and ADH4-rs1800759). Of the many possible reasons for this, none are entirely clear.
The presence or absence of Gene × Environment interactions may be partially related to differences in the strength of the associations of the ADH variants with alcohol dependence. The current evidence is inconsistent for the association of rs1800759 with alcohol-related phenotypes (Edenberg et al., 2006; Guindalini et al., 2005; Luo et al., 2005; Preuss et al., 2011), suggesting a weaker association for this variant. In our study, rs1229984 showed evidence of a main-effect-only relationship with maximum drinks and alcohol dependence symptoms. This may point to the stronger effect for rs1229984 in association with alcohol dependence, as previously reported by Bierut et al. (2012) in individuals of both European and African American ancestry. However, two studies by Meyers et al. (2015) and Sartor et al. (2014) found a significant interaction for rs1229984 and childhood adversity in predicting alcohol dependence symptoms. It may be that childhood adversity provides a stronger environmental exposure compared with religious involvement.
The functionality and location of the five markers do not appear to entirely explain why some variant-phenotype relationships were modified by religious involvement and others were not. Rs1229984, rs2066702, and rs698 are functional markers and encode different enzyme forms that metabolize ethanol at higher or lower rates (see Hurley & Edenberg, 2012). Rs1800759 is located in a 5' promoter region and is associated with the expression of ADH4 (Edenberg et al., 1999). Rs1042364, which had highly significant interaction effects with both phenotypes, is located in a 3' untranslated mRNA encoding region (Edenberg et al., 2006). It is possible that the effect of ADH4-rs1042364 was modified by religious involvement (through restricted access to alcohol and social constraints) because of the contribution of the ADH4 gene to alcohol metabolism at higher levels of consumption (Hurley & Edenberg, 2012).
Weekly or more frequent religious involvement was associated with a moderate level of protection against a higher number of maximum drinks and alcohol dependence symptoms for both ADH risk and protective variant carriers. Our results suggest a gene-by-environment relationship that is characterized by negligible phenotype differences between genotypes in a more protective environment, but increased differences under less benign conditions (i.e., plots display a fan-shaped interaction; Dick & Kendler, 2012). Likewise, Beaver et al. (2009) showed this modifying effect for religiosity in association with the DRD2 dopamine D2 receptor gene. High religiosity was protective for delinquent behaviors among both the DRD2 A-1 and A-2 allele carriers, whereas under low religiosity, A-l carriers reported higher delinquent behaviors. Luczak et al. (2003) showed that, in Asians, greater religious services attendance was protective against heavy drinking in nonflushing allele carriers but not for carriers of the flushing allele (ALDH2*2). It is likely that the flushing response was already a strong deterrent against heavy drinking.
Religious involvement may function as a protective environment against more drinking and more alcohol dependence symptoms through several mechanisms, including more restrictive social drinking norms, by providing a positive, low/no drinking social network (Ford, 2006; Ford & Kadushin, 2002), and/or by improving behavioral self-control (DeWall et al., 2014). Under Shanahan and Hofer’s (2005) social control model, religion is one source of societal constraint on individual choices and behaviors that was reported to modify genetic susceptibility. Twin studies indicate social control mechanisms for religion with other related phenotypes. For example, Koopmans et al. (1999) and Boomsma et al. (1999) showed that youths with a religious upbringing had reduced genetic influences on alcohol use initiation and disinhibition, respectively. Kendler et al. (1999) offered another putative mechanism for religion in a twin study of depression, indicating that religious involvement can be protective for depression against stressful life events. Religious involvement may, alternatively, serve as a positive environmental condition that enables individuals to effectively cope with stress, buffering the influence of risk alleles (i.e., an example of the gene–environment compensation model in Shanahan & Hofer, 2005).
To our knowledge, this is the first time that religious involvement has been examined in combination with ADH gene variants and their joint associations with alcohol-related phenotypes in adults. The current study’s large, combined sample size provides adequate power for the analysis and may reduce potential false discoveries (Duncan & Keller, 2011). In addition, using principal components to identify genetic ancestry in a regression analysis performed well in accounting for the population structure in a sample that includes subjects from different ancestry populations (Bouaziz et al., 2011). Larger racial/ethnic subsamples are needed to facilitate a more detailed analysis of religiosity and ADH gene variants in each group. The population differences in this sample for genotype frequencies, religious involvement, and alcohol phenotypes may suggest different gene–environment relationships by racial/ethnic group. The significant interaction effect between rs1042364 and religious involvement, observed in European Americans, was consistent with results from the combined sample. It is possible that this interaction is population specific, but also that it was not observed in the African American and Hispanic American samples because of small sample sizes. Similarly, the interaction for rs2066702 was observed in the combined sample but not in the subsample of African Americans, who have both a higher minor allele count at this locus (Hurley & Edenberg, 2012) and higher rates of religious participation (Brown et al., 2015; Chatters et al., 2009; Robinson et al., 2012). It is possible that this interaction was missed because of low statistical power.
The COGA sample is family based and includes several subjects from densely affected families with many affected biological family members. This may limit the generalizability of our findings to other community samples or to the general population. The public health implications are very tentative. Borders et al.’s (2010) prospective study of religion and drinking suggested the possible benefit of promoting services attendance and prayer for at-risk drinkers. Our study might extend this suggestion to ADH risk allele carriers. However, prospective studies are needed to help clarify the relationships between religious involvement, ADH gene variants, and drinking and alcohol dependence symptoms because the current study is cross-sectional and religious involvement was measured at a single time point. Religious services attendance in the past 12 months before interview may not be representative of lifetime attendance, although there is some evidence that services attendance is relatively stable over time (Kendler et al., 1997; Koenig & Vaillant, 2009). Some studies identify reductions in religious attendance from adolescence to young adulthood (Button et al., 2011), which may be less relevant to this adult sample. It is also possible that the time order between religious involvement and alcohol problems is reversed; some subjects may increase or decrease their religious involvement as a result of having problems with alcohol. Kendler et al.’s (1997) study suggested that these alternative relationships are less likely, reporting a stronger relationship between earlier church attendance and later alcohol use rather than between earlier alcohol use and later attendance.
A strength of this study was our use of robust genetic, environmental, and phenotypic variables. The mechanisms that link ADH genes to alcohol consumption and dependence are relatively well understood (Edenberg et al., 1999; Macgregor et al., 2009; McCarthy et al., 2010). Although specific markers may differ by population (e.g., Gelernter et al., 2014), there is no evidence that the biological mechanisms associated with ADH variants are different. Previous literature supports the importance of religious involvement for examining drinking behavior. We studied one aspect of religiosity (i.e., services attendance) as a limitation of our data set, but this is a complex construct, and other aspects of religion may offer additional information about this relationship. Kendler et al. (1997), for example, showed that the dimensions of religiosity are differentially associated with alcohol use; religious devotion (including services attendance) was associated with the ability to quit or maintain low levels of use, whereas traditional religious beliefs were associated with decisions to ever drink. We were not able to examine the role of spirituality. Earlier studies indicate that greater religious involvement in combination with strong spiritual beliefs provides strong protection against drinking (Brechting et al., 2010; Hodge et al., 2007; Holt et al., 2015). In addition, we investigated two phenotypes (i.e., maximum drinks and alcohol dependence symptoms), and our findings were reasonably consistent across both traits. However, some significant interactions for predicting maximum drinks (p < .033) would not be robust following corrections for multiple testing.
In summary, our findings suggest a modifying role for religious involvement, in that the associations of ADH gene variants with alcohol consumption–related phenotypes varied by the level of religious involvement that individuals reported. This relationship can be characterized by negligible phenotype differences between risk and protective genotypes under high religious involvement but larger differences at lower and no religious involvement levels. We hope this initial study will serve to generate other investigations of religious involvement, ADH and other genetic variants, and alcohol consumption and dependence. In particular, future investigations could be extended to include prospective study designs of at-risk individuals, other aspects of religiosity, and larger and separate samples of different racial/ethnic populations.
Acknowledgments
The authors thank Nathaniel Thomas for his help with preparing the article for publication.
The Collaborative Study on the Genetics of Alcoholism (COGA), principal investigators B. Porjesz, V. Hesselbrock, H. Edenberg, and L. Bierut, comprises 11 different centers: University of Connecticut (V. Hesselbrock); Indiana University (H. J. Edenberg, J. Nurnberger, Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); University of Texas Health Science Center at San Antonio (L. Almasy), Virginia Commonwealth University (D. Dick), Icahn School of Medicine at Mount Sinai (A. Goate), and Howard University (R. Taylor). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, J. McClintick, S. O’Connor, L. Wetherill, X. Xuei, Y. Liu (Indiana University); G. Chan (University of Iowa; University of Connecticut); D. Chorlian, N. Manz, C. Kamarajan, A. Pandey (SUNY Downstate); J-C Wang, M. Kapoor (Icahn School of Medicine at Mount Sinai); and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators.
We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding principal investigator and co-principal investigator of COGA. We also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, as well as P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The authors thank Kim Doheny and Elizabeth Pugh from CIDR and Justin Paschall from the NCBI dbGaP staff for valuable assistance with genotyping and quality control in developing the data set available at dbGaP. This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Center for Research Resources or NIH.
Footnotes
Research reported in this publication was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) award numbers K01AA021145 (to Karen G. Chartier, principal investigator) and K02AA018755 (to Danielle M. Dick, principal investigator). This national collaborative study is supported by National Institutes of Health (NIH) Grant U10AA008401 from the NIAAA and the National Institute on Drug Abuse (NIDA). The Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine is partially supported by National Cancer Institute Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant #UL1RR024992 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. Funding support for Genome-Wide Association Study genotyping, which was performed at The Johns Hopkins University Center for Inherited Disease Research, was provided by the NIAAA, the NIH GEI (U01HG004438), and the NIH contract “High throughput genotyping for studying the genetic contributions to human disease” (HHSN268200782096C). This article was originally presented as a poster at the World Congress of Psychiatric Genetics.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington, DC: Author; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Barrett J. C., Fry B., Maller J., Daly M. J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. doi:10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beaver K. M., Gibson C. L., Jennings W. G., Ward J. T. A gene X environment interaction between DRD2 and religiosity in the prediction of adolescent delinquent involvement in a sample of males. Biodemography and Social Biology. 2009;55:71–81. doi: 10.1080/19485560903054689. doi:10.1080/19485560903054689. [DOI] [PubMed] [Google Scholar]
- Begleiter H., Reich T., Hesselbrock V., Porjesz B., Li T.-K., Schuckit M. A., Rice J. P. The collaborative study on the genetics of alcoholism. Alcohol Health and Research World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Bierut L. J., Goate A. M., Breslau N., Johnson E. O., Bertelsen S., Fox L., Edenberg H. J. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Molecular Psychiatry. 2012;17:445–450. doi: 10.1038/mp.2011.124. doi:10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D. I., de Geus E. J., van Baal G. C., Koopmans J. R. A religious upbringing reduces the influence of genetic factors on disinhibition: Evidence for interaction between genotype and environment on personality. Twin Research. 1999;2:115–125. doi: 10.1375/136905299320565988. doi:10.1375/136905299320565988. [DOI] [PubMed] [Google Scholar]
- Borders T. F., Curran G. M., Mattox R., Booth B. M. Religiousness among at-risk drinkers: Is it prospectively associated with the development or maintenance of an alcohol-use disorder? Journal of Studies on Alcohol and Drugs. 2010;71:136–142. doi: 10.15288/jsad.2010.71.136. doi:10.15288/jsad.2010.71.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz M., Ambroise C., Guedj M. Accounting for population stratification in practice: A comparison of the main strategies dedicated to genome-wide association studies. PLoS ONE. 2011;6(12):e28845. doi: 10.1371/journal.pone.0028845. doi:10.1371/journal.pone.0028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechting E. H., Brown T. L., Salsman J. M., Sauer S. E., Holeman V. T., Carlson C. R. The role of religious beliefs and behaviors in predicting underage alcohol use. Journal of Child & Adolescent Substance Abuse. 2010;19:324–334. doi:10.1080/1067828X.2010.502494. [Google Scholar]
- Brown R. K., Taylor R. J., Chatters L. M. Race/ethnic and social-demographic correlates of religious non-involvement in America: Findings from three national surveys. Journal of Black Studies. 2015;46:335–362. doi:10.1177/0021934715573168. [Google Scholar]
- Bucholz K. K., Cadoret R., Cloninger C. R., Dinwiddie S. H., Hesselbrock V. M., Nurnberger J. I., Jr., Schuckit M. A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. doi:10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Button T. M. M., Hewitt J. K., Rhee S. H., Corley R. P., Stallings M. C. The moderating effect of religiosity on the genetic variance of problem alcohol use. Alcoholism: Clinical and Experimental Research. 2010;34:1619–1624. doi: 10.1111/j.1530-0277.2010.01247.x. doi:10.1111/j.1530-0277.2010.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button T. M. M., Stallings M. C., Rhee S. H., Corley R. P., Hewitt J. K. The etiology of stability and change in religious values and religious attendance. Behavior Genetics. 2011;41:201–210. doi: 10.1007/s10519-010-9388-3. doi:10.1007/s10519-010-9388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatters L. M., Taylor R. J., Bullard K. M., Jackson J. S. Race and ethnic differences in religious involvement: African Americans, Caribbean blacks and non-Hispanic whites. Ethnic and Racial Studies. 2009;32:1143–1163. doi: 10.1080/01419870802334531. doi:10.1080/01419870802334531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall C. N., Pond R. S., Jr., Carter E. C., McCullough M. E., Lambert N. M., Fincham F. D., Nezlek J. B. Explaining the relationship between religiousness and substance use: Self-control matters. Journal of Personality and Social Psychology. 2014;107:339–351. doi: 10.1037/a0036853. doi:10.1037/a0036853. [DOI] [PubMed] [Google Scholar]
- Dick D. M., Kendler K. S. The impact of gene-environment interaction on alcohol use disorders. Alcohol Research: Current Reviews. 2012;34:318–324. doi: 10.35946/arcr.v34.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L. E., Keller M. C. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. doi:10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Jerome R. E., Li M. Polymorphism of the human alcohol dehydrogenase 4 (ADH4) promoter affects gene expression. Pharmacogenetics. 1999;9:25–30. doi: 10.1097/00008571-199902000-00004. doi:10.1097/00008571-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Xuei X., Chen H. J., Tian H., Wetherill L. F., Dick D. M., Foroud T. Association of alcohol dehydrogenase genes with alcohol dependence: A comprehensive analysis. Human Molecular Genetics. 2006;15:1539–1549. doi: 10.1093/hmg/ddl073. doi:10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- Edlund M. J., Harris K. M., Koenig H. G., Han X., Sullivan G., Mattox R., Tang L. Religiosity and decreased risk of substance use disorders: Is the effect mediated by social support or mental health status? Social Psychiatry and Psychiatric Epidemiology. 2010;45:827–836. doi: 10.1007/s00127-009-0124-3. doi:10.1007/s00127-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C. L., Liang T., Gizer I. R. ADH and ALDH polymorphisms and alcohol dependence in Mexican and Native Americans. American Journal of Drug and Alcohol Abuse. 2012;38:389–394. doi: 10.3109/00952990.2012.694526. doi:10.3109/00952990.2012.694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C. L., Montane-Jaime K., Moore S., Shafe S., Joseph R., Carr I. G. Association of the ADHIB*3 allele with alcohol-related phenotypes in Trinidad. Alcoholism: Clinical and Experimental Research. 2007;31:216–220. doi: 10.1111/j.1530-0277.2006.00298.x. doi:10.1111/j.1530–0277.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Feighner J. P., Robins E., Guze S. B., Woodruff R. A., Jr., Winokur A., Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. doi:10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Ford J. M. Some implications of denominational heterogeneity and church attendance for alcohol consumption among Hispanics. Journal for the Scientific Study of Religion. 2006;45:253–267. doi:10.1111/j.1468-5906.2006.00304.x. [Google Scholar]
- Ford J., Kadushin C. Between sacral belief and moral community: A multidimensional approach to the relationship between religion and alcohol among whites and blacks. Sociological Forum. 2002;17:255–279. doi:10.1023/A:1016089229972. [Google Scholar]
- Gelernter J., Kranzler H. R., Sherva R., Almasy L., Koesterer R., Smith A. H., Farrer L. A. Genome-wide association study of alcohol dependence: Significant findings in African- and European-Americans including novel risk loci. Molecular Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. doi:10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. D., Agrawal A., Bucholz K. K., Madden P. A. F., Pergadia M. L., Nelson E. C., Heath A. C. Alcohol consumption indices of genetic risk for alcohol dependence. Biological Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. doi:10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindalini C., Scivoletto S., Ferreira R. G., Breen G., Zilberman M., Peluso M. A., Zatz M. Association of genetic variants in alcohol dehydrogenase 4 with alcohol dependence in Brazilian patients. American Journal of Psychiatry. 2005;162:1005–1007. doi: 10.1176/appi.ajp.162.5.1005. doi:10.1176/appi.ajp.162.5.1005. [DOI] [PubMed] [Google Scholar]
- Hasin D., Aharonovich E., Liu X., Mamman Z., Matseoane K., Carr L., Li T. K. Alcohol and ADH2 in Israel: Ashkenazis, Sephardics, and recent Russian immigrants. American Journal of Psychiatry. 2002;159:1432–1434. doi: 10.1176/appi.ajp.159.8.1432. doi:10.1176/appi.ajp.159.8.1432. [DOI] [PubMed] [Google Scholar]
- Heath A. C., Bucholz K. K., Madden P. A. F., Dinwiddie S. H., Slutske W. S., Bierut L. J., Martin N. G. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. doi:10.1017/S0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M., Easton C., Bucholz K. K., Schuckit M., Hesselbrock V. A validity study of the SSAGA—a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. doi:10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Higuchi S., Matsushita S., Imazeki H., Kinoshita T., Takagi S., Kono H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. The Lancet. 1994;343:741–742. doi: 10.1016/s0140-6736(94)91629-2. doi:10.1016/S0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- Hodge D. R., Andereck K., Montoya H. The protective influence of spiritual-religious lifestyle profiles on tobacco use, alcohol use, and gambling. Social Work Research. 2007;31:211–219. doi:10.1093/swr/31.4.211. [Google Scholar]
- Holt C. L., Roth D. L., Huang J., Clark E. M. Gender differences in the roles of religion and locus of control on alcohol use and smoking among African Americans. Journal of Studies on Alcohol and Drugs. 2015;76:482–492. doi: 10.15288/jsad.2015.76.482. doi:10.15288/jsad.2015.76.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley T. D., Edenberg H. J. Genes encoding enzymes involved in ethanol metabolism. Alcohol Research: Current Reviews. 2012;34:339–344. doi: 10.35946/arcr.v34.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Gardner C. O., Prescott C. A. Religion, psychopathology, and substance use and abuse; a multimeasure, genetic-epidemiologic study. American Journal of Psychiatry. 1997;154:322–329. doi: 10.1176/ajp.154.3.322. doi:10.1176/ajp.154.3.322. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Gardner C. O., Prescott C. A. Clarifying the relationship between religiosity and psychiatric illness: The impact of covariates and the specificity of buffering effects. Twin Research. 1999;2:137–144. doi: 10.1375/136905299320566004. doi:10.1375/twin.2.2.137. [DOI] [PubMed] [Google Scholar]
- Koenig L. B., Vaillant G. E. A prospective study of church attendance and health over the lifespan. Health Psychology. 2009;28:117–124. doi: 10.1037/a0012984. doi:10.1037/a0012984. [DOI] [PubMed] [Google Scholar]
- Konishi T., Calvillo M., Leng A.-S., Feng J., Lee T., Lee H., Wan Y.-J. Y. The ADH3*2 and CYP2E1 c2 alleles increase the risk of alcoholism in Mexican American men. Experimental and Molecular Pathology. 2003;74:183–189. doi: 10.1016/s0014-4800(03)00006-6. doi:10.1016/S0014-4800(03)00006-6. [DOI] [PubMed] [Google Scholar]
- Konishi T., Luo H. R., Calvillo M., Mayo M. S., Lin K. M., Wan Y. J. ADH1B*1, ADH1C*2, DRD2 (-141C Ins), and 5-HTTLPR are associated with alcoholism in Mexican American men living in Los Angeles. Alcoholism: Clinical and Experimental Research. 2004;28:1145–1152. doi: 10.1097/01.alc.0000134231.48395.42. doi:10.1097/01.ALC.0000134231.48395.42. [DOI] [PubMed] [Google Scholar]
- Koopmans J. R., Slutske W. S., van Baal G. C., Boomsma D. I. The influence of religion on alcohol use initiation: Evidence for genotype X environment interaction. Behavior Genetics. 1999;29:445–453. doi: 10.1023/a:1021679005623. doi:10.1023/A:1021679005623. [DOI] [PubMed] [Google Scholar]
- Li D., Zhao H., Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biological Psychiatry. 2011;70:504–512. doi: 10.1016/j.biopsych.2011.02.024. doi:10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak S. E., Corbett K., Oh C., Carr L. G., Wall T. L. Religious influences on heavy episodic drinking in Chinese-American and Korean-American college students. Journal of Studies on Alcohol. 2003;64:467–471. doi: 10.15288/jsa.2003.64.467. doi:10.15288/jsa.2003.64.467. [DOI] [PubMed] [Google Scholar]
- Luo X., Kranzler H. R., Zuo L., Lappalainen J., Yang B. Z., Gelernter J. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: Results from HWD tests and case-control association studies. Neuropsychopharmacology. 2006;31:1085–1095. doi: 10.1038/sj.npp.1300925. doi:10.1038/sj.npp.1300925. [DOI] [PubMed] [Google Scholar]
- Luo X., Kranzler H. R., Zuo L., Yang B. Z., Lappalainen J., Gelernter J. ADH4 gene variation is associated with alcohol and drug dependence: Results from family controlled and population-structured association studies. Pharmacogenetics and Genomics. 2005;15:755–768. doi: 10.1097/01.fpc.0000180141.77036.dc. doi:10.1097/01.fpc.0000180141.77036.dc. [DOI] [PubMed] [Google Scholar]
- Macgregor S., Lind P. A., Bucholz K. K., Hansell N. K., Madden P. A. F., Richter M. M., Whitfield J. B. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: An integrated analysis. Human Molecular Genetics. 2009;18:580–593. doi: 10.1093/hmg/ddn372. doi:10.1093/hmg/ddn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M., Pedersen S. L., Lobos E. A., Todd R. D., Wall T. L. ADH1B*3 and response to alcohol in African-Americans. Alcoholism: Clinical and Experimental Research. 2010;34:1274–1281. doi: 10.1111/j.1530-0277.2010.01205.x. doi:10.1111/j.1530-0277.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. L., Shmulewitz D., Wall M. M., Keyes K. M., Aharonovich E., Spivak B., Hasin D. Childhood adversity moderates the effect of ADH1B on risk for alcohol-related phenotypes in Jewish Israeli drinkers. Addiction Biology. 2015;20:205–214. doi: 10.1111/adb.12102. doi:10.1111/adb.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak L., Trocki K., Bond J. Religion and alcohol in the U.S. National Alcohol Survey: How important is religion for abstention and drinking? Drug and Alcohol Dependence. 2007;87:268–280. doi: 10.1016/j.drugalcdep.2006.07.013. doi:10.1016/j.drugalcdep.2006.07.013. [DOI] [PubMed] [Google Scholar]
- O’Connell J. R., Weeks D. E. PedCheck: A program for identification of genotype incompatibilities in linkage analysis. American Journal of Human Genetics. 1998;63:259–266. doi: 10.1086/301904. doi:10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier M., Pakstis A. J., Kidd J. R., Lee J.-F., Yin S.-J., Ko H.-C., Kidd K. K. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. American Journal of Human Genetics. 1999;64:1147–1157. doi: 10.1086/302317. doi:10.1086/302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss U. W., Ridinger M., Rujescu D., Samochowiec J., Fehr C., Wurst F. M., Zill P. Association of ADH4 genetic variants with alcohol dependence risk and related phenotypes: Results from a larger multicenter association study. Addiction Biology. 2011;16:323–333. doi: 10.1111/j.1369-1600.2010.00236.x. doi:10.1111/j.1369-1600.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., Sham P. C. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. doi:10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Bolton J. M., Rasic D., Sareen J. Exploring the relationship between religious service attendance, mental disorders, and suicidality among different ethnic groups: Results from a nationally representative survey. Depression and Anxiety. 2012;29:983–990. doi: 10.1002/da.21978. doi:10.1002/da.21978. [DOI] [PubMed] [Google Scholar]
- Saccone N. L., Kwon J. M., Corbett J., Goate A., Rochberg N., Edenberg H. J., Rice J. P. A genome screen of maximum number of drinks as an alcoholism phenotype. American Journal of Medical Genetics. 2000;96:632–637. doi: 10.1002/1096-8628(20001009)96:5<632::aid-ajmg8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Sartor C. E., Wang Z., Xu K., Kranzler H. R., Gelernter J. The joint effects of ADH1B variants and childhood adversity on alcohol related phenotypes in African-American and European-American women and men. Alcoholism: Clinical and Experimental Research. 2014;38:2907–2914. doi: 10.1111/acer.12572. doi:10.1111/acer.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. J., Hofer S. M. Social context in gene–environment interactions: Retrospect and prospect. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences, 60, Special Issue. 2005;1:65–76. doi: 10.1093/geronb/60.special_issue_1.65. doi:10.1093/geronb/60.Special_Issue_1. [DOI] [PubMed] [Google Scholar]
- Turchi C., Piva F., Solito G., Principato G., Buscemi L., Tagliabracci A. ADH4 intronic variations are associated with alcohol dependence: Results from an Italian case-control association study. Pharmacogenetics and Genomics. 2012;22:79–94. doi: 10.1097/FPC.0b013e32834d05c8. doi:10.1097/FPC.0b013e32834d05c8. [DOI] [PubMed] [Google Scholar]
- Zheng X., Levine D., Shen J., Gogarten S. M., Laurie C., Weir B. S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28:3326–3328. doi: 10.1093/bioinformatics/bts606. doi:10.1093/bioinformatics/bts606. [DOI] [PMC free article] [PubMed] [Google Scholar]