Abstract
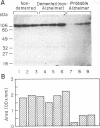
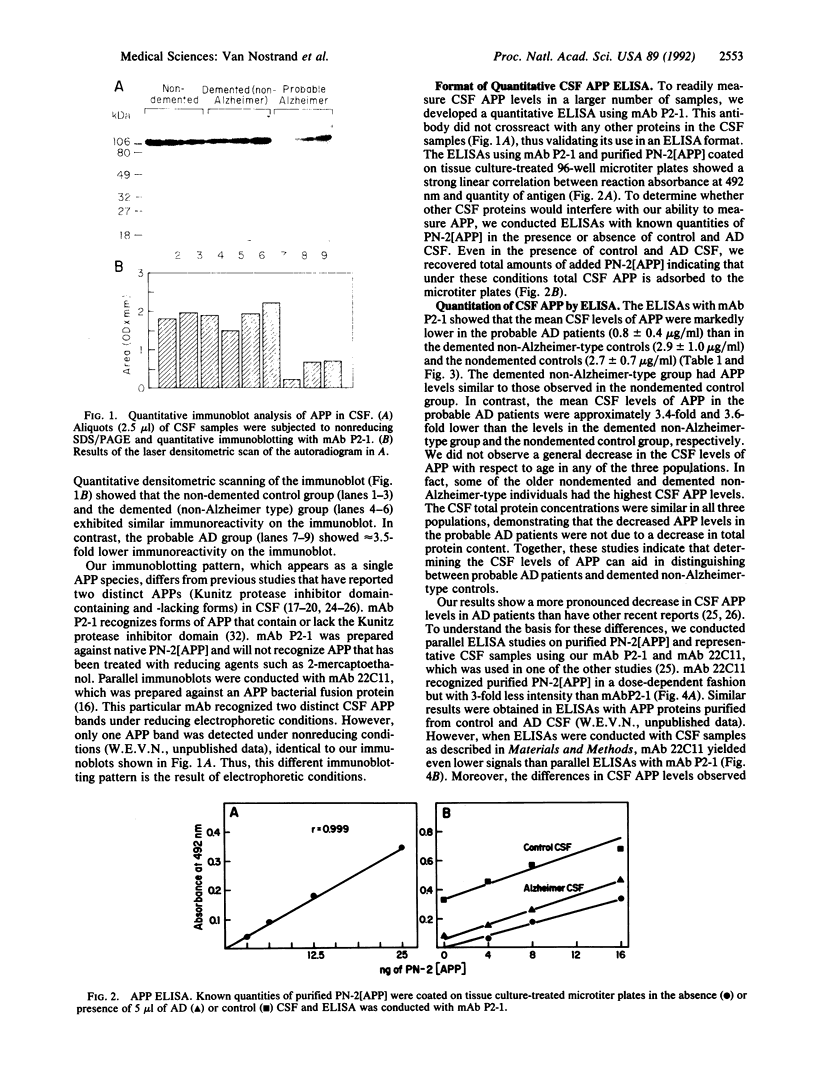
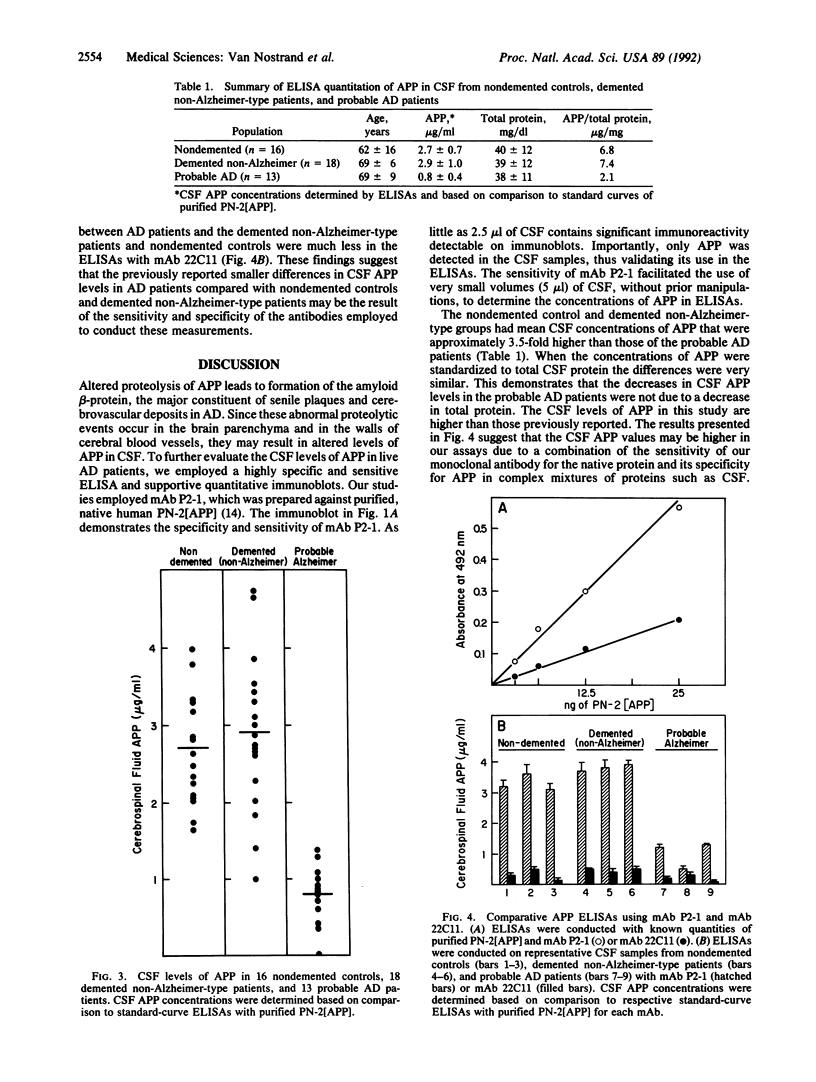
The amyloid beta-protein is deposited in senile plaques and the cerebrovasculature in Alzheimer disease (AD). Since it is derived from proteolytic processing of its parent protein, the amyloid beta-protein precursor (APP), we investigated whether levels of the secreted forms of APP are altered in cerebrospinal fluid (CSF) of AD patients. Quantitative immunoblotting studies with the anti-APP monoclonal antibody P2-1 revealed that probable AD patients had markedly lower CSF APP levels than did demented non-Alzheimer-type patients and healthy control subjects. Using antibody P2-1 in an enzyme-linked immunosorbent assay, we measured CSF levels of APP in a larger population consisting of 13 patients diagnosed with probable AD, 18 patients diagnosed with dementia (non-Alzheimer type), and 16 nondemented, healthy controls. Mean CSF levels of APP were approximately 3.5-fold lower in the live patients diagnosed with probable AD compared to the demented non-Alzheimer-type controls or the nondemented, healthy individuals. These findings suggest that abnormal metabolism of APP is reflected in the extracellular fluids of the central nervous system and that CSF levels of soluble APP provide a useful biochemical marker to assist in the clinical diagnosis of AD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Funkenstein H. H., Albert M. S., Scherr P. A., Cook N. R., Chown M. J., Hebert L. E., Hennekens C. H., Taylor J. O. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- Ghiso J., Jensson O., Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci U S A. 1986 May;83(9):2974–2978. doi: 10.1073/pnas.83.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiso J., Pons-Estel B., Frangione B. Hereditary cerebral amyloid angiopathy: the amyloid fibrils contain a protein which is a variant of cystatin C, an inhibitor of lysosomal cysteine proteases. Biochem Biophys Res Commun. 1986 Apr 29;136(2):548–554. doi: 10.1016/0006-291x(86)90475-4. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Tagliavini F., Timmers W. F., Frangione B. Alzheimer's disease amyloid precursor protein is present in senile plaques and cerebrospinal fluid: immunohistochemical and biochemical characterization. Biochem Biophys Res Commun. 1989 Aug 30;163(1):430–437. doi: 10.1016/0006-291x(89)92154-2. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Grubb A., Jensson O., Gudmundsson G., Arnason A., Löfberg H., Malm J. Abnormal metabolism of gamma-trace alkaline microprotein. The basic defect in hereditary cerebral hemorrhage with amyloidosis. N Engl J Med. 1984 Dec 13;311(24):1547–1549. doi: 10.1056/NEJM198412133112406. [DOI] [PubMed] [Google Scholar]
- Henriksson T., Barbour R. M., Braa S., Ward P., Fritz L. C., Johnson-Wood K., Chung H. D., Burke W., Reinikainen K. J., Riekkinen P. Analysis and quantitation of the beta-amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients with a monoclonal antibody-based immunoassay. J Neurochem. 1991 Mar;56(3):1037–1042. doi: 10.1111/j.1471-4159.1991.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kesslak J. P., Nalcioglu O., Cotman C. W. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991 Jan;41(1):51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Tokushima Y., Oishi K., Takahashi Y., Shiojiri S., Nakamura S., Tanaka S., Kodaira R., Ito H. Determination of amyloid beta protein precursors harboring active form of proteinase inhibitor domains in cerebrospinal fluid of Alzheimer's disease patients by trypsin-antibody sandwich ELISA. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1453–1459. doi: 10.1016/0006-291x(90)91030-v. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris J. C., Mohs R. C., Rogers H., Fillenbaum G., Heyman A. Consortium to establish a registry for Alzheimer's disease (CERAD) clinical and neuropsychological assessment of Alzheimer's disease. Psychopharmacol Bull. 1988;24(4):641–652. [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun. 1988 Oct 14;156(1):432–437. doi: 10.1016/s0006-291x(88)80859-3. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmert M. R., Usiak M., Mayeux R., Raskind M., Tourtellotte W. W., Younkin S. G. Soluble derivatives of the beta amyloid protein precursor in cerebrospinal fluid: alterations in normal aging and in Alzheimer's disease. Neurology. 1990 Jul;40(7):1028–1034. doi: 10.1212/wnl.40.7.1028. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Prior R., Mönning U., Schreiter-Gasser U., Weidemann A., Blennow K., Gottfries C. G., Masters C. L., Beyreuther K. Quantitative changes in the amyloid beta A4 precursor protein in Alzheimer cerebrospinal fluid. Neurosci Lett. 1991 Mar 11;124(1):69–73. doi: 10.1016/0304-3940(91)90824-d. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Van Nostrand W. E., Cunningham D. D. Purification of protease nexin II from human fibroblasts. J Biol Chem. 1987 Jun 25;262(18):8508–8514. [PubMed] [Google Scholar]
- Van Nostrand W. E., Farrow J. S., Wagner S. L., Bhasin R., Goldgaber D., Cotman C. W., Cunningham D. D. The predominant form of the amyloid beta-protein precursor in human brain is protease nexin 2. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10302–10306. doi: 10.1073/pnas.88.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]