Abstract Abstract
In this article, we present the rationale and design of the Sildenafil HF trial (ClinicalTrials.gov identifier: NCT02304705). We will randomize patients with heart failure and reactive pulmonary hypertension (pulmonary capillary wedge pressure > 15 mmHg, pulmonary vascular resistance > 3 Wood units) into two groups: the treatment group receiving sildenafil 20 mg 3 times a day and a matching placebo group. The duration of intervention will be 3 months. The primary outcome is 6-minute walk distance. Key features of this trial include (1) that reactive pulmonary hypertension is an inclusion criterion, (2) that patients will be enrolled regardless of left ventricular ejection fraction, and (3) that clinical stability in the 3 months preceding enrollment is not required.
Keywords: heart failure, pulmonary hypertension, sildenafil
Sildenafil is a selective inhibitor of type 5 phosphodiesterase that provides favorable effects on hemodynamics in pulmonary hypertension (PH). Currently approved for use in primary idiopathic PH, it is often utilized empirically in heart failure (HF), because this condition frequently results in PH. Such use is currently off-label. We designed a randomized, double-blind, placebo-controlled clinical trial to answer the clinical question regarding the justification of the use of sildenafil in PH secondary to HF.
Rationale. In 2007, Lewis et al.,1 from Massachusetts General Hospital, published a paper on sildenafil, a type 5 phosphodiesterase inhibitor, in HF. A single oral dose of 50 mg reduced resting and exercise pulmonary arterial pressure, systemic vascular resistance, and pulmonary vascular resistance (PVR) and increased resting and exercised cardiac index (P < 0.05 for all) without significantly altering mean arterial pressure, heart rate, or pulmonary capillary wedge pressure (PCWP). Peak V̇o2 increased (mean ± standard deviation: 15% ± 9%), and ventilatory response to CO2 output (VE/Vco2 slope [minute ventilation/CO2 production]) decreased (16% ± 5%) just 1 hour after sildenafil treatment. Improvements were confined to patients with secondary PH. The study group was only 13 patients but sufficed to demonstrate the statistical significance of the findings.
PH in HF is associated with poor outcomes. In a Mayo Clinic study,2 there was a strong, positive, graded association between pulmonary artery systolic pressure (PASP) and mortality. Increasing PASP was associated with an increased risk of death (estimated hazard ratio [HR]: 1.45 [95% confidence interval (CI): 1.13–1.85], HR: 2.07 [95% CI: 1.62–2.64] for the highest and lowest PASP tertiles, respectively). This association was independent of age, sex, comorbidities, ejection fraction (EF), and diastolic function.2
Elevated right-sided pressures in HF usually result from elevated left ventricular filling pressures. In HF, there are two major components of PH: hydrostatic and vasoreactive.3 The hydrostatic, or passive, component reflects the backward transmission of elevated left ventricular end-diastolic pressure. Therefore, pulmonary artery diastolic pressure correlates tightly with the PCWP. Normally, the pulmonary vasculature is characterized by low pressure, low resistance, and high distensibility. It can accommodate a significant increase in blood flow with a minimal elevation of pulmonary arterial pressure. When this compensatory capacity is exceeded, there is an elevation in pulmonary arterial pressure. The effective treatment for such PH is diuretic therapy or other means of fluid removal.
The vasoreactive component of PH develops with long-standing PH. It is characterized by vasospasm, vasoconstriction, and eventually morphologic changes of the pulmonary vasculature. The degree of PH is considered “out of proportion” to the PCWP. In other words, PH persists independently of the PCWP. PVR and the transpulmonary gradient (TPG) are elevated. Diuretics are, therefore, ineffective for treatment of reactive PH, and other medical treatment options are limited. In HF, reactive PH is associated with worse outcomes. In one study involving HF patients, the presence of reactive PH carried a higher risk of death (HR: 1.55 [95% CI: 1.11–2.20], P < 0.001).4
Prior clinical studies on sildenafil in HF. No widely accepted treatment option currently exists for reactive PH in HF. However, multiple studies suggest that phosphodiesterase 5 inhibitors may be an effective therapy in this setting.5-8 In particular, when patients with biventricular HF, poor right ventricular function, and increased TPG received sildenafil 20 mg 3 times a day, in addition to other HF medications, they experienced a significant decrease in pulmonary pressures, improvement in right ventricular function, and decrease in NT-proBNP (N-terminal of the prohormone brain natriuretic peptide) serum levels, as well as a substantial improvement of the 6-minute walk distance, by 91 ± 54 m.9 Importantly, there was a very modest reduction in PCWP, indicating that the reactive, and not the passive, component of PH is the principal therapeutic target for sildenafil.
Clinical studies of sildenafil in HF are summarized in Table 1. The best results were obtained when the patients were selected on the basis of presence of reactive PH, namely, increased PVR and TPG.
Table 1.
Clinical studies of sildenafil in heart failure, grouped by outcomes
| Study | Study type | Patients | N | PH | Intervention | Duration | Outcome |
|---|---|---|---|---|---|---|---|
| Positive | |||||||
| Lewis et al. 200710 | NYHA II/III/IV, EF < 40% | 34 | mPAP ≥ 25 | 25–75 mg TID | 3 months | Improved peak V̇o2 and 6-minute walk distance; reduced PVR; increased cardiac output with exercise | |
| Zakliczynski et al. 200711 | Case series | Transplant candidates | 6 | TPG > 12 and/or PVR > 2.5 WU, no reversibility with sodium nitroprusside | 50 mg BID | 1 month | Normal TPG and PVR (3 patients); acceptable responsiveness of PH to nitroprusside (2); no difference (1) |
| Guazzi et al. 200712 | NYHA II/III, EF < 45% | 46 | None | 50 mg TID | 6 months | Improved peak V̇o2, endothelial function, symptoms | |
| Jabbour et al. 200713 | Transplant candidates | 6 | None | 25–50 mg TID | 1–4 months | Decrease in TPG, PVR, and PCWP; increase in cardiac output | |
| Dumitrescu et al. 20119 | Cases series | Biventricular failure, NYHA III/IV, EF < 40%, TAPSE < 17 mm | 9 | mPAP ≥ 25, PCWP ≥ 15, TPG > 12 | 20 mg TID | 5 months | Increased 6-minute walk distance; improved echo, hemodynamics, BNP |
| Guazzi et al. 201114 | Double-blind, randomized, placebo-controlled | HF with preserved EF | 44 | PASP > 40 by echo | 50 mg TID | 12 months | Improvement in QOL, symptoms, hemodynamics, echo |
| Pons et al. 201215 | Retrospective | Transplant candidates | 15 | mPAP > 25, PVR > 2.5 WU, and/or TPG > 12 | 20–60 mg TID | 5–6 months | mPAP, PVR, and TPG decreased; all successfully transplanted |
| Reichenbach et al. 201216 | Nonrandomized, retrospective case-control | Transplant candidates | 32 | TPG > 15 | 20–60 mg TID | ∼1 year | Improved hemodynamics, clinical status, 60% transplanted |
| De Santo et al. 201217 | Prospective, nonrandomized, open-label, uncontrolled pilot trial | Transplant candidates | 31 | PVR > 6 WU, unresponsive to vasodilators | 25 mg TID, uptitrated every 2 weeks to 75 mg TID | 3 months | All successfully transplanted |
| Guazzi et al. 201118 | Prospective, randomized, placebo-controlled | NYHA II/III, EF < 40%, E/e′ > 10 | 45 | None | 50 TID | 12 months | Improved LV diastolic function and cardiac geometry, functional capacity, and clinical status |
| Guazzi et al. 201219 | Randomized, placebo-controlled | HF patients, EF < 45% | 32 | mPAP 25–35 mmHg | 50 TID | 12 months | Improved peak V̇o2, hemodynamics |
| Behling et al. 200820 | Double-blind, randomized, placebo-controlled | Outpatients with HF, EF < 40% | 19 | None | 50 TID | 4 weeks | Improved peak V̇o2; decreased PASP |
| Negative | |||||||
| Redfield et al. 201321 | Double-blind, randomized, placebo-controlled | HF with preserved EF ≥ 50% | 216 (113 sildenafil) | None | 20 mg TID for 12 weeks, then 60 mg TID for 12 weeks. | 6 months | No improvement in exercise capacity or clinical status |
| Amin et al. 201322 | Double-blind, randomized, placebo-controlled | NYHA II/III, EF < 35% | 106 | None | 25–50 mg TID | 12 weeks | No improvement in exercise capacity or clinical status |
All values for pressure, including TPG, are reported in mmHg. BID: twice a day; BNP: brain natriuretic protein; echo: echocardiography; E/e′: ratio of early transmitral flow velocity to annular velocity; EF: ejection fraction; LV: left ventricular; mPAP: mean pulmonary artery pressure; NYHA: New York Heart Association functional class; PASP: pulmonary artery systolic pressure; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; QOL: quality of life; TAPSE: tricuspid annular plane systolic excursion; TID: 3 times a day; TPG: transpulmonary gradient; WU: Wood units.
No randomized, controlled clinical trials testing sildenafil in HF selected patients on the basis of these criteria. None of the trials, therefore, utilized the full potential of phosphodiesterase 5 inhibitors in HF. Specifically, the recent multicenter, double-blind, randomized, controlled trial, RELAX, included patients with unknown pulmonary pressures. Understandably, the trial failed to demonstrate favorable effects of sildenafil.21
The ongoing SilHF trial uses very similar end points and has larger sample size than our proposed design. In our trial, we are targeting patient population with reactive PH, which is defined as PVR > 3 Wood units (WU). In the SilHF trial, PH is defined only as systolic pulmonary arterial pressure > 40 mmHg by echocardiography.23
Many physicians treating patients with advanced HF use sildenafil empirically. Phosphodiestherase 5 inhibitors for PH in HF are currently not approved by the US Food and Drug Administration (FDA) and are used as an off-label indication in patients with HF. A randomized, controlled trial including patients with the appropriate selection criteria is therefore more likely to demonstrate the benefits of sildenafil treatment in this common and important condition. Although it is true that, by itself, such a trial will not sway the FDA to change the indications for sildenafil, this may be the first step toward such change.
One specific concern about the use of sildenafil in PH secondary to HF is that this agent can cause elevation of PCWP. Unlike nitric oxide, which indeed can increase wedge pressure, sildenafil tends to decrease it.24,25
From published experience with patients who had hemodynamic characteristics similar to the inclusion criteria in the current trial, these concerns are not well justified (Table 2). Lewis et al.1,10 reported no significant change in PCWP, either at rest or with exercise, with acute or chronic sildenafil exposure. Moreover, in their study, fewer patients in the sildenafil arm required rehospitalization for HF during the trial than patients in the placebo arm. Other studies appear to corroborate these findings regarding the effect of sildenafil on PCWP.16
Table 2.
Effect of sildenafil on pulmonary capillary wedge pressure (PCWP)
| Study | PCWP at baseline, mmHg | PCWP after sildenafil, mmHg | P value | Duration |
|---|---|---|---|---|
| Lewis et al. 20071 | 14.4 ± 12.2 | 12.0 ± 2.0 | NS | 60 minutes |
| Lewis et al. 200710 | 18.0 ± 2.0 | 18.0 ± 2.0 | NS | 12 weeks |
| Dumitrescu et al. 20129 | 27.9 ± 2.3 | 20.6 ± 7.6 | 0.04 | 5 months |
| Lepore et al. 200525 | 25.0 ± 2.0 | 22.0 ± 2.0 | NS | 1 hour |
| Guazzi et al. 200426 | 18.5 ± 3.0 | 17.3 ± 2.7 | NS | 60 minutes |
| Pons et al. 201215 | 26.5 ± 12.4 | 23.3 ± 4.2 | NS | 5–6 months |
NS: not significant.
Methods
Hypothesis, objectives, and eligibility
We will enroll patients according to the following criteria.
Inclusion criteria:
-
•
≥18 years of age
-
•
known chronic HF appropriately treated with angiotensin-converting enzyme antagonists, angiotensin receptor blockers, and beta blockers, unless contraindicated, not indicated, or poorly tolerated
-
•
duration of HF on medications for at least 90 days before randomization
-
•
symptoms of HF (New York Heart Association functional classes II–IV)
-
•
indications for right heart catheterization, other than for biopsy
-
•
pulmonary artery mean pressure > 25 mmHg, PCWP > 15 mmHg, and PVR > 3 WU, as measured by right heart catheterization within 3 days of planned randomization
-
•
Patients with positive response to vasoreactivity testing, if done for clinical indications, may be included.
Exclusion criteria:
-
•
anticipated cardiac resynchronization therapy within 3 months of enrollment
-
•
hypersensitivity, allergy, or intolerable side effects of sildenafil
-
•
contraindication to sildenafil, including current nitrate therapy
-
•history of primary PH, connective-tissue disorders, severe chronic obstructive pulmonary disease (COPD), pulmonary embolism, or left-to-right shunts
-
○primary (idiopathic) PH in patients without history of HF
-
○PH in patients with systemic scleroderma
-
○PH due to congenital heart disease
-
○PH due to severe COPD or interstitial lung disease
-
○
-
•
history of heart transplant, ventricular-assist device, or any other solid-organ transplant
-
•
likely to have solid-organ transplant or any other major surgery during study enrollment/treatment period
-
•
liver cirrhosis as primary diagnosis
-
•
history of massive pulmonary embolism
-
•
acute pulmonary embolism
-
•
female subject who is pregnant, breast-feeding, or unwilling to practice an acceptable method of birth control unless postmenopausal or sterile
-
•
unreliability as a study participant, based on the investigator’s (or designee’s) knowledge of the subject (e.g., alcohol or other substance abuse, inability or unwillingness to adhere to the protocol, documented noncompliance, or vulnerable population)
-
•
current enrollment, or enrollment completed <30 days previously, in another investigational drug or device clinical study
-
•
current diagnosis of HIV or AIDS
-
•
undergoing dialysis for end-stage renal disease
-
•
end-stage liver disease comorbidities, limiting exercise tolerance
-
○
morbid obesity (body mass index > 40)
-
○
COPD with oxygen dependence
-
○
severe peripheral vascular disease with intermittent claudication
-
○
status after amputation of lower extremity(s) at any level
-
○
severe degenerative joint disease preventing normal walking
-
○
cerebrovascular accident with long-term sequelae affecting ability to walk.
-
○
Objectives. The primary objective of this study is to determine whether sildenafil for treatment of HF with reactive PH can improve exercise tolerance, measured by 6-minute walk distance. The secondary objectives of this study are to determine whether sildenafil for treatment of HF with reactive PH can improve hemodynamics, right ventricular function, or quality of life.
Design
We hypothesize that phosphodiesterase 5 inhibitors improve exercise capacity, hemodynamic and echocardiographic parameters, and quality of life in patients who have HF with secondary reactive PH. Patients over 18 years of age with HF who have PH by right heart catheterization (mean pulmonary artery pressure ≥ 25 mmHg, PCWP ≥ 15 mmHg, and PVR > 3 WU) will be eligible for randomization to sildenafil 20 mg or placebo 3 times a day for 3 months. At baseline, a 6-minute walk test and quality-of-life questionnaire will be performed (Minnesota Living with Heart Failure Score). If echocardiography was performed for clinical indications within 1 month of enrollment, those data will be used; otherwise, a new echocardiogram will be obtained. Laboratory tests are routinely performed before right heart catheterization (comprehensive metabolic panel and NT-proBNP), and results will be recorded for the study.
Participants will return for follow-up visits as needed for clinical indications. Our study coordinator will document changes of symptoms and changes in therapies and will capture clinical events such as unscheduled hospital visits. For study purposes, the only follow-up visit will take place in 3 months. At that time, an echocardiogram, right heart catheterization, 6-minute walk test, laboratory tests, and quality-of-life questionnaire will be repeated. In addition, we will evaluate for signs and symptoms of HF (dyspnea, increased cough, paroxysmal nocturnal dyspnea, peripheral edema, S3 gallop, and increased heart rate) at baseline and at the follow-up visit.
Measurements and calculations by two-dimensional echocardiography will include left ventricular end-systolic and end-diastolic dimensions, left ventricular EF (LVEF) by Simpson’s method, left atrial volume, severity of mitral and tricuspid regurgitation, velocity of tricuspid regurgitation, diameter of inferior vena cava and its variability with respirations, and right ventricular function by tricuspid annular plane systolic excursion. Measurements by right heart catheterization will include right atrial pressure; right ventricular systolic and end-diastolic pressure; pulmonary arterial systolic, diastolic, and mean pressure; and PCWP. Cardiac output and cardiac index will be calculated by both Fick and thermodilution methods.
The majority of study information will be collected from routine care per standard clinical indications. The trial design is shown on Figure 1.
Figure 1.
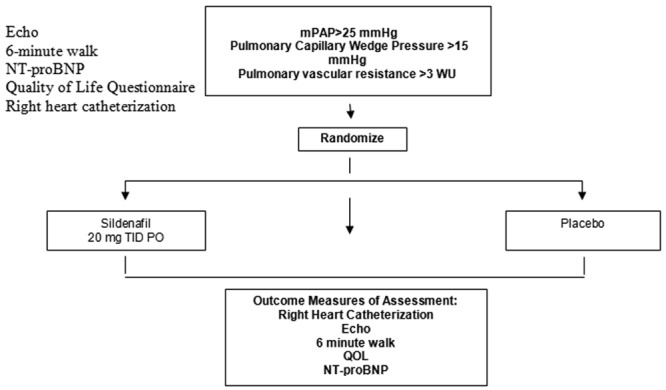
Trial design. NT-proBNP: N-terminal of the prohormone brain natriuretic peptide; mPAP: mean pulmonary arterial pressure; WU: Wood units; TID: three times/day; PO: orally (per os); QOL: quality of life.
Results
Outcomes
Our primary outcome will be an increase in 6-minute walk distance from the baseline. Our secondary outcomes are percent change in pulmonary arterial pressures (systolic, diastolic, and mean) and PVR from the baseline, percent change in cardiac index from the baseline, and percent change in tricuspid annular plane systolic excursion from the baseline.
Statistical analysis and sample size
Patients will be randomized to treatment versus placebo in a 1∶1 ratio using permuted blocks of variable size, to which the principal investigator (and the patients themselves) will be blinded. Planned sample size is 64 patients (32 per arm), for which a rationale is provided below. Data will be analyzed on an intent-to-treat basis, using methodology described below. An interim analysis will also take place after data have been acquired for approximately 32 subjects, using a temporary α of 0.01 to mitigate inflation of Type I error probability due to multiple testing. Results of the interim data analysis will be shared with a Data Safety Monitoring Board, which will advise the principal investigator on whether to continue with the study.
The planned sample size of 64 patients will provide nearly 80% power, using the final α of 0.05, to detect a difference between groups on a numerical outcome variable for which the effect size is approximately 0.7, meaning that a typical person in the treatment group would be approximately 0.7 standard deviations better than a typical person in the control group. We anticipate being able to reduce variability by considering changes from baseline to 3 months rather than just the final results at 3 months.
Each numerical outcome variable will be examined for approximate normality, and appropriate corrective action (e.g., log transformation before further data analysis) will be considered if approximate normality is untenable. A linear mixed model will be fitted, relating each outcome variable to group (between-subjects factor: treatment vs. placebo) and time (within-subjects factor: baseline vs. 3 months), with random effects for subjects and embedded linear contrasts to compare groups on change from baseline to 3 months. Although randomization is anticipated to balance groups on demographic and clinical characteristics, in the unlikely event that groups are imbalanced we may modify the linear mixed modeling to adjust for any covariates on which groups are imbalanced. Loss to follow-up is anticipated to be small, but in the unlikely event of more than 10% attrition from either group we may employ multiple imputation or another method for handling missing data. Groups will be compared on adverse events (e.g., rehospitalization) via Fisher’s exact test.
Discussion
The following features of this trial set it aside from prior studies of sildenafil in HF:
-
1
We will enroll patients with reactive PH regardless of LVEF. This has never been done before. As can be seen from Table 1, all studies specified the subset of eligible patients as having either reduced or preserved LVEF. Because the mechanism of PH in HF, as described above, is not dependent on LVEF and is in fact similar for patients with decreased EF and those with normal EF, we will include everyone eligible who meets the definition of reactive PH secondary to HF (elevated wedge pressure and elevated PVR).
-
2
Unlike prior studies,19,21,22 we will not require that patients be stable while receiving medical therapy for a set amount of time before enrollment in the study. We are trying to find additional treatment options for unstable patients with HF. Our potential study subjects are unstable by definition: they have congestion (elevated wedge pressure) and reactive PH. Because the study is randomized, differences in management, admissions, procedures, and medication adjustments will be mitigated by randomization.
Finally, we note that the two studies21,22 that showed no significant improvement with sildenafil neither measured nor estimated pulmonary arterial pressure in study subjects at enrollment. Likely, their patients represent a mix of those with and without PH. A lack of benefit from sildenafil in those studies is therefore not surprising. By including only those patients with reactive PH secondary to HF in our study, we anticipate that the benefit of sildenafil treatment in this patient population may be realized.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Lewis GD, Lachmann J, Camuso, J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007;115(1):59–66. [DOI] [PubMed]
- 2.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol 2012;59(3):222–231. [DOI] [PMC free article] [PubMed]
- 3.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail 2010;16(6):461–474. [DOI] [PubMed]
- 4.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013;1(4):290–299. [DOI] [PubMed]
- 5.Guazzi M. Sildenafil and phosphodiesterase-5 inhibitors for heart failure. Curr Heart Fail Rep 2008;5(2):110–114. [DOI] [PubMed]
- 6.Hirata K, Adji A, Vlachopoulos C, O’Rourke MF. Effect of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol 2005;96(10):1436–1440. [DOI] [PubMed]
- 7.Guazzi M, Casali M, Berti F, Rossoni G, Colonna VD, Guazzi MD. Endothelium-mediated modulation of ergoreflex and improvement in exercise ventilation by acute sildenafil in heart failure patients. Clin Pharmacol Ther 2008;83(2):336–341. [DOI] [PubMed]
- 8.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits β-adrenergic–stimulated cardiac contractility in humans. Circulation 2005;112(17):2642–2649. [DOI] [PubMed]
- 9.Dumitrescu D, Seck C, Möhle L, Erdmann E, Rosenkranz S. Therapeutic potential of sildenafil in patients with heart failure and reactive pulmonary hypertension. Int J Cardiol 2012;154(2):205–206. [DOI] [PubMed]
- 10.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007;116(14):1555–1562. [DOI] [PubMed]
- 11.Zakliczynski M, Maruszewski M, Pyka L, Trybunia D, Nadziakiewicz P, Przybylski R, Zembala M. Effectiveness and safety of treatment with sildenafil for secondary pulmonary hypertension in heart transplant candidates. Transplant Proc 2007;39(9):2856–2858. [DOI] [PubMed]
- 12.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol 2007;50(22):2136–2144. [DOI] [PubMed]
- 13.Jabbour A, Keogh A, Hayward C, Macdonald P. Chronic sildenafil lowers transpulmonary gradient and improves cardiac output allowing successful heart transplantation. Eur J Heart Fail 2007;9(6–7):674–677. [DOI] [PubMed]
- 14.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011;124(2):164–174. [DOI] [PubMed]
- 15.Pons J, Leblanc MH, Bernier M, Cantin B, Bourgault C, Bergeron S, Proulx G, et al. Effects of chronic sildenafil use on pulmonary hemodynamics and clinical outcomes in heart transplantation. J Heart Lung Transplant 2012;31(12):1281–1287. [DOI] [PubMed]
- 16.Reichenbach A, Al-Hiti H, Malek I, Pirk J, Goncalvesova E, Kautzner J, Melenovsky V. The effects of phosphodiesterase 5 inhibition on hemodynamics, functional status and survival in advanced heart failure and pulmonary hypertension: a case-control study. Int J Cardiol 2013;168(1):60–65. [DOI] [PubMed]
- 17.De Santo LS, Romano G, Maiello C, Buonocore M, Cefarelli M, Galdieri N, Nappi G, Amarelli C. Pulmonary artery hypertension in heart transplant recipients: how much is too much? Eur J Cardiothorac Surg 2012;42(5):864–869; discussion 869–870. [DOI] [PubMed]
- 18.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 2011;4(1):8–17. [DOI] [PubMed]
- 19.Guazzi M, Vicenzi M, Arena R. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: a long-term cardiopulmonary exercise testing placebo-controlled study. Eur J Heart Fail 2012;14(1):82–90. [DOI] [PubMed]
- 20.Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5′-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. J Card Fail 2008;14(3):189–197. [DOI] [PubMed]
- 21.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309(12):1268–1277. [DOI] [PMC free article] [PubMed]
- 22.Amin A, Mahmoudi E, Navid H, Chitsazan M. Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congest Heart Fail 2013;19(2):99–103. [DOI] [PubMed]
- 23.Cooper TJ, Guazzi M, Al-Mohammad A, Amir O, Bengal T, Cleland JG, Dickstein K. Sildenafil in Heart Failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail 2013;15(1):119–122. [DOI] [PubMed]
- 24.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002;105(20):2398–2403. [DOI] [PubMed]
- 25.Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, Semigran MJ. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest 2005;127(5):1647–1653. [DOI] [PubMed]
- 26.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol 2004;44(12):2339–2348. [DOI] [PubMed]


