Abstract Abstract
Endothelin receptor antagonists (ERAs) have been shown to improve the prognosis of patients with pulmonary arterial hypertension (PAH). However, the effect of the oral dual ERA bosentan on peripheral endothelial dysfunction (PED), as assessed by flow-mediated vasodilation (FMD), in patients with pulmonary hypertension is not well characterized. We investigated the effect of bosentan on PED in patients with PAH or inoperable chronic thromboembolic pulmonary hypertension (CTEPH). A total of 18 patients with PAH and 8 with CTEPH were treated with bosentan. All patients underwent FMD assessment before and after 3 months of bosentan treatment. Whereas FMD increased from 6.01% ± 2.42% at baseline to 8.07% ± 3.18% after 3 months (P < 0.0001) in patients with PAH, those with CTEPH showed no change in FMD after bosentan therapy. In addition, FMD at baseline showed no correlation with pulmonary vascular resistance (r = 0.09) or plasma brain natriuretic peptide levels (r = −0.23) in patients with PAH. Bosentan treatment ameliorated PED in patients with PAH but not in those with inoperable CTEPH. In addition, FMD did not correlate with PAH severity.
Keywords: endothelin receptor antagonists, bosentan, flow-mediated vasodilation, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension
Both pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are life-threatening diseases that are associated with poor prognoses.1,2 Endothelial dysfunction plays an important role in the pulmonary vascular remodeling of pulmonary hypertension (PH).3 In recent years, endothelin receptor antagonists (ERAs), such as bosentan, have been shown to improve exercise capacity in patients with PAH, and they are now widely used in these patients.4,5
For patients with CTEPH, pulmonary endarterectomy is the current treatment of choice, but not all patients with CTEPH are candidates for this treatment. For patients with inoperable CTEPH, pharmacologic therapy should be considered.6,7 Several uncontrolled clinical studies suggest that oral bosentan may provide hemodynamic and clinical benefits in patients with CTEPH.8,9 The efficacy of bosentan in inoperable CTEPH is unknown.
Patients with PAH exhibit impaired endothelium-dependent vasoreactivity in conduit vessels, which appears to correlate with their decreased pulmonary vascular response to vasodilators.10 Flow-mediated vasodilation (FMD) is a simple, noninvasive measure of the capacity of the endothelium11 to respond to a sudden increase in shear stress with smooth muscle relaxation and vasodilation. Low-dose bosentan (125 mg/day) has been shown to mitigate FMD-diagnosed peripheral endothelial dysfunction (PED) without affecting hemodynamic parameters or endothelial activation-related processes in scleroderma-associated vascular injury.12
Although bosentan is effective in the treatment of PAH, few data are available regarding the efficacy of bosentan treatment for FMD-diagnosed PED in patients with PAH or inoperable CTEPH. Here, we examined the effects of the oral dual ERA bosentan on endothelium-dependent vasodilation of the brachial arteries in patients with PAH or inoperable CTEPH.
Methods
Study design and population
The study was conducted prospectively at Nagoya University Hospital from October 2012 to May 2014. Patients 16–80 years old who had a clinical diagnosis of PAH or inoperable CTEPH according to the Dana Point criteria13 and who belonged to World Health Organization functional classes (WHO-FC) II–IV were eligible for enrollment. All patients with CTEPH were diagnosed by experienced surgeons as having inoperable disease because of the location or poor surgical accessibility of thrombi, patient age, or the presence of comorbidities. Exclusion criteria for all patients were (1) assignment to group 2, 3, or 5 of the Dana Point classification of PH, (2) pregnancy, (3) serum creatinine > 2.0 mg/dL, (4) history of serious chronic obstructive pulmonary disease or restrictive lung disease (vital capacity < 70%), (5) inability to walk without assistance, (6) PAH-targeted therapy, such as other ERAs, phosphodiesterase 5 inhibitors, or intravenous epoprostenol, and (7) other conditions that the physicians-in-charge judged to contraindicate enrollment because of concerns regarding patient safety.
According to the stated inclusion and exclusion criteria, we enrolled 18 patients with PAH and 8 patients with inoperable CTEPH (WHO-FC II–IV). All patients were evaluated at study entry according to institutional guidelines, including ventilation-perfusion scanning, helical computed tomography of the chest, and pulmonary angiography.
The study protocol was approved by the Ethics Review Board of Nagoya University School of Medicine (no. 1157). All participants provided their written informed consent after a physician-in-charge explained the study objectives, study protocol, possible adverse effects of the study drugs, measures for privacy protection, and procedures for study withdrawal.
Study procedures
Enrolled patients received oral bosentan at a loading dose of 62.5 mg twice daily for 4 weeks, followed by the target dose (125 mg twice daily) thereafter. Liver function tests were performed at 4-week intervals, and elevations in transaminase levels were handled according to the guidelines in the package insert for bosentan. Patients in WHO-FC IV with hemodynamic instability were treated immediately with intravenous epoprostenol when needed. At each clinic visit, patients were informed regarding all available treatment options, and all drug regimens were adjusted individually as necessary to limit side effects.
The extracted data included the diagnosis, age, sex, body mass index, underlying disease, WHO-FC, and laboratory data. Physiologic studies included electrocardiography, FMD, echocardiography, respiratory function testing, and right heart catheterization at baseline and (except for respiratory function testing and right heart catheterization) at 3 months after entry. Hematologic parameters included plasma brain natriuretic peptide (BNP), uric acid, blood urea nitrogen, serum creatinine, sodium, potassium, and liver enzymes at baseline and every 4 weeks thereafter.
Measurement of brachial artery diameter
FMD was measured according to international standards.14 The blood flow velocity and diameter of the brachial artery were evaluated with an ultrasonography system comprising a 7.5-MHz linear array transducer, appropriate software, and a novel stereotactic probe-holding device (UNEX EF 18G; Unex, Nagoya, Japan).15,16 All studies were conducted between 1:00 PM and 3:00 PM. Subjects were in a fasting state for at least 6 hours. Before FMD assessment, subjects rested in the supine position in a quiet, air-conditioned room (22°–24°C) for 30 minutes, and then imaging was initiated. The right brachial artery was scanned in longitudinal sections from 1 to 10 cm above the elbow, the skin surface was marked, and the arm was kept in the same position during the study. A pneumatic cuff placed around the forearm was inflated for 5 minutes to at least 50 mmHg above systolic pressure. The diameter of the brachial artery was scanned and recorded at baseline before cuff inflation and continuously from the release point to 2 minutes after cuff deflation to obtain the maximal diameter during reactive hyperemia. Continuous recordings of B-mode images and A-mode waves of the brachial artery in the longitudinal plane were obtained. A segment with clear near (media-adventitia) and far (intima–inner lumen) interfaces was manually determined. These border interfaces were identified automatically by means of the A-mode waves, and the diastolic diameter of the brachial artery per beat was synchronized with the electrocardiographic R-wave and tracked automatically. The diameter of the artery was measured from one media-adventitia interface to the other at end-diastole, coincident with the R-wave on the continuously recorded electrocardiogram.17 FMD was calculated as the maximal percentage increase in arterial diameter during continuous measurement of arterial diameter during the first 4.5 minutes after cuff deflation. All data were analyzed in a randomized, blinded fashion.
Statistical analysis
All data are expressed as mean ± 1 SD. Normally distributed variables were compared between the PAH and CTEPH groups with the Student t test; nonnormally distributed variables were compared with the Mann-Whitney U test, with χ2 analysis for categorical variables. To evaluate the effect of bosentan, hemodynamic parameters at baseline and those after bosentan therapy were compared with paired t tests. Changes from baseline in FMD between the PAH and CTEPH groups were assessed with unpaired t tests. Correlations were evaluated with the Pearson correlation test. All statistical analyses were performed in the SPSS 17.0 software package (SPSS, Chicago). A P value of <0.05 was considered statistically significant.
Results
Overall, the age (mean ± 1 SD) of the patients (5 males, 21 females) was 55 ± 10 years, their mean pulmonary arterial pressure (mPAP) at baseline was 47 ± 12 mmHg, and their FMD was 6.02% ± 2.25%. The sex and mean age and body mass index at baseline of patients with PAH did not differ from those of patients with CTEPH (Table 1). In addition, there were no significant differences in uric acid, mPAP, pulmonary vascular resistance (PVR), mixed venous oxygen saturation, cardiac output, or cardiac index between the two groups. Plasma BNP levels at baseline were significantly higher (P = 0.02) in patients with PAH than in those with CTEPH.
Table 1.
Baseline characteristics
| All (n = 26) | PAH (n = 18) | CTEPH (n = 8) | P | |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 55 ± 7 | 55 ± 6 | 0.89 |
| Male, no. (%) | 5 (19) | 4 (22) | 1 (13) | 0.50 |
| BMI | 24.07 ± 6.71 | 24.19 ± 8.02 | 23.84 ± 3.04 | 0.87 |
| WHO-FC II/III/IV, no. | 3/17/6 | 2/12/4 | 1/5/2 | 0.97 |
| I/CHD/porto/CTD, no. | 8/2/2/6 | 8/2/2/6 | … | … |
| Laboratory | ||||
| BNP, median (range), pg/mL | 273 (85–310) | 344 (98–546) | 115 (26–134) | 0.02 |
| Uric acid, mg/dL | 6.8 ± 2.1 | 7.0 ± 2.2 | 6.2± 1.9 | 0.37 |
| Cardiac catheterization | ||||
| PAWP, mmHg | 9.9 ± 3.8 | 10.2 ± 4.0 | 9.1 ± 3.3 | 0.46 |
| Systolic PAP, mmHg | 77 ± 21 | 75 ± 23 | 80 ± 14 | 0.52 |
| Diastolic PAP, mmHg | 29 ± 10 | 30 ± 10 | 26 ± 8 | 0.33 |
| Mean PAP, mmHg | 47 ± 12 | 47 ± 14 | 47 ± 8 | 0.92 |
| PVR, Wood units | 10.15 ± 6.61 | 10.94 ± 7.68 | 8.37 ± 2.78 | 0.23 |
| RAP, mmHg | 7.12 ± 4.07 | 7.11 ± 4.51 | 7.13 ± 3.14 | 0.99 |
| Svo2, % | 62.7 ± 8.4 | 63.5 ± 9.0 | 60.9 ± 6.9 | 0.43 |
| Cardiac output, L/min | 4.46 ± 1.70 | 4.34 ± 1.92 | 4.69 ± 1.31 | 0.60 |
| Cardiac index, L/min/m2) | 2.76 ± 0.90 | 2.72 ± 0.96 | 2.86 ± 0.86 | 0.71 |
Unless otherwise noted, data are mean ± SD. BMI: body mass index; BNP: brain natriuretic peptide; CTEPH: chronic thromboembolic pulmonary hypertension; I/CHD/porto/CTD: idiopathic PAH/PAH with congenital heart disease/portopulmonary PAH/PAH with collagen tissue disease; PAH: pulmonary arterial hypertension; PAP: pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; Svo2: mixed venous oxygen saturation; WHO-FC: Word Health Organization functional class.
At 3 months after the initiation of bosentan therapy, 16 (89%) patients with PAH and 7 (88%) patients with CTEPH were receiving the maximal dose of bosentan (125 mg twice daily), and the remaining patients, 2 (11%) with PAH and 1 (12%) with CTEPH, were receiving bosentan at a dose of 62.5 mg twice daily. Bosentan was well tolerated by all patients throughout the follow-up period, and we did not observe cardiovascular events or drug-related liver dysfunction.
Parameters during FMD, plasma BNP level, and tricuspid regurgitation peak gradient (TRPG) at baseline and after bosentan therapy are shown in Table 2, and the correlation between these parameters and FMD at baseline is shown in Table 3. Briefly, baseline FMD showed no significant correlation with any of the parameters evaluated, including PVR and plasma BNP levels, in both the PAH and CTEPH groups. At baseline, FMD, heart rate, and TRPG did not differ significantly between the PAH and CTEPH groups, but systolic and diastolic blood pressures were significantly lower in the PAH group than in the CTEPH group. After bosentan treatment, FMD did not differ between the PAH and CTEPH groups, and systolic blood pressure remained significantly lower (P = 0.035) in patients with PAH. Systolic and diastolic blood pressures, heart rate, and TRPG at baseline did not differ from those after bosentan treatment in both the PAH and CTEPH groups. Plasma BNP level was significantly decreased after bosentan treatment in patients with PAH (P = 0.046) but not in those with CTEPH. FMD increased after bosentan treatment in patients with PAH (baseline: 6.01% ± 2.42%; after 3 months: 8.07% ± 3.18%; P < 0.0001) but not in those with CTEPH (baseline: 6.04% ±1.95%; after 3 months: 6.21% ± 3.15%; P = 0.91; Fig. 1).
Table 2.
Parameters during FMD measurements, BNP, and TRPG at baseline and after bosentan treatment
| PAH | CTEPH | P (PAH vs. CTEPH, unpaired t) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After bosentan | P (paired t) | Baseline | After bosentan | P (paired t) | Baseline | After bosentan | |
| FMD, % | 6.01 ± 2.42 | 8.07 ± 3.18 | <0.001 | 6.04 ± 1.95 | 6.21 ± 3.15 | 0.910 | 0.98 | 0.193 |
| Heart rate, bpm | 82.2 ± 14.8 | 81.3 ± 15.8 | 0.884 | 81.3 ± 15.8 | 72.8 ± 10.9 | 0.111 | 0.88 | 0.134 |
| Systolic BP, mmHg | 108.3 ± 19.4 | 109.4 ± 19.7 | 0.831 | 124.6 ± 12.2 | 131.1 ± 22.3 | 0.562 | 0.017 | 0.035 |
| Diastolic BP, mmHg | 72.2 ± 14.0 | 69.3 ± 9.6 | 0.634 | 82.6 ± 8.4 | 74.6 ± 7.4 | 0.057 | 0.031 | 0.138 |
| BNP, pg/mL | 344 ± 354 | 249 ± 315 | 0.046 | 115 ± 109 | 116 ± 141 | 0.976 | 0.021 | 0.150 |
| TRPG, mmHg | 77.7 ± 25.5 | 68.4 ± 23.5 | 0.123 | 74.9 ± 23.7 | 67.3 ± 26.8 | 0.332 | 0.794 | 0.922 |
Data are mean ± SD. BNP: brain natriuretic peptide; BP: blood pressure; CTEPH: chronic thromboembolic pulmonary hypertension; FMD: flow-mediated vasodilation; PAH: pulmonary arterial hypertension; TRPG: tricuspid regurgitation peak gradient.
Table 3.
Correlation between various parameters and flow-mediated vasodilation (FMD)
| PAH | CTEPH | |||
|---|---|---|---|---|
| r | P | r | P | |
| Age, years | −0.447 | 0.063 | −0.266 | 0.524 |
| Systolic BP, mmHg | −0.311 | 0.209 | 0.297 | 0.476 |
| Heart rate, bpm | −0.062 | 0.807 | 0.272 | 0.514 |
| BMI | 0.380 | 0.120 | −0.444 | 0.270 |
| Mean PAP, mmHg | 0.072 | 0.785 | 0.036 | 0.932 |
| Cardiac index | 0.309 | 0.213 | 0.180 | 0.669 |
| Plasma BNP, pg/mL | −0.225 | 0.370 | −0.329 | 0.426 |
| Svo2, % | 0.068 | 0.801 | −0.359 | 0.382 |
| PVR, Wood units | −0.040 | 0.875 | −0.188 | 0.655 |
BMI: body mass index; BNP: brain natriuretic peptide; BP: blood pressure; CTEPH: chronic thromboembolic pulmonary hypertension; PAH: pulmonary arterial hypertension; PAP: pulmonary arterial pressure; PVR: pulmonary vascular resistance; Svo2, mixed venous oxygen saturation
Figure 1.
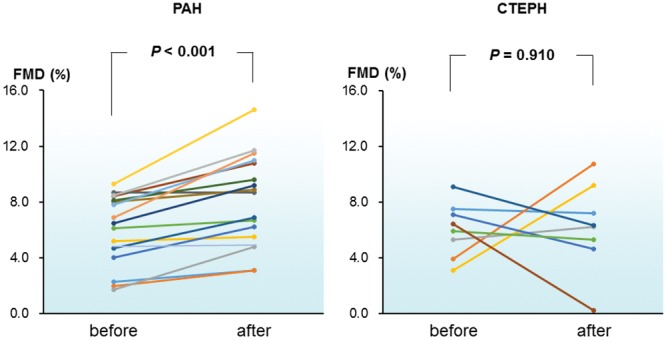
Flow-mediated vasodilation (FMD) in individual patients with pulmonary arterial hypertension (PAH; left) or inoperable chronic thromboembolic pulmonary hypertension (CTEPH; right) before (baseline) and after 3 months of treatment with bosentan.
We compared the bosentan-associated improvement in FMD in the subgroups of patients who had PAH with collagen tissue disease (CTD), PAH associated with other comorbidities, or idiopathic PAH (Fig. 2). The improvement in FMD was similar between PAH with and without CTD. FMD significantly increased after bosentan treatment in patients with idiopathic PAH or PAH associated with other comorbidities (n = 12; baseline [mean ± SEM]: 5.78% ± 0.20%; after 3 months: 7.68% ± 0.23%; P < 0.001) and in those in which PAH was associated with CTD (n = 6; baseline: 6.48% ± 0.43%; after 3 months: 8.83% ± 0.68%; P = 0.023).
Figure 2.
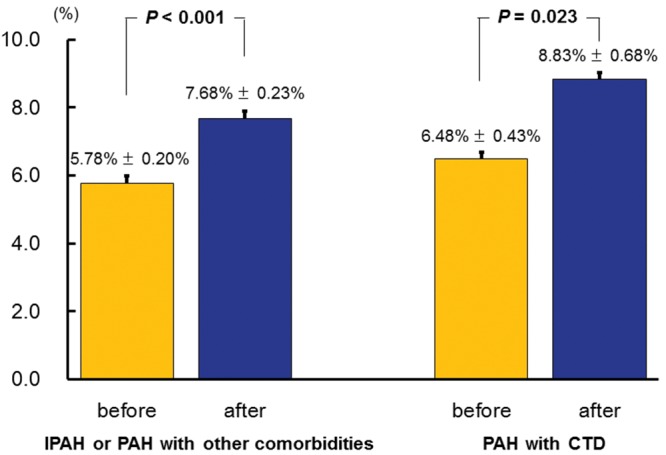
Flow-mediated vasodilation (FMD, mean + SEM) before (baseline; gold bars) and after (blue bars) 3 months of bosentan treatment in patients with idiopathic pulmonary arterial hypertension (IPAH) or PAH with other comorbidities (left) or patients who had PAH with collagen tissue disease (CTD; right).
Discussion
Twice-daily treatment with the oral dual ERA bosentan significantly increased FMD in our patients with PAH but not in those with inoperable CTEPH. In addition, FMD was not associated with the severity of PAH, in terms of PVR and plasma BNP. Taken together, these data show that bosentan improves peripheral endothelial function in patients with PAH but not in those with CTEPH.
Effect of bosentan on peripheral endothelial function in PAH compared with that in CTEPH
In this study, baseline hemodynamic parameters (determined through right heart catheterization) did not differ between the PAH and CTEPH groups. In addition, neither group of patients showed clinical progression of disease during the 3-month follow-up period. However, bosentan therapy increased FMD from baseline levels in patients with PAH but not in those with CTEPH. In addition, the improvement in FMD was similar between PAH patients with CTD and those without.
The precise mechanisms underlying the endothelial dysfunction in patients with PAH or CTEPH remain unknown. Increasing evidence indicates that oxidative stress plays an important role in the mechanisms of endothelial dysfunction in cardiovascular diseases.18 For example, patients with PH show altered production of various endothelial vasoactive mediators, including nitric oxide (NO), prostacyclin, endothelin 1, serotonin, and thromboxane.3
In accordance with our results, Wolff et al.10 described the iloprost-associated alleviation of impaired peripheral endothelial function in severe idiopathic PAH. In contrast to bosentan’s lack of effect, the favorable effects of sildenafil in CTEPH may involve the improvement of endothelial function.19 Our results suggest that the effect of bosentan on peripheral endothelial function might depend on the etiology underlying the PH, but additional investigation is needed to confirm this hypothesis.
FMD measures the NO-dependent vasodilatation of the brachial artery in response to shear stress due to reperfusion after temporary restriction of blood flow. The dependence on NO is depleted in both the pulmonary vascular bed20 and the systemic circulation21 in patients with idiopathic PAH. Surprisingly, FMD did not reflect the severity of the baseline hemodynamic state in either PAH or CTEPH in our study. Similarly, Wolff et al.10 found no association between endothelial dysfunction and hemodynamic measurements, but the peripheral endothelium–dependent vasoreactivity correlated with the pulmonary vascular response to inhaled iloprost in idiopathic PAH. In contrast, Peled et al.22 showed a weak correlation between mPAP and the peripheral arterial tone ratio by measuring the dilatation response in forearm blood flow after brachial arterial occlusion during noninvasive plethysmography. In our study, the plasma BNP level was decreased after bosentan treatment in patients with PAH but not in those with CTEPH. We were unable to determine whether the difference in the effects of bosentan between PAH and CTEPH was related to the underlying pathoetiology of the disease or the degree of hemodynamic improvement in individual cases. Further investigation is needed to resolve this issue. Our patients’ FMD values were discordant with the severity of PAH before the treatment. However, our observations suggest that the posttreatment increase in FMD was associated with hemodynamic improvement in patients with PAH, such that serial observation of FMD might be a useful marker of therapeutic effects.
Current treatment for PAH and inoperable CTEPH
The current treatment algorithms for PAH23,24 recommend an ERA or a phosphodiesterase type 5 inhibitor as the initial treatment for PAH of WHO-FC II or III. Bosentan became available in Japan in 2005 and since then has been used as a front-line therapy for Japanese patients with PAH. Despite this prolonged usage, the therapeutic effect of bosentan on PED as measured by FMD is poorly understood in both PAH and CTEPH.
On the other hand, riociguat has a dual mode of action, acting both in synergy with endogenous NO and by directly stimulating soluble guanylate cyclase independently of NO availability.25,26 Although riociguat improved exercise capacity and PVR in patients with PAH27 and CTEPH,28 the utility of other PAH-targeted drug therapies in inoperable CTEPH is currently unknown. In one study of PAH, PVR decreased over time with bosentan therapy but increased in the placebo group.29 The only randomized, placebo-controlled clinical trial of the safety and efficacy of targeted medical treatment with bosentan for CTEPH (the BENEFiT trial) revealed a significant drop in PVR but no change in the 6-minute walking distance, WHO-FC, or time to clinical worsening in patients with inoperable CTEPH who received bosentan for a 16-week period.30 In a meta-analysis and review, bosentan therapy was associated with improvements in the hemodynamics and exercise capacity of patients with CTEPH.7 The differences between bosentan and riociguat in CTEPH suggest that the NO-soluble guanylate cyclase–cyclic guanosine monophosphate pathway has a role in the pathologic features underlying CTEPH, including impairments in NO-mediated production of endogenous cyclic guanosine monophosphate and in progressive remodeling of the remaining perfused areas of the pulmonary vascular bed.31,32 Investigations into the efficacy of new PAH-targeted therapies, such as the soluble guanylate cyclase stimulator riociguat, for PED in CTEPH are needed. The effect of bosentan on PED in CTEPH had been unknown until this study, which showed that bosentan does not reduce PED in inoperable CTEPH. However, our study has several limitations, including a small patient population and a brief follow-up period. In addition, controlled data on mortality and time to clinical worsening in our patients with PAH and CTEPH are needed.
Conclusion
Bosentan therapy improved FMD in patients with PAH but not those with inoperable CTEPH. In addition, FMD was not correlated with PAH severity. Therefore, FMD is useful for assessing the effects of therapeutics on peripheral endothelial function in patients with PAH. Additional investigations are needed to confirm our results.
Acknowledgments
We express our sincere appreciation to all the patients, collaborating physicians, and other medical staff for their important contributions to this study.
Source of Support: Nil.
Conflict of Interest: AH and TK belong to a department endowed by Actelion Pharmaceuticals Japan. All other authors: none declared.
References
- 1.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 2010;35(5):1079–1087. [DOI] [PMC free article] [PubMed]
- 2.Jenkins D, Mayer E, Screaton N, Madani M. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev 2012;21(123):32–39. [DOI] [PMC free article] [PubMed]
- 3.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109(2):159–165. [DOI] [PubMed]
- 4.Rubin LJ, Badesch DB, Barst RJ, Galiè N, Black CM, Keogh A, Pulido T, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346(12):896–903. [DOI] [PubMed]
- 5.McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galiè N, Rainisio M, Simonneau G, Rubin LJ. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J 2005;25(2):244–249. [DOI] [PubMed]
- 6.Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015;24(136):272–282. [DOI] [PMC free article] [PubMed]
- 7.Becattini C, Manina G, Busti C, Gennarini S, Agnelli G. Bosentan for chronic thromboembolic pulmonary hypertension: findings from a systematic review and meta-analysis. Thromb Res 2010;126(1):e51–e56. [DOI] [PubMed]
- 8.Hoeper MM, Kramm T, Wilkens H, Schulze C, Schäfers HJ, Welte T, Mayer E. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest 2005;128(4):2363–2367. [DOI] [PubMed]
- 9.Hughes R, George P, Parameshwar J, Cafferty F, Dunning J, Morrell NW, Pepke-Zaba J. Bosentan in inoperable chronic thromboembolic pulmonary hypertension. Thorax 2005;60(8):707. [DOI] [PMC free article] [PubMed]
- 10.Wolff B, Lodziewski S, Bollmann T, Opitz CF, Ewert R. Impaired peripheral endothelial function in severe idiopathic pulmonary hypertension correlates with the pulmonary vascular response to inhaled iloprost. Am Heart J 2007;153(6):1088.e1–1088.e7. [DOI] [PubMed]
- 11.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340(8828):1111–1115. [DOI] [PubMed]
- 12.Sfikakis PP, Papamichael C, Stamatelopoulos KS, Tousoulis D, Fragiadaki KG, Katsichti P, Stefanadis C, Mavrikakis M. Improvement of vascular endothelial function using the oral endothelin receptor antagonist bosentan in patients with systemic sclerosis. Arthritis Rheum 2007;56(6):1985–1993. [DOI] [PubMed]
- 13.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54(1 Suppl.):S43–S54. [DOI] [PubMed]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39(2):257–265. [DOI] [PubMed]
- 15.Kabutoya T, Hoshide S, Ogata Y, Iwata T, Eguchi K, Kario K. The time course of flow-mediated vasodilation and endothelial dysfunction in patients with a cardiovascular risk factor. J Am Soc Hypertens 2012;6(2):109–116. [DOI] [PubMed]
- 16.Inaba H, Takeshita K, Uchida Y, Hayashi M, Okumura T, Hirashiki A, Yoshikawa D, et al. Recovery of flow-mediated vasodilatation after repetitive measurements is involved in early vascular impairment: comparison with indices of vascular tone. PLoS ONE 2014;9:e83977. doi:10.1371/journal.pone.0083977. [DOI] [PMC free article] [PubMed]
- 17.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 2010;55(5):1075–1085. [DOI] [PMC free article] [PubMed]
- 18.White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA 1994;91(3):1044–1048. [DOI] [PMC free article] [PubMed]
- 19.Rossi R, Nuzzo A, Lattanzi A, Coppi F, Modena MG. Sildenafil improves endothelial function in patients with pulmonary hypertension. Pulm Pharmacol Ther 2008;21(1):172–177. [DOI] [PubMed]
- 20.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. New Engl J Med 1995;333(4):214–221. [DOI] [PubMed]
- 21.Cella G, Bellotto F, Tona F, Sbarai A, Mazzaro G, Motta G, Fareed J. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest 2001;120(4):1226–1230. [DOI] [PubMed]
- 22.Peled N, Bendayan D, Shitrit D, Fox B, Yehoshua L, Kramer MR. Peripheral endothelial dysfunction in patients with pulmonary arterial hypertension. Respir Med 2008;102(12):1791–1796. [DOI] [PubMed]
- 23.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62(25 Suppl.):D51–D59. [DOI] [PubMed]
- 24.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62(25 Suppl.):D60–D72. [DOI] [PubMed]
- 25.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, Weissmann N, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009;33(4):785–792. [DOI] [PubMed]
- 26.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed]
- 27.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. New Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 28.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. New Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 29.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358(9288):1119–1123. [DOI] [PubMed]
- 30.Jaïs X, D’Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008;52(25):2127–2134. [DOI] [PubMed]
- 31.Lang IM, Klepetko W. Chronic thromboembolic pulmonary hypertension: an updated review. Curr Opin Cardiol 2008;23(6):555–559. [DOI] [PubMed]
- 32.McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007;93(9):1152–1158. [DOI] [PMC free article] [PubMed]


