Abstract Abstract
Radiotherapy as a primary treatment for thoracic malignancies induces deleterious effects, such as acute or subacute radiation-induced lung injury (RILI). Although the molecular etiology of RILI is controversial and likely multifactorial, a potentially important cellular target is the lung endothelial cytoskeleton that regulates paracellular gap formation and the influx of macromolecules and fluid to the alveolar space. Here we investigate the central role of a key endothelial cytoskeletal regulatory protein, the nonmuscle isoform of myosin light chain kinase (nmMLCK), in an established murine RILI model. Our results indicate that thoracic irradiation significantly augmented nmMLCK protein expression and enzymatic activity in murine lungs. Furthermore, genetically engineered mice harboring a deletion of the nmMLCK gene (nmMLCK−/− mice) exhibited protection from RILI, as assessed by attenuated vascular leakage and leukocyte infiltration. In addition, irradiated wild-type mice treated with two distinct MLCK enzymatic inhibitors, ML-7 and PIK (peptide inhibitor of kinase), also demonstrated attenuated RILI. Taken together, these data suggests a key role for nmMLCK in vascular barrier regulation in RILI and warrants further examination of RILI strategies that target nmMLCK.
Keywords: bronchoalveolar lavage, nonmuscle myosin light chain kinase, radiation-induced lung injury
Radiotherapy, a primary treatment for thoracic malignancies, is often limited by deleterious dose-limiting side effects that manifest themselves as acute or subacute lung injury 6–24 weeks after radiation exposure.1 Although the exact molecular mechanisms of radiation-induced lung injury (RILI) are controversial and likely multifactorial, a potentially important cellular target in RILI is the lung endothelium, a functionally dynamic tissue that regulates influx of macromolecules and fluid between the vascular compartment and the lung interstitial space.2 Disruption of endothelial cell (EC) barrier integrity is considered a key feature of pulmonary inflammation in general and RILI in particular, resulting in increased vascular permeability and alveolar flooding. Thus, stabilization or enhanced restoration of lung vascular endothelial integrity is a critical step in preventing or reducing acute or subacute RILI, as we have previously demonstrated.2
We have also reported the role of the nonmuscle myosin light chain kinase isoform (nmMLCK) as an essential element of the inflammatory responses, with variants in the MYLK gene contributing to acute lung injury (ALI), ventilation-induced lung injury (VILI) susceptibility, and asthma susceptibility.3,4 Nonmuscle MLCK is a calcium/calmodulin-dependent kinase critically involved in catalyzing the phosphorylation of myosin light chain (MLC), leading to cell contraction and vascular EC barrier disruption, which indicates a critical role for nmMLCK in regulating vascular permeability.5 We have also reported that genetic variants (single-nucleotide polymorphisms) in MYLK confer significant susceptibility to sepsis-6 or trauma-induced7 ALI, in addition to contributing to the risk of severe asthma, another inflammatory lung disorder, in African Americans.3,4,8 Nonmuscle MLCK is activated by various permeability-enhancing factors, such as inflammatory cytokines and chemokines mediating cell contraction, formation of cell-cell and cell-matrix gaps, and vascular permeability.9
We studied the role of nmMLCK in RILI development and evaluated the therapeutic potential of MLCK inhibitors in alleviating RILI, utilizing our previously characterized murine model of RILI.2,10 Our results indicate that single-fraction thoracic radiation (20 Gy) significantly augments nmMLCK protein expression and MLC phosphorylation in wild-type (WT) mice lungs, which peaked 3 days after radiation. Furthermore, mice with nmMLCK deletion were protected from RILI, as assessed by vascular leak, leukocyte infiltration, and histological evidence of inflammation and injury. Correspondingly, radiated WT mice treated with MLCK inhibitors ML-7 or PIK (peptide inhibitor of kinase, an MLCK inhibitory oligopeptide) also demonstrated attenuated RILI. Taken together, these data suggests a key role for nmMLCK in vascular barrier regulation in RILI and warrant further examination of RILI strategies that target nmMLCK.
Methods
Reagents
Antibodies against nmMLCK, MLC, and phosphorylated MLC (p-MLC) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ML-7 and β-actin antibody were purchased from Sigma (St. Louis, MO). Horseradish peroxidase–conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Cell Signaling (Danvers, MA). PIK (or MLCK inhibitor peptide 18) was purchased from Tocris Bioscience (Bristol, United Kingdom).
Murine model of RILI
All experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee (IACUC). C57BL/6 (20–25-g) mice 8–10 weeks old were purchased from Jackson Laboratory (Bar Harbor, ME), and nmMLCK−/− mice were provided as gifts from D. M. Watterson11 and bred according to approved IACUC protocol. Mice were anesthetized with ketamine (100 mg/kg) and acepromazine (1.5 mg/kg) and administered radiation (20 Gy) to the thorax, as we described previously.2 Briefly, a 5-mm-thick lead block was used to shield the rest of the animal while the thorax, between the clavicles and below the sternum, was irradiated with a 250-kV x-ray beam at a dose rate of 2 Gy/minute using an orthovoltage animal irradiator. Body weight was measured for individual mice. Select mice were treated with MLCK inhibitor ML-7 (15 μg/mouse, every other day) or PIK (0.25 mg/mouse, every other day) or vehicle control via intraperitoneal injection, beginning 1 week before irradiation and continuing for up to 6 weeks afterward. The mice were then killed, and indices of lung vascular leak and inflammation were assessed via bronchoalveolar lavage (BAL) fluid protein levels and cell counts, as previously described.2 Lungs were harvested for further evaluation.
BAL fluid analysis
BAL was performed after irradiation, as we have previously described.2 Briefly, at the termination of each experiment, mice were euthanized by exsanguination under anesthesia according to the IACUC protocol. Both lungs were lavaged with 1 mL of cold Hank’s buffered saline solution (HBSS). The BAL fluid collected was centrifuged at 500 g for 20 minutes at 4°C, and the supernatant was removed and recentrifuged at 12,000 g for 10 minutes for supernatant collection. The cell pellet was resuspended in cold HBSS for determination of total cell count and cell differential on slides prepared by cytocentrifugation (Cytospin 3; Shandon Instruments, Pittsburgh, PA) and Diff Quik staining (Dade Behring, Dugen, Switzerland). The protein concentration in BAL supernatant was determined by a protein assay (Bio-Rad, Hercules, CA).
Lung histology and immunohistochemistry
Lungs collected from radiated mice were fixed in 10% formalin for at least 48 hours, and lung paraffin sections were used for immunohistochemistry and stained with nmMLCK primary antibody (catalog no. sc-25428, Santa Cruz Biotechnology) using the avidin-biotin-peroxidase method. Briefly, lung sections (4–5 μm thick) were deparaffinized and rehydrated with graded ethanol. After 30 minutes incubation at room temperature with Tris-buffered saline (TBS) containing 3% normal serum or casein for blocking, the slides were incubated with nmMLCK antibody overnight at 4°C, followed by 3 washes in TBS and further incubation with a streptavidin-conjugated secondary antibody, followed by reaction with substrate and chromagen (diaminobenzidine [DAB]). Slides were then counterstained with hematoxylin.
Western blotting of nmMLCK, total-MLC, and p-MLC protein in lung tissue
Lung tissues were homogenized in a polytron with a buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA (ethylene glycol tetraacetic acid), 5 mM β-glycerophosphate, 1 mM MgCl2, 1% Triton X-100, 1 mM sodium orthovanadate, 10 µg/mL protease inhibitors, 1 µg/mL aprotinin, 1 µg/mL leupeptin, and 1 µg/mL pepstatin. Lysates were centrifuged at 500 g for 5 minutes at 4°C, equal amounts of protein (40 µg) were loaded onto 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and Western blotting was performed according to standard protocols.12 Nonmuscle MLCK (catalog no. sc-365352, Santa Cruz Biotechnology), and p-MLC (catalog no. 3675, Cell Signaling) were purchased.
Quantitative real-time polymerase chain reaction (RT-PCR) confirmation of gene change
Quantification of the selected transcripts was performed by relative quantitative RT-PCR with SYBR Green RT-PCR assays and the CFX384 Real-time PCR system (Bio-Rad). The complementary DNAs (cDNAs) were generated from 1 µg of total RNA with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The resulting cDNA was subjected to a 40-cycle PCR amplification using manufacturer’s protocol. Three replicates were run for each gene for each sample in a 384-well plate. The relative quantitation method (ΔΔCt) was used (against internal control GAPDH), with the relative level of the messenger RNA (mRNA) for the gene of interest calculated. The significance of the SYBR Green data was calculated; results were considered significant when P < 0.05.
Statistical analysis
Two-way analysis of variance (ANOVA) was used to compare the means of data from two or more different experimental groups. If significant difference was present by ANOVA (P < 0.05), a least significant differences test was performed post hoc. Subsequently, differences between groups were considered statistically significant when P values were <0.05.
Results
Lung radiation induces nmMLCK expression and MLC phosphorylation
Nonmuscle MLCK is crucial in driving cytoskeletal rearrangement via phosphorylating MLCs, leading to cell contraction and EC barrier dysfunction. To examine radiation-induced alterations in MLCK expression and activity, C57BL/6J (WT) mice were radiated with a 20-Gy single-fraction thoracic radiation dose, and expression levels of nmMLCK protein and mRNA levels were examined 6 weeks after radiation. Both immunohistochemistry and Western blotting data demonstrated significantly increased nmMLCK expression levels 6 weeks after radiation in mouse lungs compared to lungs of nonradiated WT controls, suggesting that nmMLCK is influenced by RILI exposure (Fig. 1A, 1B). Similarly, nmMLCK activity was rapidly increased after radiation (1 day; Fig. 1C), reflected by increased levels of MLC phosphorylation, beginning 1 day after radiation, peaking at 3 days, and dissipating over 6 weeks after radiation. Correspondingly, mRNA expression of nmMLCK was upregulated significantly in radiated lungs 6 weeks after radiation (Figs. 1D, S1), findings also observed in lung microarray data collected from a Gene Expression Omnibus (GEO) data set (GEO data set ID: GSE41789; Fig. S2).13
Figure 1.
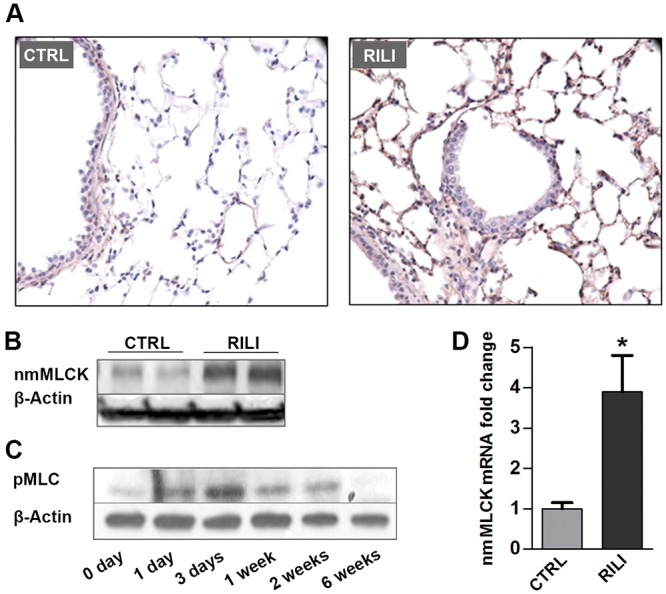
Nonmuscle myosin light chain kinase (nmMLCK) is upregulated in murine radiation-induced lung injury (RILI) lungs. C57BL/6J mice were exposed to a single-fraction thoracic radiation (20 Gy), and lung tissues were examined for nmMLCK expression and activity. A, Lung immunohistochemistry staining for nmMLCK; B, lung protein Western blot analysis for nmMLCK; C, lung protein phosphorylated MLC (pMLC) Western blot analysis (with different time points after radiation); D, quantitative polymerase chain reaction analysis for nmMLCK messenger RNA (mRNA). *P < 0.05 compared to nonradiated samples. n = 5–6. CTRL: control.
Mice with targeted deletion of nmMLCK are protected from RILI
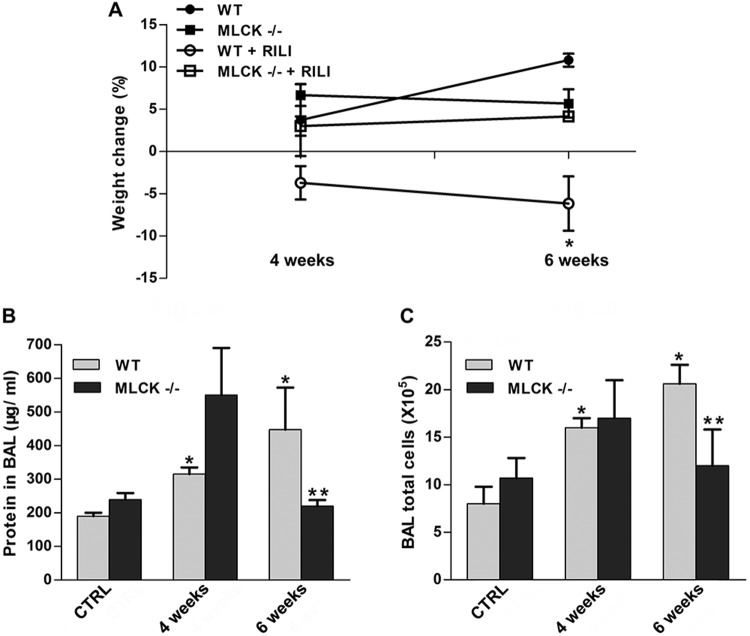
Utilizing a previously characterized preclinical model of RILI,2 C57BL/6 (WT) and nmMLCK−/− mice were exposed to a single dose of whole-thoracic radiation (20 Gy) to assess RILI in terms of BAL protein (reflecting vascular leak) and inflammatory cell influx (reflecting inflammation). Radiation induced significant increases in the levels of protein and total cells in BAL fluid of WT mice, compared to nonradiated respective controls. In contrast, nmMLCK−/− mice were protected from RILI in terms of vascular leak, inflammation, and percentage body weight loss, compared to age-matched radiated WT mice (Fig. 2).
Figure 2.
The nmMLCK−/− mice are protected from radiation-induced lung injury (RILI). nmMLCK−/− mice and wild-type (WT) mice received thoracic radiation (20 Gy), and bronchoalveolar lavage (BAL) fluid was extracted/examined for inflammatory profile. A, Body weight of the corresponding mice was examined 2 weeks, 4 weeks, or 6 weeks after radiation. *P < 0.05 between WT+RILI and other groups. n = 5–6. B, C, BAL protein levels (B) and total cell counts (C) were analyzed. *P < 0.05 compared to WT-control (CTRL) group. **P < 0.05 compared to the WT–6-week group. n = 5–6.
Protective effects of inhibitors of MLC kinase activity on RILI
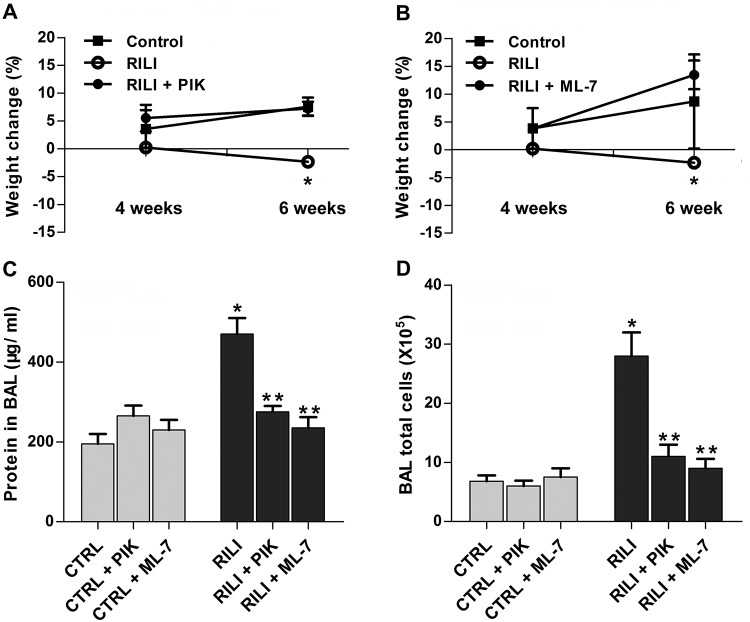
Our previous in vitro and in vivo studies demonstrated beneficial effects of the membrane-permeant inhibitor of MLC kinase activity (PIK, a highly specific peptide inhibitor of MLCK)14 by inhibiting MLC kinase activity and MLC phosphorylation.15 ML-7, an nmMLCK chemical inhibitor,16 was also adapted to define nmMLCK function. Radiated mice were administered PIK (0.25 mg/mouse) or ML-7 (15 μg/mouse) every other day for 6 weeks in this RILI model. Both PIK- and ML-7-treated mice were protected from RILI, compared to untreated radiated controls, as evidenced by BAL protein content and total BAL cellularity (Fig. 3A–3C).
Figure 3.
Protective effects of nonmuscle myosin light chain kinase (nmMLCK) inhibitors ML-7 and PIK (peptide inhibitor of kinase) on radiation-induced lung injury (RILI). Mice were treated with MLCK inhibitor ML-7 (15 μg/mouse, every other day), PIK (0.25 mg/mouse, every other day), or vehicle control (CTRL) via intraperitoneal injection beginning 1 week before irradiation and continuing for a period of up to 6 weeks. A, B, Body weight of the corresponding mice was examined 2 weeks, 4 weeks, or 6 weeks after radiation. A, *P < 0.05 between RILI and RILI+PIK groups. n = 5–6. B, *P < 0.05 between RILI and RILI+ML-7 groups. n = 5–6. C, D, Bronchoalveolar lavage (BAL) protein levels (C) and total cell counts (D). *P < 0.05 between CTRL and RILI groups. **P < 0.05 compared to RILI group. n = 5–6.
Discussion
The molecular mechanisms underlying the development of RILI remain enigmatic and controversial, impeding the identification of novel therapeutic targets. To date, therapeutic strategies have largely been designed to ameliorate the acute effects of radiation via neutralizing proinflammatory cytokines or attenuating inflammatory cell infiltration. As we have shown that endothelial and epithelial barrier integrity is perturbed in murine RILI,2,10 we hypothesized that preservation of vascular barrier integrity is an effective therapeutic strategy in RILI as well as in other acute lung injury syndromes.15,17 By facilitating formation of a paracellular gap, nmMLCK plays a central role in EC cytoskeletal rearrangement during lung injury.18 We have previously reported that nmMLCK knockout mice, as well as mice treated with an inhibitory peptide to reduce MLCK activity, were protected against VILI.15 Furthermore, our studies identified MYLK gene variants (single-nucleotide polymorphisms) that confer susceptibility to sepsis, sepsis- and trauma-induced ALI, and increased risk of severe asthma in African Americans.3,4 In this study, we report that nmMLCK contributes to RILI by modulating MLC phosphorylation induced in radiated mouse lungs.
The striking paucity of mechanistic information that addresses RILI pathogenesis magnifies the importance of appropriate preclinical models of human RILI. We employed our established RILI model to define the role of nmMLCK in RILI pathogenesis. Murine RILI, followed by a single clinically relevant radiation dose (18–25 Gy) over a clinically relevant time (∼12 weeks), exhibits increased vascular leak (BAL protein) and leukocyte infiltration (BAL cell count), two critical parameters to assess acute lung inflammation.
Nonmuscle MLCK is upregulated in lung tissue by radiation consistently (Figs. 1D, S2), from a combination of sources: endothelium/epithelium (Fig. 1A) and infiltrated inflammatory leukocytes.19 Furthermore, we also tested the mRNA level of two major nmMLCK splice variants, nmMLCK1 and nmMLCK2, which were induced after 6 weeks of radiation treatment (Fig. S1). Regulation of nmMLCK transcription is possibly mediated by the increased generation of reactive oxygen species (ROSs) evoked by radiation-induced injury. We have identified multiple antioxidant response elements located in nmMLCK core promoter (−2.5 kb to transcription start site), suggesting the activation of nmMLCK expression by ROS-dependent pathways. In addition, we have found that Nrf2 knockout mice exhibit a lower expression of nmMLCK (data not shown), which also supports that nmMLCK levels are maintained by Nrf2 positively. Although inhibitors of nmMLCK and nmMLCK knockout mice exhibited similar RILI protection effects, we believe that the MLC phosphorylation is biphasic. Nonmuscle MLCK is activated via a calmodulin-dependent mechanism in the acute phase (minutes to hours),20 and nmMLCK is kept upregulated in an ROS-Nrf2-dependent manner in the delayed phase (days to weeks). This biphasic mechanism makes nmMLCK inhibitors consistent and reliable for RILI.
Nonmuscle MLCK knockout mice were significantly protected from RILI (Fig. 2), similar to our previous findings in acute respiratory distress syndrome and VILI murine models, suggesting a highly conserved function of nmMLCK in regulation of EC barrier integrity during inflammatory stimuli. Interestingly, this protection effect in nmMLCK knockout mice was not present at an early time point (4 weeks in Fig. 2B, 2C), which suggests a complex role of nmMLCK in barrier disruption15 and restoration.21 Importantly, pharmacological inhibitors of nmMLCK (ML-7 and PIK)22,23 effectively attenuated RILI, similar to the effects observed in nmMLCK knockout mice at a delayed time point, suggesting that regardless of its dual role in endothelial barrier regulation, nmMLCK is an effective therapeutic target of RILI, a devastating and life-threatening syndrome.
Besides playing the central role in endothelial barrier regulation in response to stimuli,5 nmMLCK is also essential for neutrophil transmigration and epithelial barrier dysfunction during lung injury response.19,22 It would be interesting to characterize nmMLCK function in neutrophil-epithelial interaction with RILI in our future work. Remarkably, it is demonstrated that p21-activated kinase and extracellular signal-regulated kinase are critical activators of MLC involved in vascular permeability regulation during inflammation development.24 Those results provided another avenue for identifying the therapy targets of RILI.
In summary, this concise study characterizes the upregulation of nmMLCK expression and activity in RILI and suggests a key role for nmMLCK in vascular barrier regulation in RILI. Further examination of RILI strategies that target nmMLCK is warranted.
Appendix. Supplementary figures
Figure S1.
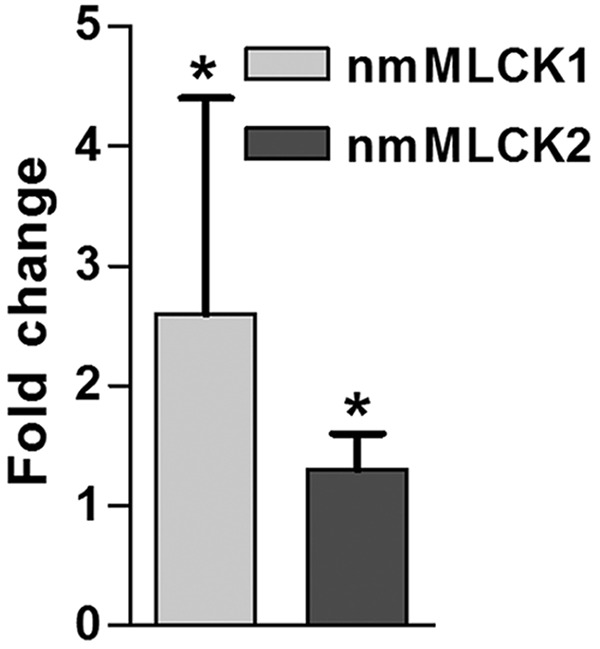
Messenger RNA (mRNA) expression level of two nonmuscle myosin light chain kinase (nmMLCK) variants in radiated lungs after radiation. Compared to control, both nmMLCK1 and nmMLCK2 were increased in mRNA level after 6 weeks of radiation. *P < 0.05. n = 5.
Figure S2.
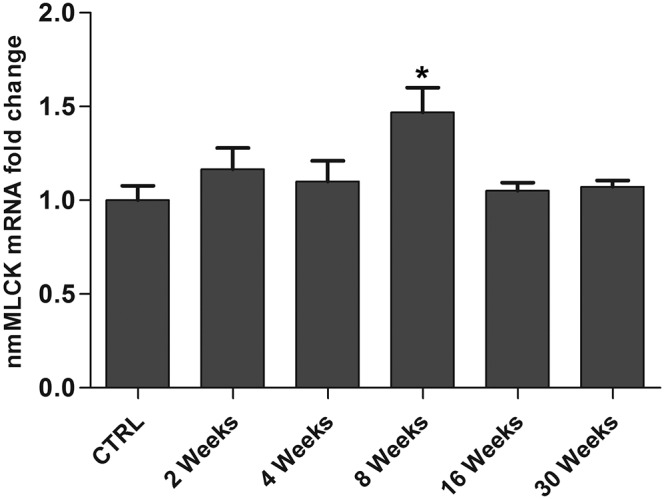
Time-dependent nonmuscle myosin light chain kinase (nmMLCK) expression in murine lungs after radiation. Gene expression in lung tissue from mice (female C57 mice aged 10 weeks) irradiated to 17.5 Gy were analyzed by Affymetrix Mouse430 2.0 microarray chip (Gene Expression Omnibus data set ID: GSE41789), and relative nmMLCK (1425504_at) levels were analyzed compared to untreated controls. *P < 0.05 compared to control (CTRL). mRNA: messenger RNA.
Source of Support: These studies were supported by National Institutes of Health grants P01HL58064 (JGNG), P01HL126609 (JGNG), R01HL125615 (JGNG), P01HL98050 (JGNG), and R01HL91899 (JGNG and TW). These studies are also supported by Veterans Administration Health System grant 1IK2BX001477 (LH) and a Parker B. Francis Fellowship (TW).
Conflict of Interest: None declared.
Supplements
Appendix (504KB, pdf)
References
- 1.Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 2000;10(4):296–307. PubMed PMID:11040330. [DOI] [PubMed]
- 2.Mathew B, Huang Y, Jacobson JR, Berdyshev E, Gerhold LM, Wang T, Moreno-Vinasco L, et al. Simvastatin attenuates radiation-induced murine lung injury and dysregulated lung gene expression. Am J Respir Cell Mol Biol. 2011;44(3):415–422. doi:10.1165/rcmb.2010-0122OC. PubMed PMID:20508068; PubMed Central PMCID:PMC3095940. [DOI] [PMC free article] [PubMed]
- 3.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol 2007;31(4):296–305. doi:10.1002/gepi.20210. PubMed PMID:17266121. [DOI] [PubMed]
- 4.Acosta-Herrera M, Pino-Yanes M, Ma SF, Barreto-Luis A, Corrales A, Cumplido J, Pérez-Rodríguez E, et al. Fine mapping of the myosin light chain kinase (MYLK) gene replicates the association with asthma in populations of Spanish descent. J Allergy Clin Immunol 2015;136(4):1116–1118. doi:10.1016/j.jaci.2015.04.025. PubMed PMID:26025125. [DOI] [PMC free article] [PubMed]
- 5.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001;91(4):1487–1500. PubMed PMID:11568129. [DOI] [PubMed]
- 6.Gao L, Grant AV, Rafaels N, Stockton-Porter M, Watkins T, Gao P, Chi P, et al. Polymorphisms in the myosin light chain kinase gene that confer risk of severe sepsis are associated with a lower risk of asthma. J Allergy Clin Immunol 2007;119(5):1111–1118. doi:10.1016/j.jaci.2007.03.019. PubMed PMID:17472811. [DOI] [PubMed]
- 7.Christie JD, Ma SF, Aplenc R, Li M, Lanken PN, Shah CV, Fuchs B, Albelda SM, Flores C, Garcia JG. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med 2008;36(10):2794–2800. PubMed PMID:18828194. [DOI] [PubMed]
- 8.Wang T, Zhou T, Saadat L, Garcia JG. A MYLK variant regulates asthmatic inflammation via alterations in mRNA secondary structure. Eur J Hum Genet 2015;23(6):874–876. doi:10.1038/ejhg.2014.201. PubMed PMID:25271083. [DOI] [PMC free article] [PubMed]
- 9.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 2010;87(2):272–280. doi:10.1093/cvr/cvq144. PubMed PMID:20479130; PubMed Central PMCID:PMC2895546. [DOI] [PMC free article] [PubMed]
- 10.Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, Gerhold LM, et al. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J 2011; 25(10):3388–3400. doi:10.1096/fj.11-183970. PubMed PMID:21712494; PubMed Central PMCID:PMC3177585. [DOI] [PMC free article] [PubMed]
- 11.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA 2003;100(10):6233–6238. doi:10.1073/pnas.1031595100. PubMed PMID:12730364; PubMed Central PMCID:PMC156355. [DOI] [PMC free article] [PubMed]
- 12.Wang T, Wang L, Moreno-Vinasco L, Lang GD, Siegler JH, Mathew B, Usatyuk PC, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol 2012;9:35. doi:10.1186/1743-8977-9-35. PubMed PMID:22931549; PubMed Central PMCID:PMC3489700. [DOI] [PMC free article] [PubMed]
- 13.Citrin DE, Shankavaram U, Horton JA, Shield W III, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst 2013;105(19):1474–1484. doi:10.1093/jnci/djt212. PubMed PMID:24052614; PubMed Central PMCID:PMC3787909. [DOI] [PMC free article] [PubMed]
- 14.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 2002;123(1):163–172. PubMed PMID:12105845. [DOI] [PubMed]
- 15.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol 2011;44(1):40–52. doi:10.1165/rcmb.2009-0197OC. PubMed PMID:20139351; PubMed Central PMCID:PMC3028257. [DOI] [PMC free article] [PubMed]
- 16.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 2003;371(Pt. 1):199–204. doi:10.1042/BJ20021535. PubMed PMID:12534346; PubMed Central PMCID:PMC1223271. [DOI] [PMC free article] [PubMed]
- 17.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol 2010;43(4):394–402. doi:10.1165/rcmb.2009-0223OC. PubMed PMID:19749179; PubMed Central PMCID:PMC2951871. [DOI] [PMC free article] [PubMed]
- 18.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 1995;163(3):510–522. doi:10.1002/jcp.1041630311. PubMed PMID:7775594. [DOI] [PubMed]
- 19.Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating β2 integrins. Nat Immunol 2008;9(8):880–886. doi:10.1038/ni.1628. PubMed PMID:18587400; PubMed Central PMCID:PMC2553242. [DOI] [PMC free article] [PubMed]
- 20.Holzapfel G, Wehland J, Weber K. Calcium control of actin-myosin based contraction in triton models of mouse 3T3 fibroblasts is mediated by the myosin light chain kinase (MLCK)-calmodulin complex. Exp Cell Res 1983;148(1):117–126. PubMed PMID:6605252. [DOI] [PubMed]
- 21.Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, et al. Abl tyrosine kinase phosphorylates nonmuscle myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell 2010;21(22):4042–4056. doi:10.1091/mbc.E09-10-0876. PubMed PMID:20861316; PubMed Central PMCID:PMC2982111. [DOI] [PMC free article] [PubMed]
- 22.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 2006;116(10):2682–2694. doi:10.1172/JCI29218. PubMed PMID:17016558; PubMed Central PMCID:PMC1578628. [DOI] [PMC free article] [PubMed]
- 23.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA 2010;107(18):8237–8241. doi:10.1073/pnas.0908869107. PubMed PMID:20404178; PubMed Central PMCID:PMC2889526. [DOI] [PMC free article] [PubMed]
- 24.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: βPIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell 2007;18(6):2346–2355. doi:10.1091/mbc.E06-07-0584. PubMed PMID:17429073; PubMed Central PMCID:PMC1877103. [DOI] [PMC free article] [PubMed]