Summary
Clodronate belongs to Bisphosphonates family and it has been studied especially for osteoporosis treatment, Paget’s disease, osteolytic metastases, hypercalcemia malignancy and some childhood skeletal diseases. Besides the osteoporosis treatment, it has been successfully used for treating tumoral osteolysis and for bone localization of multiple myeloma, hypercalcemia malignancy, primary hyperparathyroidism, Paget’s disease and algodystrophy.
Filipponi study showed a statistically significant reduction of the incidence of vertebral fractures after 4 years of treatment with clodronate, intravenously administered at a dose of 200 mg every three weeks.
Frediani study, published in 2003 on BONE, proved the clodronate efficacy in the prevention of fractures caused by glucocorticoid-induced osteoporosis (GIO). Clodronate doses of 800 mg/day per os and 100 mg i.m./week are substantially equivalent, because the oral absorption is about 1,9%. A higher efficacy on BMD was documented in various works, especially in cohorts of patients with a greater fracture risk, using higher doses (1600 mg per os). This has led to the hypothesis of using clodronate 200 mg i.m. formulation. Clodronate is an osteoporosis drug that can be assumed in different doses (100 mg i.m./week, clodronate 200 mg i.m. every 2 weeks) considering the risk band, identified by algorithms (FRAX o DeFRA), by BMD and by the presence of at least one risk factor. That means that it is possible to envisage a differentiated use of clodronate adapting the doses to the fracture risk and to the severity of pain symptoms, thus promoting a greater adherence to the therapy. To conclude clodronate is helpful in reducing fracture risk, is safe, well tolerated, and has a good rate cost/effectiveness in patients with fracture risk over 7% established with FRAX.
Keywords: bisphosphonates, clodronate, BMD, prevention fractures, fracture risk reduction, GIO, adherence, cost-effectiveness
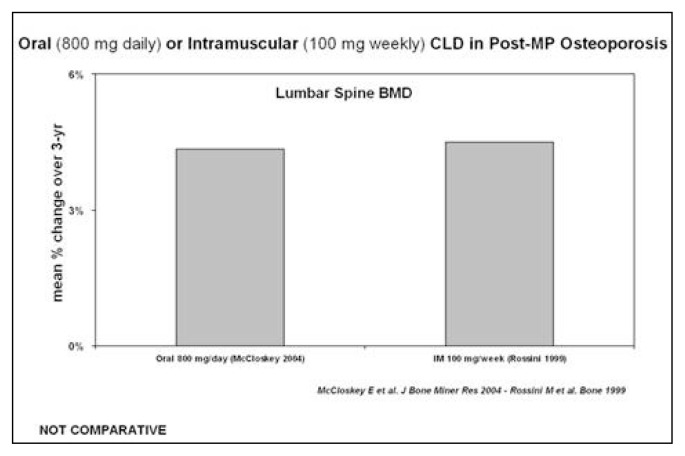
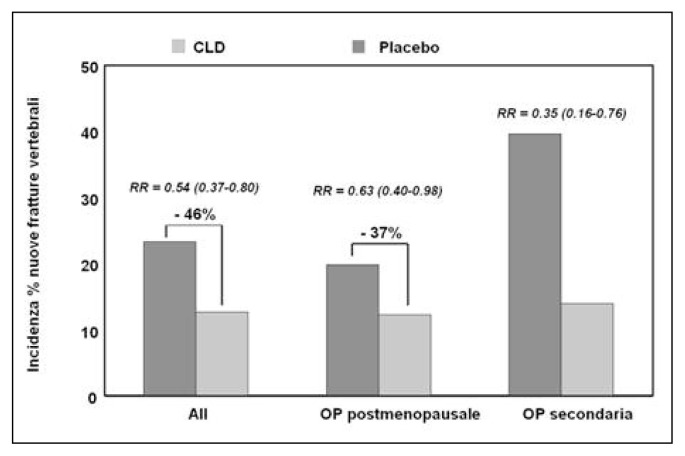
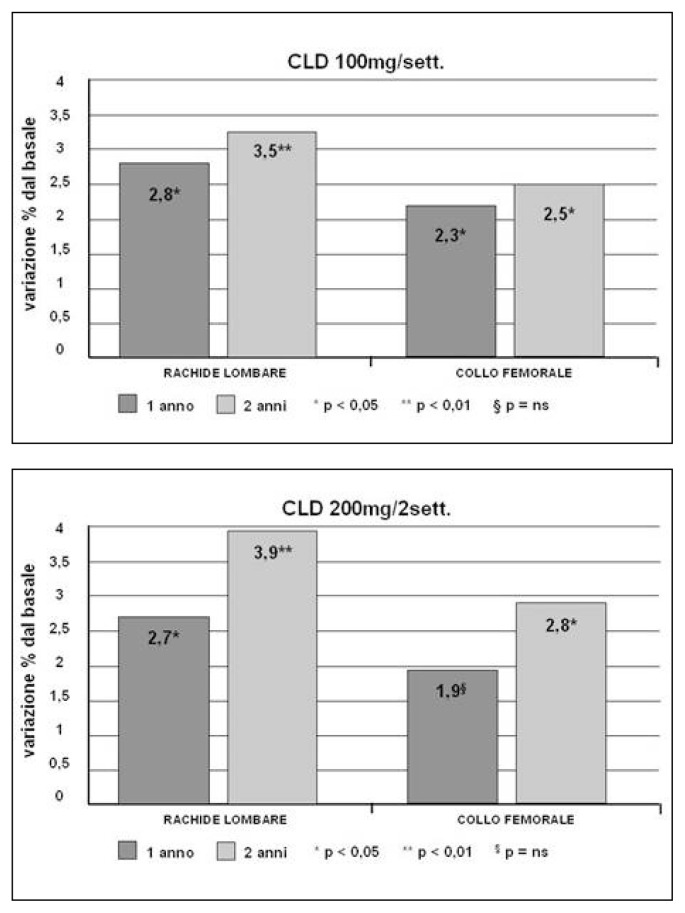
The discovery of the pharmacological activity of bisphosphonates, caused by a high affinity to hydroxyapatite, and of a possible use in the treatment of skeletal diseases, goes back to the end of 1960. Bisphosphonates have been studied especially for osteoporosis treatment, Paget’s disease, osteolytic metastases, hypercalcemia malignancy and some childhood skeletal diseases. Millions of people in the world, especially post-menopausal women, are now taking bisphosphonates. Bisphosphonates, besides the medicinal use, are utilized for their chelating metal properties, in prosthetic surgery for stabilizing nano-particles and as scintigraphic tracer in many osteo-articular diseases. Clodronate was one of the first bisphosphonates to be synthesized and used in the medical field. It has been a subject of study for 30 years, uninterruptedly. The interest in this molecule is due to various reasons, such as: the ability to inhibit osteoclastic resorption, to increase BMD, to lower the risk of vertebral and non-vertebral fractures and to exert an analgesic effect, independent of the anti-fracture effectiveness, on inflammatory pain, neuropathic and neoplastic, especially caused by bone metastases from breast and prostate cancer. Clodronate proved to have an anti-inflammatory and anti-arthritic activity, both in animal models and humans. In patients suffering from breast cancer, clodronate provided evidence of effectiveness in lowering the incidence of bone and visceral metastases of 50%, while significantly increasing survival. Clodronate was the first osteoporosis drug to be administered at intervals of 8 or 15 days and for this reason and its good tolerability, it has been widely appreciated from the start, when there was no consolidated data in literature about its anti-fracture efficacy. It is available in oral packs (400 mg) and parental packs (100, 200 and 300 mg); the most used administration is the parental one. Besides the osteoporosis treatment, it has been successfully used for treating tumoral osteolysis and for bone localization of multiple myeloma, hypercalcemia malignancy, primary hyper-parathyroidism, Paget’s disease and algodystrophy. As for the osteoporosis treatment, it has been proved that the weekly administration of 100 mg i.m. clodronate determines, after 1–3 years of therapy, an increase of the bone mass of 4% at vertebral level (Figure 1) and of about 3% at femoral level. Del Puente et al. tested the efficacy of 100 mg i.m./week clodronate on a group of patients, who were non-responders for many reasons to the alendronate oral therapy, documenting a statistically significant increase of BMD after a year of observation. Filipponi et al. showed a statistically significant reduction of the incidence of vertebral fractures after 4 years of treatment with clodronate, intravenously administered at a dose of 200 mg every three weeks. Mc-Closkey et al. showed, in two double-blind studies that lasted 3 years and were published respectively in 2004 and in 2007 on JBMR, a statistically significant reduction of the relative risk of vertebral fractures (46%) in women suffering from both post-menopausal osteoporosis and secondary osteoporosis, treated with oral clodronate at a dose of 800 mg/day (Figure 2). In the Frediani study, published in 2003 on BONE, the efficacy of clodronate is also proved in the prevention of fractures caused by glucocorticoid-induced osteoporosis. Clodronate doses of 800 mg/day per os and 100 mg i.m./week are substantially equivalent, because the oral absorption is about 1,9%. A higher efficacy on bone mass was documented in various works, especially in cohorts with a greater fracture risk, using higher doses (1600 mg per os) and this has led to the hypothesis of using clodronate 200 mg i.m. formulation. In a first study, Frediani proved the densitometric equivalence between clodronate 200 mg i.m. every 14 days and clodronate 100 mg i.m. every 7 days and subsequently, in a second study and in an off-label use, the higher densitometric efficacy of 200 mg i.m./week compared to 100 mg i.m./week. This finding was evident especially at femoral level, place where the drug had previously seemed ineffective at preventing femoral fractures, because it did not lead to adequate bone mass increases (Figures 3, 4). It can be assumed that, in relation to the risk band, identified by algorithms (FRAX o DeFRA) by BMD and by the presence of at least one risk factor, it is possible to envisage a differentiated use of clodronate 100 mg i.m./week and clodronate 200 mg i.m. every 2 weeks, adapting the doses to the fracture risk and to the severity of pain symptoms, thus promoting a greater adherence to the therapy. In recent years, with the introduction of algorithms that select patients at a high fracture risk at ten years, there is much talk about the cost/benefit relationship and pharmaco-economic models that calculate the intervention threshold on the basis of the drug cost, the monitoring, the anti-fracture efficacy, the quality of life and how much a community can and wants to spend. About this, a sub-analysis of McCloskey’s study, conducted on 3974 patients aged over 75, shows that clodronate is most effective on patients with a higher fracture risk and, in another McCloskey’s study, that the intervention threshold is “cost-effective” when the fracture risk is about 7–10%. In conclusion, it is possible to say that clodronate is effective in preventing fractures, it is well tolerated, it has a good therapeutic adherence and a great cost/benefit relationship; in the end, it should be reminded that its administration after a fracture does not prevent the normal mineralization of the bone callus but, on the contrary, it improves the degree of calcification.
Figure 1.
Lumbar BMD variations with clodronate i.m. in patients with post-menopausal osteoporosis.
Figure 2.
Reduction of incidence of vertebral fractures after 3 years of treatment with oral clodronate in patients with osteoporosis (McCloskey, 2004).
Figures 3 and 4.
Clodronate effects on lumbar and femoral neck BMD at 1 and 2 years of treatment in patients with post-menopausal osteoporosis (Frediani, 2011).
References
- Frediani B, et al. Intramuscular CLD prevents vertebral fractures in GIOP-Arthritis. Bone. 2003 [Google Scholar]
- McCloskey E, et al. CLD Reduces Vertebral Fracture Risk in Women With Postmenopausal or Secondary Osteoporosis: 3-yr RCT. J Bone Miner Res. 2004 doi: 10.1359/JBMR.040116. [DOI] [PubMed] [Google Scholar]
- McCloskey E, et al. Oral (800 mg daily) or Intramuscular (100 mg weekly) CLD in Post-MP Osteoporosis. J Bone Miner Res. 2004 [Google Scholar]
- McCloskey EV, Beneton M, Charlesworth D, et al. Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis: Results of a double-blind, placebo-controlled randomized study. J Bone Miner Res. 2007;22:135–141. doi: 10.1359/jbmr.061008. [DOI] [PubMed] [Google Scholar]
- Filipponi P, Cristallini S, Rizzello E, et al. Cyclical intravenous clodronate in postmenopausal osteoporosis: Results of a long-term clinical trial. Bone. 1996;18:179–184. doi: 10.1016/8756-3282(95)00442-4. [DOI] [PubMed] [Google Scholar]
- Frediani B. Effects of two administration schemes of intramuscular clodronic acid on bone mineral density. A randomized, open-label, parallel-group study. Clin Drug Investig. 2011;31:43–50. doi: 10.2165/11539990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McCloskey EV, Johansson H, Oden A, et al. Ten-year fracture probability identifies women who will benefit from clodronate therapy - additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int. 2009;20:811–817. doi: 10.1007/s00198-008-0786-9. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–S413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- Frediani B, Cavalieri L, Cremonesi G. Clodronic acid formulations available in Europe and their use in osteoporosis. A review. Clin Drug Invest. 2009;29:359–379. doi: 10.2165/00044011-200929060-00001. [DOI] [PubMed] [Google Scholar]
- Dando TM, Wiseman LR. Clodronate: A review of its use in the prevention of bone metastases and the management of skeletal complications associated with bone metastases in patients with breast cancer. Drugs Aging. 2004;21:949–962. doi: 10.2165/00002512-200421140-00005. [DOI] [PubMed] [Google Scholar]
- Muratore, et al. Clinical utility of clodronate in the prevention and management of osteoporosis in patients intolerant of oral bisphosphonates. Drug Des Devel Ther. 2011;5:445–454. doi: 10.2147/DDDT.S12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelzner P, Kunze A, Henzgen S, et al. High-dose clodronate therapy prevents joint destruction in chronic antigen-induced arthritis of the rat but inhibits bone formation at the axial skeleton. Inflamm Res. 2000;49:424–433. doi: 10.1007/s000110050611. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Motegi K, Iwakura Y, et al. Involvement of interleukin-1 in the inflammatory actions of aminobisphosphonates in mice. Br J Pharmacol. 2000;130:1646–1654. doi: 10.1038/sj.bjp.0703460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinoi V, Brandi ML. Clodronate: Mechanisms of action on bone remodelling and clinical use in osteometabolic disorders. Expert Opin Pharmacother. 2002;3:1643–1656. doi: 10.1517/14656566.3.11.1643. [DOI] [PubMed] [Google Scholar]
- Del Puente A, Scognamiglio A, Itto E, et al. Intramuscular clodronate in nonresponders to oral alendronate therapy for osteoporosis. J Rheumatol. 2000;27:1980–1983. [PubMed] [Google Scholar]
- Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- Ringe JD, Möller G. Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol Int. 2009;30:213–221. doi: 10.1007/s00296-009-0940-5. [DOI] [PubMed] [Google Scholar]