Abstract
Background and aims
Peripancreatic fat necrosis occurs frequently in necrotising pancreatitis. Distinguishing markers from mediators of severe acute pancreatitis (SAP) is important since targeting mediators may improve outcomes. We evaluated potential agents in human pancreatic necrotic collections (NCs), pseudocysts (PCs) and pancreatic cystic neoplasms and used pancreatic acini, peripheral blood mononuclear cells (PBMC) and an acute pancreatitis (AP) model to determine SAP mediators.
Methods
We measured acinar and PBMC injury induced by agents increased in NCs and PCs. Outcomes of caerulein pancreatitis were studied in lean rats coadministered interleukin (IL)-1β and keratinocyte chemoattractant/growth-regulated oncogene, triolein alone or with the lipase inhibitor orlistat.
Results
NCs had higher fatty acids, IL-8 and IL-1β versus other fluids. Lipolysis of unsaturated triglyceride and resulting unsaturated fatty acids (UFA) oleic and linoleic acids induced necro-apoptosis at less than half the concentration in NCs but other agents did not do so at more than two times these concentrations. Cytokine coadministration resulted in higher pancreatic and lung inflammation than caerulein alone, but only triolein coadministration caused peripancreatic fat stranding, higher cytokines, UFAs, multisystem organ failure (MSOF) and mortality in 97% animals, which were prevented by orlistat.
Conclusions
UFAs, IL-1β and IL-8 are elevated in NCs. However, UFAs generated via peripancreatic fat lipolysis causes worse inflammation and MSOF, converting mild AP to SAP.
INTRODUCTION
Agents elevated in body fluids of patients with severe acute pancreatitis (SAP) and animal models of acute pancreatitis (AP)1, 2 include adipokines,3, 4 proteases like trypsin,5–8 unsaturated fatty acids (UFAs) such as oleic and linoleic acid (LA)1, 9, 10 or cytokines including interleukin (IL)-1β,11 IL-6,12–15 IL-8,12, 15, 16 monocyte chemotactic protein-1 (MCP-1)17 and tumour necrosis factor-alpha (TNF-α).13 Scoring systems to risk stratify AP include these.17–20 Clinically, however, SAP markers versus mediators are indistinguishable as causality is difficult to establish while studying human AP in isolation. Moreover, human SAP is independent of aetiology21–24 (with the exception of hypertriglyceridemic AP25–28), and SAP can occur with minimal pancreatic necrosis.29–31
Significance of this study.
What is already known on this subject
Peripancreatic necrosis may be associated with severe acute pancreatitis (SAP).
Cytokines and unsaturated fatty acids (UFAs) are increased in human and animal models of acute pancreatitis.
It is unclear whether these are markers versus mediators of SAP.
What are the new findings?
Pancreatic necrotic collections have higher interleukin (IL)-1β, IL-8 and UFA levels than pancreatic cystic neoplasms.
UFAs at concentrations less than in necrotic collections but not IL-1β and IL-8 cause necro-apoptosis.
Peripancreatic fat lipolysis causes multisystem injury independent of pancreatic necrosis.
How might it impact on clinical practice in the foreseeable future?
The distinction between a marker and mediator of SAP is made clear in this study.
It supports that targeting peripancreatic fat necrosis independent of pancreatic necrosis may improve SAP outcomes.
It supports that targeting lipolytic generation of UFAs, but not cytokines, may improve SAP outcomes.
In contrast, severity of animal models of AP is based on the aetiology (eg, bile salt vs caerulein32, 33) causing severe necrosis alone or with worse outcomes,33 and therapies are considered relevant based on their efficacy in aetiologically different models. This rationale and lack of distinction between marker and mediators may partly explain the limited benefit noted in several clinical trials of AP, targeting proteases,34–43 reactive oxygen species44 and inflammatory mediators45 and mixed outcomes noted in the few case reports of anti-TNF therapy,46–50 though these targets seem scientifically sound based on animal models of AP. Interestingly, hereditary pancreatitis due to cationic trypsinogen (PRSS1) gene mutations, despite its high penetrance in initiating AP does not cause necrotising pancreatitis (NP) or AP-related mortality.51 Similarly, while cytokines are elevated in SAP, some studies show cytokines do not induce SAP-associated outcomes,52–56 and others have shown IL-657 and TNF-α58 to be protective in AP. These controversies support the need to look for alternate targets, which are mediators of SAP rather than its markers.
Several epidemiological studies suggest obesity59–66 or increased intra-abdominal fat is associated with SAP.63, 67–69 These fat depots may undergo necrosis during AP and this has been linked to SAP for more than a hundred years.70 Peripancreatic fat necrosis is in the spectrum of necrotising pancreatitis,71, 72 is a part of the revised Atlanta criteria,72 radiographic severity scoring systems (eg, Schroeder and Balthazar scoring)3, 73 and correlates with worse outcomes during AP.74, 75 However, it is unknown whether this has a causal role or is a mere marker of severity.
Triglyceride, which forms the bulk of adipocytes that are increased in obesity,76–78 is a good substrate for the lipases released basolaterally during pancreatitis79–82 as noted in human disease.83, 84 In vitro studies by Mossner et al85 more than two decades ago showed a protective effect of lipase inhibition in vitro but not in vivo AP models.86 Thus, it remains unclear whether it is the exaggerated baseline proinflammatory state87–89 or some other acute mechanism that worsens AP outcomes in obesity.
We recently showed intrapancreatic lipase inhibition reduces necrosis and improves outcomes of biliary AP exacerbated by intrapancreatic fat.90 Thus, the observations that (i) AP outcomes in humans unlike rodent models are independent of aetiology, (ii) AP, associated with peripancreatic necrosis in humans, is severe for unclear reasons and (iii) cytokines have an unclear role in SAP outcomes prompted us to study the mechanisms of worse outcomes in human AP.
To do so, we initially determined the agents that are increased in human pancreatic necrotic collections (NC) that are known to have both pancreatic and peripancreatic necrosis.71, 72 A PubMed search failed to pull up guidelines or an authoritative text on criteria that may be used to conclusively prove causality in human disease. We thus applied Koch’s postulates (http://ocp.hul.harvard.edu/contagion/koch.html) of causality to determine mediators of SAP, whereby the mediator should (A) be present at high levels in an affected source (NC) compared with controls, that is, pancreatic cystic neoplasms (PCN); (B) be isolated from NCs in pure form; (C) convert mild AP in lean rodents to SAP when added in a pure form and (D) be re-isolated in this setting (equivalent to re-isolating the microorganism). We also went a step further to study the effect of preventing mediator generation on outcomes of AP.
We chose a pharmacological approach over a genetic approach since pancreatic lipases have a redundant role,91 dual lipase knockouts are embryonically lethal92 and us using of the mildest AP model, that is, caerulein AP in rats, to study exacerbating factors. Our results highlight the potential role of acute peripancreatic fat lipolysis in worsening outcomes of AP and help distinguish markers from mediators of SAP, along with suggesting a potential approach to improve SAP outcomes.
METHODS
Human pancreatic fluid collections
Samples were collected as a part of protocols described previously.90 One of the original 15 NC fluid was too small in volume for the detailed analysis done in this study. Information collected included age, gender, body mass index (BMI), primary diagnosis, disease duration at the time of collection and modality of collection (ie, surgical or endoscopic). Sample collection predated the revised Atlanta criteria.72 For purposes of the study, the clinician classified samples as NCs (collections with solid debris, n=14) or pseudocysts (PCs) (fluid collections, n=11). In our study, all PCs were at least 3 months old. We chose PCN as the non-inflammatory control group (n=10); these included intra-pancreatic mucinous neoplasm, serous cystadenomas and mucinous cystic neoplasms. Minimum disease duration at intervention from the time of diagnosis of AP was 4 weeks, consistent with established guidelines.93, 94
Animal work
Wistar rats (Charles River L, Wilmington, Massachusetts, USA) were acclimatised for at least two days prior to use. They were housed with a 12 h light/dark cycle, at temperatures ranging from 21 to 25°C, fed standard laboratory chow and allowed to drink ad libitum. Caerulein was purchased from Bachem (King of Prussia, Pennsylvania, USA). Other agents were procured as mentioned in relevant sections below.
Rat acinar cells were prepared fresh and used as described previously.95–97 Results reported for in vitro studies are from five experiments carried out in duplicates. For these in vitro studies, fatty acids were dissolved as described previously1 and cytokines (IL-1β and keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO); Peprotech Rocky Hill, New Jersey, USA) were dissolved as per the manufacturer’s instructions. Caerulein pancreatitis was induced in rats with two doses of 20 μg/kg of caerulein administered intraperitoneally, for three consecutive days as described previously.97, 98 This dose has been shown to reliably cause hyperamylasemia and induce oedema and inflammation of the pancreas.97, 98 Equivalent doses to this have been used by different groups.99, 100 Rats were sacrificed 3 days after the induction of pancreatitis. For caerulein pancreatitis with IL-1β and KC/GRO (the rat homologue of IL-8), these cytokines were dissolved as per the manufacturer’s instruction and a total of six doses were administered at 200 ng/kg/dose and 6.0 μg/kg/dose, respectively, two times per day by tail vein for three consecutive days. These were given along with the caerulein injections (20 μg/kg/dose of caerulein administered intraperitoneally, two times per day, for 3 days). The daily doses are based on the mean serum concentrations of IL-1β (367 ng/L) and IL-8 (12 μg/L) in the severe glyceryl trilinoleate (GTL) model published previously.90 These doses cumulatively (ie, 1200 ng/kg and 36 μg/kg) exceed the median concentrations accumulated over several weeks in the NCs, which are 729 and 497 ng/L, respectively. Doses in these ranges have been used previously in rats53, 101–103 These animals were sacrificed 2–4 h after the last injection. For caerulein pancreatitis in rats coadministered triolein or triolein+orlistat, a single dose of 3 mL (3% body weight) triolein (TCI, Philadelphia, Pennsylvania, USA) or triolein+orlistat (Cayman Chemical, Ann Arbor, Michigan, USA, 50 mg/kg dissolved in the triolein) was given intraperitoneally 2 h after the first dose of caerulein. Preliminary triolein dosing studies using 2% and 1% body weight were done on five animals per group (see online supplementary figure S3). Studies for this article had 8–12 animals in each group for the lung lavages and histology, with a total of 16–28 for mortality studies. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, Pennsylvania, USA) and the Mayo Clinic (Scottsdale, Arizona, USA).
Graphical depiction
Box and whisker plots were used unless there were <8 samples/group, in which case bar graphs were used. Box and whisker plots show the mean (dotted line), median (solid line), 25th and 75th centiles (two boxes) and 10th and 90th centiles (whiskers). Tukey–Kramer test when significant is depicted as ‘*’in human studies. Summary statistics includes means±SEM or 95% CIs and/or medians and IQRs. All significance levels were evaluated at the two-tailed, p<0.05 level. For animal studies, all groups were compared by analysis of variance (ANOVA) to control. An ‘*’indicates p<0.05 versus controls (Con) groups. † indicates p<0.05 between treatments with and without the lipase inhibitor orlistat. Other methods are detailed in the online supplementary section.
Results
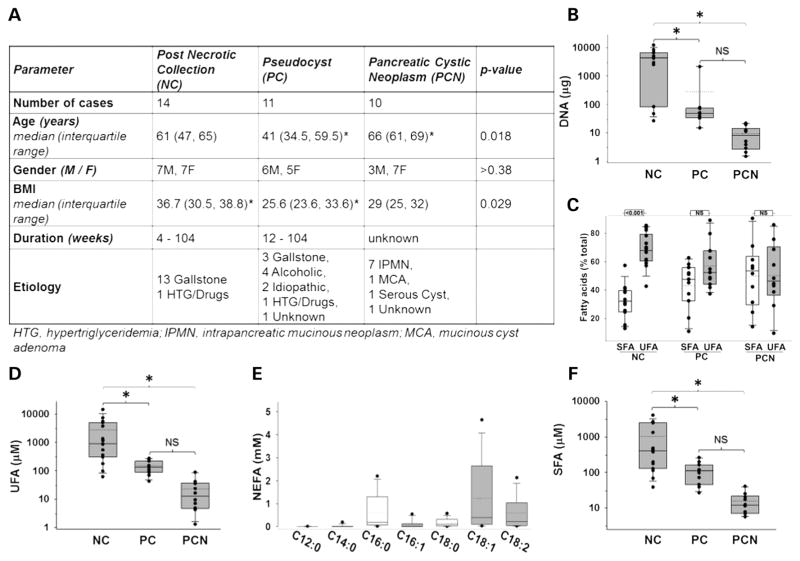
NCs occur in obese patients and are enriched in UFAs, IL-1β and IL-8
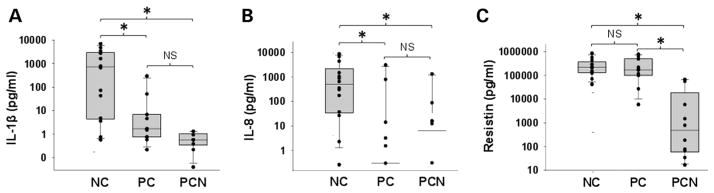
Patients with NCs had a higher BMI than those with PCs (figure 1A). Biliary pancreatitis accounted for 13 of the 14 NCs. NCs had the highest DNA concentration (a marker of dead or exfoliated cells; figure 1B), UFAs (figure 1C, D) and SFAs (figure 1C, F). Of these, oleic (C18:1, 1235±412 μM), palmitic (C16:0, 613±214 μM), linoleic (C18:2, 586±185 μM) and stearic (C18:0, 170±46 μM) acids (figure 1E) were the main non-esterified fatty acid (NEFA). NCs also had the highest IL-1β and IL-8 (figure 2A, B), and resistin was higher in both NCs and PCs compared with PCNs (figure 2C). However, IL-6, MCP-1, nerve growth factor and HGF were similar among all groups (see online supplementary figure S1). The same agents as those increased in NCs (except IL-8) were elevated on combining all inflammatory collections (NCs and PCs) in comparison to non-inflammatory ones (PCNs) (see online supplementary figure S2).
Figure 1.
Necrotic collections (NCs) occur in obese patients and are enriched in DNA and unsaturated fatty acids (UFAs). (A) Table showing biometric data, disease duration at the time of intervention and aetiology of pancreatic fluid collections. Patients with pancreatic cystic neoplasms (PCNs) were older than and those with NCs had higher body mass index (BMI) than those with pseudocysts (PCs). p Values for age and BMI (one-way analysis of variance) and gender (two-tailed Fishers exact test) are mentioned. * indicates groups significantly different (p<0.05) from each other. Box plots showing (B) DNA, (C) proportion of UFAs, (D) amounts of UFA, (E) non-esterified fatty acid (NEFA), (F) SFAs in NC, PC and PCN. * indicates groups significantly different (p<0.05), and ‘NS’ those not significantly different from each other.
Figure 2.
Necrotic collections (NCs) are enriched in interleukin (IL)-1β, IL-8 and resistin. Box plots showing (A) IL-1β, (B) IL-8 and (C) resistin levels in log scale being elevated in NC and pseudocysts (PC) compared with pancreatic cystic neoplasms (PCN). * indicates groups significantly different (p<0.05), and ‘NS’ those not significantly different from each other.
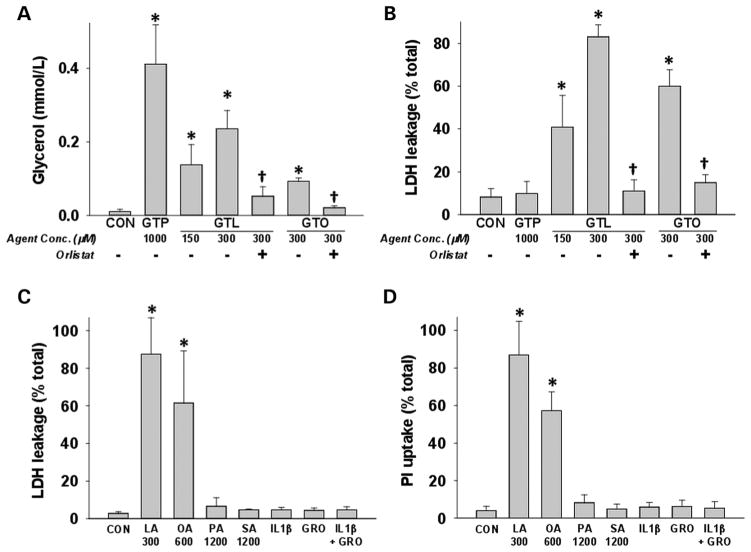
UFAs, but not other agents, enriched in NCs or PCs induce necrosis
Lipolysis of triglycerides by pancreatic lipases was measured by glycerol generation after incubating acini with glyceryl tripalmitate (GTP), a saturated triglyceride, or the unsaturated triglycerides (UTGs), GTL or glyceryl trioleate (GTO) alone, or with the lipase inhibitor orlistat. Despite glycerol generation from GTL, GTO and GTP (figure 3A), significantly increased cell injury quantified by lactate dehydrogenase (LDH) leakage was only noted in the presence of GTL and GTO (figure 3B). Both lipolysis and cell injury were prevented by orlistat. To identify agents mediating acinar injury, we incubated pancreatic acini with agents increased in NCs or PCs at relevant concentrations and quantified LDH leakage or propidium iodide (PI, figure 3C, D) uptake after 5 h. The UFAs oleic acid (OA) and LA, at half the concentrations noted in NCs, caused acinar necrosis, while SFAs (palmitic acid (1.2 mM) and stearic acid (1.2 mM)), cytokines IL-1β and KC/GRO (the rat homologue of IL-8) alone or in combination, all of which were more than twofold concentrations in NCs, did not. The same was shown to be true for resistin recently.104
Figure 3.
Unsaturated fatty acids generated via triglyceride lipolysis induce acinar cell death: (A) glycerol concentrations in medium from acini incubated with saturated (glyceryl tripalmitate (GTP)) and unsaturated triglycerides (glyceryl trilinoleate (GTL)), glyceryl trioleate (GTO)) are reduced by orlistat. Only GTL and GTO cause cell injury quantified as (B)% lactate dehydrogenase (LDH) leakage, which is prevented by orlistat (50 μM). Acinar necrosis measured at 5 h as %LDH leakage (C) or as propidium iodide uptake (D) induced by various agents elevated in necrotic collections (NCs) and pseudocysts. Only oleic (OA) and linoleic (LA) acids induced necrosis at half their concentrations in NCs. Palmitic acid, stearic acid (SA) and inflammatory cytokines (10 ng/mL each) did not cause necrosis. These are more than two times the concentrations in NCs. * indicates groups significantly different (p<0.05) from control, and † indicates significant reduction of measured parameter upon lipase inhibition by orlistat versus without orlistat. Numbers on X-axis represent micromolar amounts.
Peripancreatic UTG lipolysis, unlike cytokines increased in NCs, worsens AP independent of pancreatic necrosis and acute inflammatory cell infiltration
We have recently shown intrapancreatic UTG lipolysis to cause severe pancreatic necrosis.90 To understand the role of peripancreatic fat and discriminate the role of this acute lipotoxicity from that of cytokines in AP outcomes, we compared caerulein pancreatitis outcomes in lean rats coadministered IL-1β and KC/GRO with those coadministered triolein alone or with orlistat. The triolein dose (3% body weight) was based on (A) fat exceeding 30% body weight in obesity,105 (B) visceral fat averaging >3% body weight in obese humans106, 107 and (C) preliminary dosing studies on mortality and organ failure (see online supplementary figure S3). The cytokine doses chosen, as detailed in the methods, are relevant to those in NCs and the serum cytokine concentrations found in the severe GTL model published previously.90
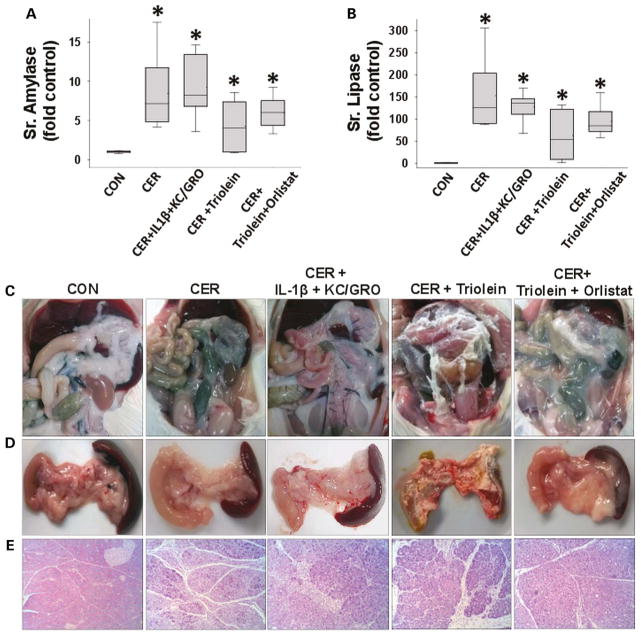
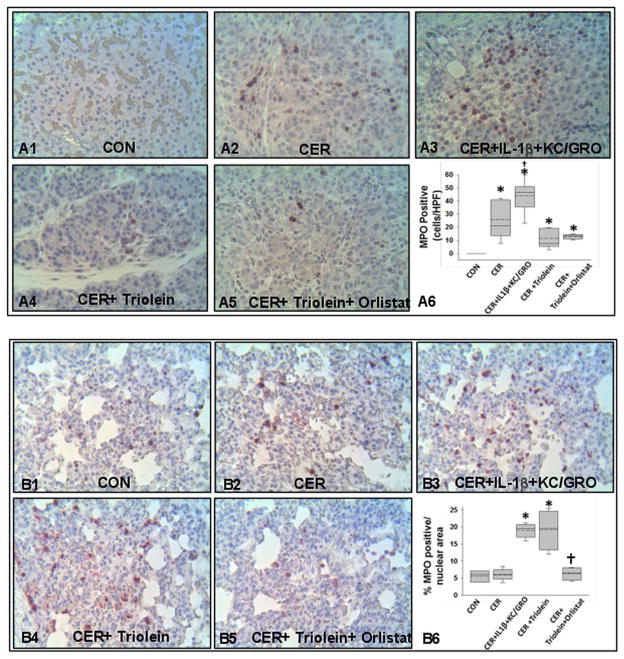
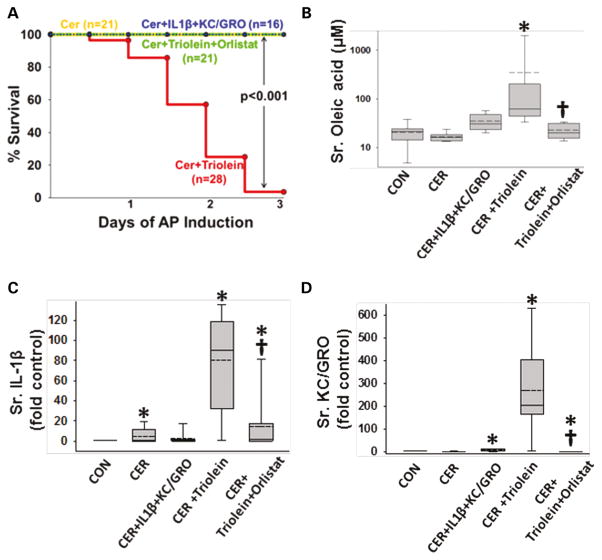
Administration of IL-1β and KC/GRO with caerulein caused a febrile response (38.5±0.5°C vs37.2±0.4°C in controls, p<0.001) consistent with the effects of IL-1β described previously.53, 102, 103 The parameters of induction of pancreatitis, that is, increase in serum amylase or lipase more than threefold above controls used in humans108 were similar in all groups (figure 4A, B). However, lipase activity in the ascites of rats with pancreatitis was increased, which was prevented in the orlistat-treated group (see online supplementary figure S4), supporting a local effect on peripancreatic lipolysis. At the time of necropsy, the triolein-treated group had strands of saponified fat in the peritoneal cavity and around the pancreas (figure 4C, D), while those coadministered triolein with orlistat had a thick whitish fluid coating, consistent with the appearance of unhydrolysed triolein. On histology, there was no evidence of pancreatic necrosis histologically among any of the pancreatitis groups (figure 4E). While cytokine coadministration did increase infiltration of myeloperoxidase (MPO)-positive cells into the pancreas (figure 5A3, A6) and lungs (figure 5B3, B6) compared with caerulein alone (figure 5A2, B2), it did not cause mortality in caerulein AP (figure 6A). In contrast rats coadministered triolein had 97% mortality, increased serum oleate, IL-1β and KC/GRO (Figure 6A–D). All these were dramatically improved in the group coadministered orlistat along with the triolein. Triolein administration alone did not result in induction of pancreatitis or any of the changes described above (see online supplementary figure S5).
Figure 4.
During caerulein pancreatitis, coadministration of interleukin (IL)-1β with keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO), or intraperitoneal triolein alone or with orlistat does not affect pancreatitis initiation or cause pancreatic necrosis: serum amylase (A) and lipase (B) were similarly increased more than threefold above normal in all groups with caerulein pancreatitis at 1 day. Fat stranding in the peritoneal cavity (C) and surface of pancreata (D) in animals coadministered triolein with caerulein (CER) was prevented by orlistat, with remnant unhydrolysed triolein appearing as a thick whitish liquid coating. (E) Histologically there was no evidence of necrosis in any group. Please note ductal metaplasia-like appearance in survivors up to 3 days.
Figure 5.
Interleukin (IL)-1β+keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) and triolein coadministration results in worse local and systemic inflammation during caerulein (CER) pancreatitis. Immunohistochemistry images for myeloperoxidase (MPO) in the pancreas (A1–5) and lungs (B1–5), with each group mentioned at the bottom of the image. While caerulein pancreatitis increased MPO-positive cells compared with controls (A2, A6*), IL-1β+KC/GRO coadministration caused a further increase (A3, A6 †), compared with caerulein alone. Systemically, in the lungs, IL-1β+KC/GRO coadministration (B3) and triolein coadministration (B4) significantly increased MPO-positive cells compared with caerulein pancreatitis alone (B2) or other groups (B1,4,5). Orlistat treatment significantly reduced (B6, †) the lung MPO increased noted in the CER+ triolein group.
Figure 6.
Lipolysis of triolein unlike interleukin (IL)-1β+keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) coadministration results in a worse cytokine response and mortality in caerulein (CER) pancreatitis. (A) Kaplan–Meier curves showing time course of mortality in the triolein coadministered group (red), which is prevented by orlistat (green) and is absent in other groups. Number per group is mentioned in parenthesis. Serum oleate (B), IL-1β (C) and KC/GRO (D) are significantly increased in the acute pancreatitis (AP) group coadministered triolein and reduced by orlistat. Multiple comparisons were done using one-way analysis of variance. Significant (p<0.05) difference from controls is denoted with an *; † over the CER+triolein+orlistat group indicates significant difference from CER+triolein.
Lipotoxic cell death mediated by UFAs results in multisystem organ failure
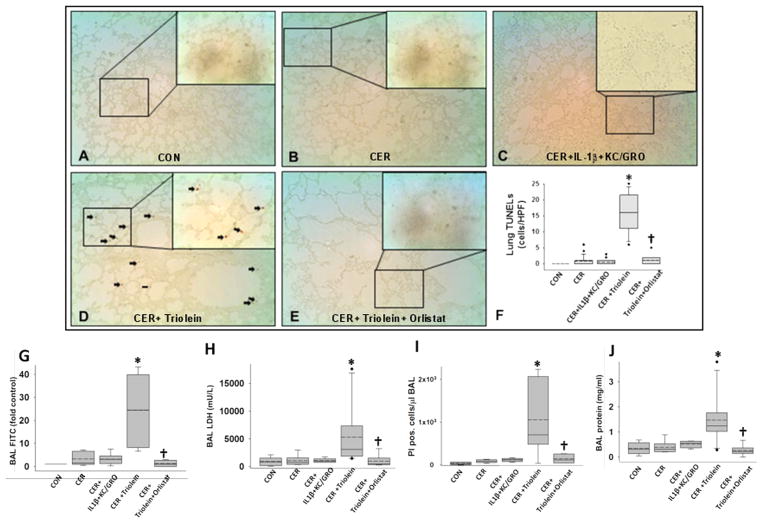
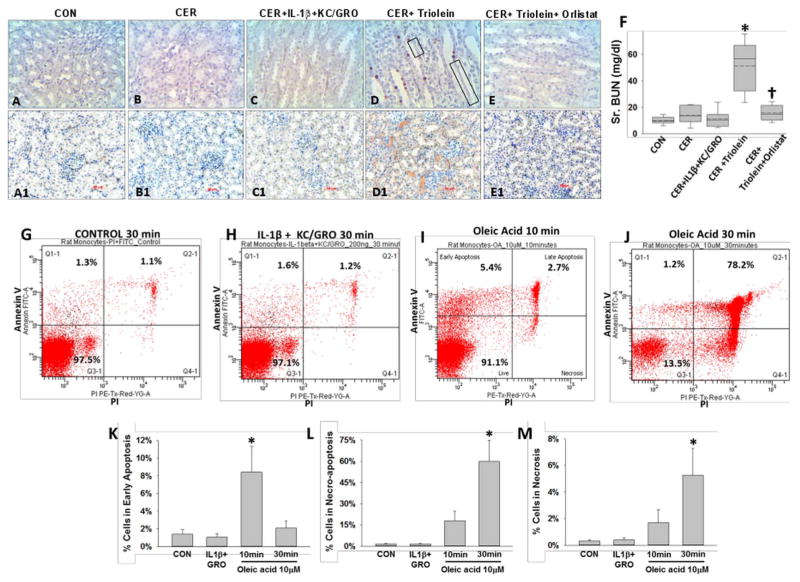
Mortality from SAP in humans can occur with multisystem organ failure (MSOF) independent of necrosis.72, 74 Thus, we looked for evidence of kidney failure and lung injury. Rats with pancreatitis coadministered triolein had respiratory failure evidenced by a reduction of oxygen saturation from 97±1% at baseline to 89.3±0.9% (p<0.01) 2 h prior to sacrifice. This was associated with an increase in terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive cells in the lungs (figure 7A–F), as previously shown in lipotoxic acute respiratory distress syndrome.1, 109–112 The bronchoalveolar lavage of the triolein coadministered group had an increase in fluorescein isothiocyanate leakage dextran, LDH leakage, protein and PI-positive cells (* in figure 7G–J). All these detrimental changes in parameters of lung function and lung injury were absent in all other groups and completely prevented in the rats coadministered orlistat († in figure 7E–J). The kidneys of the caerulein+GTO group had kidney injury evidenced by TUNEL-positive tubular cells, loss of tubular cells (boxes in figure 8D), kidney injury molecule-1 (KIM-1) positivity in the renal tubules (figure 8D1) and a higher serum blood urea nitrogen (figure 8F), consistent with renal failure. These adverse outcomes were prevented by orlistat (figure 8E, E1, F [†]). The administration of triolein alone (see online supplementary figure S5) or the cytokines along with caerulein (figures 7C and 8C, C1 and graphs in figures 7 and 8) did not result in these adverse outcomes. Peripheral blood mononuclear cells (PBMCs) treated with a fraction of serum OA concentrations (10 μM, figure 8I, J) had a large increase in apoptosis initially, which progressed to predominantly necro-apoptosis. IL-1β and KC/GRO at more than two times serum concentrations did not cause this (figure 8H). These observations suggest that acute lipolytic generation of UFAs from peripancreatic fat worsens outcomes of AP by UFA-mediated necro-apoptotic distant organ injury.
Figure 7.
Lipolysis of triolein unlike interleukin (IL)-1β+keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) coadministration results in lung injury during caerulein (CER) pancreatitis: images of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) of lungs (A–E) with each group mentioned at the bottom of the image. There was significantly more positive staining in the lungs (D) of CER+triolein group (*), which is prevented by orlistat (E and F), depicted as †. Bronchoalveolar lavage (BAL) fluid was analysed for fluorescein isothiocyanate (FITC) fluorescence (G), lactate dehydrogenase (LDH; H), total protein (I) or stained with propidium iodide, and the fluorescent cells were counted (J). Significant (p<0.05) difference from controls is denoted with an *; † over the CER+triolein+orlistat group indicates significant difference from CER +triolein.
Figure 8.
Lipolysis of triolein unlike interleukin (IL)-1β+keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) coadministration results in necro-apoptotic injury and renal failure during caerulein (CER) pancreatitis: images of terminal deoxynucleotidyl transferase dUTP nick end labelling (A–E) and immunohistochemistry for kidney injury molecule-1 (A1–E1) in kidneys, with each group mentioned above the image. There was positive staining in the CER+triolein group, sloughing of renal tubular cells (black boxes) along with renal failure evidenced by an increase in serum blood urea nitrogen (BUN) (F, *), which is prevented by orlistat depicted as †. Rat peripheral blood mononuclear cells from the control group (G), those treated with IL-1b+KC/GRO (200 ng/mL each for 30 min; H), 10 μM oleic acid (OA) for 10 min (I) or 30 min (J) were stained for annexin V and propidium iodide (PI) and analysed by flow cytometry. OA significantly increased (*) early apoptosis at 10 min (K), which progressed to predominantly necro-apoptosis by 30 min (L) with progressively increasing necrosis (M).
DISCUSSION
In this study, we initially found NCs to have high levels of UFAs, IL-1β, IL-8 and resistin, which remained significant after adjusting for age, gender and BMI. Peripancreatic necrosis is a part of necrotising pancreatitis.71, 72 Having previously shown that intrapancreatic UTG lipolysis worsens pancreatic necrosis,90 in this study we tested whether lipolysis of extra-pancreatic UTG or cytokines caused MSOF independent of pancreatic necrosis. Applying Koch’s postulates to sort out which agent most closely fulfils these by worsening AP to SAP, we identified UFAs (with oleate as the prototype, online supplementary figure S6) as being the best candidates for being SAP mediators. The acute conversion of caerulein AP to lethal disease by peripancreatic UTG lipolysis in lean rats suggests acute UFA toxicity worsens AP and not the baseline or exaggerated proinflammatory response of obesity. Using the mild caerulein model, we also learn that like in human disease,21–24 outcomes of AP can be unrelated to aetiology, with a modifier such as lipotoxicity. Lastly, we learn that peripancreatic fat necrosis can worsen outcomes independent of pancreatic necrosis by distant organ injury. Recent clinical literature 3, 74, 75, 113 and the revised Atlanta criteria72 accept peripancreatic necrosis as a risk factor for SAP. These parallels we note have mechanistic, translational and therapeutic relevance, and are discussed below.
Peripancreatic fat necrosis causes SAP in the absence of pancreatic necrosis
Early mortality in human SAP may occur with minimal pancreatic necrosis.29, 30, 31 Traditional animal models such as the taurocholate model however relate severity to local necrosis.33 Similarly, the mouse caerulein AP is regarded as severe compared with rat AP114 since mice have more necrosis.33 In the current study, we note peripancreatic lipolysis of triolein to cause mortality in caerulein pancreatitis without pancreatic necrosis. This phenomenon, which replicates the picture in human SAP, is prevented by lipase inhibition.
Unsaturated fatty acid lipotoxicity due to visceral fat lipolysis mediates MSOF in SAP
We note the UFA OA to be the most abundant fatty acid in NCs. UFAs inhibit mitochondrial complexes I and V.1 While we noted early caspase 3/7 activation in acini, this was not sustained (results not shown), perhaps due to concurrent ATP depletion104, 115, 116 triggering the necrosis noted at 5 h. Noting this, and the dual annexin V and PI staining of PBMCs, TUNEL positivity in lungs and renal tubules (which are also KIM-1 positive), the likely mode of injury induced by UFAs is necro-apoptotic.
Serum oleate concentrations in rats coadministered triolein and caerulein (350±294 μM) are similar to patients with SAP with complications (614±146 μM).117 An increase in serum C18 UFAs (mainly oleate), similar to what we note, was reported in dogs with AP.118 Patients with SAP with complications also had serum free fatty acid >1400 μM,117 which is similar to what we noted in our study (1421±851 μM). We have previously shown elevated UFAs in biliary SAP90 and IL-12, 18 induced AP.1 UFAs cause organ failure including acute lung injury,109–112 renal tubular toxicity,119, 120 renal failure110, 121 and hypocalcaemia.121 This spectrum of endpoints secondary to UFAs and the findings of this study support UFA generation as a target to improve SAP outcomes. The published evidence mentioned above, along with this study showing that MSOF in SAP results from triolein hydrolysis to oleate and not triolein alone (see online supplementary figure S5), brings this two-step phenomenon (ie, increased UTG (in fat)+lipase (in AP)→high UFA→AP with MSOF (ie, SAP)) to closely replicate the third postulate of Koch as shown in online supplementary figure S6. Such two-step fulfilment would be relevant to therapy in diseases such as cholangitis, where neither biliary obstruction alone nor bacteria entering the biliary tract alone (such as post choledochojejunostomy) would cause cholangitis, which would only happen when the two are combined such as after recurrence of a stricture.
The same mode of initiating pancreatitis may have different outcomes
While severity of traditional animal models is attributed to the aetiology,33 outcomes in human AP are unrelated to the aetiology.21–24, 93 While all groups in our study fulfil the diagnostic criteria for AP,108 the improved outcomes in the triolein+ orlistat group with AP were unrelated to serum amylase or lipase, but associated with reduced lipase activity in ascites, supporting the notion that improved outcomes may result from targeting the modifier, that is, lipase-mediated UFA generation from the hydrolysable peripancreatic UTG in visceral fat and not the primary insult. Interestingly, this resonates with hypertriglyceridemic (HTG) pancreatitis being the exceptional AP aetiology that is typically severe,25–28, 122 though excluding the patients with HTG from our analysis in figures 1 and 2 did not change the results.
Serum cytokines are markers of AP severity
Predictive models for SAP often use serum cytokines.11–17, 123 In agreement with the trends noted in patients and consistent with UFAs being proinflammatory,111, 124, 125 cytokines were higher in NCs (also enriched in UFAs) and in rodents administered triolein during AP. These cytokines were reduced by lipase inhibition consistent with previous studies showing cytokine upregulation occurs secondary to UFAs.1 This observation, the lack of cell death in acinar cells and PBMCs treated with IL-1β +KC/GRO, the mild course in the IL-1β+KC/GRO groups (despite higher MPOs and a febrile response), suggests that the increased inflammatory response may be a marker but is not a mediator of the adverse events resulting in SAP. This is supported by previous studies showing cytokine levels follow the course of lipotoxicity and normalise on lipase inhibition.1, 90 Similarly, mice administered IL-6 do not develop organ failure126 and obesity-associated SAP is unaffected in IL-6 knockout (KO) mice.126 Additionally, the elevation of IL-6 and TNF-α in response to intravenous UFAs111 supports UFAs as drivers of inflammation. The cytokines themselves may potentially have a protective role in AP as shown in AP models using IL-6 KO mice57 or on neutralising TNF-α.58 Similarly, IL-8/KC/GRO/CXCL1 mediated neutrophil influx is protective in other models of lung injury.127–129
The human part of this study is limited by its small size, analysis of a limited number of cytokines/chemokines and the lack of data on agents degraded over the course of the illness before intervention was clinically indicated. In addition, the endoscopist or the surgeon characterised collections as NCs or PCs, and since sample collection predated revised Atlanta criteria, we were unable to apply the definitions detailed in the revised criteria. However, this is unlikely to influence our conclusions since all NCs were walled off at the time of intervention (consistent with the definition of walled-off pancreatic necrosis) and grouping NCs and PCs together as being postinflammatory showed similarly high UFAs and cytokines vs PCNs (see online supplementary figure S2), the causality of which was explored in animal and in vitro studies. While we do not have information on underlying chronic pancreatitis in patients with PC, all PCs in our study were present for at least 12 weeks. Thus, the potential misclassification of a small proportion of these collections is unlikely to have significantly influenced our primary finding of UFA-induced lipotoxicity since both NCs and PCs alone or together had higher UFAs compared with PCNs and both IL-1β and IL-8 were analysed mechanistically, even when IL-8 was not significantly different in PCs from PCNs. Lack of information on which fluid collections were infected is also a limitation of this study. Infections are likely to predominantly affect cytokine concentrations. However, the cytokines increased in PCs and/or NCs did not worsen outcomes in our in vitro or animal experiments. Conversely, microbes infecting NP do not produce pancreatic lipases, and UFAs as a consequence, which result in acinar necrosis. Therefore, our observations regarding the role of UFA-induced lipotoxicity are unlikely to be affected by infections.
In summary, human pancreatic necrosis collections are enriched in UFAs and cytokines. Peripancreatic fat necrosis resulting in UFA generation causes MSOF and mortality, converting mild AP to SAP independent of pancreatic necrosis. Therefore, targeting UFA-mediated lipotoxicity, rather than cytokines such as IL-1β or IL-8, may be a better option to improve AP outcomes, especially in the context of obesity.
Supplementary Material
Acknowledgments
Funding This project was supported by grant number RO1DK092460 (VPS) and the Clinical Translational Science Institute (CTSI) supported by the National Institutes of Health through grant numbers UL1RR024153 and UL1TR000005 (VPS, SN). This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory that is supported in part by award P30CA047904. Funding was also provided by a startup package from the University of Pittsburgh, Department of Medicine (VPS).
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2014-308043).
Competing interests None.
Ethics approval Institutional Review Board at the University of Pittsburgh Medical Center (IRB number PRO10120148) and Washington University Medical Center and the Committee for Oversight of Research Involving the Dead at the University of Pittsburgh Medical Center.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors VPS and SN designed and conceptualised the study. Acquisition of data was carried out by PN, KP, CD, RNT, CDO, SN, KL, RB, JC, AS, GIP, AK, DCW, JPD, RAC, CA, DJ, FMM and DY. Analysis and interpretation of data was done by PN, KP, CD, SN, JPD, MDC, RP, RAC, CA, DJ, FMM, DY and VPS. Manuscript was drafted by AS, SN, MDC, RP, DY and VPS, while AS, MDC, RP, AK, DCW, FMM, DY, SN, VPS performed critical revision of the manuscript for important intellectual content. Statistical analysis was done by PN, KP, RNT, MDC, DY and VPS. Funding was obtained by SN and VPS, and the entire study was supervised by VPS
References
- 1.Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra10. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sennello JA, Fayad R, Pini M, et al. Interleukin-18, together with interleukin-12, induces severe acute pancreatitis in obese but not in nonobese leptin-deficient mice. Proc Natl Acad Sci USA. 2008;105:8085–90. doi: 10.1073/pnas.0804091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffler A, Hamer O, Dickopf J, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. Am J Gastroenterol. 2010;105:2474–84. doi: 10.1038/ajg.2010.278. [DOI] [PubMed] [Google Scholar]
- 4.Schaffler A, Hamer OW, Dickopf J, et al. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. Am J Gastroenterol. 2011;106:957–67. doi: 10.1038/ajg.2010.503. [DOI] [PubMed] [Google Scholar]
- 5.Geokas MC, Rinderknecht H, Brodrick JW, et al. Studies on the ascites fluid of acute pancreatitis in man. Am J Dig Dis. 1978;23:182–8. doi: 10.1007/BF01073198. [DOI] [PubMed] [Google Scholar]
- 6.Buchler M, Malfertheiner P, Uhl W, et al. Gabexate mesilate in the therapy of acute pancreatitis. Multicenter study of tolerance of a high intravenous dose (4 g/day) Med Klin (Munich) 1988;83:320–4. 52. [PubMed] [Google Scholar]
- 7.Berling R, Borgstrom A, Ohlsson K. Peritoneal lavage with aprotinin in patients with severe acute pancreatitis. Effects on plasma and peritoneal levels of trypsin and leukocyte proteases and their major inhibitors. Int J Pancreatol. 1998;24:9–17. doi: 10.1007/BF02787525. [DOI] [PubMed] [Google Scholar]
- 8.Renner IG, Rinderknecht H, Douglas AP. Profiles of pure pancreatic secretions in patients with acute pancreatitis: the possible role of proteolytic enzymes in pathogenesis. Gastroenterology. 1978;75:1090–8. [PubMed] [Google Scholar]
- 9.Domschke S, Malfertheiner P, Uhl W, et al. Free fatty acids in serum of patients with acute necrotizing or edematous pancreatitis. Int J Pancreatol. 1993;13:105–10. doi: 10.1007/BF02786078. [DOI] [PubMed] [Google Scholar]
- 10.Panek J, Sztefko K, Drozdz W. Composition of free fatty acid and triglyceride fractions in human necrotic pancreatic tissue. Med Sci Monit. 2001;7:894–8. [PubMed] [Google Scholar]
- 11.Hirota M, Nozawa F, Okabe A, et al. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas. 2000;21:141–6. doi: 10.1097/00006676-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Messmann H, Vogt W, Falk W, et al. Interleukins and their antagonists but not TNF and its receptors are released in post-ERP pancreatitis. Eur J Gastroenterol Hepatol. 1998;10:611–17. doi: 10.1097/00042737-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on acute pancreatitis. Crit Care Med. 1999;27:749–55. doi: 10.1097/00003246-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845–53. doi: 10.3748/wjg.v16.i15.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoun E, Chen J, Reighard D, et al. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology. 2009;9:777–85. doi: 10.1159/000214191. [DOI] [PubMed] [Google Scholar]
- 16.Daniel P, Lesniowski B, Mokrowiecka A, et al. Circulating levels of visfatin, resistin and pro-inflammatory cytokine interleukin-8 in acute pancreatitis. Pancreatology. 2010;10:477–82. doi: 10.1159/000276986. [DOI] [PubMed] [Google Scholar]
- 17.Regner S, Appelros S, Hjalmarsson C, et al. Monocyte chemoattractant protein 1, active carboxypeptidase B and CAPAP at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology. 2008;8:42–9. doi: 10.1159/000114866. [DOI] [PubMed] [Google Scholar]
- 18.Gregoric P, Sijacki A, Stankovic S, et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology. 2010;57:349–53. [PubMed] [Google Scholar]
- 19.Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675–84. doi: 10.3748/wjg.14.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papachristou GI, Whitcomb DC. Predictors of severity and necrosis in acute pancreatitis. Gastroenterol Clin North Am. 2004;33:871–90. doi: 10.1016/j.gtc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Dai CY, Hou NJ, et al. Etiology, severity and recurrence of acute pancreatitis in southern taiwan. J Formos Med Assoc. 2006;105:550–5. doi: 10.1016/S0929-6646(09)60149-2. [DOI] [PubMed] [Google Scholar]
- 22.Sekimoto M, Takada T, Kawarada Y, et al. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin MJ, Chen H, Luo B, et al. Severe acute pancreatitis in the elderly: etiology and clinical characteristics. World J Gastroenterol. 2008;14:2517–21. doi: 10.3748/wjg.14.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidarsdottir H, Moller PH, Thorarinsdottir H, et al. Acute pancreatitis: a prospective study on incidence, etiology, and outcome. Eur J Gastroenterol Hepatol. 2013;25:1068–75. doi: 10.1097/MEG.0b013e3283640fc8. [DOI] [PubMed] [Google Scholar]
- 25.Buch A, Buch J, Carlsen A, et al. Hyperlipidemia and pancreatitis. World J Surg. 1980;4:307–14. doi: 10.1007/BF02393387. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Munoz JE, Malfertheiner P, Ditschuneit HH, et al. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol. 1991;10:261–7. [PubMed] [Google Scholar]
- 27.Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–61. doi: 10.3748/wjg.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloret Linares C, Pelletier AL, Czernichow S, et al. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas. 2008;37:13–2. doi: 10.1097/MPA.0b013e31816074a1. [DOI] [PubMed] [Google Scholar]
- 29.Fu CY, Yeh CN, Hsu JT, et al. Timing of mortality in severe acute pancreatitis: experience from 643 patients. World J Gastroenterol. 2007;13:1966–9. doi: 10.3748/wjg.v13.i13.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnovale A, Rabitti PG, Manes G, et al. Mortality in acute pancreatitis: is it an early or a late event? Jop. 2005;6:438–44. [PubMed] [Google Scholar]
- 31.Mutinga M, Rosenbluth A, Tenner SM, et al. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28:91–5. doi: 10.1385/IJGC:28:2:091. [DOI] [PubMed] [Google Scholar]
- 32.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–16. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 33.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–93. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Andriulli A, Caruso N, Quitadamo M, et al. Antisecretory vs. antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. Jop. 2003;4:41–8. [PubMed] [Google Scholar]
- 35.Andriulli A, Leandro G, Clemente R, et al. Meta-analysis of somatostatin, octreotide and gabexate mesilate in the therapy of acute pancreatitis. Aliment Pharmacol Ther. 1998;12:237–45. doi: 10.1046/j.1365-2036.1998.00295.x. [DOI] [PubMed] [Google Scholar]
- 36.Asang E. Changes in the therapy of inflammatory diseases of the pancreas. A report on 1 year of therapy and prophylaxis with the kallikrein- and trypsin inactivator trasylol (Bayer) Langenbecks Arch Klin Chir Ver Dtsch Z Chir. 1960;293:645–70. [PubMed] [Google Scholar]
- 37.Buchler M, Malfertheiner P, Uhl W, et al. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology. 1993;104:1165–70. doi: 10.1016/0016-5085(93)90288-n. [DOI] [PubMed] [Google Scholar]
- 38.Chen HM, Chen JC, Hwang TL, et al. Prospective and randomized study of gabexate mesilate for the treatment of severe acute pancreatitis with organ dysfunction. Hepatogastroenterology. 2000;47:1147–50. [PubMed] [Google Scholar]
- 39.Park KT, Kang DH, Choi CW, et al. Is high-dose nafamostat mesilate effective for the prevention of post-ERCP pancreatitis, especially in high-risk patients? Pancreas. 2011;40:1215–19. doi: 10.1097/MPA.0b013e31822116d5. [DOI] [PubMed] [Google Scholar]
- 40.Seta T, Noguchi Y, Shimada T, et al. Treatment of acute pancreatitis with protease inhibitors: a meta-analysis. Eur J Gastroenterol Hepatol. 2004;16:1287–93. doi: 10.1097/00042737-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Trapnell JE, Rigby CC, Talbot CH, et al. Proceedings: Aprotinin in the treatment of acute pancreatitis. Gut. 1973;14:828. [PubMed] [Google Scholar]
- 42.Trapnell JE, Rigby CC, Talbot CH, et al. A controlled trial of Trasylol in the treatment of acute pancreatitis. Br J Surg. 1974;61:177–82. doi: 10.1002/bjs.1800610303. [DOI] [PubMed] [Google Scholar]
- 43.Trapnell JE, Talbot CH, Capper WM. Trasylol in acute pancreatitis. Am J Dig Dis. 1967;12:409–12. doi: 10.1007/BF02241945. [DOI] [PubMed] [Google Scholar]
- 44.Abbasinazari M, Mohammad Alizadeh AH, Moshiri K, et al. Does allopurinol prevent post endoscopic retrograde cholangio-pancreatography pancreatitis? A randomized double blind trial. Acta Med Iran. 2011;49:579–83. [PubMed] [Google Scholar]
- 45.Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–9. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maser EA, Deconda D, Lichtiger S, et al. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6:1112–16. doi: 10.1016/j.cgh.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 47.Zeitz J, Huber M, Rogler G. Serious course of a miliary tuberculosis in a 34-year-old patient with ulcerative colitis and HIV infection under concomitant therapy with infliximab. Med Klin (Munich) 2010;105:314–18. doi: 10.1007/s00063-010-1046-2. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez-Fernandez SG, Tremoulet AH. Infliximab treatment of pancreatitis complicating acute kawasaki disease. Pediatr Infect Dis J. 2012;31:1087–9. doi: 10.1097/INF.0b013e31826108c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triantafillidis JK, Cheracakis P, Hereti IA, et al. Acute idiopathic pancreatitis complicating active Crohn’s disease: favorable response to infliximab treatment. Am J Gastroenterol. 2000;95:3334–6. doi: 10.1111/j.1572-0241.2000.03332.x. [DOI] [PubMed] [Google Scholar]
- 50.Fefferman DS, Alsahli M, Lodhavia PJ, et al. Re: Triantafillidis –Acute idiopathic pancreatitis complicating active Crohn’s disease: favorable response to infliximab treatment. Am J Gastroenterol. 2001;96:2510–11. doi: 10.1111/j.1572-0241.2001.04070.x. [DOI] [PubMed] [Google Scholar]
- 51.Rebours V, Boutron-Ruault MC, Jooste V, et al. Mortality rate and risk factors in patients with hereditary pancreatitis: uni- and multidimensional analyses. Am J Gastroenterol. 2009;104:2312–17. doi: 10.1038/ajg.2009.363. [DOI] [PubMed] [Google Scholar]
- 52.Wang LZ, Su JY, Lu CY, et al. Effects of recombinant human endothelial-derived interleukin-8 on hemorrhagic shock in rats. Zhongguo Yao Li Xue Bao. 1997;18:434–6. [PubMed] [Google Scholar]
- 53.Morimoto K, Morimoto A, Nakamori T, et al. Cardiovascular responses induced in free-moving rats by immune cytokines. J Physiol. 1992;448:307–20. doi: 10.1113/jphysiol.1992.sp019043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wogensen L, Jensen M, Svensson P, et al. Pancreatic beta-cell function and interleukin-1 beta in plasma during the acute phase response in patients with major burn injuries. Eur J Clin Invest. 1993;23:311–19. doi: 10.1111/j.1365-2362.1993.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Ballou LR, Morham SG, et al. Cyclooxygenase-2 mediates the febrile response of mice to interleukin-1beta. Brain Res. 2001;910:163–73. doi: 10.1016/s0006-8993(01)02707-x. [DOI] [PubMed] [Google Scholar]
- 56.Bhargava R, Janssen W, Altmann C, et al. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PLoS ONE. 2013;8:e61405. doi: 10.1371/journal.pone.0061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuzzocrea S, Mazzon E, Dugo L, et al. Absence of endogenous interleukin-6 enhances the inflammatory response during acute pancreatitis induced by cerulein in mice. Cytokine. 2002;18:274–85. doi: 10.1006/cyto.2002.0883. [DOI] [PubMed] [Google Scholar]
- 58.Guice KS, Oldham KT, Remick DG, et al. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. J Surg Res. 1991;51:495–9. doi: 10.1016/0022-4804(91)90171-h. [DOI] [PubMed] [Google Scholar]
- 59.Abu Hilal M, Armstrong T. The impact of obesity on the course and outcome of acute pancreatitis. Obes Surg. 2008;18:326–8. doi: 10.1007/s11695-007-9298-5. [DOI] [PubMed] [Google Scholar]
- 60.Papachristou GI, Papachristou DJ, Avula H, et al. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–85. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 61.Porter KA, Banks PA. Obesity as a predictor of severity in acute pancreatitis. Int J Pancreatol. 1991;10:247–52. doi: 10.1007/BF02924162. [DOI] [PubMed] [Google Scholar]
- 62.Shin KY, Lee WS, Chung DW, et al. Influence of obesity on the severity and clinical outcome of acute pancreatitis. Gut Liver. 2011;5:335–9. doi: 10.5009/gnl.2011.5.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Leary DP, O’Neill D, McLaughlin P, et al. Effects of abdominal fat distribution parameters on severity of acute pancreatitis. World J Surg. 2012;36:1679–85. doi: 10.1007/s00268-011-1414-y. [DOI] [PubMed] [Google Scholar]
- 64.Sempere L, Martinez J, de Madaria E, et al. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–64. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 65.Evans AC, Papachristou GI, Whitcomb DC. Obesity and the risk of severe acute pancreatitis. Minerva Gastroenterol Dietol. 2010;56:169–79. [PubMed] [Google Scholar]
- 66.Chen SM, Xiong GS, Wu SM. Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta-analysis. J Dig Dis. 2012;13:244–51. doi: 10.1111/j.1751-2980.2012.00587.x. [DOI] [PubMed] [Google Scholar]
- 67.Sadr-Azodi O, Orsini N, Andren-Sandberg A, et al. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133–9. doi: 10.1038/ajg.2012.381. [DOI] [PubMed] [Google Scholar]
- 68.Yashima Y, Isayama H, Tsujino T, et al. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46:1213–18. doi: 10.1007/s00535-011-0430-x. [DOI] [PubMed] [Google Scholar]
- 69.Funnell IC, Bornman PC, Weakley SP, et al. Obesity: an important prognostic factor in acute pancreatitis. Br J Surg. 1993;80:484–6. doi: 10.1002/bjs.1800800426. [DOI] [PubMed] [Google Scholar]
- 70.Hotchkiss LW., VIII Acute pancreatitis with very extensive fat necrosis. Ann Surg. 1912;56:111–17. doi: 10.1097/00000658-191207000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman ML, Werner J, van Santvoort HC, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176–94. doi: 10.1097/MPA.0b013e318269c660. [DOI] [PubMed] [Google Scholar]
- 72.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 73.Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–6. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 74.Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612–19. doi: 10.1038/ajg.2011.438. [DOI] [PubMed] [Google Scholar]
- 75.Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098–103. doi: 10.1016/j.cgh.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 76.Ren J, Dimitrov I, Sherry AD, et al. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008;49:2055–62. doi: 10.1194/jlr.D800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas LW. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci. 1962;47:179–88. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- 78.Garaulet M, Hernandez-Morante JJ, Lujan J, et al. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- 79.Cosen-Binker LI, Binker MG, Wang CC, et al. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–51. doi: 10.1172/JCI34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fallon MB, Gorelick FS, Anderson JM, et al. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863–72. doi: 10.1016/0016-5085(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 81.Gaisano HY, Lutz MP, Leser J, et al. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lam PP, Cosen Binker LI, Lugea A, et al. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic. 2007;8:605–17. doi: 10.1111/j.1600-0854.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 83.Aho HJ, Sternby B, Kallajoki M, et al. Carboxyl ester lipase in human tissues and in acute pancreatitis. Int J Pancreatol. 1989;5:123–34. doi: 10.1007/BF02924413. [DOI] [PubMed] [Google Scholar]
- 84.Kloppel G, Dreyer T, Willemer S, et al. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409:791–803. doi: 10.1007/BF00710764. [DOI] [PubMed] [Google Scholar]
- 85.Mossner J, Bodeker H, Kimura W, et al. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int J Pancreatol. 1992;12:285–96. doi: 10.1007/BF02924368. [DOI] [PubMed] [Google Scholar]
- 86.Kimura W, Meyer F, Hess D, et al. Comparison of different treatment modalities in experimental pancreatitis in rats. Gastroenterology. 1992;103:1916–24. doi: 10.1016/0016-5085(92)91452-a. [DOI] [PubMed] [Google Scholar]
- 87.Machado RM, Nakandakare ER, Quintao EC, et al. Omega-6 polyunsaturated fatty acids prevent atherosclerosis development in LDLr-KO mice, in spite of displaying a pro-inflammatory profile similar to trans fatty acids. Atherosclerosis. 2012;224:66–74. doi: 10.1016/j.atherosclerosis.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 88.Alam I, Lewis K, Stephens JW, et al. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. 2007;8:119–27. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 89.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durgampudi C, Noel P, Patel K, et al. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. Am J Pathol. 2014;184:1773–84. doi: 10.1016/j.ajpath.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–16. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 92.Miller R, Lowe ME. Carboxyl ester lipase from either mother’s milk or the pancreas is required for efficient dietary triglyceride digestion in suckling mice. J Nutr. 2008;138:927–30. doi: 10.1093/jn/138.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 94.Tenner S, Baillie J, Dewitt J, et al. American college of gastroenterology guidelines: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15. 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 95.Singh VP, Saluja AK, Bhagat L, et al. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–95. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh VP, Saluja AK, Bhagat L, et al. Serine protease inhibitor causes F-actin redistribution and inhibition of calcium-mediated secretion in pancreatic acini. Gastroenterology. 2001;120:1818–27. doi: 10.1053/gast.2001.24883. [DOI] [PubMed] [Google Scholar]
- 97.Singh VP, McNiven MA. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell. 2008;19:2339–47. doi: 10.1091/mbc.E07-11-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhagat L, Singh VP, Song AM, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–5. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 99.Leja-Szpak A, Jaworek J, Tomaszewska R, et al. Melatonin precursor; L-tryptophan protects the pancreas from development of acute pancreatitis through the central site of action. J Physiol Pharmacol. 2004;55:239–54. [PubMed] [Google Scholar]
- 100.Wisner J, Green D, Ferrell L, et al. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988;29:1516–23. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frevert CW, Huang S, Danaee H, et al. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–44. [PubMed] [Google Scholar]
- 102.Coelho MM, Luheshi G, Hopkins SJ, et al. Multiple mechanisms mediate antipyretic action of glucocorticoids. Am J Physiol. 1995;269:R527–35. doi: 10.1152/ajpregu.1995.269.3.R527. [DOI] [PubMed] [Google Scholar]
- 103.Strijbos PJ, Hardwick AJ, Relton JK, et al. Inhibition of central actions of cytokines on fever and thermogenesis by lipocortin-1 involves CRF. Am J Physiol. 1992;263:E632–6. doi: 10.1152/ajpendo.1992.263.4.E632. [DOI] [PubMed] [Google Scholar]
- 104.Acharya C, Cline RA, Jaligama D, et al. Fibrosis Reduces Severity of Acute-on-Chronic Pancreatitis in Humans. Gastroenterology. 2013;145:466–75. doi: 10.1053/j.gastro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choh AC, Demerath EW, Lee M, et al. Genetic analysis of self-reported physical activity and adiposity: the Southwest Ohio Family Study. Public Health Nutr. 2009;12:1052–60. doi: 10.1017/S1368980008003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Demerath EW, Reed D, Choh AC, et al. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity (Silver Spring) 2009;17:2060–6. doi: 10.1038/oby.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–44. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 109.Hussain N, Wu F, Zhu L, et al. Neutrophil apoptosis during the development and resolution of oleic acid-induced acute lung injury in the rat. Am J Respir Cell Mol Biol. 1998;19:867–74. doi: 10.1165/ajrcmb.19.6.3118. [DOI] [PubMed] [Google Scholar]
- 110.Wu RP, Liang XB, Guo H, et al. Protective effect of low potassium dextran solution on acute kidney injury following acute lung injury induced by oleic acid in piglets. Chin Med J (Engl) 2012;125:3093–7. [PubMed] [Google Scholar]
- 111.Inoue H, Nakagawa Y, Ikemura M, et al. Molecular-biological analysis of acute lung injury (ALI) induced by heat exposure and/or intravenous administration of oleic acid. Leg Med (Tokyo) 2012;14:304–8. doi: 10.1016/j.legalmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Lai JP, Bao S, Davis IC, et al. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol. 2009;156:189–200. doi: 10.1111/j.1476-5381.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bakker OJ, van Santvoort H, Besselink MG, et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475–80. doi: 10.1136/gutjnl-2012-302870. [DOI] [PubMed] [Google Scholar]
- 114.Mareninova OA, Sung KF, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–81. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 115.Voronina SG, Barrow SL, Simpson AW, et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology. 2010;138:1976–87. doi: 10.1053/j.gastro.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samad A, James A, Wong J, et al. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. J Biol Chem. 2014;289:23582–95. doi: 10.1074/jbc.M114.589440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230–6. doi: 10.1159/000055816. [DOI] [PubMed] [Google Scholar]
- 118.Lazaro EJ, Bose AK, Spraggins R, et al. Lipid alterations in acute pancreatitis. Can J Surg. 1978;21:270–1. [PubMed] [Google Scholar]
- 119.Moran JH, Nowak G, Grant DF. Analysis of the toxic effects of linoleic acid, 12, 13-cis-epoxyoctadecenoic acid, and 12,13-dihydroxyoctadecenoic acid in rabbit renal cortical mitochondria. Toxicol Appl Pharmacol. 2001;172:150–61. doi: 10.1006/taap.2001.9149. [DOI] [PubMed] [Google Scholar]
- 120.Ishola DA, Jr, Post JA, van Timmeren MM, et al. Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int. 2006;70:724–31. doi: 10.1038/sj.ki.5001629. [DOI] [PubMed] [Google Scholar]
- 121.Dettelbach MA, Deftos LJ, Stewart AF. Intraperitoneal free fatty acids induce severe hypocalcemia in rats: a model for the hypocalcemia of pancreatitis. J Bone Miner Res. 1990;5:1249–55. doi: 10.1002/jbmr.5650051210. [DOI] [PubMed] [Google Scholar]
- 122.Scherer J, Singh VP, Pitchumoni CS, et al. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ueda T, Takeyama Y, Yasuda T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41:158–65. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 124.Kosaka K, Suzuki K, Hayakawa M, et al. Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol Cell Biochem. 1994;139:141–8. doi: 10.1007/BF01081737. [DOI] [PubMed] [Google Scholar]
- 125.Zhao F, Wang W, Fang Y, et al. Molecular mechanism of sustained inflation in acute respiratory distress syndrome. J Trauma Acute Care Surg. 2012;73:1106–13. doi: 10.1097/TA.0b013e318265cc6f. [DOI] [PubMed] [Google Scholar]
- 126.Pini M, Rhodes DH, Castellanos KJ, et al. Role of IL-6 in the resolution of pancreatitis in obese mice. J Leukoc Biol. 2012;91:957–66. doi: 10.1189/jlb.1211627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsai WC, Strieter RM, Wilkowski JM, et al. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–40. [PubMed] [Google Scholar]
- 128.Mehrad B, Wiekowski M, Morrison BE, et al. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am J Respir Crit Care Med. 2002;166:1263–8. doi: 10.1164/rccm.200204-367OC. [DOI] [PubMed] [Google Scholar]
- 129.Batra S, Cai S, Balamayooran G, et al. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188:3458–68. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.