Abstract
Objective
Ovarian carcinoma (OC) is rare in young women and the fraction of early onset OC attributable to inherited mutations in known OC genes is uncertain. We sought to characterize the fraction of OC that is heritable in women diagnosed with ovarian, fallopian tube, or peritoneal carcinoma at forty years of age or younger.
Methods
We sequenced germline DNA from forty-seven women diagnosed with OC at age 40 or younger ascertained through a gynecologic oncology tissue bank or referred from outside providers using BROCA, a targeted capture and massively parallel sequencing platform that can detect all mutation classes. We evaluated 11 genes associated with ovarian carcinoma (BARD1, BRCA1, BRCA2, BRIP1, MLH1, MSH2, MSH6, PALB2, PMS2, RAD51D, and RAD51C) and additional candidate genes in DNA repair (ATM, BAP1, CHEK2, MRE11A, NBN, PTEN, TP53). We counted only clearly damaging mutations.
Results
Damaging mutations in OC genes were identified in 13 of 47 (28%) subjects, of which 10 (77%) occurred in BRCA1 and one each occurred in BRCA2, MSH2, and RAD51D. Women with a strong family history were no more likely to have an OC gene mutation (8/17, 47%) than those without a strong family history (9/30, 30%, P = 0.35). Additionally, damaging mutations in non-OC genes were identified, one in NBN and one in CHEK2.
Conclusions
A high proportion of young women with invasive OC have mutations in BRCA1, and a smaller fraction have mutations in other known OC genes. Family history was not associated with mutation status in these early onset cases.
Keywords: Inherited, Young, Ovarian, Carcinoma, BRCA1, BRCA2
1. Introduction
Of the approximately 22,000 cases of ovarian cancer that are diagnosed each year in the United States, about 2700 occur in women age forty-four or younger [1,2]. However, many of the early onset cases are germ cell or stromal malignancies and the proportion of epithelial ovarian cancer or ovarian carcinoma (OC) occurring in women under 40 years is <10%, with a median age of diagnosis in the early 60s [2]. In many solid malignancies, very early onset is a hallmark of hereditary predisposition [3,4]. However, most unselected OC series that evaluated for genetic predisposition have included few young women [2,5,6,7,8,9], leading to an incomplete understanding of the hereditary factors underlying early onset OC.
Studies of unselected women with OC suggest that inherited damaging mutations in BRCA1 and BRCA2 account for 13%–15% of all OC cases [5,6,7,9]. Women with BRCA1 mutations are on average about 10 years younger than non-mutation carriers or those with BRCA2 mutations [9,10]. A smaller subset of the inherited mutations that predispose to OC fall under the definition of Lynch syndrome, also called hereditary non-polyposis colorectal cancer. Lynch syndrome is caused by mutations in the DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2 [11]. OC patients with Lynch syndrome are often younger than average at diagnosis, with a median age at diagnosis of 43 years [11,12].
In addition to BRCA1, BRCA2, and the Lynch syndrome genes, additional genes associated with hereditary OC have been recently identified including RAD51C, RAD51D, and BRIP1 [5,13,14,15,16,17,18,19]. The contribution of these genes to early onset OC has not been previously evaluated. One study of OC in women under 30 identified no BRCA1 or BRCA2 mutations and two possibly pathogenic MLH1 mutations [20].
Another study of 52 women with OC age forty or younger found a BRCA1 or BRCA2 mutation in 19% (10/52; 8 BRCA1, 2 BRCA2, 95% confidence interval 11–32%) [21], similar to the contribution of BRCA1 and BRCA2 mutations in all-ages cohorts [5,6,7,8,21]. We sought to define the contribution of BRCA1, BRCA2, Lynch syndrome, and recently identified or suspected OC genes including RAD51C, RAD51D, BRIP1, PALB2, and BARD1, as well as other known breast cancer genes in women with early onset OC.
2. Methods
All patients in this study were diagnosed with ovarian, fallopian tube, or peritoneal carcinoma at age 40 years or younger and provided informed consent on a protocol approved by the institutional review board. Patients had either enrolled in genomics research at the time of diagnosis at the University of Washington or were referred by outside providers to the study based on an early age of diagnosis. Patients were excluded if they had OC diagnosed at the time of planned risk-reducing surgery. A “strong family history” was defined as a relative with OC, a relative with breast cancer <50 years of age, or two relatives with breast cancer at any age.
Germline DNA was extracted from blood and sequenced using BROCA, a targeted capture, massively parallel sequencing test developed at the University of Washington [22]. For this study, we sequenced ATM, BAP1, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, MLH1, MSH2, MSH6, MRE11A, NBN, PALB2, PMS2, PTEN, RAD51C, RAD51D, and TP53. 1 µg of DNA was used to create paired-end libraries with ~200 base pair (bp) inserts, hybridized to a custom pool of oligonucleotides for the genomic regions of interest using the SureSelectXT enrichment system on a Bravo liquid handling instrument (Agilent). Samples were then barcoded and sequenced on a HiSeq (Illumina) with 2 × 101 bp paired end reads and a 7 bp index read. Sequencing reads were aligned to the human reference genome (hg19) using BWA (Burrows–Wheeler alignment). Variants were identified using GATK37 and Pindel after indel realignment and base quality recalibration. Variants from low quality (≤50) and low depth of coverage regions (<5 reads) were filtered out. Single nucleotide variants, insertions and deletions, and copy number variations were detected as previously described [22]. Missense mutations were only included if proven to be deleterious (i.e., BRCA1 C61G). All mutations were validated with Sanger sequencing.
Patients were classified as having an OC gene mutation if they had a damaging mutation in one of 11 genes: BRCA1, BRCA2, BRIP1, RAD51C, RAD51D, PALB2, BARD1, MSH2, MLH1, PMS2, and MSH6. Mutations in other candidate genes were not assumed to be causative.
Secondary mutations that restore BRCA2 were evaluated in one case as previously described [23].
3. Results
A total of 47 young women with OC were evaluated, 38 from consecutive enrollment at the University of Washington and 9 from outside referrals. Eleven cases from the UW series were previously reported (Supplemental Table S1) [5,13]. Table 1 compares the clinical characteristics of University of Washington patients to those of the outside referral patients. Eight of 9 outside cases had been previously tested and all but one were negative for BRCA1 and BRCA2 mutations at the time of referral. In this young population of women with OC, histology other than high-grade serous was relatively common (Table 2) and accounted for 26 (55%) of the carcinomas, including 2 undifferentiated (4%), 9 endometrioid (19%), 5 clear cell (11%), 3 mucinous (6%) and 7 low grade serous carcinomas (15%). No mutations were identified amongst low grade serous and mucinous carcinomas. Endometrioid OC had the highest mutation rate (4/9, 44%) and the most diverse gene distribution (2 BRCA1, 1 MSH2, 1 RAD51D, Table 2).
Table 1.
Clinical characteristics of study patients by mode of referral.
| UW tissue bank | Outside referral | |
|---|---|---|
| Number of subjects | 38 | 9 |
| Median age at dx (range) | 37 (27–40) | 37 (28–40) |
| Strong family historya | 11 (29.7%) | 6 (75%) |
| WTb | 25 (65.8%) | 7 (77.8%) |
| BRCA mutationc | 10 (26.3%) | 1 (11.1%) |
| Mutations in non-BRCA OC genesc | 2 (5.3%) | 0 |
| Mutations in other genesd | 1 (2.6%) | 1 (11.1%) |
| Histology | ||
| HG serous | 15 (39.5%) | 6 (66.7%) |
| undifferentiated carcinoma | 2 (5.3%) | 0 |
| LG serous | 6 (15.8%) | 1 (11.1%) |
| Endometrioid | 7 (18.4%) | 2 (22.2%) |
| Clear cell | 5 (13.1%) | 0 |
| Mucinous | 3 (7.9%) | 0 |
| FIGO stagee | ||
| Stage I | 6 (17.1%) | 1 (11.1%) |
| Stage II | 5 (14.3%) | 0 |
| Stage III | 21 (60%) | 6 (66.7%) |
| Stage IV | 3 (8.6%) | 2 (22.2%) |
Abbreviations:
UW = University of Washington, dx = diagnosis, WT = wildtype, OC = ovarian carcinoma, HG serous= high grade serous (grade 2 or 3), LG serous= low grade serous (grade 1).
A relative with OC, a relative with br ca <50 years of age, or two relatives with br ca at any age.
No damaging mutations detected in any genes tested.
MSH2 and RAD51D.
CHEK2 and NBN.
Stage was not available for three cases.
Table 2.
Clinical characteristics of study patients by mutation status.
| N | Genetic status | Mutation breakdown | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OC genes | Other genes | ||||||||
| WTa | MUTb | BRCA1 | BRCA2 | MSH2 | RAD51D | CHEK2 | NBN | ||
| Number of subjects | 47 | 32 | 13 | 10 | 1 | 1 | 1 | 1 | 1 |
| Median age at dx (range) | 37 (27–40) | 36 (27–40) | 38 (31–40) | 37.5 (31–40) | 40 | 38 | 34 | 40 | 38 |
| Strong family historyc | 17 | 9 (52.9%) | 8 (47.1%) | 7 (41.2%) | 1 (5.9%) | 0 | 0 | 0 | NA |
| Personal hx of br ca | 2 | 0 | 2 (100%) | 2 (100%) | 0 | 0 | 0 | 0 | 0 |
| Optimally debulked (<1 cm)d | |||||||||
| Yes | 32 | 22 (68.8%) | 10 (31.2%) | 7 (21.9%) | 1 (3.1%) | 1 (3.1%) | 1 (3.1%) | 1 | 1 |
| No | 6 | 5 (83.3%) | 1 (16.7%) | 1 (16.7%) | 0 | 0 | 0 | 0 | 0 |
| Histology | |||||||||
| HG serous | 21 | 14 (66.7%) | 7 (33.3%) | 6 (28.6%) | 1 (4.8%) | 0 | 0 | 0 | 0 |
| Undifferentiated carcinoma | 2 | 1 (50%) | 1 (50%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 |
| LG serous | 7 | 7 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endometrioid | 7 | 3 (42.9%) | 4 (57.1%) | 2 (22.2%) | 0 | 1 (11.1%) | 1 (11.1%) | 1 (11.1%) | 1 (11.1%) |
| Clear cell | 5 | 4 (80%) | 1 (20%) | 1 (20%) | 0 | 0 | 0 | 0 | 0 |
| Mucinous | 3 | 3 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FIGO stagee | |||||||||
| Stage I | 5 | 4 (80%) | 1 (20%) | 0 | 0 | 0 | 1 (14.3%) | 1 (14.3%) | 1 (14.3%) |
| Stage II | 5 | 3 (60%) | 2 (40%) | 1 (20%) | 0 | 1 (20%) | 0 | 0 | 0 |
| Stage III | 27 | 20 (74.1%) | 7 (25.9%) | 6 (22.2%) | 1 (3.7%) | 0 | 0 | 0 | 0 |
| Stage IV | 5 | 3 (60%) | 2 (40%) | 2 (40%) | 0 | 0 | 0 | 0 | 0 |
Abbreviations:
OC = ovarian carcinoma, WT = wildtype, MUT = mutant, dx = diagnosis, NA = data not available, hx = history, br ca = breast carcinoma, HG serous = high grade serous (grade 2 or 3), LG serous = low grade serous (grade 1).
No damaging mutations detected in any genes tested.
Damaging mutation detected in an “OC” gene.
A relative with OC, a relative with br ca < 50 years of age, or two relatives with br ca at any age.
Two BRCA1, 5 WT have unknown debulking status.
Stage was not available for three cases.
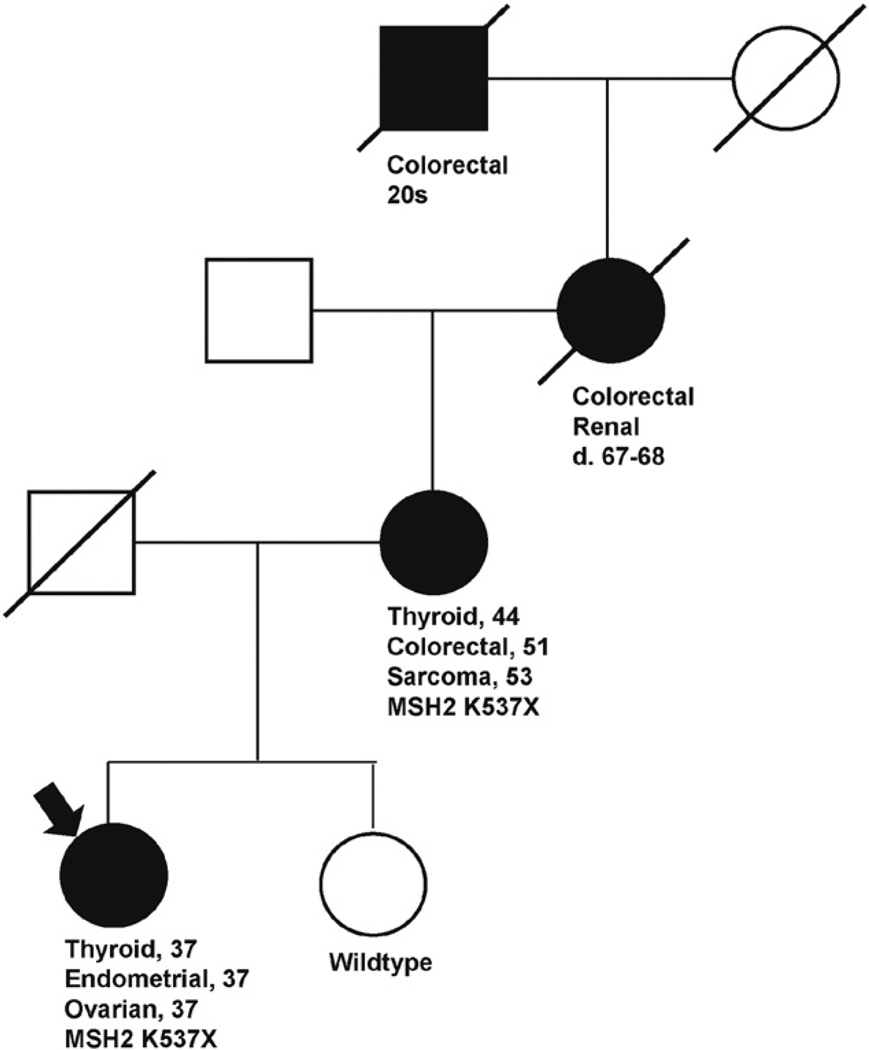
Damaging mutations in OC genes were identified in 12 of 38 (32%) of UW subjects, of which 10 (83%) occurred in BRCA1 or BRCA2, and one each occurred in the non-BRCA OC genes MSH2 and RAD51D. The RAD51D 5810delA frameshift mutation was previously reported by Wickramanayake et al. and occurred in an individual with synchronous endometrioid ovarian and endometrial carcinomas [13]. This individual had insufficient knowledge of her family history of cancer to generate a pedigree. The subject with the MSH2 mutation meets criteria for Lynch syndrome based on revised Amsterdam criteria (Fig. 1).
Fig. 1.
Pedigree of MSH2 mutation carrier diagnosed with OC at age 37 (arrow). Cancers and age of diagnosis are presented for relatives with Lynch-related cancers shaded in black. This family meets Amsterdam revised criteria for Lynch syndrome.
In contrast to the young cases enrolled at diagnosis without previous genetic testing, 8 of the 9 outside referrals had been previously tested for BRCA1 and BRCA2 mutations. No mutations were found in the previously untested patient. Only one BRCA2mutation was found. It had been previously detected in that patient. This mutation (BRCA2 L2653P), initially classified as a variant of unknown significance (VUS), was reclassified to “favor deleterious” by Myriad Genetics at the time of study entry. Loss of the wildtype allele was identified in her primary tumor consistent with the deleterious reclassification. Notably, a reversion to wildtype sequence was identified in her recurrent tumor, at which time she demonstrated progression during treatment with a PARP inhibitor.
In addition to mutations in one of the 11 OC genes, one damaging mutation was identified in a known breast cancer gene, CHEK2 (CHEK2 c.758_761del ACTG), in a patient with endometrioid OC, and a damaging mutation in the breast cancer gene NBN (NBN c.1550_1551insA) was identified in another patient with endometrioid OC. These were considered to be incidental mutations as these genes do not demonstrate a higher mutation rate in our larger series of OC unselected by age compared to population controls (24).
Women with a strong family history were not significantly more likely to have an OC gene mutation (8/17, 47%) compared to those without a strong family history (9/30, 30%, P = 0.35). BRCA1 was the most commonly mutated gene, accounting for 10/13 (77%) OC gene mutations. Two of the 47 patients had a personal history of breast cancer, and both had BRCA1 mutations.
4. Discussion
Our data suggest that BRCA1 mutations account for the vast majority of inherited damaging mutations in young OC patients and early onset OC has a higher BRCA1 mutation rate compared to OC unselected for age. Not counting the 8 cases referred after previous BRCA testing, we found a BRCA1 mutation in 25.6% (10/39) of patients compared to 11.1% when we evaluated 360 women unselected for age (40/360, P = 0.02) [5]. This BRCA1 mutation rate also exceed that found in other series unselected for age including 8% (107/1342, P = 0.002) in Zhang et al. and 9.6% (20/209, P = 0.02) in Pal et al. [6,7]. In a study of OC patients age 40 and younger, Arts-de Jong et al. found a BRCA1 mutation rate of 15.4% (8/52) patients [21]. Our data are consistent with a study we have completed on 1345 women enrolled in clinical trials for the primary therapy of stages III and IV OC, GOG 218 and GOG 262 [24]. In this cohort, 36/1345 (2.7%) women were under the age of 40, and, in that group, 12/36 (33.3%) had germline mutations in BRCA1 [24]. Together, these data demonstrate that very early onset OC is associated with inherited mutations in BRCA1 but only rarely in other OC genes.
Only 1 BRCA2 mutation was identified in our cohort (2.1%). These data are consistent with prospective registry data of BRCA1 and BRCA2 mutation carriers demonstrating that 4% of BRCA1 carriers will have OC by the age of 40 (including cancers identified at the time of risk-reducing surgery) but diagnosis before 40 is very rare in BRCA2 mutation carriers [10]. Mutations in other, non-BRCA OC genes in our young cohort were relatively uncommon, with 2 of 47 (4.2%, 95% confidence interval 1.3–14.3%), patients having a mutation in another OC gene, a similar proportion to the rate we previously found in women unselected for age of diagnosis [5]. Recently, the Ovarian Cancer Association Consortium have reported on OC patients that were sequenced for mutations in RAD51C, RAD51D, PALB2, BRIP1, and BARD1 [25,26]. Only 2 of the approximately 150 that were under age 40 had mutations in these genes, however this rate may be artificially low as the sequencing method was less sensitive and could not detect copy number changes [25,26]. In the GOG 218 and GOG 262 series, there were no mutations in women under age 40 in non-BRCA genes associated with OC (0/36) [24].
A strong family history did not increase the likelihood of identifying an OC mutation in our cohort, though previous history of breast cancer was associated with an increased likelihood of BRCA1 mutations. This result differs from that of a previous study of young OC patients which found that those with one or more first degree relatives with a history of breast and/or ovarian carcinoma had a high probability of carrying a BRCA1 or BRCA2 mutation [21]. However, their definition of “strong family history” was more restrictive than ours, possibly explaining the increased likelihood of identifying a damaging mutation.
Young women with OC in this series had a similar histological distribution compared to previous population studies (Table 2) [6,7,27], but we did have a high fraction (15%) of low grade serous OC, in which we identified no germline OC mutations. Interestingly, the highest mutation rate (44%) and greatest genetic diversity (BRCA1, MSH2, RAD51D), was identified in cases with endometrioid OC. However, the limited number of endometrioid OC (n = 9) preclude definitive conclusions about early onset endometrioid OC.
Our study included only 2women <30 years of age, neither of whom had identifiable mutations. We have too few cases in that age range to speak to the very early onset cases. However, our data demonstrate that BRCA1 mutations are common in women with OC diagnosed in their 30s. Stratton et al. assessed BRCA1, BRCA2, MLH1, and MSH2 mutations in 98 women diagnosed with OC at less than 30 years of age, but only half of the BRCA2 gene was sequenced [20]. These authors found no BRCA1 or BRCA2 mutations and two “probable” mutations in MLH1 (K618T and D304H), of which one, D304H may be pathogenic. The MLH1 K618T has been reclassified as a benign variant (class I on InSiGHT database April 22, 2015 http://insight-group.org/variants/database/). MLH1 D304H is a very rare variant not reported in >66,000 alleles on ExAC (http://exac.broadinstitute.org/gene/ENSG00000076242) that is highly conserved, predicted to be pathogenic (score 1.0 on PolyPhen2, http://genetics.bwh.harvard.edu/pph2/index.shtml) and for which another missense substitution at the same amino acid (D304V) is known to be pathogenic. Furthermore, that patient had endometrioid OC and early onset transitional cell carcinoma of the bladder, a history consistent with Lynch syndrome. Thus, for OC diagnosed less than age 30, hereditary mutations appear rare and may be limited to Lynch syndrome genes.
In this series, we detected one deleterious mutation in NBN and one in CHEK2. Mutations in these two genes are detected relatively often in commercial testing panels. However, larger, case–control studies are needed to determine the relative risk for OC of deleterious mutations in these genes. Our data in a larger series unselected for age do not implicate these genes in the pathogenesis of OC [24]. Providers should exercise caution in ascribing a cause and effect relationship to OC for damaging mutations in these genes and others not proven to associate with OC, regardless of the wording of the test report.
A weakness of this study is its small sample size. However, invasive OC is very rare in women under age 40. In Zhang et al.'s study of 1342 women with OC in Ontario, only 9 invasive cases occurred in women younger than 40 years [6]. Young women are more often diagnosed with borderline neoplasms, which are unlikely to have an inherited predisposition [28]. Reassuringly, our mutation distribution is consistent with young cases from a series unselected for age [24].
In conclusion, a high proportion of women 40 years or younger with invasive OC have mutations in BRCA1, and only a small fraction have mutations in other known OC genes. The remaining cases are likely to be sporadic, but we cannot exclude the possibility that other genes that predispose to early OC have not yet been discovered.
Supplementary Material
HIGHLIGHTS.
Women with ovarian carcinoma age ≤40 have a high rate of BRCA1 mutations.
Deleterious germline mutations were also identified in BRCA2, MSH2, and RAD51D.
The family history did not predict mutation status in these young patients.
Acknowledgments
Financial support: Supported by NIH/NCI grants R01CA131965 (ES), P50CA083636 (ES), the Ovarian Cancer Research Fund (ES, BN), Wendy Feuer Fund for the Prevention and Treatment of Ovarian Cancer (ES), the Cori and Tony Bates Novel Technologies for Cancer Prevention Fund (ES), and the Marsha Rivkin Center for Ovarian Cancer Research (BN).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
The following is the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2015.12.017.
Contributor Information
Sarah S. Bernards, Email: sarahb18@uw.edu.
Barbara M. Norquist, Email: bnorquis@uw.edu.
Maria I. Harrell, Email: maribel@uw.edu.
Kathy J. Agnew, Email: kagnew@uw.edu.
Ming K. Lee, Email: mlee@uw.edu.
Tom Walsh, Email: twalsh@uw.edu.
Elizabeth M. Swisher, Email: swishere@uw.edu.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. (2014) http://dx.doi.org/10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Factsheets: Ovary Cancer. Bethesda, MD: National Cancer Institute; [accessed 5-11-2015]. http://seer.cancer.gov/statfacts/html/ovary.html. [Google Scholar]
- 3.Shuch B, Vourganti S, Ricketts CJ, Middleton L, Peterson J, Merino MJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J. Clin. Oncol. 2014 Feb 10;32(5):431–437. doi: 10.1200/JCO.2013.50.8192. http://dx.doi.org/10.1200/JCO.2013.50.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(c) National Comprehensive Cancer Network, Inc., 2015. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast and/or Ovarian Cancer Genetic Assessment V.1.2015. All rights reserved. http://www.nccn.org (15 May 2015, date last accessed). NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
- 5.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. U. S. A. 2011 Nov 1;108(44):18032–18037. doi: 10.1073/pnas.1115052108. http://dx.doi.org/10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Royer R, Li S, McLaughlin JR, Rosen B, Risch HA, et al. Frequencies of BRCA1 and BRCA2 mutations among 1342 un-selected patients with invasive ovarian cancer. Gynecol. Oncol. 2011 May 1;121(2):353–357. doi: 10.1016/j.ygyno.2011.01.020. http://dx.doi.org/10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005 Dec 15;104(12):2807–2816. doi: 10.1002/cncr.21536. http://dx.doi.org/10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 8.Pal T, Akbari MR, Sun P, Lee J-H, Fulp J, Thompson Z, et al. Frequency of mutations in mismatch repair genes in a population-based study of women with ovarian cancer. Br. J. Cancer. 2012 Nov 6;107(10):1783–1790. doi: 10.1038/bjc.2012.452. http://dx.doi.org/10.1038/bjc.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation—positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J. Clin. Oncol. 2012 Jul 20;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. http://dx.doi.org/10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014 May 20;32(15):1547–1553. doi: 10.1200/JCO.2013.53.2820. http://dx.doi.org/10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu KH, Daniels M. Endometrial and ovarian cancer in women with Lynch syndrome: update in screening and prevention. Familial Cancer. 2013 Jun;12(2):273–277. doi: 10.1007/s10689-013-9664-5. http://dx.doi.org/10.1007/s10689-013-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson P, Bützow R, Lynch HT, Mecklin JP, Järvinen HJ, Vasen HF, et al. The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecol. Oncol. 2001 Aug;82(2):223–228. doi: 10.1006/gyno.2001.6279. http://dx.doi.org/10.1006/gyno.2001.6279. [DOI] [PubMed] [Google Scholar]
- 13.Wickramanayake A, Bernier G, Pennil C, Casadei S, Agnew KJ, Stray SM. Loss of function germline mutations in RAD51D in women with ovarian carcinoma. Gynecol. Oncol. 2012 Dec;127(3):552–555. doi: 10.1016/j.ygyno.2012.09.009. http://dx.doi.org/10.1016/j.ygyno.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat. Genet. 2011 Aug 7;43(9):879–882. doi: 10.1038/ng.893. http://dx.doi.org/10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat. Genet. 2011 Oct 2;43(11):1104–1107. doi: 10.1038/ng.955. http://dx.doi.org/10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 16.Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010 May;42(5):410–414. doi: 10.1038/ng.569. http://dx.doi.org/10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 17.Coulet F, Fajac A, Colas C, Eyries M, Dion-Minière A, Rouzier R, et al. Germline RAD51C mutations in ovarian cancer susceptibility. Clin. Genet. 2013 Apr;83(4):332–336. doi: 10.1111/j.1399-0004.2012.01917.x. http://dx.doi.org/10.1111/j.1399-0004.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- 18.Pelttari LM, Heikkinen T, Thompson D, Kallioniemi A, Schleutker J, Holli K, et al. RAD51C is a susceptibility gene for ovarian cancer. Hum. Mol. Genet. 2011 Aug 15;20(16):3278–3288. doi: 10.1093/hmg/ddr229. http://dx.doi.org/10.1093/hmg/ddr229. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Dicks E, Ramus SJ, Tyrer JP, Intermaggio MP, Hayward J, et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J. Clin. Oncol. 2015 Aug 10; doi: 10.1200/JCO.2015.61.2408. http://dx.doi.org/10.1200/JCO.2015.61.2408 (pii: JCO.2015.61.2408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratton JF, Thompson D, Bobrow L, Dalal N, Gore M, Bishop DT, et al. The genetic epidemiology of early-onset epithelial ovarian cancer: a population-based study. Am. J. Hum. Genet. 1999 Dec;65(6):1725–1732. doi: 10.1086/302671. http://dx.doi.org/10.1086/302671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arts-de Jong M, Manders CM, Hoogerbrugge N, Ligtenberg MJL, Massuger LF, de Hullu JA, et al. Added value of family history in counseling about risk of BRCA1/2 mutation in early-onset epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2013 Oct;23(8):1406–1410. doi: 10.1097/IGC.0b013e3182a1cf71. http://dx.doi.org/10.1097/IGC.0b013e3182a1cf71. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T, Lee MK, Casadei S, Thornton AM, Stray SM, Pennil C, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc. Natl. Acad. Sci. U. S. A. 2010 Jul 13;107(28):12629–12633. doi: 10.1073/pnas.1007983107. http://dx.doi.org/10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 2011 Aug 1;29(22):3008–3015. doi: 10.1200/JCO.2010.34.2980. http://dx.doi.org/10.1200/JCO.2010.34.2980 (Epub 2011 Jun 27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. doi: 10.1001/jamaoncol.2015.5495. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Dicks E, Ramus SJ, Tyrer JP, Intermaggio MP, Hayward J, et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J. Clin. Oncol. 2015 Sep 10;33(26):2901–2907. doi: 10.1200/JCO.2015.61.2408. http://dx.doi.org/10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J. Natl Cancer Inst. 2015 Aug 27;107(11) doi: 10.1093/jnci/djv214. http://dx.doi.org/10.1093/jnci/djv214 (pii: djv214. Print 2015 Nov.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boger-Megiddo I, Weiss NS. Histologic subtypes and laterality of primary epithelial ovarian tumors. Gynecol. Oncol. 2005 Apr;97(1):80–83. doi: 10.1016/j.ygyno.2004.11.054. http://dx.doi.org/10.1016/j.ygyno.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Gotlieb WH, Chetrit A, Menczer J, Hirsh-Yechezkel G, Lubin F, Friedman E, et al. Demographic and genetic characteristics of patients with borderline ovarian tumors as compared to early stage invasive ovarian cancer. Gynecol. Oncol. 2005 Jun;97(3):780–783. doi: 10.1016/j.ygyno.2005.02.022. http://dx.doi.org/10.1016/j.ygyno.2005.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.