Abstract
Objective
Although there has been major progress in gout imaging, no gout classification criteria currently include advanced imaging techniques. The objective of this study was to examine the usefulness of imaging modalities in the classification of gout when compared to monosodium urate (MSU) crystal confirmation as the gold standard, in order to inform development of new gout classification criteria.
Methods
We systematically reviewed the published literature concerning the diagnostic performance of plain film radiography, magnetic resonance imaging, ultrasound (US), conventional computed tomography, and dual energy computed tomography (DECT). Only studies with MSU crystal confirmation as the gold standard were included. When more than one study examined the same imaging feature, the data were pooled and summary test characteristics were calculated.
Results
Eleven studies (9 manuscripts and 2 meeting abstracts) satisfied the inclusion criteria. All were set in secondary care, with mean gout disease duration of at least 7 years. Three features were examined in more than one study: the double contour sign (DCS) on US, tophus on US, and MSU crystal deposition on DECT. The pooled (95% CI) sensitivity and specificity of US DCS were 0.83 (0.72–0.91) and 0.76 (0.68–0.83) respectively, of US tophus were 0.65 (0.34–0.87) and 0.80 (0.38–0.96) respectively, and of DECT were 0.87 (0.79–0.93) and 0.84 (0.75–0.90) respectively.
Conclusions
US and DECT show promise for gout classification but the few studies to date have mostly been in patients with longstanding, established disease. The contribution of imaging over clinical features for gout classification criteria requires further examination.
Keywords: gout, classification criteria, ultrasound, dual energy computed tomography, imaging
INTRODUCTION
Classification criteria are necessary to ensure relative homogeneity of participants in clinical research, including clinical trials and epidemiological studies.[1] The definitive classification of gout relies upon the microscopic identification of monosodium urate (MSU) crystals in synovial fluid or from tophi.[2] However, examination of synovial fluid may not be practical for all studies such as those with an epidemiological focus. Therefore, clinical classification criteria also exist for gout. The most widely used clinical classification criteria are the 1977 American Rheumatology Association (ARA) preliminary classification criteria of acute arthritis of primary gout.[3, 4]
The 1977 ARA clinical criteria included two plain radiography features; asymmetric swelling within a joint, and subcortical cysts without erosions.[4] Since 1977, major advances have been made in the imaging of gout, and new imaging modalities have become more widely available and commonly used in clinical practice.[5] Inclusion of such imaging tests, if they can distinguish gout from not-gout, may be helpful in the clinical classification of gout. However, it remains unclear how accurate and useful available imaging modalities are for the classification of gout, particularly when compared to the microscopic confirmation of MSU crystals as the gold standard test.
The objective of this study was to examine the usefulness of imaging modalities in the classification of symptomatic gout when compared to MSU crystal confirmation as the gold standard. We systematically reviewed the published literature concerning the diagnostic performance of plain film radiography (X-ray), magnetic resonance imaging (MRI), ultrasound (US), conventional computed tomography (CT), and dual energy computed tomography (DECT). This systematic review was performed to inform the development of new classification criteria for gout.[2]
METHODS
Literature Search
A systematic search was performed by a medical librarian using Ovid Medline, PubMed, Embase, and Cochrane databases from January 1946 to March 2014. Search terms included gout, podagra, crystal arthrop$, toph$, imaging, arthrography, radiography, ultrasound, radiograph, plain x-ray, magnetic resonance imaging, MRI, Tomography, CT, dual energy CT, DECT. (Complete search strategy listed in Supplementary File 1). Articles were excluded from the search if they were not published in the English language, did not involve human subjects, or were case reports (as these reports did not include comparator patients and thus would not meet the inclusion criteria as described below). We also searched the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) meetings for relevant abstracts from 2007–2013. All abstracts with “gout” in the title or body were reviewed.
Review of Literature
After the initial searches were completed, AO reviewed all the resulting titles and abstracts. Citations were excluded if the title or abstract was not relevant to the goals of the review. Full manuscripts of the remaining citations were reviewed by AO. Review articles were excluded but references within review articles were searched to ensure adequate capture of all relevant articles. When not enough information was provided in the abstract or manuscript, authors were emailed to obtain further data.
Selection Criteria
Inclusion criteria were: a) studies examining the diagnostic performance of an imaging modality (X-ray, MRI, US, CT or DECT) in gout, b) inclusion of at least two groups of patients where one group had gout, c) gout was confirmed by the presence of MSU crystals in joint fluid. The article or abstract also had to include either the raw results (positive versus negative imaging features for each group), or specificity and sensitivity. Exclusion criteria were: a) use of clinical criteria or physician- or patient-report for classification of gout instead of MSU crystal confirmation, b) lack of a control or comparison group, c) cases with asymptomatic hyperuricaemia, or d) insufficient information provided to calculate sensitivity and specificity.
Data Extraction and Quality Assessment
Data were extracted from manuscripts by AO and ND using a standardized data abstraction tool. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool was then applied by AO and ND. When there were differences in QUADAS scores between AO and ND, WT served as a third reviewer to settle discrepancies. The QUADAS is a 14-item scale designed to assess the quality of studies of diagnostic accuracy included in systematic reviews.[6]
Meta-Analysis
When more than one study examined the same imaging feature, the data were pooled and summary test characteristics were calculated from the hierarchical summary receiver-operating characteristic (HSROC) curve model of Rutter and Gatsonis implemented in R software version 0.5.5.[7, 8] Summary receiver operating characteristic curves were then generated. In generating the HSROC, we did not assume similar thresholds across studies since a “positive” test depended on observer judgment rather than objective measurement.
Results were compiled using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Standards for Reporting of Diagnostic Accuracy (STARD) guidelines.[9, 10]
RESULTS
Study Identification
A total of 1171 manuscripts and 88 abstracts were reviewed (Figure 1). Among manuscripts identified, 884 were excluded after review of the title and abstract, 338 were excluded after review of the paper, and 1 duplicate was excluded. Among ACR and EULAR meeting abstracts identified, 88 were excluded after review and additional information was sought in 3. Of these, only 1 response was received and this abstract was excluded as the classification of gout cases was based on 1977 ARA clinical criteria rather than MSU crystal confirmation. A total of 11 studies were included in the analysis: 9 full length manuscripts, [11–19] and 2 meeting abstracts.[20, 21] Seven studies examined US, three studies examined DECT and one examined X-ray features of the sternomanubrial joint.
Figure 1. Search results.
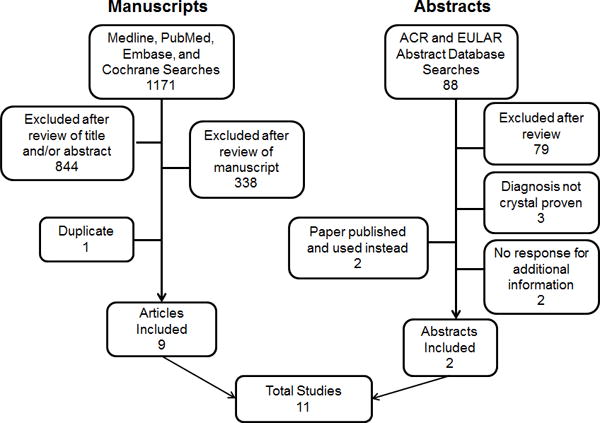
Ovid Medline, PubMed, Embase, and Cochrane databases were searched using the search strategy in the appendix. In addition, proceedings from the American College of Rheumatology (ACR) and European Union League Against Rheumatism (EULAR) annual meetings from 2007–2012 were searched for relevant abstracts.
Quality Assessment
Overall, the included studies met most of the quality indicators of the QUADAS tool (Figure 2 and Table S1). The most common quality issues were unreported time between arthrocentesis confirming MSU crystals (reference test) and the performance of the imaging (index) test and lack of reporting of withdrawals or uninterpretable results.
Figure 2. Methodologic quality as assessed using the QUADAS tool.

The vertical access contains the individual quality metrics and the horizontal access reflects the proportion of studies meeting these criteria (in green). Yellow signifies that it was unclear whether the study met the quality metric (usually because it was reported) and red signifies that the study specifically did not meet that metric.
Patient and Study Characteristics
Study characteristics are shown in Table 1. Most studies were single center (with exception of Naredo et al[14]) case-control or cross-sectional studies comparing gout to other types of arthritis. Patients were generally referred to the study with joint swelling and were recruited from secondary care clinics. In the four studies that reported disease duration, the mean duration of gout ranged from 7 to 13 years. However, half of the patients in one study (Bongartz et al[19]) had symptom duration of <6 weeks. In most studies, both active joints and inactive joints were included in the analysis. Arthrocentesis was performed in all patients with gout, although it was often not clear when the arthrocentesis occurred relative to the imaging test. Only half of the studies reported performing arthrocentesis in the control/comparator patients.
Table 1.
Patient Characteristics
| Gout Patients | Comparator Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Population | Dates | N | Disease duration, years (mean) |
Age, years (mean) |
N | Age, years (mean) |
Arthrocentesis | Conditions |
| Ultrasound | ||||||||||
| Ottaviani 2012 | Prospective Case-Control | Hospital outpatients with rheumatic disease | 11/2008–10/2010 | 53 | 9.2 | 59.7 | 50 | 59.5 | Yes | CPPD, RA, OA, PsA |
| Lamers-Karnebeek 2014 | Prospective Cross-Sectional | Patients presenting with mono- or oligoarthritis | NR | 26 | NR | 63.5 | 28 | NR | Yes | CPPD, ReA, PsA, OA, PMR, UA, Lofgren’s Syndrome, Gout with neg MSU (2 patients) |
| Thiele 2007 | Retrospective Case-Control | Rheumatology clinic patients with unclear diagnosis | 11/2003–12/2004 | 23 | NR | 58.6 | 23 | NR | No | CPPD, RA, sarcoidosis, OA, FMS, PsA, bursitis, tendinitis, Inflammatory oligoarthritis NOS, lateral epicondylitis, muscle fiber tear |
| Naredo 2013 | Prospective Case-Control | Rheumatology and general practice clinics | NR | 91 | 7 | 56.4 | 42 | 56.6 | NR | RA, SpA, healthy |
| Nalbant 2003 | Prospective Case-Control | Patients with subcutaneous nodules and rheumatic disease attending rheumatology clinics | 5/2001–10/2001 | 10 | 10.7 | 61.3 | 13 | 56.5 | NR | RA |
| Ponce 2009† | Prospective Cross-Sectional | Patients with joint effusion | NR | 13 | NR | NR | 88 | NR | Yes | OA, RA, SpA, UIA, CTD, CPPD, bursitis, AVN, hemorrhagic joint |
| Bergner 2013† | Cross-Sectional | Patients undergoing arthrocentesis | NR | 39 | NR | NR | 74 | NR | Yes | CPPD, Non-crystal arthropathy |
| Dual Energy Computed Tomography | ||||||||||
| Glazebrook 2011 | Retrospective Cross-sectional | Patients with arthralgia and potential gout | 4/2008–2/2010 | 12* | NR | NR | 19 | NR | Yes | CPPD, possible diagnoses of RA, seronegative IA, and CTS |
| Bongartz 2014 | Prospective Case-Control | Patients with joint pain or swelling in rheumatology procedure clinic | 10/2010–9/2012 | 40 | NR** | 62.1 | 41 | 58.7 | Yes | OA, RA, septic arthritis, CTD, CPPD, unknown |
| Choi 2012 | Prospective Case-Control | Clinic patients with arthritis | 12/2009–6/2011 | 40 | 13 | 62 | 40 | 53 | NR | RA, PsA, OA, UIA, AS |
| Plain Radiography | ||||||||||
| Parker 1984 | Retrospective Cross-sectional | Patients undergoing routine lateral CXR | 1978–1980 | 20 | NR | 60.8 | Healthy: 69 Arthritis: 88 |
Healthy: 52.6 Arthritis: 58 |
NR | Among those with arthritis: RA, ReA, AS, PsA, CPPD, PMR, DISH |
43 patients included in study initially but only 12 were found to have MSU crystals.
20 patients had symptom duration <6 weeks.
Refers to an abstract.
Abbreviations: NR=not reported, RA=Rheumatoid Arthritis, SpA=Spondyloarthropathy, ReA=Reactive Arthritis, AS=Ankylosing Spondylitis, PsA=Psoriatic Arthritis, OA=Osteoarthritis, UIA=Undifferentiated Inflammatory Arthritis, CPPD=Calcium Pyrophosphate Disease, PMR=Polymyalgia Rheumatica, DISH=Diffuse Idiopathic Skeletal Hyperostosis, AVN=Avascular Necrosis, CTD=Connective Tissue Disease, CXR=chest radiograph
Imaging Features
A variety of imaging features were examined in the studies included (Table 2). There was also substantial variation in the joints examined in each study (Table 2). In the studies examining US, most of the sonographers were rheumatologists with training in musculoskeletal US (5/7 studies; two studies did not report the sonographer’s training). Four of seven US studies utilized sonographers blinded to the patient’s diagnosis, one study had one blinded and one unblinded sonographer, and two studies did not report whether the sonographer was blinded. In all three DECT studies, the images were interpreted by musculoskeletal radiologists who were blinded to the diagnosis.
Table 2.
Study Characteristics
| Study | Training | Blinded | Features Examined | Joints Included | Active Joints Only | Arthrocentesis of all imaged joints |
|---|---|---|---|---|---|---|
| Ultrasound | ||||||
| Ottaviani 2012 | Rheumatologists trained in MSK US | Reader 1: No Reader 2: Yes |
Double contour sign and tophus at MTP, knee, MCP | Bilateral MTP1, MTP2, knees, MCP2, MCP3 (10 joints total) | No | No |
| Lamers-Karnebeek 2014 | Rheumatologists (2 trainees, 2 established) | Yes | Double contour sign and tophus at MTP1, knee, wrist, ankle, MCP, elbow | Knee, MTP1, wrist, ankle | No | No |
| Thiele 2007 | Rheumatologist trained in MSK US Second rheumatologist with limited training |
Yes | Double contour sign MTP effusion Power Doppler of synovium |
Humeral head, humero-radial joint, MCP joints, knee, MTP1 | Yes | No |
| Naredo 2013 | Rheumatologists trained in MSK US | Yes | Double contour sign Intra-articular, intra-bursal, or tendon/ligament hyperechoic aggregates or hyperechoic linear band |
Bilateral elbow, radiocarpal, midcarpal, ulnar-carpal, first through fifth MCP, knee, tibiotalar, talonavicular, and first MTP, wrist extensor and flexor tendons, quadriceps tendon, patellar tendon, ankle retromalleolar medial and lateral tendons, ankle extensor tendons, Achilles tendon, and medial and lateral collateral ligaments of the knee, deep infrapatellar bursa, retrocalcaneal bursa and gastrocnemius, semimembranosus bursae | No | No |
| Nalbant 2003 | Rheumatologist trained in MSK US | NR* | Nodule characteristics: density (homogenous or heterogenous), hypoechoic, hyperechoic, post acoustic shadow, adjacent cortical bone irregularity, adjacent bursitis. | Sites of nodule involvement | Yes | No |
| Ponce 2009† | Not reported | Yes | Fluid characteristics: cloudy, anechoic, cloudy, mixed, dotted, corpuscular, granular | Knees, shoulders, elbows, ankles, MCPs, Baker cysts | Yes | Yes |
| Bergner 2013† | NR | NR | Double contour sign, synovitis, hypervascularization | knees, small finger or toe joints, elbows, ankles, shoulders, wrists | Yes | Yes |
| Dual Energy Computed Tomography | ||||||
| Glazebrook 2011 | 2 MSK Radiologists | Yes | MSU crystal deposition | Affected joint | Yes | No |
| Bongartz 2014 | 2 MSK Radiologists | Yes | MSU crystal deposition | Affected joint* | Yes | Yes |
| Choi 2012 | MSK Radiologist | Yes | MSU crystal deposition | All peripheral joints (elbows, wrists, hands, knees, ankles and feet) | No | No |
| Plain Radiography | ||||||
| Parker 1984 | Rheumatologist and Radiologist | Yes | Inflammatory bone changes Proliferative bone changes Joint fusion |
Sternomanubrial joints | No | No |
MSK=musculoskeletal, MSU=monosodium urate, US=musculoskeletal ultrasound, MCP=metacarpophalangeal joint, MTP= metatarsophalangeal joint, NR=not reported
Refers to an abstract,
A secondary analysis in Bongartz et al. examine all joints but this analysis was not included in the meta-analysis.
Pooled results
Only three imaging features were examined in more than one study: the double contour sign (DCS) on US, presence of tophus on US, and MSU crystal deposition on DECT. Pooled results are presented in Table 3. The pooled (95% CI) sensitivity and specificity of DCS were 0.83 (0.72–0.91) and 0.76 (0.68–0.83) respectively. The pooled (95% CI) sensitivity and specificity for tophus on US were 0.65 (0.34–0.87) and 0.80 (0.38–0.96) respectively. DECT had pooled (95% CI) sensitivity and specificity of 0.87 (0.79–0.93) and 0.84 (0.75–0.90). The summary ROC curves are shown in Figure 3.
Table 3.
Meta-analysis Results
| Individual Study Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| True Positive | False Positive | False Negative | True Negative | Sensitivity | Specificity | Sensitivity | Specificity | AUC | |
| Ultrasound: Double Contour Sign | |||||||||
|
| |||||||||
| Ottaviani 2012 | NR | NR | NR | NR | 0.67 | 0.98 | 0.83 (0.72–0.91) |
0.76 (0.68–0.83) |
0.84 |
| Lamers-Karnebeek 2014 | 20 | 7 | 6 | 21 | 0.77 | 0.75 | |||
| Thiele 2007 | 34 | 0 | 3 | 26 | 0.92 | 1.00 | |||
| Naredo 2013 | 68 | 7 | 23 | 35 | 0.75 | 0.83 | |||
| Bergner 2013 | 36 | 21 | 3 | 53 | 0.92 | 0.72 | |||
|
| |||||||||
| Ultrasound: Tophus | |||||||||
|
| |||||||||
| Ottaviani 2012 | NR | NR | NR | NR | 0.74 | 1.00 | 0.65 (0.34–0.87) |
0.80 (0.38–0.96) |
0.75 |
| Lamers-Karnebeek 2014 | 5 | 2 | 21 | 26 | 0.19 | 0.93 | |||
| Thiele 2007 | 27 | 0 | 10 | 26 | 0.73 | 1.00 | |||
| Naredo 2013 | 78 | 11 | 13 | 31 | 0.86 | 0.74 | |||
| Nalbant 2003 | 15 | 3 | 5 | 17 | 0.75 | 0.85 | |||
|
| |||||||||
| Dual Energy Computed Tomography: MSU Crystal Deposition | |||||||||
|
| |||||||||
| Glazebrook 2011 | 12 | 4 | 0 | 15 | 1 | 0.79 | 0.87 (0.79–0.93) |
0.84 (0.75–0.90) |
0.90 |
| Bongartz 2014 | 36 | 7 | 4 | 34 | 0.90 | 0.83 | |||
| Choi 2012 | 34 | 3 | 6 | 37 | 0.93 | 0.78 | |||
Abbreviations: MSU=monosodium urate, NR=not reported, AUC=area under the curve
Figure 3. Hierarchical summary receiver operator curves (HSROC).
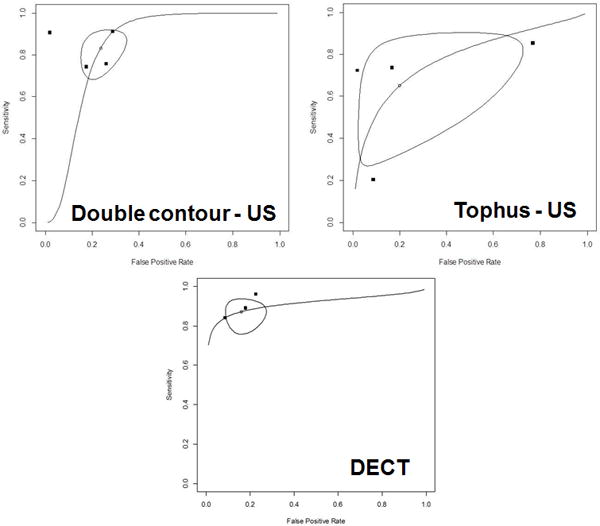
Hierarchical summary receiver operating characteristic curve for a) ultrasound double contour sign, b) tophi on ultrasound, and c) DECT. The closed points represent the individual studies in the review. The open point represents the pooled sensitivity and specificity estimate and the enclosed shape represents the bivariate 95% confidence interval for the pooled sensitivity and specificity estimate.
DISCUSSION
In this systematic review and meta-analysis, we found 11 studies examining the accuracy of imaging features for the classification of gout. Relatively few studies met the inclusion criteria requiring MSU crystal confirmation as the gold standard and the inclusion of a comparison group without gout. The three imaging findings examined in the pooled analysis had similar pooled specificity, and pooled sensitivity was high for both DCS and DECT but lower for US identification of tophi. The results available suggest that US and DECT may be useful to include in revised gout clinical classification criteria.
The value of each modality for classification of gout in terms of sensitivity and specificity in comparison to MSU crystal proven gout as the gold standard (rather than ACR criteria or physician diagnosis) has not previously been explored in a meta-analysis. Three previous systematic reviews have examined the usefulness of ultrasound as an outcome tool in gout. Chowalloor et al[22] and Ottaviani et al[23] provided an extensive review of the features of gout reported in US studies to date but did not focus on the diagnostic or classification properties of these features and did not perform a meta-analysis. Mathieu et al performed a systematic literature review and meta-analysis of the prevalence of ultrasound characteristics in gout.[24]
However, in examining the test properties of ultrasound, none of these reviews specifically restricted the gold standard to demonstration of MSU crystals. This is important because comparison of a new test to a reference standard that may or may not be accurate can lead to inflation or deflation of the sensitivity and specificity of the index test.
Interpretation of the results reported in this study requires some important considerations. First, the patients studied had been diagnosed with gout for an average of at least 7 years in those studies reporting length of disease. These imaging modalities may perform differently in patients with early gout. It is this population of patients with earlier gout, most often without tophi, for which an accurate imaging technique would be most useful. Thus, further studies are needed to address this population. It is also important to note that we excluded studies examining the use of imaging modalities in patients with asymptomatic hyperuricemia only, as the proposed new classification criteria will apply to people with symptomatic disease, rather than those with asymptomatic hyperuricaemia and/or asymptomatic MSU crystal deposition.[2] Therefore, studies examining the use of imaging modalities to determine risk of symptomatic gout or the presence of subclinical gout in patients with asymptomatic hyperuricemia were beyond the scope of this review.
A further issue when considering imaging for gout classification is the observation that all the studies involved patients in secondary care rheumatology clinics. Patients recruited from secondary care setting may have more complex and severe gout than those treated in primary care. Gout is mostly managed within primary care, and a key property of new classification criteria for gout is that they should be applicable to patients within a range of research settings, including primary care.[2, 25]
We used MSU crystal identification as the gold standard but even this test has some variability when performed by different investigators.[26] However, this is the best gold standard available. Additionally, not all joints included in these imaging analyses were sites at which arthrocentesis had been performed. We do not believe this should substantially affect the results, particularly as this mirrors current clinical practice in which a patient is diagnosed or classified as having gout when multiple joints are inflamed but MSU crystals are identified on arthrocentesis from one joint. Finally, there may be a risk of misclassification bias in that not all comparator patients underwent arthrocentesis to confirm their “control” status.
The methods employed by the included studies were, in general, satisfactory. However, the majority of studies included study utilized a case-control design. Such designs may exaggerate the diagnostic properties (sensitivity and specificity). Future studies may consider cross-sectional designs in which patients for whom the clinical question is “does this patient have gout?” are referred for participation. This type of design was implemented in some of the studies included.[12, 18, 20, 21] Finally, there was great variability in the study protocols used and the sites that were imaged. Standardization of the methodology used for both ultrasound and DECT are needed. One of the goals of Naredo et al was to examine optimum sites for inclusion in US studies.[14] At present, it is similarly unclear which sites are optimal for DECT imaging, and also which scanner settings are most appropriate to achieve optimal sensitivity and specificity for urate deposition.[27]
In summary, although imaging modalities such as ultrasound and DECT show promise in the classification of symptomatic gout, the studies to date have been small and have primarily involved people with longstanding, established disease. Determination of whether these imaging modalities should be included in the revised ACR/EULAR classification criteria for gout will occur at a consensus meeting adjacent to EULAR in Paris, France in June 2014. Future studies aiming to determine the usefulness of imaging modalities in the diagnosis of symptomatic gout should focus on patients with recent onset joint pain and swelling, and should use MSU crystal identification as the gold standard when determining test characteristics. Additional studies are also needed to determine which imaging modalities are optimal and to examine the relative contribution of imaging modalities over clinical elements to the classification of gout in clinical situations including primary care.
Supplementary Material
Acknowledgments
This study was funded by the American College of Rheumatology and the European Union League Against Rheumatism. We thank Janet Joyce for performing the literature search and Yihui Connie Jiang for administrative support.
FUNDING STATEMENT
Dr. Ogdie is supported by NIH K23AR063764. Dr Dalbeth is supported by the Health Research Council of New Zealand.
Footnotes
LICENSE FOR PUBLICATION
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or nonexclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
COMPETING INTERESTS
None declared.
CONTRIBUTOR STATEMENT
All authors assisted in study conception, design and interpretation. Drs. Ogdie, Dalbeth and Taylor reviewed the articles to be included in the review. Drs. Ogdie and Dalbeth extracted the data. Drs. Ogdie, Dalbeth, Taylor and Weatherall performed the data analysis. Dr. Ogdie wrote the first draft of the manuscript and all of the authors reviewed and edited the manuscript.
References
- 1.Singh J, Solomon DH, Dougados M, et al. Classification and Response Criteria Subcommittee of the Committee on Quality Measures, American College of Rheumatology. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006;55(3):348–52. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 2.Dalbeth N, Fransen J, Jansen TL, et al. New classification criteria for gout: a framework for progress. Rheumatology (Oxford) 2013;52(10):1748–53. doi: 10.1093/rheumatology/ket154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik A, Schumacher H, Dinnella J, Clayburne G. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15(1):22–4. doi: 10.1097/RHU.0b013e3181945b79. [DOI] [PubMed] [Google Scholar]
- 4.Wallace S, Robinson H, Masi A, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 5.Girish G, Glazebrook K, Jacobson J. Advanced imaging in gout. Am J Roentgenol. 2013;201(3):515–25. doi: 10.2214/AJR.13.10776. [DOI] [PubMed] [Google Scholar]
- 6.Whiting P, Rutjes A, Reitsma J, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology. 2003;25:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doebler Philippe. Meta-Analysis of Diagnostic Accuracy (2013) 2014 Apr 30; R package version 0.5.5. [Google Scholar]
- 8.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 9.Bossuyt P, Reitsma J, Bruns D, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Family Practice. 2004;21:4–10. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Burns L, Shojania K, et al. Dual energy CT in gout: a prospective validation study. Ann Rheum Dis. 2012;71(9):1466–71. doi: 10.1136/annrheumdis-2011-200976. [DOI] [PubMed] [Google Scholar]
- 12.Glazebrook K, Guimarães L, Murthy N, et al. Identification of intraarticular and periarticular uric acid crystals with dual-energy CT: initial evaluation. Radiology. 2001;261(2):516–24. doi: 10.1148/radiol.11102485. [DOI] [PubMed] [Google Scholar]
- 13.Nalbant S, Corominas H, Hsu B, et al. Ultrasonography for assessment of subcutaneous nodules. J Rheumatol. 2003;30(6):1191–5. [PubMed] [Google Scholar]
- 14.Naredo E, Uson J, Jiménez-Palop M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203487. Epub. [DOI] [PubMed] [Google Scholar]
- 15.Ottaviani S, Richette P, Bardin T, et al. Ultrasonography in gout: a case-control study. Clin Exp Rheumatol. 2012;30(4):499–504. [PubMed] [Google Scholar]
- 16.Parker V, Malhotra C, Ho GJ, Kaplan S. Radiographic appearance of the sternomanubrial joint in arthritis and related conditions. Radiology. 1984;153(2):343–7. doi: 10.1148/radiology.153.2.6333045. [DOI] [PubMed] [Google Scholar]
- 17.Thiele R, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford) 2007;46(7):1116–21. doi: 10.1093/rheumatology/kem058. [DOI] [PubMed] [Google Scholar]
- 18.Lamers-Karnebeek F, van Riel P, Jansen T. Additive value for ultrasonographic signal in a screening algorithm for patients presenting with acute mono-/oligoarthritis in whom gout is suspected. Clin Rheumatol. 2014;33(4):555–559. doi: 10.1007/s10067-014-2505-6. [DOI] [PubMed] [Google Scholar]
- 19.Bongartz T, Glazebrook K, Kavros S, et al. Dual-Energy Computed Tomography for the diagnosis of gout: an accuracy and diagnostic yield study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-205095. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergner R, Peters L, Schmitt V, et al. Arthrosonographic findings in crystal arthropathies. Ann Rheum Dis. 2013;72(Suppl 3):713. [Google Scholar]
- 21.Ponce A, Surís X, Cerdà D, et al. Echographic Patters of Synovial Fluid: can they predict the results of microscopic cell counts? Ann Rheum Dis. 2009;68(Suppl 3):330. [Google Scholar]
- 22.Chowalloor P, Keen H. A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Ann Rheum Dis. 2013;72(5):638–45. doi: 10.1136/annrheumdis-2012-202301. [DOI] [PubMed] [Google Scholar]
- 23.Ottaviani S, Bardin T, Richette P. Usefulness of ultrasonography for gout. Joint Bone Spine. 2012;79(5):441–5. doi: 10.1016/j.jbspin.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Mathieu S, Pereira B, Couderc M, Soubrier M. Usefulness of ultrasonography in the diagnosis of gout: a meta-analysis. Ann Rheum Dis. 2013;72(10):e23. doi: 10.1136/annrheumdis-2013-204108. [DOI] [PubMed] [Google Scholar]
- 25.Dalbeth N. Management of gout in primary care: challenges and potential solutions. Rheumatology (Oxford) 2013;52(9):1549–50. doi: 10.1093/rheumatology/ket215. [DOI] [PubMed] [Google Scholar]
- 26.Berendsen D, Janesen TL, Taylor W, et al. A critical appraisal of the competence of crystal identification by rheumatologists. EULAR Poster Presentation. 2013 Abstract 629. [Google Scholar]
- 27.Dalbeth N, Choi HK. Dual-energy computed tomography for gout diagnosis and management. Curr Rheumatol Rep. 2013;15(1):301. doi: 10.1007/s11926-012-0301-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


