Abstract
The age-adjusted prevalence of peripheral arterial disease in the US population has been estimated to approach 12%. The clinical consequences of occlusive peripheral arterial disease include pain on walking (claudication), pain at rest, and loss of tissue integrity in the distal limbs; the latter may ultimately lead to amputation of a portion of the lower extremity. Surgical bypass techniques and percutaneous catheter-based interventions may successfully reperfuse the limbs of certain patients with peripheral arterial disease. In many patients, however, the anatomic extent and distribution of arterial occlusion is too severe to permit relief of pain and healing of ischemic ulcers. No effective medical therapy is available for the treatment of such patients, for many of whom amputation represents the only hope for alleviation of symptoms. The ultimate failure of medical treatment and procedural revascularization in significant numbers of patients has led to attempts to develop alternative therapies for ischemic disease. These strategies include administration of angiogenic cytokines, either as recombinant protein or as gene therapy, and more recently, to investigations of stem/progenitor cell therapy. The purpose of this review is to provide an outline of the preclinical basis for angiogenic and stem cell therapies, review the clinical research that has been done, summarize the lessons learned, identify gaps in knowledge, and suggest a course toward successfully addressing an unmet medical need in a large and growing patient population.
Keywords: angiogenesis, genetic therapy, microvessels, progenitor cell, stem cells, vasculogenesis
Since Judah Folkman first described the process of angiogenesis in 1971, much has been learned about the cells, the extracellular factors, and the signaling pathways that modulate the angiogenesis, arteriogenesis, and vasculogenesis.1 Promising preclinical studies led to trials of angiogenic factors and cell therapies for peripheral arterial disease (PAD) in the last 2 decades.2,3 Early stage trials have provided evidence for safety and bioactivity; however, the field still awaits a positive phase 3 pivotal trial. In this review, we will provide an outline regarding the vascular response to ischemia in animal models and in humans. We will discuss what has been learned from the clinical trials of cell therapy and angiogenic factors for PAD. We will conclude with recommendations for those who wish to develop regenerative therapies in the treatment of PAD.
Vascular Response to Metabolic Demand
Ischemia defines a state in which blood flow is insufficient to meet metabolic demands. This condition occurs regularly, but transiently, in healthy humans when they begin to exercise.4 In response to exercise, homeostatic mechanisms are activated so that vascular supply increases to match metabolic demand.5 Specifically, the tissue requirements for oxygen and nutrients are matched by the inflow of oxygenated blood. In addition, venous and lymphatic outflow balance the arterial inflow, so as to remove metabolites from active tissues. As the tissue becomes more metabolically active, arterial inflow and venous/lymphatic outflow increase.6 For example, at rest, the skeletal muscles of the leg require a blood flow of ≈5 mL/min per milligram of tissue. With physical exertion, such as running on a treadmill, blood flow to the lower limbs may increase 5-fold to match metabolic demand.5 This vascular response is tightly regulated by neuronal, hormonal, endothelial, and metabolic mechanisms.
Functional Responses to Metabolic Demand
In the exercising leg muscle, the arterioles dilate, reducing vascular resistance. This vasodilation is largely because of accumulation of tissue metabolites in the active muscle. Chief among these vasodilator metabolites is adenosine, which stimulates purinergic receptors on vascular smooth muscle to induce relaxation.4,6 Adenosine accumulation is because of the increased utilization of adenosine triphosphate during muscular contraction. Thus vascular resistance (and blood flow) is tightly coupled to metabolic activity. Other factors which accumulate with active metabolism, and which can also induce a direct vasodilation of the vascular smooth muscle, include extracellular potassium, hydrogen ions, and lactic acid.7 Humoral, nervous, and endothelial regulation play less of a role in regulating microvascular tone during exercise. However, these factors predominantly regulate the response of the conduit and collateral arteries that subserve the active tissue.
As arterioles relax in the exercising leg muscle, vascular resistance drops, increasing blood flow through the larger conduit arteries of the leg. This increase in flow causes an increase in vascular shear stress. This tractive force of blood flow stimulates the endothelium to release several vasodilator factors, including nitric oxide, prostacyclin, and endothelium-dependent hyperpolarizing factor.8 The conduit arteries then vasodilate to accommodate the increase in blood flow. Other factors contribute secondarily to vasodilation of the limb conduit arteries. These include a reduction in sympathetic nerve outflow to the limb arteries (thereby reducing any vasoconstriction because of α-adrenergic receptors) and an increase in circulating epinephrine (which stimulates a vasodilation mediated by β-adrenergic receptors on the vascular smooth muscle).7,9
Structural Responses to Metabolic Demand
In addition to the acute functional response to exercise, there are chronic vascular adaptations to regular exercise.10,11 Preclinical models indicate that regular exercise can increase microvascular density, which seems to be because of the activation of angiogenic mechanisms (described below).12,13 In addition, regular exercise can increase the diameter of conduit arteries, in part because of enhanced endothelium-dependent vasodilation and increased sensitivity of the vascular smooth muscle to vasodilatory factors.14,15 Furthermore, a positive remodeling of the conduit arteries because of structural changes in the vessel wall has been described.16,17 The positive remodeling is under the influence of arteriogenic mechanisms (described below).
Impaired Vascular Response and Tissue Ischemia
There are multiple mechanisms that impair the functional and structural responses of the vasculature to metabolic demands of the tissue. Cardiovascular risk factors, such as hyperlipidemia, diabetes mellitus, hypertension, and tobacco use, each impair endothelium-dependent vasodilation.18,19 Their effect is in part because of reduced generation and increased degradation of vascular nitric oxide.20 Notably, even in the absence of occlusive arterial disease, a functional impairment in endothelial vasodilator function may reduce exercise capacity. In hypercholesterolemic apolipoprotein E–deficient mice, limb blood flow during exercise is reduced, and exercise capacity (as judged by time on the treadmill) is reduced.21 Similarly, in the absence of PAD, exercise capacity is impaired in humans with cardiovascular risk factors.22 Treatment of risk factors (as with statins or converting enzyme inhibitors) can improve endothelium-dependent vasodilation and enhance exercise capacity.23,24
Of course, in patients with PAD, the major cause of demand/supply mismatch is a structural, rather than a functional, abnormality in the vessel wall.25 Specifically, the primary problem is the hemodynamically significant atherosclerotic disease that impedes flow. Impaired vasoreactivity plays little or no role in the diseased vascular conduits. For example, in the femoral artery of PAD patients, intra-arterial infusions of vasodilators acting on the endothelium or directly on the vascular smooth muscle have no effect because of the severe and circumferential atherosclerosis, fibrosis, and calcification typically observed in the limb arteries of these patients.26 This may explain the failure of most vasodilator therapies to improve functional capacity in individuals with PAD.
That being said, the microvasculature as well as the collateral channels of the ischemic limb are not directly involved by atherosclerosis. Accordingly, any impairment of their endothelial and vascular smooth muscle response may contribute to vascular resistance in the limb. Thus, excessive vascular tone, as induced by exogenous catecholamines, may aggravate tissue ischemia in PAD.27 In patients with ischemic heart failure requiring inotropic support, the coexistence of PAD may lead to the complications of skin necrosis and gangrene.28 In patients with mild PAD, administration of vasoconstrictor agents for relief of migraine headaches has been reported to worsen claudication.29
Microvascular flow may be further impaired by emboli from conduit vessels upstream. It is likely that in the atherosclerotic vasculature of the PAD patient, microemboli composed of platelets and leukocytes are chronically bombarding the microvasculature. When this embolic burden is greater (such as with the rupture of an atherosclerotic plaque in the aorta or a large conduit vessel), it may have cutaneous manifestations.30 Such an event may occur spontaneously, or after vascular interventions such as femoral artery cannulation for cardiac catheterization. Typically, one observes splinter hemorrhages in the nailbeds in minor cases; areas of cyanosis in the toes and a livedo reticularis pattern in the feet, legs, and buttocks in moderate cases; and in severe cases, a decline in renal function (because of associated renal atheroembolism), hypotension, impaired cognition, and severe ischemia and pain in the toes.31
Structural Responses to Chronic Ischemia
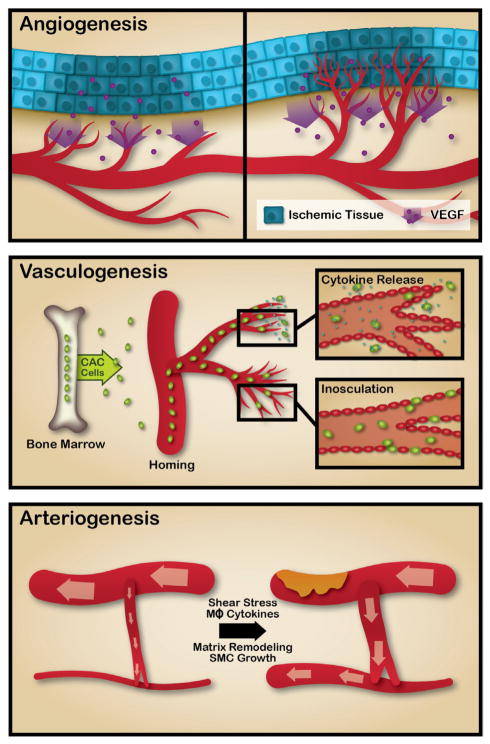
When the vascular response to metabolic demands is chronically insufficient, structural alterations in the vasculature are initiated.32 These structural alterations are because of angiogenesis, arteriogenesis, and adult vasculogenesis. Angiogenesis describes an expansion of the microvasculature, because of sprouting of endothelial cells (ECs) from pre-existing capillaries, followed by their proliferation, migration, and capillary formation.33 By contrast, arteriogenesis describes the remodeling of existing collateral channels, so that they can deliver more blood flow to the limb.34 Finally, adult vasculogenesis describes the incorporation of circulating cells into the regenerating microvasculature.35
Angiogenesis
Angiogenesis is triggered by reduced oxygen delivery to the tissue and is largely under the control of the transcriptional factor hypoxia-inducible factor-1 alpha (HIF-1 α) generated by cells in ischemic tissue33 (although circulating cells that home to sites of ischemia, such as monocytes that generate angiogenic cytokines, may contribute; see below). With reduced tissue oxygen pressure, HIF-1α becomes more stable because it contains an oxidation dependent degradation domain. This domain contains 2 prolyl residues that are hydroxylated by prolyl hydroxylase, which leads to the ubiquitination and destruction of HIF-1α. However, under hypoxic conditions, prolyl hydroxylase activity is reduced and HIF-1α accumulates. This transcriptional factor translocates to the nucleus to orchestrate the response to ischemia, by activating the gene expression of vascular endothelial growth factor (VEGF), erythropoietin, and glycolytic enzymes. Subsequently, VEGF induces angiogenesis, characterized by capillary sprouting, EC migration, proliferation, and luminogenesis to generate new capillaries (Figure).
Figure. Angiogenesis is triggered by reduced oxygen delivery to the tissue, which induces the elaboration by ischemic cells of angiogenic factors such as vascular endothelial growth factor (VEGF).
Angiogenesis is characterized by capillary sprouting, endothelial cell migration, proliferation, and luminogenesis to generate new capillaries. Adult vasculogenesis is mediated by the action of circulating cells such as endothelial progenitor cells (EPCs). EPCs are a heterogenous population of cells, largely of hematopoietic lineage, and are characterized by cell-surface antigen markers including CD34, CD133, and VEGF receptor 2 (VEGFR-2). These circulating cells contribute to the expansion of the microvasculature via multiple mechanisms, secretion of paracrine factors playing a prominent role. A small subset of these cells seem to incorporate into the vasculature (inosculation). Arteriogenesis is a positive remodeling of pre-existing collateral channels in the limb. There is little to no flow through these narrow, high resistance channels in healthy individuals. However, when major conduits become severely narrowed or occluded, more flow becomes directed through the collateral channels. Under the influence of vascular shear stress, the diameter of these channels increase. This positive remodeling seems to be because of endothelial factors, as well as infiltrating macrophages. The remodeling process is characterized by dynamic restructuring of the extracellular matrix with degradation and synthesis; vascular smooth muscle cell apoptosis as well as proliferation, which lead to an increased diameter and thickness of the vessel.
Arteriogenesis
By contrast, arteriogenesis is a positive remodeling of pre-existing collateral channels in the limb.34 There is little to no flow through these narrow, high resistance channels in a normal individual. However, when major conduits become severely narrowed or occluded, more flow becomes directed through the collateral channels. Under the influence of vascular shear stress, the diameter of these channels increase. This positive remodeling seems to be because of endothelial factors, as well as infiltrating macrophages. The remodeling process is characterized by dynamic restructuring of the extracellular matrix with degradation and synthesis; vascular smooth muscle cell apoptosis as well as proliferation, which lead to an increased diameter and thickness of the vessel.36 Monocytes and macrophages play a major role in this remodeling process, as they are recruited sites of arteriogenesis and generate growth factors, metalloproteinases, chemokines, and nitric oxide that may contribute to arteriogenesis.36 Finally, it is possible that arteriogenesis may occur de novo in the absence of pre-existing collateral channels, but there is no evidence for such a phenomenon in humans.
Vasculogenesis
Finally, adult vasculogenesis differs from angiogenesis and arteriogenesis in that new blood vessels are formed at least in part by the action of endothelial progenitor cells (EPCs).37 EPCs are largely of hematopoietic lineage and are characterized by cell-surface antigen markers including CD34, CD133, and VEGF receptor-2.38–41 EPCs originate from the bone marrow and can circulate the blood and localize in regions of blood vessel formation and facilitate microvasculature regeneration by paracrine or direct mechanisms.42–46 They are recruited by VEGF and stromal-derived factor 1 generated by the ischemic tissue. These circulating cells contribute to the expansion of the microvasculature via multiple mechanisms, with their secretion of paracrine factors playing a prominent role. In addition, a small subset of these cells seem to be of endothelial lineage potential. This subset of EPCs seem to incorporate into the vasculature of the ischemic region to contribute to the vascular response.42
The contribution of progenitor cells to new blood vessel formation has been the subject of controversy. The most definitive studies have used genetic markers, bone marrow replacement, and parabiosis models to document the existence of circulating cells that contribute to angiogenesis in the ischemic limb.47 With respect to negative studies, these may be related to the fidelity of the engineered markers. In any event, the fidelity of murine models to human physiology is well established to be variable at best. In this regard, there are data from human studies that provide clear evidence of the role of circulating cells in vascular repair.48,49 These data, generated by examining hearts from patients who had undergone sex-mismatched transplants, reveal that extracardiac cells contribute between 14% and ≈40% of the EC population in the donor heart, with evidence that the contribution was greatest in the microcirculation and precipitated by injury signals.
In animal models of PAD, there is strong evidence that multiple mechanisms contribute to the vascular response to acute and chronic ischemia.50–52 In PAD patients, these mechanisms are not as robust, or may not even be operative to any significant degree, as discussed below. Furthermore, there is great heterogeneity between patients in their vascular response to chronic ischemia. Most notably, clinicians are well aware that patients with the same degree of arterial occlusive disease in the named conduits may have dramatically different impairments in their functional capacity. For example, one patient with an occluded superficial femoral artery may be much less symptomatic than another, because of robust generation of geniculate collaterals. These collaterals connect branches of the deep femoral artery with those of the popliteal artery, thus providing a biological bypass around the occluded superficial femoral artery.
In patients with PAD, the number of EPCs is reduced.53 This may be because of an adverse effect of cardiovascular risk factors on their mobilization or self-renewal, as the risk factor burden is known to be inversely correlated to the numbers of these circulating cells. Furthermore, the function of these cells are impaired in PAD, as assessed by their ability to form colonies and to incorporate into pre-existing vascular networks ex vivo.54
Trials of Angiogenic Therapies
Preclinical models of PAD have been developed to test angiogenic therapies. The murine hindlimb ischemia model is most commonly used. In this model, the superficial femoral artery is ligated proximally and distally, and the segment in between the ligatures is excised. The common femoral artery is also ligated, to obstruct flow to the deep femoral artery. Typically, Bl6 mice are used over the age of 12 weeks, as younger animals recover so quickly and completely that angiogenic agents cannot be easily tested. The angiogenic agents or vehicle are most frequently injected directly into the ischemic muscle of the limb. The effect on blood flow is followed noninvasively by laser Doppler spectroscopy, and angiogenic response can be quantified by microscopic examination of stained cross-sections of the tissue. In some studies, functional capacity is also assessed (eg, by treadmill testing). In other studies, strains of mice are used that have greater sensitivity to ischemia. For example, the experimental procedure described above, when performed in the BalbC mouse, causes severe ischemia and extensive gangrene. This difference between strains in ischemic response has led to new insights regarding the determinants of critical limb ischemia (CLI) as discussed below. In addition, rabbits and swine are also used as models for PAD, but less frequently.
The preclinical studies provided proof-of-concept that exogenous administration of several cytokines could enhance angiogenesis, arteriogenesis, and adult vasculogenesis in ischemic limbs, improve limb blood flow, and reduce tissue loss. Promising preclinical studies with VEGF, fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and HIF-1α led to clinical trials in PAD which are briefly described below (Table 1).
Table 1.
Selected Clinical Trials of Gene Therapy Products for Critical Limb Ischemia
| Reference (Trial) | Product | Dosage | Delivery Method | n | Rutherford Class | Follow-Up Duration | Outcomes: Bioactivity Parameters Improved |
|---|---|---|---|---|---|---|---|
| Fibroblast growth factor | |||||||
| Belch et al55 (TAMARIS) | FGF-1; plasmid | 8×0.5 mg injections for 4 sessions | IM | 525 | Class 5–6 | 12 mo | No changes in major amputation or death rates |
| Comerota et al152 | FGF-1; plasmid | Dose escalation; 1×500, 1×1000, 1×2000, 1×4000, 1×8000, 1×16 000 mcg; or 2×500, 2×1000, 2×4000, 2×8000 mcg | IM | 51 | Class 4–6 | 6 mo | Decrease in pain, aggregate ulcer size; Increased TcPO2, ABI |
| Nikol et al153 (TALISMAN-201) | FGF-1; plasmid | 8×0.5 mg injections for 4 sessions | IM | 125 | No option CLI | 12 mo | Decreased amputation rate; no changes in ulcer healing |
| Lederman et al56 (TRAFFIC) | FGF-2; recombinant | 30 mcg/kg single dose or 30 mcg/kg double dose (day 1+day 30) | IA | 190 | Class 2–3 | 3 mo | Increase peak walking time at 90 days; no change in quality of life or ABI |
| Vascular endothelial growth factor | |||||||
| Rajagopalan et al58 (RAVE) | VEG121; adenovirus | Low dose 4×109 pu; high dose 4×1010 pu; 20 injections | IM | 105 | Class 1–3 | 6 mo | No changes in peak walking time or ABI; increased peripheral edema |
| Baumgartner et al154 | VEGF165; plasmid | 4000 mcg | IM | 9 | Class 4–6 | 10 wk | Increased vascularity on digital subtraction angiography (DSA); healing of ischemic ulcers; proliferation of endothelial cells on pathology |
| Makinen et al155 | VEG165; plasmid and adenovirus | 2×1010 pfu VEGF-Ad; or 2 mg/2mL VEGF plasmid | IA | 56 | Class 1–6 | 3 mo | Increased vascularity on DSA |
| Kusumanto et al156 | VEGF165; plasmid | 2 mg each for 2 session | IM | 54 | Class 4–6 | 100 days | No changes in amputation rates or rest pain; improvements in skin ulceration, ABIs, TBIs |
| Hypoxia-inducible factor 1-alpha | |||||||
| Rajagopalan et al60 | HIF-1alpha; adenovirus | Dose escalation; 1×108 to 2×1011 viral particles | IM | 34 | No option CLI | 12 mo | No serious adverse events attributable to study treatment; no amputations in 2 highest dose groups; complete rest pain resolution in 14 of 32 patients and complete ulcer healing in 5 of 18 patients |
| Creager et al59 | HIF-1alpha; adenovirus | 2×109; 2×1010; 2×1011 viral particles; or placebo | IM | 289 | Class 1–3 | 12 mo | No significant differences in peak walking time, claudication onset time, ABI, or quality of life |
| Developmental endothelial locus-1 | |||||||
| Grossman et al157 | Del-1; plasmid | 42 mg plasmid or placebo over 21 injections in each leg (84 mg per subject) | IM | 105 | PAD and stable exercise-limiting IC | 12 mo | Improvement in peak walking time, ABI, claudication onset time, and quality of life but no difference between control group |
| Hepatocyte growth factor | |||||||
| Morishita et al63 | HGF; plasmid | Either 2 or 4 mg (4 or 8 injections) | IM | 22 | Fontaine IIb, III, or IV, | 2 yr | ABI, pain relief, and ulcer healing were improved in the majority of patients |
| Powell et al158 (HGF-STAT) | HGF; plasmid | 0.4 mg×8 for 3 sessions (low); 4 mg×8 for 2 sessions (mid); 4 mg×8 for 3 sessions (high) | IM | 104 | Class 4–6 | 12 mo | Increased TcPO2 at 6 mo in high-dose group; no changes in amputation, death, ulcer size, wound healing, ABI, TBI |
| Powell et al159 (HGF-0205) | HGF; plasmid | 0.5 mg×8 for 3 sessions | IM | 27 | Class 5–6 | 6 mo | No change in wound healing or amputation; increase in TBI at 6 mo; increase rest pain improvement |
| Shigematsu et al160 | HGF; plasmid | 0.5 mg×8 for 2 sessions | IM | 40 | Class 4–5 | 3 mo | Increased reduction in ulcer size; increased quality of life; no change in rest pain or ABI |
| Henry et al161 | HGF (VM202); plasmid | Dose escalation 2–16 mg | IM | 12 | No option CLI | 12 mo | Increase in median ABI/TBI; decrease in pain (visual analog scale) |
| Gu et al162 | HGF (VM202); plasmid | 4 mg; 8 mg; 12 mg; 16 mg | IM | 21 | Class 4–6 | 3 mo | Increase in mean ABI and TcPO2; decrease in pain; improved wound healing |
ABI indicates ankle brachial index; Del-1, developmental endothelial locus-1; CLI, critical limb ischemia; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor-1 alpha; IA, intra-arterial; IC, intermittent claudication; IM, intramuscular; PAD, peripheral artery disease; TBI, toe brachial index; TcPO2, transcutaneous oxygen pressure; and VEGF, vascular endothelial growth factor.
Fibroblast Growth Factor
The first randomized clinical trial of angiogenic therapy was with recombinant human basic FGF for the treatment of intermittent claudication (IC). In this study, basic FGF was administered weekly by intravenous infusions of 2 μg/kg for 6 sequential weeks (total dose 12 μg/kg).55 The primary efficacy end point was change in peak walking time on a graded exercise treadmill protocol. The study was stopped prematurely after treatment of the first 24 subjects because of clinically significant proteinuria in 5 of the 16 subjects who received systemic basic FGF. The small sample size limited evaluation of the predefined efficacy end points; however, there was no significant difference between the treatment and control groups for any of the measures of efficacy. The importance of this study is that it affirmed the view that systemic administration of angiogenic therapy should be limited in time and space to reduce the risk of side effects. Subsequent studies used infusions directly into limb arteries56 or injections of the angiogenic agents into the limb musculature to reduce the risk of systemic side effects. In the TRAFFIC (Therapeutic Angiogenesis with FGF-2 for Intermittent Claudication) study, intra-arterial administration of recombinant FGF-2 increased peak walking time at 90 days. A repeat infusion at 30 days was no better than one infusion. The findings of TRAFFIC supported the concept of therapeutic angiogenesis as a potential treatment strategy for PAD, but the intra-arterial use of FGF-2 has not been pursued in phase III studies. A phase 1/2a study of FGF gene therapy delivered intramuscularly using a non-transmissible recombinant Sendai virus has been completed,57 and a phase 2a study is underway in patients with IC.
Vascular Endothelial Growth Factor
The Regional Angiogenesis with Vascular Endothelial growth factor (RAVE) study was a randomized clinical trial assessing the benefit of an adenoviral construct encoding VEGF121 (AdVEGF) in patients with primarily unilateral exercise-limiting IC.58 Study subjects were randomized to low-dose (4×109 particle units) or high-dose (4×1010 particle units) administered as 20 intramuscular injections to the symptomatic leg in a single session. This study was not encouraging because the primary efficacy end point, change in peak walking time at 12 weeks, did not differ between the placebo, low-dose, or high-dose groups.
Hypoxia-Inducible Factor-1a
As described above, HIF-1α is a transcriptional factor that orchestrates the tissue response to hypoxia. The WALK study tested the efficacy of an adenoviral construct encoding a constitutively active form of HIF-1α in patients with IC.59 Previously, a small pilot study in patients with CLI suggested that the gene therapy was well-tolerated.60 In a subsequent randomized clinical trial involving over 400 claudicants, patients received 20 injections of the construct into each limb, at one study visit. The primary end point was the change in treadmill exercise time at 6 months by comparison to baseline (before the injections). Regrettably, this well-conducted large randomized clinical trial was negative because there was no difference between the active and placebo groups, which each improved ≈25% in maximal treadmill time.
Developmental Endothelial Locus-1
The angiomatrix protein developmental endothelial locus-1 induces a potent angiogenic response, through coordinate upregulation of the integrins ανβ5 and ανβ3.61 The therapeutic potential of this angiogenic protein was tested using a plasmid-expressing developmental endothelial locus-1.62 One hundred subjects with bilateral IC were randomized to bilateral intramuscular delivery of the plasmid or placebo. The study drug was administered at one visit, being delivered as 42 intramuscular injections in both lower extremities. There was no difference between the placebo and plasmid treated groups in the primary end point, peak walking time at day 90 post-treatment, continuing the trend of negative randomized clinical trials for angiogenic therapies in PAD.
Hepatocyte Growth Factor
A phase I/IIa open-label trial was conducted using HGF in the form of a plasmid DNA construct and administered by intramuscular injection of naked plasmid DNA.63 Study subjects had moderate to severe PAD (Fontaine IIb, III, or IV) secondary to atherosclerosis or Buerger disease. HGF plasmid (2 or 4 mg) was injected into the calf or distal thigh (in 4 or 8 injection sites). Four weeks after the initial injection, the dose was repeated, giving study subjects a total of 4 or 8 mg of HGF plasmid. The 2 month follow-up of these individuals revealed that surrogate markers [ankle-brachial index (ABI); pain relief; ulcer healing] were improved in the majority of patients. No significant side effects of the gene therapy were reported at 6 months. At 2-year follow-up, the improvements were largely maintained.64 A global phase III study is now underway to study the efficacy of intramuscular injection of this therapy in patients with CLI.
Summary of Angiogenic Therapies for PAD
Despite substantial evidence of efficacy in preclinical studies, as well as some promising phase I/IIa human trials, larger randomized clinical trials of angiogenic therapies for ICor CLI have been negative. The discordance between the early developmental studies and the larger randomized clinical trials may be because of problems with the animal model. Most of the proof-of-concept preclinical studies have been in murine models of hindlimb ischemia. Typically, this model is a surgically induced ischemia in the absence of any cardiovascular risk factors, other than age (most often older C57 Bl6J mice are used because younger mice of this strain rapidly recover hindlimb blood flow to normal levels, as a result of the generation of collateral vessels and robust endogenous angiogenesis). Obviously, the typical murine model is different from PAD patient that are entered into clinical trials. The addition of cardiovascular risk factors to murine models may improve their predictive value. For example, diabetes mellitus is common in patients with PAD and is known to impair angiogenesis. Diabetes mellitus disrupts many processes required for angiogenesis, such as the elaboration of nitric oxide, VEGF, and microRNA-regulating angiogenic processes.65 Thus, in the absence of clinically relevant risk factors and disease, any benefit of an angiogenic agent in a mouse model may not be informative in humans. Murine models of hindlimb ischemia which incorporate cardiovascular risk factors, and vascular obstruction because of atherosclerosis, might provide more predictive results for potential angiogenic therapies in PAD.
It is also important to consider the strain differences in the response to ischemia. In response to the surgical induction of hindlimb ischemia, the C57Bl6 mice have a perfusion defect (the severity increasing with the age of the mouse) but rarely any tissue loss. By contrast, the BalbC strain manifests a profound ischemia, with gangrene and limb necrosis, after the same experimental procedure.66 The vulnerability of this strain has been traced to a genetic difference in a quantitative trait locus on chromosome 7 harboring 37 genes.67 The genetic difference in this strain may mediate reduced angiogenesis and collateral formation and increased sensitivity of the skeletal muscle to ischemia.68 Notably, the BalbC mice express lower levels of microRNA 93 in their ischemic skeletal muscle.69 This microRNA regulates multiple genes that modulate cell proliferation and apoptosis and may play a critical role in perfusion recovery from hindlimb ischemia. An understanding of the genetics of ischemic response is likely to yield novel insights that may provide new therapeutic directions.
Furthermore, there is insufficient knowledge regarding the methods for administration of the angiogenic therapy. For any angiogenic agent, there are many unknowns regarding the dose, the frequency of administration, and method of delivery in patients with PAD. Should angiogenic therapy be administered to the parenchyma of the tissue (ie, the ischemic skeletal muscle) so as to enhance angiogenesis within the tissue? Or should the therapy be targeted to the vicinity of the named conduits and to the proximity of known routes for collateral vessels? For example, the geniculate collaterals are supplied by branches of the deep femoral artery and these collaterals reconstitute the popliteal artery in the setting of superficial femoral artery occlusion. Could angiogenic/arteriogenic therapy targeted to these vessels enhance perfusion by increasing collateral flow?
With respect to the trials of angiogenic/arteriogenic therapies for CLI, the great majority of past trials were not informed by the recent concept of angiosomes. Angiosomes comprise a segment of tissue composed of the skin, subcutaneous tissue, muscle, and bone perfused by a specific artery and drained by a specific vein.70 In the foot, there are 6 angiosomes: 3 of which are supplied by the posterior tibial artery, 2 by the peroneal artery (n=2), and one by the anterior tibial artery. A recent retrospective review suggests that revascularization efforts (surgical or endovascular) are more likely to be successful when targeted to the vessel supplying the affected angiosome in the foot.71 Future trials of angiogenic/arteriogenic therapies for CLI will likely incorporate this concept.
In this regard, in the development of new PAD therapies, more attention should be paid to therapeutic arteriogenesis. Whereas the microvascular phenomenon of angiogenesis may be sufficient to improve perfusion to the small tissue volume of the ischemic murine hindlimb, the metabolic activity and much larger volume of the human leg require larger conduits that provide adequate inflow. The growth factor granulocyte macrophage-colony stimulating factor (GM-CSF) is known to increase circulating monocytes and has been tested in clinical trials. Regrettably, in the largest study in patients with IC, multiple subcutaneous administrations of GM-CSF over 14 days did not improve walking distance and ABIs.72 Furthermore, GM-CSF therapy may increase the risk of acute coronary events.73 Clearly, new insights into the mechanisms of arteriogenesis are needed so as to develop effective methods to therapeutically manipulate arteriogenesis. Some basic research insights from preclinical studies that may be represent therapeutic targets include endothelial-derived synectin as well as the Dll4-Notch signaling pathway.74,75
Trials of Cell-Based Therapies
Asahara’s discovery of the EPC subpopulation in 1997 marked the dawn of cell therapy as a therapeutic approach to vascular disease.76 Evidence suggested that these cells are derived from the bone marrow and are recruited into the circulating blood where they home to sites of ischemia. Since then, preclinical studies have made a compelling case for pursuing the use of these and other progenitor cells in trials for cardiovascular regeneration. In general, preclinical studies support the notion that a fraction of bone marrow–derived mononuclear cells (BM-MNCs) support angiogenesis and vasculogenesis.
Parenthetically, the widely used term EPC is now embedded into the vernacular of vascular biology; however, it is recognized that the term is shorthand for a much more complex biology. The surface markers that are commonly used for identification of human EPCs include markers that are not specific for endothelial lineage, such as CD133 and kinase insert domain receptor.77 In addition, methods for harvesting, purifying, and culturing EPCs are not standardized. Thus, semantic confusion is compounded by methodological variation. Within this population of cells, there are true endothelial progenitors that can incorporate into the vascular network, whereas hematopoietic progenitors may contribute by secreting angiogenic cytokines. In cell culture, EPCs may form early-outgrowth and late-outgrowth colonies. Cells derived from the former colonies are clearly not of endothelial origin, expressing markers of hematopoietic lineage, and are morphologically distinct from late-outgrowth cells, which grow in a cobblestone pattern reminiscent of ECs. At present, there are no surface markers that clearly distinguish early endothelial progenitors; the best approach currently is to define endothelial lineage functionally (ie, by the ability of true EPCs to inosculate into a new or pre-existing vascular network).
In addition, mature ECs might be recruited into the circulation, and home to sites of ischemia, so as to increase capillary density.47 In a parabiotic model, hindlimb ischemia led to the incorporation of circulating c-kit+CD45− progenitors in the microvasculature of the ischemic limb. Bone marrow replacement studies revealed that about half of these cells were derived from the bone marrow and about half were not. The authors proposed that these cells were ECs that were recruited into the blood, and circulated to the site of hindlimb ischemia, presumably under the influence of homing signals such as stromal-derived factor 1.
To date, over 50 clinical studies have been reported on the treatment of peripheral artery disease with progenitor cells.2,78 Studies have included patients of varying PAD severity, including those with IC to those CLI. Select past and ongoing studies include those with less severe ischemia (ie, patients with IC, Rutherford class 1–3).79,80 However, the majority of clinical trials of cell therapy for PAD have primarily focused on CLI patients (Rutherford class 4–6) in small phase I or II studies. Since the first pilot clinical study to evaluate treatment of peripheral vascular disease with stem cell therapy in 2002,81 therapeutic details such as patient selection, effective cell type selection and processing, optimal dosage, and delivery route continue to be characterized.
A variety of cell types have been studied as potential PAD treatments, including unselected BM-MNC or peripheral blood MNC (PB-MNC), marker-specific cells selected from the marrow or blood, mesenchymal stem cells (MSCs), and adipose tissue–derived regenerative cells. Discussed below are the clinical studies investigating cell therapies for peripheral vascular disease treatment.
Bone Marrow–Derived Mononuclear Cells (Tables 2 and 3)
Table 2.
Randomized Controlled Trial Studies Studying Unselected BM-MNC or PB-MNC for Critical Limb Ischemia
| Reference (Trial) | Cell Type | Cell Dose | Delivery Method | Subject No. | Indication | Follow-Up | Outcome |
|---|---|---|---|---|---|---|---|
| Bone marrow–derived mononuclear cells | |||||||
| Van Tongeren et al98 | BM-MNC | 109 cells (included 106 CD34+ cells) | IA+IM 15:IM 12:IA+IM |
27 | No option CLI | 12 mo | Significant improvements for both tx arms in ABI, pain score, pain-free walking distance with cell therapy compared with baseline. No significant difference in limb salvage rates between tx arms |
| Walter et al163 (PROVASA) | BM-MNC | 108 cells (included 106 CD34+) Dose escalation: single dose or double dose |
IA | 40 | CLI RC 4–6 | 6 mo | Significant improvements in ulcer healing, rest pain with cell therapy in this multicenter, double-blind, randomized-start clinical trial. No significant improvements found in ABI or limb salvage |
| Iafrati et al88 (BMAC) | BM-MNC | 109 cells | IM | 48 | RC 4–5 | 3 mo | Improvement in amputation, pain, quality of life, Rutherford classification, and ABI compared with control |
| Benoit et al87 (BMAC) | BM-MNC | 109 cells | IM | 48 | RC 4–5 | 6 mo | Lower amputation rates compared with placebo |
| Prochazka et al89 | BM-MNC | Unspecified | IM | 96 | RC 4–6 | 4 mo | Significant improvements in limb salvage rates and ABI |
| Peripheral blood–derived mononuclear cells | |||||||
| Huang et al90 | PB-MNC | 108 (≈0.4% CD34+) cells | IM | 28 | RC 4–6 | 3 mo | Significant improvements in ABI, ulcer healing, Laser Doppler blood perfusion, angiographic scores, limb salvage with cell therapy compared with randomized control |
| Ozturk et al94 | PB-MNC | 107 | IM | 40 | RC 4–6 | 3 mo | Improved ABIs, pain scores |
| Mohammadzadeh et al93 | PB-MNC | 107 | IM | 21 | CLI, DM | 3 mo | Improved ABIs, amputation rates |
ABI indicates ankle brachial index; BM-MNC, bone marrow–derived mononuclear cell; CLI, critical limb ischemia; DM, diabetes mellitus; IA, intra-arterial; IM, intramuscular; PB-MNC, peripheral blood–derived mononuclear cell; and RC, Rutherford classification.
Table 3.
Randomized Controlled Trials Comparing Cell Types for Critical Limb Ischemia
| Reference (Trial) | Cell Types | Cell Dose | Delivery Method | Subject No. | Indication | Follow-Up | Outcome |
|---|---|---|---|---|---|---|---|
| Peripheral blood–derived mononuclear cells | |||||||
| Tateishi-Yuyama et al81 (TACT trial) | BM- vs PB-MNCs | 109 BM- or PB-MNC’s (the BM-MNC infusion included 107 CD34+ cells) | IM gastroc | 45 | RC 4–6 | 24 wk | Significant improvements in ABI, TcPO2, rest pain, pain-free walking time with BM-MNC compared with placebo (pilot study), as well as with BM- MNC compared with PB-MNC (randomized control trial) |
| Matoba et al95 (TACT trial) | BM- vs PB-MNCs | 109 BM-MNC vs PB-MNC’s as control (the BM-MNC infusion included 107 CD34+ cells) | IM | 115 | RC 4–6, 74 ASO 41 TAO |
3 yr | Significant improvements in leg pain, walking distance, ulcer size at 2 yrs with BM-MNC compared with PB-MNC. No significant difference in ABI or TcPO2 detected |
| Huang et al79 | BM- vs PB-MNCs | 109 G-CSF– mobilized PB-MNC (includes 108 C34+ cells) vs 108 BM-MNC (includes 107 Cd34+ cells) | IM | 150 | Any RC; ASO | 3 mo | Significant improvements in ABI, skin temperature, rest pain with PB-MNC compared with BM-MNC. No significant difference in TcPO2, pain-free walking distance or amputation rates detected |
| Kamata et al96 | BM- vs PB-MNCs | 108 BM-MNC or PB-MNC (included 106 CD34+ cells in each group) Note: PB-MNC were not mobilized |
IM | 6 | RC 4–6 Vasculitic |
1 mo | Significant improvements in rest pain (both treatment groups) with both BM-MNC and PB-MNC therapy |
| Lu et al125 | BM-MSC vs BM-MNC | Unspecified | IM | 41 | CLI, DM, RC 5–6 | 6 mo | BM-MNC and BM-MSC associated with improved ABIs, TcO2, and pain-free walking time. Collateral score, faster ulcer healing with BM-MSC>BM-MNC>placebo |
ABI indicates ankle brachial index; ASO, arteriosclerosis obliterans; BM-MNC, bone marrow derived mononuclear cell; BM-MSC, bone marrow derived mesenchymal stem cell; CLI, critical limb ischemia; DM, diabetes mellitus; IA, intra-arterial; IM, intramuscular; PB-MNC, peripheral blood derived mononuclear cell; RC, Rutherford classification; TAO, thromboangiitis obliterans; and TcPO2, transcutaneous oxygen pressure
Mononuclear cells, harvested from either bone marrow or blood, are comprised of a heterogeneous mix of blood cells, nonhematopoietic stromal cells such as MSCs and EPCs, and these cells secrete a variety of proangiogenic cytokines.42,82
The first report of an autologous stem cell therapy for the treatment of ischemic limbs was through the TACT (Therapeutic Angiogenesis using Cell Transplantation) study using unselected MNCs from both the bone marrow (BM-MNC) and peripheral blood (PB-MNC) for the treatment of CLI.81 The study reported improved rest pain, transcutaneous oxygen pressure (TcPO2), ABI, and pain-free walking distance by comparison to the control group (saline injection) at 6 months. Subsequent studies have investigated the safety and feasibility of autologous unselected BM-MNCs in ischemic limb treatment, primarily in small case series.2,78 Initial findings suggested clinical benefits of intramuscularly administered unselected BM-MNCs for ischemic limb treatment, including improved ABI, pain-free walking distance/time, wound healing, limb salvage, and Rutherford class.83–86 Recent studies have provided additional supportive evidence in randomized, controlled trials.87–89 These studies of BM-MNC administered intramuscularly to patients with severe limb ischemia report clinical improvement including lower amputation rates and improved ABI when compared with control treated subjects. While promising, these studies are not definitive.
Peripheral Blood–Derived Mononuclear Cell (Tables 2 and 3)
MNCs harvested from peripheral blood have also been actively examined in autologous cell therapy for ischemic limb treatment.81,90–94 PB-MNCs are mobilized through granulocyte-colony stimulating factor injection, and cell collection from blood rather than bone marrow harvesting offers faster hematologic recovery and eliminates anesthetic medication use and anemia risk. Collective clinical findings of small case series show significant improvements in ABI, pain scale, walking distance, and limb salvage compared with baseline. Randomized trials of PB-MNCs have confirmed these early clinical improvements (3 months) in ABI and pain, although amputation rates did not differ between those treated with unselected PB-MNC versus the control group.90–94 One study reported significantly fewer patients facing major amputation of a limb in the group infused with PB-MNC versus those in the control group at the conclusion of the studied.93
BM-MNC Versus PB-MNC
To date, 4 randomized controlled trials have directly compared BM-MNC with PB-MNC.79,81,95,96 The first study, by Tateishi-Yuyama et al,81 used CLI patients with bilateral ischemia as their own controls to directly compare the therapeutic effects of BM-MNC and PB-MNC in CLI patients out to 1 year. They showed that BM-MNC infusion resulted in significant improvements in ABI, TcPO2, rest pain, pain-free walking time compared with both the placebo group, and with those infused with PB-MNC. Notably, the dose of cells administered to the PB-MNC group was substantially lower, providing evidence that a cell dose threshold must be reached to achieve bioactivity. Subsequent comparative studies of similar sample size have reported inconsistent clinical outcomes. Although Huang et al reported that at 3 months, those treated with PB-MNC showed improved ABI and rest pain compared with patients receiving BM-MNC, Matoba et al, reporting on long-term follow-up of the TACT study, reported significant improvements in pain, walking distance, and ulcer size at 2 years in those infused with BM-MNC compared with those treated with PB-BMC, perhaps reflecting the same influence of dose as seen in the initial publication.79,95 One small open-label study reported that CLI vasculitis patients significantly improved in rest pain at 1 month with either BM-MNC or PB-MNC treatment.96
MNC Delivery Through Intramuscularly/Intra-arterialy
In addition to identifying suitable cell sources for PAD treatment, the influence of the administration route also needs to be defined. Although the studies described above have primarily evaluated the therapeutic effects of intramuscular administration of MNCs from either bone marrow or blood, the potential of intra-arterial MNC delivery is also being explored.97–99 Intramuscular delivery is hypothesized to result in a transient cell depot directly within the ischemic tissue site to allow both local paracrine activity as well as incorporation of cells into the neovasculature. Intra-arterial delivery is thought to direct stem cells into viable peri-ischemic zones with sufficient oxygen and nutrient content for cell function support. Intra-arterialy delivered BM-MNC to ischemic limbs have been shown to result in increased capillary densities and improved ABI and pain-free walking distance at 1 year.100,101 The PROVASA (Intraarterial Progenitor Cell Transplantation of Bone Marrow Mononuclear Cells for Induction of Neovascularization in Patients With Peripheral Arterial Occlusive Disease Study) study represents the only multicenter, randomized, controlled trial investigating intra-arterial BM-MNC therapy in CLI patients to date.99 The PROVASA study demonstrated dose-dependent ulcer healing improvement and rest pain reduction in those treated with intra-arterialy administered BM-MNC compared with the placebo group. However, ABI or limb salvage rates did not differ between the groups. To date, only Lenk et al102 have studied the effects of intra-arterialy administered PB-MNC on patients with end-stage PAD, in which those treated with unselected PB-MNC significantly improved in ABI and TcPO2 from baseline to 3 months.
Several smaller studies have reported on the clinical outcomes of concurrently delivering BM-MNC via intramus-cularly and intra-arterialy, and, collectively, these studies provide evidence of the early and late clinical gains including ABI, ulcer healing, pain-free walking distance, and limb salvage.97,103–105 In a randomized study of 27 no-option CLI patients, Van Tongeren et al98 showed a nonsignificant trend toward lower amputation rates in the intramuscular+intra-arterial group compared with the intramuscular group. Hence, larger studies are need to clarify further the optimal route of therapeutic cell delivery. The JUVENTAS (Intra-arterial Stem Cell Therapy for Patients With Chronic Limb Ischemia) study has reported on the clinical design of evaluating 110 to 160 CLI patients treated with intra-arterialy administered BM-MNC, but outcomes of the study have yet to be reported.106
Marker-Selected MNCs (Table 4)
Table 4.
Randomized Controlled Trials Studying Marker-Selected Cells for Critical Limb Ischemiaa
| Reference (Trial) | Cell Type(s) | Cell Dose | Delivery Method | Subject No. | Indication | Follow-Up | Outcome |
|---|---|---|---|---|---|---|---|
| Losordo et al115 (ACT34-CLI) | PB-CD34+ | Dose escalation: Placebo, 105 (n=7), 106 (n=9) | IM | 28 | RC 4–5 | 12 mo | Nonsignificant improvement in amputation rates at 12 mo (P =0.058) with increased dose cell therapy compared with placebo-control |
| Perin et al122 (CLI-001) | ALDHbr cells (CD34+, 133+, 14+,117+) vs BM-MNC | Unspecified | IM | 21 | RC 4–5 | 6 mo | Dose-related nonsignificant trend t reduced amputation rate |
| Powell et al117 (RESTORE-CLI) | TRC (CD90+, 14+, 45+, 105+/14+/45+ cells) | 107–108 | IM | 86 | RC 4–6 | 12 mo | Decreased treatment failure at 12 mo (major amputation of the injected leg, all-cause mortality, doubling of total wound surface area from baseline, or de novo gangrene) |
| Kirana et al118 | TRC, BM-MNC | 107 TRC 107 BM-MNC |
IM/IA | 24 | RC 4–6 | 12 mo | No difference between cell therapies |
ALDHbr cells indicates aldehyde dehydrogenase (bright) cells; BM-MNC, bone marrow–derived mononuclear cell; IA, intra-arterial; IM, intramuscular; PB-MNC, peripheral blood–derived mononuclear cell; RC, Rutherford classification; and TRC, tissue repair cells.
There is still debate regarding the definition of the EPC. Not in question is the reality that the ECs that line the blood vessels and compose the capillaries at birth do not survive for 100 years. Similarly, the monthly growth of a vascular endometrium for ≥30 years seems to be by a means other than existing blood vessels. It seems that only a small subset of EPCs is of true endothelial lineage in humans. These endothelial colony-forming cells can form vascular structures in vivo but are rare, only 1 to 2 per 100 million MNC.107 Another subset of EPCs, which are more common, is of hematopoietic lineage. These EPCs share the same surface markers (CD31, CD105, CD144, CD146, von Willebrand factor, kinase insert domain receptor, and ulex europaeus agglutinin 1 lectin) and incorporate acetylated low-density lipoprotein, but they express the myeloid surface markers CD45 and CD14 and have other features of the monocyte/macrophage phenotype. Some of these cells may contribute to angiogenesis not by incorporating into the vasculature but by secreting angiogenic cytokines and matrix metalloproteinases.108,109 Still other bone marrow–derived cells can form pericytes, which associate with and stabilize endothelial networks.110
EPCs are a subpopulation of MNC, which can be isolated based on surface markers such as the progenitor marker CD34. Evidence suggests that a percentage of CD34 cells can differentiate into mature ECs and that CD34 cells in general facilitate vascular repair in various ischemic tissues.111,112 Preclinical studies of isolated human EPC transplantation in ischemic hindlimb in mice have demonstrated increased perfusion and higher limb salvage rate.113 Clinical safety and feasibility of CD34+ cells for ischemic limb treatment have been evaluated in a dose escalation trial of granulocyte-colony stimulating factor–mobilized peripheral blood CD34+ cells (3 doses: 105, 5×105, 106) administered by intramuscular injection.114 Any dose of CD34+ administered resulted in a total efficacy score improvement at 3 months, exhibited by pain and ulcer size reduction, and increases in toe brachial pressure index, TcPO2, and pain-free walking distance. Longer term clinical benefits were also reported in a 28-patient, randomized, double-blinded, controlled, dose-escalation study in which granulocyte-colony stimulating factor–mobilized selected CD34+ cells were administered by intramuscularly (ACT-34 CLI [Autologous Cell Therapy-34 Critical Limb Ischemia] trial).115 The study showed that at 12 months, amputation incidence was lower in the combined cell-treated groups (doses of 105 or 106 cells per kg body weight) compared with the control group (P=0.054). Additionally, each dose group trended toward improved amputation-free survival at 6 and 12 months.
A mix of multiple cell lineages for PAD treatment have also been studied. Ixmyelocel-T or tissue repair cells consists of CD90+ cells [primarily of mesenchymal stem and progenitor cells and CD45+ cells (endothelial stem and progenitor cells)] harvested from the bone marrow and expanded in culture. In a randomized phase 2 trial (RESTORE-CLI [Use of Tissue Repair Cells (TRCs-Autologous Bone Marrow Cells) in Patients with Peripheral Arterial Disease to Treat Critical Limb Ischemia]), tissue repair cell administered intramuscularly to CLI patients resulted in a significantly prolonged time to treatment failure and trend toward increased amputation-free survival at 1 year.116,117 A randomized trial comparing tissue repair cells with unselected BM-MNCs (REVIVE-CLI [An Efficacy and Safety Study of Ixmyelocel-T in Patients With Critical Limb Ischemia]) was, unfortunately, recently terminated because of slow enrollment and a change in strategic focus of the sponsor. In a small randomized trial, Kirana et al118 reported nonsignificant trends toward improved ABI, TcPO2, and wound healing in patients treated with BMC or tissue repair cell, but no differences between the 2 groups were observed.
Another heterogeneous mix of MNC derived proangiogenic cell populations has been explored using high aldehyde dehy-drogenase (ALDH) activity. ALDH is an oxidizing enzyme highly expressed in both embryonic and adult stem cells, and BM-MNCs selected for high ALDH activity express CD34+, CD133+, CD14+, and CD117+.119,120 Preclinically, treatment of mice ischemic hind limbs with BM-MNCs selected for high ALDH activity led to perfusion recovery and increased blood vessel density.121 A phase 1, randomized, controlled trial compared unselected BM-MNCs to BM-MNCs selected for high ALDH in the treatment of CLI, demonstrated significant ABI improvement in both groups. However, no differences were observed between the 2 groups, and neither treated group showed significant improvement in ischemic ulcer grade or TcPO2 compared with the control group.122 An ongoing study of ALDH bright cells is being conducted by the National Institutes of Health–sponsored Cardiovascular Cell Therapy Research Network.123
Mesenchymal Stem Cells
Mesenchymal stem cells have been pursued as a potential candidate for PAD cell therapy because of their multi-potent, paracrine, transdifferentiation, and immunosuppressive effects.124 Preclinical findings showed that bone marrow–derived mesenchymal stem cells (BM-MSCs) injected into ischemic hind limbs of rats led to greater perfusion and capillary density increase than BM-MNC administration. A randomized controlled trial comparing BM-MSCs to BM-MNCs was conducted on 41 CLI patients with diabetes mellitus and demonstrated significantly improved pain-free walking time in both groups at 6 months.125 Additionally, the BM-MSC infusion led to significantly greater collateral blood vessel scores than BM-MNC injection. All patients in the cell therapy group experienced ulcer healing, but those injected with BM-MSCs healed by 8 weeks while those receiving BM-MNCs showed healing by 12 weeks. These findings may suggest that BM-MSCs may offer faster wound healing compared with unselected BM-MNCs.
Other Cell Types for PAD Therapy
A combinatorial approach of infusing multiple cell types in ischemic areas has also been studied. A small phase I investigation of 10 patients with severe limb ischemia examined the effects of concurrently delivering BM-MNC (30×108) and BM-MSC (30×106) administered intramuscularly. Patients receiving the combination cell therapy reported significant ABI improvement as early as 1 month, which lasted out to the last follow-up at 10 months. Trends toward improved walking time, TcPO2, and quality of life were also observed.
Allogeneic MSCs have also been explored for PAD treatment, in an attempt to leverage their immune-modulating capabilities. In a phase I study of 20 CLI patients, intramuscular administration of allogeneic BM-MSC treatment led to a significantly increased ABI compared with the placebo group at 6 months.126
In addition to allogeneic MSCs, various cell types such as adipose-derived regenerative cells, induced pluripotent stem cells, and nuclear reprogrammed stem cells are currently being explored for proangiogenic potential for PAD treatment in preclinical models. Clinical studies using these cell sources are now required to determine whether these cell sources provide advantages compared with prior cell types.127–131
Summary of Cell Therapies for PAD
Since the publication of the TACT study, there have been ≈50 reported therapeutic cell trials in patients with PAD. Two thirds of these studies have been in patients with CLI as defined by rest pain or tissue necrosis, and the other 1/3 in patients with symptomatic PAD (ie, IC). Most of the trials have used BM-MNCs, with fewer using granulocyte-colony stimulating factor–mobilized PB-MNCs and fewer still using a specific cell type. Most of the trials have used intramuscular administration to the calf and thigh (10–80 sites) using a total of 1–10×107 CD34+ cells, although the dose of CD34 cells is only rarely prespecified. End points have included ankle-brachial pressure index, transcutaneous oxygen level, Laser Doppler perfusion, pain-free or maximal walking distance, relief of rest pain, or healing of ischemic ulcers.
Using these end points, modest signals of efficacy has been reported in most trials, but the confidence intervals are large. Most trials enrolled relatively small numbers of patients, followed for 1 or 2 years. Furthermore, in most trials, the characterization of the therapeutic cells and the evaluation of dose have both been limited. Often, there is no functional assessment of the cells. Together, these 3 phenomena, ie, lack of information about the drug, its dose or it’s potency, are challenges to the interpretation of the studies, and opportunities for future development, which appears warranted based on early signals of bioactivity.
As noted above, whether there is persistence of these cells in the ischemic tissues is controversial, with human and animal evidence of incorporation of progenitor cells in the vasculature,132,133 while other studies have failed to document these findings. Moreover, the mechanism(s) of their therapeutic effect (if any) remains to be fully understood. Preclinical animal studies suggest that progenitor cells can integrate into the ischemic tissue neovasculature and can secrete paracrine factors to increase the density and perfusion of the microvasculature.42,134,135 In addition, these transplanted progenitor cells secrete exosomes containing proteins, RNAs, and microRNAs that stimulate both receptor-mediated and genetic mechanisms and contribute to a significant component of the paracrine effect of progenitor cell transplantation.136,137 As a result, almost 10 years later, we do not know for certain if cell therapy in PAD is useful, or do we understand the mechanism of any clinical benefit. Furthermore, in the case of autologous adult stem cells, the conditions that give rise to PAD (eg, diabetes mellitus and tobacco exposure) may also decrease the number and function of the therapeutic cells.138 Despite this theoretical limitation, early clinical studies have provided evidence for safety and bioactivity/benefit. Augmenting the potency of autologous progenitors139 represents one potential means of overcoming the possible detriment conferred by the existence of comorbid conditions. Alternatively, allogeneic progenitor cells (such as mesenchymal stromal cells derived from adipose or placental tissue) may represent another possible approach, although these cells will encounter difficulties because of shortened tissue residence time, which may limit their effect.140 Although these allogeneic cells are easier to procure and amenable to tissue banking, there is little clinical or preclinical evidence of their efficacy. Nevertheless, the potential limitations of adult stem/progenitor cells provide a rationale for deriving and testing therapeutic cells from other sources.
The typical approach to isolate BM-MNCs or PB-MNCs for PAD cell therapy trials generate a heterogeneous population of cells that have not been completely characterized.76,141–143 It is possible that a greater effect size and more persistent benefit of cell therapy could be realized with a more precisely defined cell population. Although the use of multiple surface markers to isolate subpopulations of therapeutic cells is attractive scientifically, the use of a single surface marker for cell selection is a practical concession in terms of technical and cost factors. If multiple marker–selected cells are shown to have significant benefit compared with single maker cells, these technical challenges will have to be overcome.
Greater clarity is needed regarding the composition and fate of the cells that are used for cardiovascular cell therapy. Cells aspirated or mobilized from the bone marrow are a heterogeneous population of cells that may promote EC survival and proliferation, contribute to network formation, stabilize newly formed vessels, enhance myocardial cell survival, and incorporate into ischemic tissue. Improved characterization of the administered cells and dose–response relationships could provide insights into which cell types might be associated with these physiological processes, and with improvements in clinical end points. Greater knowledge about the therapeutic subset of cells could also provide guidance about the dose and frequency of administration required for optimum effects. The therapeutic product needs much better definition, so as to allow improved quality control, and monitoring of variation because of methodological or patient differences. New methodologies to comprehensively characterize cell therapies such as single cell mass cytometry might be used to comprehensively characterize the cell product.144 Quantification of these cellular subsets and their response to hypoxia may be more predictive of clinical responses. In addition, the study of cryopreserved cells is needed to permit multiple dosing which, logically, should any augment clinical benefits induced by a single administration. There is a long successful track record of the use of cryopreserved cells for hematopoetic cell transplant, and this history should provide guidance for the field of cardiovascular cell therapy.
It is also necessary to apply contemporary imaging methodologies to gain more insight into the distribution and fate of the injected cells and their effect on limb perfusion and anatomy. For example, molecular imaging using cells that are genetically modified (eg, with constructs encoding thymidine kinase) for positron emission tomography have been developed and are now in clinical trials for Food and Drug Administration approval.145,146 New developments in MRI, such as dynamic contrast-enhanced imaging, combined with innovative 4-dimensional algorithms, may provide greater precision of limb perfusion measurements to improve the ability to detect changes related to angiogenesis and arteriogenesis induced by therapeutic cell therapy. It is important to note that the regional differences in limb ischemia are dependent on the variability of the arterial anatomy and heterogeneity of the disease from one patient to the next. It is conceivable that the benefit of cell delivery might be enhanced by directing the cell injections to ischemic regions of the calf and thigh, as mapped by magnetic resonance perfusion imaging. One of the major challenges to developing therapies for CLI has been the lack of a reliable surrogate that is predictive of clinical success (ie, wound healing, pain relief, and avoidance of amputation). The development of a method of quantifying perfusion in the limb could, therefore, represent a breakthrough in the field.
Beyond Adult Stem Cell Therapies
In the case of PAD, there are preclinical data, and small clinical trials with adult progenitor cells, indicating that benefit may be achieved simply by injecting therapeutic cells into the ischemic muscle. However, to restore maximal levels of perfusion, it is likely that large diameter, low resistance conduits will be necessary. It is unlikely that injections of cells could create this benefit. An additional approach would be to provide the cell therapy as a biological conduit, incorporated into a cylindrical bioengineered matrix or decellularized cadaveric vessels. These bioengineered conduits would serve to replace autologous saphenous vein in patients that have insufficient or diseased veins.
An alternative approach would be to use vascular cells derived from embryonic stem cells or induced pluripotent stem cells. The generation of these cells and their differentiation into vascular cells have been previously reviewed.147 In brief, both embryonic stem cell–derived or induced pluripotent stem cell–derived ECs, when injected into the ischemic hindlimb, can enhance capillary density and improve blood flow in pre-clinical models.127,130,148,149 These effects seem to be because of paracrine substances released by the cells, rather than their direct incorporation into the microvasculature of the ischemic limb. Whereas these studies are promising, the complex nature of stem cells and their derivatives presents several challenges. The use of human embryonic stem cells is confounded by ethical issues as well as the immune barrier. Whereas autologous induced pluripotent stem cell–derived cells avoid these issues, there remains concern about the possibility for teratoma formation. Cell populations derived from different sources or donors may not have the same safety and activity profiles, despite the use of the same processing methods on each population. Moreover, alterations to cell processing methods may lead to unintended changes in the safety and activity profile. The cells may also undergo genetic or epigenetic changes during cell culture, during isolation, expansion, or in cold storage. These factors are some of the liabilities when attempting to generate any stable, renewable, and consistent cell product.
Accordingly, another exciting approach is to use small molecules or growth factors to modify resident stem cells, or somatic cells, in a therapeutic manner. In this way, it may be possible to avoid the vagaries of therapy with exogenous cells. Proof-of-concept has been obtained in preclinical studies for therapeutic transdifferentiation. Specifically, several investigators have used viral vectors encoding lineage factors to transdifferentiate nonvascular cells into ECs.150,151 Based on our previous work that optimal activation of innate immune signaling can increase epigenetic plasticity,127 we have used this property of cells to systematically change their phenotype. This knowledge has permitted us to develop a small molecule cocktail that induces human fibroblasts to transdifferentiate into induced ECs.128 The human induced ECs are identical to genuine ECs as assessed by immunohistochemical markers, functional assays, and RNA seq transcriptional profiles. They can self-assemble into functional capillaries in vivo. In the murine model of PAD, injections of these cells into the ischemic hindlimb improves limb blood flow together with an increase in capillary density.128 Finally, the transdifferentiation cocktail can induce human fibroblasts to metamorphose into ECs in vivo, in a matrigel plug assay in immunodeficient mice. This proof-of-concept for vascular transdifferentiation in vivo provides encouragement for translational development.
Summary and Conclusion
It is disappointing that after extensive preclinical investigation, and after 2 decades of clinical trials of angiogenic or cell therapies, we have no Food and Drug Administration–approved therapies for PAD in either class. Nevertheless, we have gained great insights into the mechanisms of vascular regeneration and the response to ischemia. Furthermore, we have some understanding of the flaws in the preceding trials that may permit improved trial design in the future. Based on the foregoing information, we have the following recommendations:
The field needs a more practical regulatory roadmap. In particular, for CLI, the Food and Drug Administration has indicated that a demonstration of amputation-free survival will be required for approval. This is a high bar that discourages new drug development for this indication. Furthermore, this criterion does not recognize the real benefit of pain relief and wound healing for these individuals. Pain relief and wound healing in patients with CLI, in the absence of an adverse safety signal, should be approvable (and reimbursable) end points. A robust dialogue between regulatory bodies, physicians, and industry, with input from patients, is necessary to strike an appropriate balance between global risk (disease risk, potential risk of the therapy) and possible benefit.
There is a critical need for the application of novel imaging technologies to understand mechanisms of response and to indicate the likelihood of success. For example, MRI of perfusion and anatomy may provide evidence of enhanced perfusion and arteriogenesis. Molecular imaging with positron emission tomography probes may provide new insights into the distribution and fate of angiogenic agents or administered cells.
Preclinical models are challenged by the fact that the human leg is unique in form and function, and in the setting of PAD exists in a unique environment. Preclinical models may benefit by incorporating more features of PAD if this increases their predictive value. Investigators working with preclinical models should consider incorporating cardiovascular risk factors and chronic structural disease of the limb arteries into their models. Primate models remain the closest approximation to human atherosclerosis; however, they are difficult to use for financial, technical, and political reasons.
These improved models may provide critically needed information regarding the correct dose, duration, delivery method, for angiogenic or cell therapies. For example, should angiogenic therapy be targeted to the skeletal muscle, or directed along the course of the conduit vessels or collaterals?
Improvements to cell therapy will benefit from a more precise characterization of cellular subsets in the therapeutic product. At a basic level, it is necessary to understand which cell or cells being administered are the putative therapeutic agent, and to define the dose. A more comprehensive understanding of mechanisms of benefit and the contributions of different cellular subsets to the therapeutic effect are also required. Single cell analyses (eg, mass cytometry or RNA seq) may better define these subsets.
In clinical trials of cell therapies, a systematic approach to characterizing the study population and cell product, and the cell processing, dose, frequency, and delivery methods will likely add to the advancement of the field. A consensus statement on best practices for conducting and reporting cell therapy trials would improve the quality of studies, allow comparison of different approaches, and advance the field.
Angiogenic therapies have not yet fulfilled their early preclinical promise. This may be because of many factors including deficiencies in preclinical models, and insufficient information about drug dose, duration, and delivery. Newer strategies, such as therapeutic transdifferentiation, may revitalize this arena. In any event, in the development of an effective angiogenic agent, one must also address safety concerns related to pathological angiogenesis. For example, many diabetic patients with PAD also have retinal neovascularization that might be adversely affected by an angiogenic therapy. Accordingly, it is likely that any angiogenic therapy (small molecules to cells) will be safer if its delivery is constrained in space and time.
In the meantime, lacking any approved angiogenic or cell therapy for PAD, the best way to enhance collateral formation and angiogenesis may be regular exercise. Because the patient with PAD typically has coexisting coronary artery disease, these individuals should be under the care of a cardiologist or vascular specialist, who can supervise a stress test to detect myocardial ischemia, and initiate medications to reduce cardiovascular risk, before implementing a supervised exercise program.
Nonstandard Abbreviations and Acronyms
- ABI
ankle-brachial index
- ALDH
aldehyde dehydrogenase
- BM-MNC
bone marrow–derived mononuclear cell
- BM-MSC
bone marrow–derived mesenchymal stem cell
- CLI
critical limb ischemia
- EC
endothelial cell
- EPC
endothelial progenitor cell
- FGF
fibroblast growth factor
- G-CSF
granulocyte-colony stimulating factor
- HGF
hepatocyte growth factor
- HIF-1α
hypoxia-inducible factor 1-alpha
- IC
intermittent claudication
- MSC
mesenchymal stem cell
- PAD
peripheral arterial disease
- PB-MNC
peripheral blood–derived mononuclear cell
- TcPO2
transcutaneous oxygen pressure
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
This work was supported in part by National Institutes of Health grants to J.P. Cooke (U01 HL100397, U01 HL099997, and 1UM1 HL113456). Dr Cooke is an inventor on patents owned by Stanford University related to the generation of nuclear reprogramming to pluripotency and transdifferentiation. Dr Losordo is an employee of NeoStem, which is developing stem cell therapies for cardiovascular application.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112:1288–1302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 4.Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol (1985) 2009;107:429–437. doi: 10.1152/japplphysiol.00331.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ, Casey DP. Muscle blood flow, hypoxia, and hypoperfusion. J Appl Physiol (1985) 2014;116:852–857. doi: 10.1152/japplphysiol.00620.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985) 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 8.Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol (1985) 2011;110:687–694. doi: 10.1152/japplphysiol.00787.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–1144. [PubMed] [Google Scholar]
- 12.Pereira F, de Moraes R, Tibiriçá E, Nóbrega AC. Interval and continuous exercise training produce similar increases in skeletal muscle and left ventricle microvascular density in rats. Biomed Res Int. 2013;2013:752817. doi: 10.1155/2013/752817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki J. Microvascular angioadaptation after endurance training with L-arginine supplementation in rat heart and hindleg muscles. Exp Physiol. 2005;90:763–771. doi: 10.1113/expphysiol.2005.031138. [DOI] [PubMed] [Google Scholar]
- 14.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol. 2011;300:H664–H669. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 15.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerman M, Cannan M, Charles R, Higano M, Stuart H, Nishimura M, Rick A, Holmes M, Jr, David R. Coronary vascular remodeling in association with endothelial dysfunction. Am J Cardiol. 1998;81:1105–1109. doi: 10.1016/s0002-9149(98)00135-0. [DOI] [PubMed] [Google Scholar]
- 17.Lanzer P. Mastering Endovascular Techniques: A Guide to Excellence. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 18.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 19.Nair N, Oka RK, Waring LD, Umoh EM, Taylor CB, Cooke JP. Vascular compliance versus flow-mediated vasodilation: correlation with cardiovascular risk factors. Vasc Med. 2005;10:275–283. doi: 10.1191/1358863x05vm633oa. [DOI] [PubMed] [Google Scholar]
- 20.Cooke M, John P, Dzau M, Victor J. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell AJ, Niebauer J, Lin PS, Tsao PS, Bernstein D, Cooke JP. Hypercholesterolemia impairs exercise capacity in mice. Vasc Med. 2009;14:249–257. doi: 10.1177/1358863X08100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heffernan KS, Karas RH, Mooney PJ, Patel AR, Kuvin JT. Pulse wave amplitude is associated with brachial artery diameter: implications for gender differences in microvascular function. Vasc Med. 2010;15:39–45. doi: 10.1177/1358863X09349523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2000:Cd001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease) J Am Coll Cardiol. 2006;47:e1–e192. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 27.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Inglis SC, Hermis A, Shehab S, Newton PJ, Lal S, Davidson PM. Peripheral arterial disease and chronic heart failure: a dangerous mix. Heart Fail Rev. 2013;18:457–464. doi: 10.1007/s10741-012-9331-1. [DOI] [PubMed] [Google Scholar]
- 29.Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. 2004;15:1197–1207. doi: 10.1097/01.RVI.0000137978.15352.C6. [DOI] [PubMed] [Google Scholar]
- 30.Halperin JL. Evaluation of patients with peripheral vascular disease. Thromb Res. 2002;106:V303–V311. doi: 10.1016/s0049-3848(01)00366-8. [DOI] [PubMed] [Google Scholar]
- 31.Donohue KG, Saap L, Falanga V. Cholesterol crystal embolization: an atherosclerotic disease with frequent and varied cutaneous manifestations. J Eur Acad Dermatol Venereol. 2003;17:504–511. doi: 10.1046/j.1468-3083.2003.00710.x. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 33.Moeller BJ, Cao Y, Vujaskovic Z, Li CY, Haroon ZA, Dewhirst MW. The relationship between hypoxia and angiogenesis. Semin Radiat Oncol. 2004;14:215–221. doi: 10.1016/j.semradonc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis) J Pathol. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 36.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 38.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schäfer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 39.Ito A, Nomura S, Hirota S, Suda J, Suda T, Kitamura Y. Enhanced expression of CD34 messenger RNA by developing endothelial cells of mice. Lab Invest. 1995;72:532–538. [PubMed] [Google Scholar]
- 40.Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 43.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of cxcr4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 45.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 46.Asahara T. Cell therapy and gene therapy using endothelial progenitor cells for vascular regeneration. Handb Exp Pharmacol. 2007:181–194. doi: 10.1007/978-3-540-68976-8_8. [DOI] [PubMed] [Google Scholar]
- 47.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 48.Rupp S, Koyanagi M, Iwasaki M, Bauer J, von Gerlach S, Schranz D, Zeiher AM, Dimmeler S. Characterization of long-term endogenous cardiac repair in children after heart transplantation. Eur Heart J. 2008;29:1867–1872. doi: 10.1093/eurheartj/ehn223. [DOI] [PubMed] [Google Scholar]
- 49.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 50.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 53.Morishita T, Uzui H, Nakano A, Mitsuke Y, Geshi T, Ueda T, Lee JD. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metallo-proteinase. J Atheroscler Thromb. 2012;19:149–158. doi: 10.5551/jat.10074. [DOI] [PubMed] [Google Scholar]
- 54.Jung C, Rafnsson A, Shemyakin A, Böhm F, Pernow J. Different sub-populations of endothelial progenitor cells and circulating apoptotic progenitor cells in patients with vascular disease and diabetes. Int J Cardiol. 2010;143:368–372. doi: 10.1016/j.ijcard.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 55.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E TAMARIS Committees and Investigators. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 56.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH TRAFFIC Investigators. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 57.Yonemitsu Y, Matsumoto T, Itoh H, et al. DVC1-0101 to treat peripheral arterial disease: a Phase I/IIa open-label dose-escalation clinical trial. Mol Ther. 2013;21:707–714. doi: 10.1038/mt.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 59.Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, Annex BH, Hiatt WR. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–1773. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 60.Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM, Goldman CK, McEllin K, Kelly R, Chronos N. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115:1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 61.Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajagopalan S, Olin JW, Young S, Erikson M, Grossman PM, Mendelsohn FO, Regensteiner JG, Hiatt WR, Annex BH. Design of the Del-1 for therapeutic angiogenesis trial (DELTA-1), a phase II multicenter, double-blind, placebo-controlled trial of VLTS-589 in subjects with intermittent claudication secondary to peripheral arterial disease. Hum Gene Ther. 2004;15:619–624. doi: 10.1089/104303404323142060. [DOI] [PubMed] [Google Scholar]
- 63.Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31:713–720. doi: 10.1161/ATVBAHA.110.219550. [DOI] [PubMed] [Google Scholar]
- 64.Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Sawa Y, Kaneda Y, Ogihara T, Morishita R. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2012;32:2503–2509. doi: 10.1161/ATVBAHA.111.244632. [DOI] [PubMed] [Google Scholar]
- 65.Leeper NJ, Cooke JP. MicroRNA and mechanisms of impaired angiogenesis in diabetes mellitus. Circulation. 2011;123:236–238. doi: 10.1161/CIRCULATIONAHA.110.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 67.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 2008;117:1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McClung JM, McCord TJ, Keum S, Johnson S, Annex BH, Marchuk DA, Kontos CD. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol. 2012;180:2156–2169. doi: 10.1016/j.ajpath.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazarika S, Farber CR, Dokun AO, Pitsillides AN, Wang T, Lye RJ, Annex BH. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127:1818–1828. doi: 10.1161/CIRCULATIONAHA.112.000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40:113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 71.Bosanquet DC, Glasbey JC, Williams IM, Twine CP. Systematic review and meta-analysis of direct versus indirect angiosomal revascularisation of infrapopliteal arteries. Eur J Vasc Endovasc Surg. 2014;48:88–97. doi: 10.1016/j.ejvs.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 72.van Royen N, Schirmer SH, Atasever B, et al. START Trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation. 2005;112:1040–1046. doi: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- 73.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 74.Cristofaro B, Shi Y, Faria M, et al. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140:1720–1729. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moraes F, Paye J, Mac Gabhann F, Zhuang ZW, Zhang J, Lanahan AA, Simons M. Endothelial cell-dependent regulation of arteriogenesis. Circ Res. 2013;113:1076–1086. doi: 10.1161/CIRCRESAHA.113.301340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 77.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta NK, Armstrong EJ, Parikh SA. The current state of stem cell therapy for peripheral artery disease. Curr Cardiol Rep. 2014;16:1–9. doi: 10.1007/s11886-013-0447-2. [DOI] [PubMed] [Google Scholar]
- 79.Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–1342. doi: 10.1160/th07-02-0137. [DOI] [PubMed] [Google Scholar]
- 80.Losordo DL, Henry TD, Kibbe MR, Krichavsky M, Mendelsohn F. Abstract 13688: Randomized, double-blind, placebo-controlled pilot trial of autologous cd34+ cell therapy for severe intermittent claudication: interim results. Circulation. 2011;124:A13688. [Google Scholar]
- 81.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 82.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duong Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, Fiessinger JN, Cavazzana-Calvo M, Aiach M, Emmerich J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–846. doi: 10.1038/modpathol.2008.48. [DOI] [PubMed] [Google Scholar]
- 84.Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Sueda T, Goto C, Matsubara H, Murohara T, Yoshizumi M. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 85.Motukuru V, Suresh KR, Vivekanand V, Raj S, Girija KR. Therapeutic angiogenesis in Buerger’s disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cells. J Vasc Surg. 2008;48:53S–60S. doi: 10.1016/j.jvs.2008.09.005. discussion 60S. [DOI] [PubMed] [Google Scholar]
- 86.Saito Y, Sasaki K, Katsuda Y, Murohara T, Takeshita Y, Okazaki T, Arima K, Katsuki Y, Shintani S, Shimada T, Akashi H, Ikeda H, Imaizumi T. Effect of autologous bone-marrow cell transplantation on ischemic ulcer in patients with Buerger’s disease. Circ J. 2007;71:1187–1192. doi: 10.1253/circj.71.1187. [DOI] [PubMed] [Google Scholar]
- 87.Benoit E, O’Donnell TF, Jr, Iafrati MD, Asher E, Bandyk DF, Hallett JW, Lumsden AB, Pearl GJ, Roddy SP, Vijayaraghavan K, Patel AN. The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design. J Transl Med. 2011;9:165. doi: 10.1186/1479-5876-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iafrati MD, Hallett JW, Geils G, Pearl G, Lumsden A, Peden E, Bandyk D, Vijayaraghava KS, Radhakrishnan R, Ascher E, Hingorani A, Roddy S. Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg. 2011;54:1650–1658. doi: 10.1016/j.jvs.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 89.Procházka V, Gumulec J, Jalůvka F, Salounová D, Jonszta T, Czerný D, Krajča J, Urbanec R, Klement P, Martinek J, Klement GL. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19:1413–1424. doi: 10.3727/096368910X514170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 91.Ishida A, Ohya Y, Sakuda H, Ohshiro K, Higashiuesato Y, Nakaema M, Matsubara S, Yakabi S, Kakihana A, Ueda M, Miyagi C, Yamane N, Koja K, Komori K, Takishita S. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. 2005;69:1260–1265. doi: 10.1253/circj.69.1260. [DOI] [PubMed] [Google Scholar]
- 92.Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmés A, Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg. 2010;24:287–294. doi: 10.1016/j.avsg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 93.Mohammadzadeh L, Samedanifard SH, Keshavarzi A, Alimoghaddam K, Larijani B, Ghavamzadeh A, Ahmadi AS, Shojaeifard A, Ostadali MR, Sharifi AM, Amini MR, Mahmoudian A, Fakhraei H, Aalaa M, Mohajeri-Tehrani MR. Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mono-nuclear cells in diabetic patients with critical limb ischaemia. Exp Clin Endocrinol Diabetes. 2013;121:48–53. doi: 10.1055/s-0032-1311646. [DOI] [PubMed] [Google Scholar]
- 94.Ozturk A, Kucukardali Y, Tangi F, et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications. 2012;26:29–33. doi: 10.1016/j.jdiacomp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H TACT Follow-up Study Investigators. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 96.Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, Ikeda U, Shimada K, Yoshio T, Okazaki H, Minota S. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford) 2007;46:882–884. doi: 10.1093/rheumatology/kel436. [DOI] [PubMed] [Google Scholar]
- 97.Malyar NM, Radtke S, Malyar K, Arjumand J, Horn PA, Kröger K, Freisinger E, Reinecke H, Giebel B, Brock FE. Autologous bone marrow mononuclear cell therapy improves symptoms in patients with end-stage peripheral arterial disease and reduces inflammation-associated parameters. Cytotherapy. 2014;16:1270–1279. doi: 10.1016/j.jcyt.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Van Tongeren RB, Hamming JF, Fibbe WE, Van Weel V, Frerichs SJ, Stiggelbout AM, Van Bockel JH, Lindeman JH. Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mono-nuclear cells: a clinical trial in patients with advanced limb ischemia. J Cardiovasc Surg (Torino) 2008;49:51–58. [PubMed] [Google Scholar]
- 99.Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, Zeiher AM PROVASA Investigators. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 100.Cobellis G, Silvestroni A, Lillo S, Sica G, Botti C, Maione C, Schiavone V, Rocco S, Brando G, Sica V. Long-term effects of repeated autologous transplantation of bone marrow cells in patients affected by peripheral arterial disease. Bone Marrow Transplant. 2008;42:667–672. doi: 10.1038/bmt.2008.228. [DOI] [PubMed] [Google Scholar]
- 101.Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Pérez-Camacho I, Marcos-Sánchez F, Hmadcha A, Soria B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20:1629–1639. doi: 10.3727/096368910X0177. [DOI] [PubMed] [Google Scholar]
- 102.Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, Schmidt A, Scheinert D, Biamino G, Emmrich F, Schuler G, Hambrecht R. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26:1903–1909. doi: 10.1093/eurheartj/ehi285. [DOI] [PubMed] [Google Scholar]
- 103.Bartsch T, Brehm M, Zeus T, Kögler G, Wernet P, Strauer BE. Transplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM-PAD study) Clin Res Cardiol. 2007;96:891–899. doi: 10.1007/s00392-007-0569-x. [DOI] [PubMed] [Google Scholar]
- 104.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 105.Franz RW, Shah KJ, Johnson JD, Pin RH, Parks AM, Hankins T, Hartman JF, Wright ML. Short- to mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vasc Endovascular Surg. 2011;45:398–406. doi: 10.1177/1538574411405545. [DOI] [PubMed] [Google Scholar]
- 106.Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC JUVENTAS Study Group; SMART Study Group. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010;52:843–849. 849.e1. doi: 10.1016/j.jvs.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 107.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 108.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 109.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 110.Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- 111.Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 113.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 115.Losordo DW, Kibbe MR, Mendelsohn F, et al. Autologous CD34+ Cell Therapy for Critical Limb Ischemia Investigators. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E, Velazquez O, Marston WA, Bartel RL, Longcore A, Stern T, Watling S. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011;54:1032–1041. doi: 10.1016/j.jvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20:1280–1286. doi: 10.1038/mt.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D, Gastens MH, Quast T, Negrean M, Stirban OA, Nandrean SG, Götting C, Minartz P, Kleesiek K, Tschoepe D. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66:384–393. doi: 10.1111/j.1742-1241.2011.02886.x. [DOI] [PubMed] [Google Scholar]
- 119.Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- 120.Balber AE. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 2011;29:570–575. doi: 10.1002/stem.613. [DOI] [PubMed] [Google Scholar]
- 121.Capoccia BJ, Robson DL, Levac KD, Maxwell DJ, Hohm SA, Neelamkavil MJ, Bell GI, Xenocostas A, Link DC, Piwnica-Worms D, Nolta JA, Hess DA. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perin EC, Silva G, Gahremanpour A, Canales J, Zheng Y, Cabreira-Hansen MG, Mendelsohn F, Chronos N, Haley R, Willerson JT, Annex BH. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2011;78:1060–1067. doi: 10.1002/ccd.23066. [DOI] [PubMed] [Google Scholar]
- 123.Perin EC, Murphy M, Cooke JP, et al. Cardiovascular Cell Therapy Research Network. Rationale and design for PACE: patients with intermittent claudication injected with ALDH bright cells. Am Heart J. 2014;168:667–673. doi: 10.1016/j.ahj.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan J, Tie G, Xu TY, Cecchini K, Messina LM. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev. 2013;9:360–372. doi: 10.1007/s12015-013-9433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 126.Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 128.Marino G, Moraci M, Armenia E, Orabona C, Sergio R, De Sena G, Capuozzo V, Barbarisi M, Rosso F, Giordano G, Iovino F, Barbarisi A. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res. 2013;185:36–44. doi: 10.1016/j.jss.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 129.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki Ki, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R. Endothelial cells derived from human ipscs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wong WT, Huang NF, Botham CM, Sayed N, Cooke JP. Endothelial cells derived from nuclear reprogramming. Circ Res. 2012;111:1363–1375. doi: 10.1161/CIRCRESAHA.111.247213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asahara T, Krasinski K, Chen D, Sullivan A, Kearney M, Silver M, Li T, Isner JM. Circulating endothelial progenitor cells in peripheral blood incorporate into re-endothelialization after vascular injury. Circulation. 1997;96:I–725. [Google Scholar]
- 133.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 134.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 135.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 136.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 138.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 139.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C, Muhly-Reinholz M, Carmona G, Urbich C, Zeiher AM, Dimmeler S. Sustained persistence of transplanted pro-angiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res. 2008;103:1327–1334. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]
- 141.Barber CL, Iruela-Arispe ML. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr Res. 2006;59:26R–32R. doi: 10.1203/01.pdr.0000203553.46471.18. [DOI] [PubMed] [Google Scholar]
- 142.Brixius K, Funcke F, Graf C, Bloch W. Endothelial progenitor cells: a new target for the prevention of cardiovascular diseases. Eur J Cardiovasc Prev Rehabil. 2006;13:705–710. doi: 10.1097/01.hjr.0000221862.34662.31. [DOI] [PubMed] [Google Scholar]
- 143.Kalka C, Baumgartner I. Gene and stem cell therapy in peripheral arterial occlusive disease. Vasc Med. 2008;13:157–172. doi: 10.1177/1358863x08088616. [DOI] [PubMed] [Google Scholar]
- 144.Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hema-topoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yaghoubi SS, Wu L, Liang Q, Toyokuni T, Barrio JR, Namavari M, Satyamurthy N, Phelps ME, Herschman HR, Gambhir SS. Direct correlation between positron emission tomographic images of two reporter genes delivered by two distinct adenoviral vectors. Gene Ther. 2001;8:1072–1080. doi: 10.1038/sj.gt.3301490. [DOI] [PubMed] [Google Scholar]
- 147.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–I54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staudacher DL, Sela Y, Itskovitz-Eldor J, Flugelman MY. Intra-arterial injection of human embryonic stem cells in athymic rat hind limb ischemia model leads to arteriogenesis. Cardiovasc Revasc Med. 2011;12:228–234. doi: 10.1016/j.carrev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 150.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ, Cooke JP. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131:300–309. doi: 10.1161/CIRCULATIONAHA.113.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooke JP. Therapeutic transdifferentiation: a novel approach for vascular disease. Circ Res. 2013;112:748–750. doi: 10.1161/CIRCRESAHA.113.301053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Comerota AJ, Throm RC, Miller KA, Henry T, Chronos N, Laird J, Sequeira R, Kent CK, Bacchetta M, Goldman C, Salenius JP, Schmieder FA, Pilsudski R. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–936. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- 153.Nikol S, Baumgartner I, Van Belle E, et al. TALISMAN 201 investigators. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 154.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 155.Mäkinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Ylä-Herttuala S. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 156.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17:683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 157.Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF, Weiss RJ, Kandzari DE, Kleiman N, Anderson RD, Gottlieb D, Karlsberg R, Snell J, Rocha-Singh K. Results from a phase II multi-center, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153:874–880. doi: 10.1016/j.ahj.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 158.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 159.Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH HGF-0205 Trial Investigators. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg. 2010;52:1525–1530. doi: 10.1016/j.jvs.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 161.Henry TD, Hirsch AT, Goldman J, Wang YL, Lips DL, McMillan WD, Duval S, Biggs TA, Keo HH. Safety of a non-viral plasmid-encoding dual isoforms of hepatocyte growth factor in critical limb ischemia patients: a phase I study. Gene Ther. 2011;18:788–794. doi: 10.1038/gt.2011.21. [DOI] [PubMed] [Google Scholar]
- 162.Gu Y, Zhang J, Guo L, Cui S, Li X, Ding D, Kim JM, Ho SH, Hahn W, Kim S. A phase I clinical study of naked DNA expressing two isoforms of hepatocyte growth factor to treat patients with critical limb ischemia. J Gene Med. 2011;13:602–610. doi: 10.1002/jgm.1614. [DOI] [PubMed] [Google Scholar]
- 163.Walter D, Krankenberg H, Balzer J, Kalka C, Baumgartner I, Schluter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, Zeiher AM. Intra-arterial administration of bone marrow mononuclear cells in patients with critical limb ischemia—a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]