Abstract
The colonic mucosa protects itself from the luminal content by secreting mucus that keeps the bacteria at a distance from the epithelium. For this barrier to be effective, the mucus has to be constantly replenished which involves exocytosis and expansion of the secreted mucins. Mechanisms involved in regulation of mucus exocytosis and expansion are poorly understood, and the aim of this study was to investigate whether epithelial anion secretion regulates mucus formation in the colon. The muscarinic agonist carbachol was used to induce parallel secretion of anions and mucus, and by using established inhibitors of ion transport, we studied how inhibition of epithelial transport affected mucus formation in mouse colon. Anion secretion and mucin exocytosis were measured by changes in membrane current and epithelial capacitance, respectively. Mucus thickness measurements were used to determine the carbachol effect on mucus growth. The results showed that the carbachol-induced increase in membrane current was dependent on NKCC1 co-transport, basolateral K+ channels and Cftr activity. In contrast, the carbachol-induced increase in capacitance was partially dependent on NKCC1 and K+ channel activity, but did not require Cftr activity. Carbachol also induced an increase in mucus thickness that was inhibited by the NKCC1 blocker bumetanide. However, mice that lacked a functional Cftr channel did not respond to carbachol with an increase in mucus thickness, suggesting that carbachol-induced mucin expansion requires Cftr channel activity. In conclusion, these findings suggest that colonic epithelial transport regulates mucus formation by affecting both exocytosis and expansion of the mucin molecules.
Keywords: Colon, Mucus, Anion secretion, Cftr, Capacitance, Exocytosis
Introduction
The colonic mucosa is a dynamic structure capable of alternating between absorption and secretion to fine tune ion and water homeostasis and maintains a protective barrier against invasion of the abundant luminal bacteria [22, 35, 40]. Coordinated secretion of ions, fluid and mucus each contribute to the maintenance of this barrier. Anion secretion has an especially important role by creating the driving force for fluid secretion and by ensuring the right ionic milieu required for formation of a normal mucus layer [21, 48]. In the small intestine and in the airways, bicarbonate secretion via the cystic fibrosis transmembrane conductance regulator (CFTR) channel plays a key role in this process, as reflected by the dramatic changes in the physical properties of mucus in patients with cystic fibrosis (CF) and animal models of CF [12, 28]. The mucus differs between the small intestine and the large intestine. In the murine colon, the mucus layer is built up by two layers: an inner layer that is devoid of bacteria and an outer layer that is the habitat of the commensal flora [40]. In the small intestine, the mucus layer forms a more loose and permeable structure, and upon removal of the loose mucus gel, only a very thin discontinuous mucus layer remains [1, 16, 24, 28].
The role of ion transport in mucus formation can be viewed as consisting of two separate processes; firstly, the effect of ion transport on mucin granule exocytosis, and secondly, the role of ion transport in mucus hydration and expansion. The interaction between epithelial ion secretion and mucus physiology is to some extent understood in the airways and in the small intestine, where anion transport is implicated in both exocytosis and expansion of the secreted mucins [7, 8, 28]. However, how anion transport affects mucus secretion and expansion in the colon remains unknown.
Ulcerative colitis is a relatively common chronic disorder characterised by periods of severe colonic inflammation. The disease has a characteristically recurrent behaviour, with alternating periods of exacerbations and remissions. One of the main hallmarks of acute inflammation is mucus depletion and severely impaired electrogenic transport [25, 47]. In addition, there are studies pointing towards defects in mucus expansion during active disease, which links a defective ion transport with altered mucus properties [32]. Further implications for the role of colon mucus in regulation of the colonic barrier can be found in a recent study showing that mucus secretion from the colonic crypts protects against ischemia-induced tissue damage by physically removing the bacteria that have accessed the colonic crypts [27]. Thus, mucus is both constitutively released to maintain the mucus layer and induced in response to pathophysiological stimuli.
The aim of the present study was to characterise the interaction between secretagogue-induced epithelial anion secretion (chloride and/or bicarbonate) and colonic mucus formation (exocytosis and expansion). We used a recently described ex vivo setup to study mucus growth in isolated colonic specimens [29] and Ussing chamber measurements of net membrane current and epithelial capacitance to monitor anion secretion and mucus exocytosis. Carbachol (CCh), a muscarinic agonist, was used to stimulate anion and mucin secretions, and by using established inhibitors of epithelial transport, we investigated the relation between the membrane current and epithelial capacitance responses. CCh acts by increasing intracellular Ca2+, a response pathway also activated by natural in vivo stimuli, such as contacts with bacteria [57]. Our results support that the CCh-induced change in epithelial capacitance reflects compound exocytosis of mucins. The exocytosis process required functional NKCC1 co-transport and basolateral K+ channel activity, but was independent of Cftr mediated transport. In contrast, a functional Cftr channel was essential for the CCh-induced effect on mucus expansion which may be due to the loss of Cftr-mediated bicarbonate secretion.
Materials and methods
Ethical information
All animal studies were approved by the local animal ethics committee in Gothenburg, Sweden. Animals were anaesthetized by inhalation of isoflurane (Isoba® Vet, Schering plough, USA) and euthanized by cervical dislocation.
Experimental design
Five series of experiments were conducted. We first tested the hypothesis that CCh-induced changes in membrane capacitance can be used as a marker for mucin exocytosis. This was done by comparing the capacitance response to various secretagogues (forskolin, prostaglandin E2 (PGE2), ATP and CCh) in two cell lines, one enterocyte model and one goblet cell containing model. To verify that the capacitance response reflected exocytosis, the responses to CCh and PGE2 were tested in the presence of exocytosis inhibitors. In the second series, we studied the impact of vesicular trafficking of Cftr on the capacitance response using wild-type (WT) and delF508 Cftr mice known to have impaired vesicular trafficking of Cftr [52]. In the third series, we studied the capacitance response to CCh in mice lacking the major intestinal gel forming mucin, mucin 2 (Muc2). In the fourth series, we investigated how known inhibitors of the ion transport machinery affected the net membrane current response and the capacitance response to CCh. In the fifth series, we studied the effect of CCh on mucus growth in WT and F508del Cftr mouse distal colon.
Cell culture
Caco-2 and LS513 cells (American Type Culture Collection, ATCC, Manassas, USA) were cultured in RPMI media containing 15 vs. 10 % foetal calf serum, 2 mML-glutamine, 100 units/ml penicillin G sodium and 100 μg/ml streptomycin (Lonza, Basel, Switzerland). The cells were plated on snapwell tissue culture inserts (0.4 μm pores, 1.13 cm in diameter; Corning Life Sciences, Kennebuck, USA) at a density of 7.5×104 cells/well and cultured for 21–24 days to allow the cells to differentiate.
Mouse tissue
The experiments were performed using male WT C57Bl/6 mice, Muc2−/−, Muc2+/− and Cftrtm1eur (F508del Cftr) (CF) mice all on C57Bl/6 background [19, 59, 60, 62]. The animals were between 10 and 15 weeks old. To increase the survival of the homozygous mutant Cftrtm1eur mice, the mice were given an osmotic laxative in the drinking water (PEG4000 18 mM, KCl 10 mM, Na2SO4 40 mM, NaHCO3 84 mM and NaCl 25 mM). The animals were transferred to normal tap water 3 days prior to the experiments. All animals had free access to water and chow.
Ussing chamber
The distal colon (approximately 2 cm) was dissected, flushed with ice-cold oxygenised (95 % O2 and 5 % CO2) Krebs buffer to remove colonic contents and kept on ice for 30 min. The tissue was then opened along the mesenteric border and mounted in the Ussing chamber (exposed area 0.45 cm2, chamber volume 10 ml). The LS513 and Caco-2 cells were directly transferred from the culture incubator and mounted in the Ussing chamber (exposed area 1.00 cm2, chamber volume 5 ml). The apical side of the epithelium was bathed in Krebs-mannitol buffer, and the basolateral side was bathed in Krebs-glucose buffer. The solutions were constantly gassed with 95 % O2 and 5 % CO2 at a temperature of 37 °C, pH 7.4.
Transepithelial potential difference (PD) was measured once every minute during the whole experiment with a pair of matched Ag/Ag calomel electrodes (Radiometer, Copenhagen, Denmark) placed in saturated KCl and connected to the mucosal and serosal sides via a pair of agar bridges (0.9 % NaCl/4 % agar) (Marine Bioproducts, Canada). After mounting the tissue, PD was allowed to stabilise for 20 min to achieve steady-state conditions. Epithelial resistance (Rp), net membrane current (Im) and epithelial capacitance (Cp) were measured using square pulse analysis as described previously [30, 36].
Briefly, 5 V, 2 ms pulses were generated by a square pulse generator (Medimet, Gothenburg, Sweden) via a current limiting resistor connected to a platinum electrode and applied across the tissue sample. The mean voltage response curve from 20 measurements was calculated, and a linear fit was applied to the mean graph resulting in the voltage at time 0. Rp, Cp and Im were assessed simultaneously in 4.5-min intervals. When using the square pulse method, the obtained Rp and Cp values represent the epithelial resistance and capacitance, and the subepithelial tissue and the resistance of the muscle layers are not included in the measurements. Because of this, our Rp values are lower compared to the total tissue resistance that is obtained from traditional short circuit current measurements [10]. The lower Rp obtained by the square pulse method results in higher baseline Im. Studies have shown that the membrane capacitance is independent of baseline muscle tone as baseline capacitance was comparable in full thickness and stripped rat colon [53].
All Ussing chamber experiments were designed as follows: mounting of the tissue, 20 min equilibration period, 30 min baseline recordings, 30 min exposure to inhibitors (bumetanide, BaCl2, charybdotoxin (CTX), GlyH-101 or primaquine) or control recordings and 30 min exposure to forskolin, PGE2, CCh or ATP. All inhibitors remained in the solution during secretagogue stimulation. All substances except ATP and GlyH-101 were added to the serosal side. In the substitution experiments, chloride and bicarbonate-free buffers were added to the luminal and serosal side at the start of the experiment. Nocodazole was also added at the start of the experiment.
The Im response to CCh followed a biphasic pattern with a rapid increase in Im that peaked 2 min after addition followed by a lower sustained plateau phase. The Cp response showed a monophasic pattern that peaked 9.5 min after addition. Due to the rapid time frame of the Im response, the first data collection point was matched to coincide with the peak response to Im (2 min after addition of CCh), and the following recordings were made in 4.5-min intervals. The experiments were performed on full thickness tissue which is expected to affect the drug access to the tissue. However, we have previously shown that the PD response to CCh peaks 2 min after addition in stripped mouse distal colon, showing that the presence or absence of the longitudinal muscle layer does not affect the time frame of the CCh response [29].
Mucus thickness measurements
Measurements of mucus thickness were performed as described previously [29]. Briefly, the distal colon was dissected, flushed with ice-cold Krebs buffer and kept on ice for 30 min. The specimen was opened along the mesenteric border, the longitudinal muscle layer was removed by blunt dissection and the tissue was divided into two parts and studied in parallel. Transepithelial PD was measured once every minute using a pair of matched Ag/Ag calomel electrodes (Radiometer, Copenhagen, Denmark) connected to the tissue via agar bridges (0.9 % NaCl, 4 % agar) (Marine Bioproducts, Canada). To visualise the colonic mucus layer, a suspension of activated charcoal particles was added to the apical surface and allowed to sediment down to the top of the mucus layer. The thickness of the mucus layer was assessed by measuring the distance between the mucus surface and the epithelial surface using a micropipette connected to a micromanipulator and a digimatic indicator. The thickness of the mucus layer was measured in 15-min intervals for a total of 60 min.
In the F508del Cftr mice, the viscoelastic properties of the mucus are known to be altered resulting in a more dense mucus layer in the small intestine [28]. The altered mucus properties in the F508del Cftr mice could potentially affect the outcome of the mucus measurements. However, since the charcoal particles do not penetrate either the WT or F508del Cftr colonic mucus, we do not think that the altered viscoelastic properties of the F508del Cftr colonic mucus affect the outcome of our measurements.
CCh (1 mM) was added to the serosal side after 30 min incubation, and the response was measured 15 and 30 min after stimulation. In the inhibitory experiments, bumetanide (0.1 mM) was added to the serosal side at the start of the experiment followed by the addition of CCh at t30. We have previously shown that stimulation with CCh (1 mM) results in a significant increase in mucus growth rate during the first 15 min following addition of CCh (0–15 min post-stimulation) and that the growth rate returns to the baseline level during the following 15 min (15–30 min post-stimulation) [29].
Histology
Tissue specimens were fixed in Carnoy’s methanol fixative (60 % dry methanol, 30 % chloroform and 10 % glacial acetic acid) followed by washing in methanol and embedding in paraffin. Four-micrometer-thick sections were dewaxed, hydrated and stained with Alcian blue and periodic acid Schiff’s reagent (PAS), as described previously [49]. Pictures were obtained using an Eclipse E1000 (Nikon, Tokyo, Japan) microscope.
Drugs and buffer composition
CCh, BaCl2, CTX, PGE2, ATP, acetazolamide and primaquine (Sigma-Aldrich, Steinheim, Germany) were dissolved in water. Forskolin and bumetanide (Sigma-Aldrich, Steinheim, Germany) were dissolved in ethyl alcohol, whilst Gly-H 101 (Merck, Darmstadt, Germany) and nocodazole (Sigma-Aldrich, Steinheim, Germany) were dissolved in DMSO. The Krebs buffer had the following composition in millimoles: NaCl 115.8, CaCl2 1.3, KCl 3.6, KH2PO4 1.4, NaHCO3 23.1 and MgSO4 1.2 (Merck, Darmstadt, Germany). The Krebs-mannitol buffer also contained Na-pyruvate (5.7 mM) (Sigma-Aldrich, Steinheim, Germany), Na-L-glutamate (5.1 mM) (Merck, Darmstadt, Germany) and D-mannitol (10 mM) (Sigma-Aldrich, Steinheim, Germany) and the Kreb-glucose buffer contained Na-pyruvate (5.7 mM), Na-L-glutamate (5.1 mM) and D-glucose (10 mM) (Sigma-Aldrich, Steinheim, Germany). In the chloride-free experiments, NaCl, KCl and CaCl2 were replaced with equimolar concentrations of Na-gluconate and K-gluconate and twice the concentration of Ca-gluconate (Sigma-Aldrich, Steinheim, Germany) to compensate for the chelation of calcium by gluconate. pH was set to 7.4 using acetic acid. In the bicarbonate-free experiments, NaHCO3 was replaced with equimolar concentration of NaH2PO4 (Merck, Darmstadt, Germany), the solution was gassed with 100 % O2 and the carbonic anhydrase was inhibited by acetazolamide (1 mM).
Statistics
Data are presented as mean±standard error of the mean (SEM). Single comparison between two groups was made using the Student’s t test. Comparisons between two groups over time were made using a two-way ANOVA with Bonferroni’s post hoc test, and comparisons between three groups or more were made using a one-way ANOVA with a Dunnet’s post hoc test for comparisons between controls and other experimental groups, respectively, or Bonferroni’s post hoc to compare differences between all groups. Due to the large spread in the magnitude of the membrane current response to the respective secretagogues in the cell culture Ussing experiments, the statistical analysis in Table 1 was performed on log-transformed data. All other statistical analyses were performed on non-transformed data. A p value <0.05 was considered statistically significant.
Table 1.
Membrane current response to Forskolin, PGE2, CCh and ATP in LS513 and Caco-2 cells
| ΔIm, μA/cm2 | Ctrl | Forskolin (10 μM) | PGE2 (10 μM) | CCh (1 mM) | ATP (3 mM) |
|---|---|---|---|---|---|
| LS513 | 0.1±0.1 | 324±53*** | 176±41*** | 15±4*** | 332±42*** |
| Caco-2 | 0.2±0.2 | 52±8*** | 25±4*** | 1.6±0.7 | 10±1*** |
LS513 cells: n=6 in all groups except forskolin, n=5. Caco-2 cells: ctrl n=6, forskolin n=9, ATP, n=6, CCh n=5, and PGE2 n=2. Data are presented as mean±SEM
p<0.001 (compared to the respective Ctrl group)
Results
Real-time mucus exocytosis simultaneously with ion secretion
Epithelial capacitance has previously been used to study mucus exocytosis in cultured epithelial cells [5, 11]. To determine whether epithelial capacitance could be used to monitor mucus secretion in our setup, we compared two cell lines: (1) the enterocyte-like cell line Caco-2 which produces minimal amounts of gel-forming mucins (analysed by immunohistochemistry) and (2) the goblet cell line LS513 which produces both MUC2 and MUC5AC [43]. Since we were specifically interested in mucus exocytosis, we used these cells to determine which type of stimuli that induced a capacitance response in the goblet cell line, but not in the enterocyte cell line. Forskolin and PGE2 were used to active cAMP-mediated secretion, and ATP and CCh were used to induce Ca2+-mediated secretion. The results showed that forskolin and PGE2 induced a significant increase in Cp in both cell lines (Fig. 1a–d), whereas CCh and ATP only induced a Cp response in the LS513 cells (Fig. 1e–h).
Fig. 1.
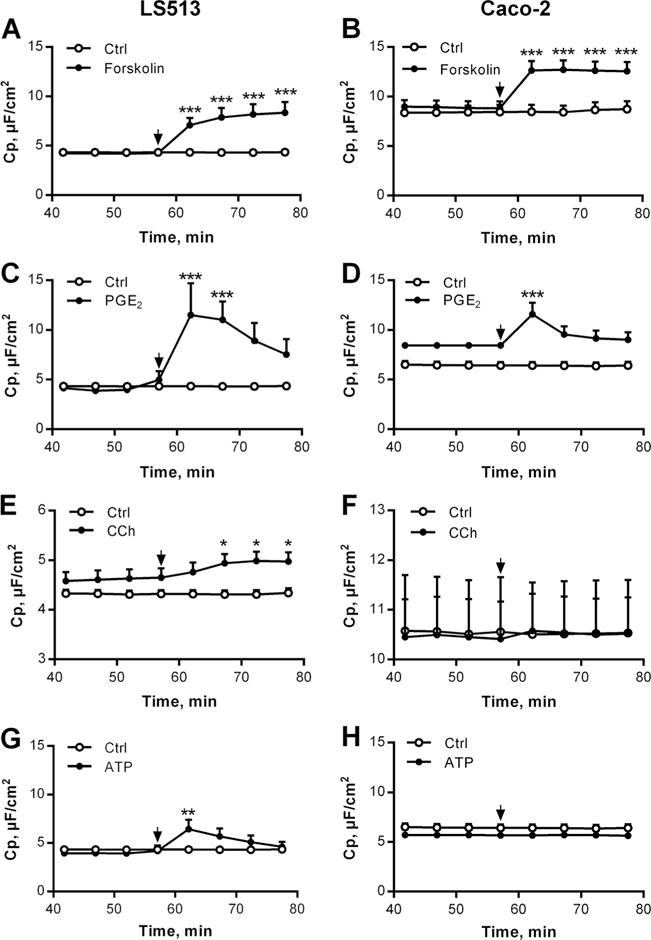
Capacitance recordings in LS513 (left panel) and Caco-2 cells (right panel) during control (Ctrl) conditions and after stimulation with secretagogues. a LS513 Forskolin (10 μM, n=5). b Caco-2 Forskolin (10 μM, n=9). c LS513 PGE2 (10 μM, n=6). d Caco-2 PGE2 (10 μM, n=2). e LS513 CCh (1 mM, n=6). f Caco-2 CCh (1 mM, n=5). g LS513 ATP (3 mM, n=6). h Caco-2 ATP (3 mM, n=5). LS513 Ctrl, n=7; Caco-2 Ctrl, n=6. Data are presented as mean±SEM, *p<0.05; **p<0.01; ***p<0.001. The arrows mark the time point when the drugs were added. Baseline values: Rp (Ω×cm2), Im (μA/cm2). LS513: Forskolin: Rp: 296±55, Im: 7±1; PGE2: Rp: 88±33, Im: 5±4; CCh: Rp: 121±13, Im: 6±2; ATP: Rp: 185±30, Im: 7±1; Ctrl: Rp: 266±52, Im: 5±1. Caco-2: Forskolin: Rp: 152±18, Im: 19±4; PGE2: Rp: 177±6, Im: 15±4; CCh: Rp: 89±9, Im: 24±4; ATP: Rp: 339±33, Im: 6±1; Ctrl to forskolin: Rp: 144±23, Im: 20±6; Ctrl to PGE2 and ATP: Rp: 196±20, Im: 12±2; Ctrl to CCh: Rp: 126±22, Im: 22±6
In parallel with the capacitance measurements, we also measured the effect of the respective substances on the membrane current (Im). Forskolin, PGE2 and ATP induced a significant increase in Im in both the LS513 and Caco-2 cells whereas CCh only induced a significant increase in Im in the LS513 cells (Table 1). Thus, the Im response to CCh and the Cp responses to CCh and ATP were only seen in the culture system containing goblet cells.
The capacitance response to carbachol and PGE2 is sensitive to exocytosis inhibitors
To confirm that the capacitance response indeed corresponded to exocytosis, we pretreated the LS513 cells with nocodazole, an inhibitor of microtubule polymerization, or with primaquine, an inhibitor of vesicle formation, and studied the Cp response to CCh and PGE2. Pretreatment with nocodazole significantly reduced the Cp response to CCh (Fig. 2a) and PGE2 (Fig. 2b), whereas primaquine had a partial, but not significant, effect on the PGE2 response. These results suggest that both CCh and PGE2 induce exocytosis of already existing vesicles and that the process is dependent on microtubule polymerisation.
Fig. 2.
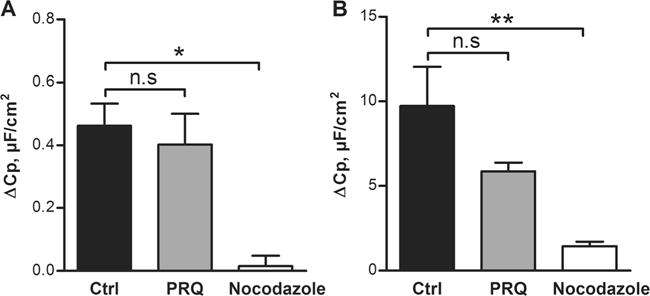
Effect of exocytosis inhibitors on the capacitance response to carbachol (CCh) and PGE2 in LS513 cells. a Delta increase in Cp after stimulation with CCh in ctrl, primaquine (PRQ) (0.3 mM) or nocodazole (0.01 mM) treated cells. b Delta increase in Cp after stimulation with PGE2 in ctrl, PRQ (0.3 mM) or nocodazole (0.01 mM) treated cells. n=6 in all groups; data are presented as mean±SEM, *p<0.05; **p<0.01. Baseline values: Rp (Ω×cm2), Cp (μF/cm2), Im (μA/cm2). CCh Ctrl: Rp: 84±24, Cp: 4.3±0.5, Im: 27±12. CCh PRQ: Rp: 80±11, Cp: 3.6±0.1, Im: 6.4±3.4. CCh nocodazole: Rp: 97±8, Cp: 4.1±0.1, Im: 9±2. PGE2 Ctrl: Rp: 88±33, Cp: 4.3±0.4, Im: 5±5. PGE2 PRQ: Rp: 91±28, Cp: 4.5±0.2, Im: 9±4. PGE2 nocodazole: Rp: 97±17, Cp: 4.2±0.2, Im: 9±2
F508del Cftr mice respond to CCh with a normal increase in capacitance but fail to exhibit a capacitance response to forskolin
The cell line experiments confirmed that the capacitance responses to CCh and ATP were only present in the goblet cell line and that the CCh and PGE2 responses reflect exocytosis of already formed vesicles. Since these experiments do not differentiate between the contribution of vesicular trafficking and mucin granule exocytosis, we proceeded by studying the response to CCh and forskolin in WT and F508del Cftr mice that have impaired vesicular trafficking of the Cftr channel [52]. We chose to continue with CCh and forskolin since ATP does not induce a secretory response in mouse colon, whilst CCh is a potent inducer of both anion and mucus secretions, and forskolin has previously been used to study trafficking of the Cftr channel [14, 29, 54, 56]. The results showed that stimulation of the colonic tissue with forskolin (10 μM) induced a significant increase in Cp in WTcolon, but not in the F508del Cftr colon (Fig. 3a). In contrast, both the WT and the F508del Cftr tissue responded to CCh with a significant increase in Cp (Fig. 3b). The F508del Cftr mice have a 10 % residual Cftr activity [31], and to ensure that the intact Cp response to CCh was not due to the remaining Cftr activity, we repeated the experiments in the presence of the Cftr inhibitor GlyH-101. The results showed that the Cp response to CCh was still present in the F508del Cftr (CF) mice in the presence of apical GlyH-101 (50 μM), and the magnitude of the response did not differ from that of the WT mice (WT: Δ2.9±0.4 μF/cm2 vs. CF+GlyH101: Δ4.9±0.9 μF/cm2, p>0.05). Thus, the Cp response to forskolin appears to reflect insertion of Cftr into the apical plasma membrane, whereas Cftr translocation to the apical membrane does not appear to contribute to the Cp response to CCh.
Fig. 3.
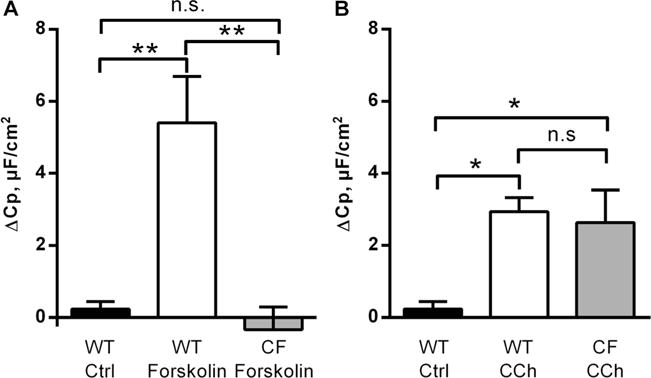
Carbachol- (CCh) and forskolin-induced effect on capacitance in wild-type (WT) and F508del Cftr (CF) colon. a Delta increase in Cp after stimulation with forskolin (10 μM) in WT and CF colon. b Delta increase in Cp after stimulation with CCh (1 mM) in WT and CF colon. WT Ctrl, n=6; WT forskolin, n=6; CF forskolin, n=4; WT CCh n=7; CF CCh n=7. Data are presented as mean±SEM, *p<0.05; **p<0.01. Baseline values: Rp (Ω×cm2), Cp (μF/cm2), Im (μA/cm2). WT Ctrl: Rp: 36±4, Cp: 20±1, Im: 227±41. WT forskolin: Rp: 43±4, Cp: 22±1, Im: 220±50. CF forskolin: Rp: 37±3, Cp: 20±3, Im: 59±7. WT CCh: Rp: 40±2, Cp: 19±1, Im: 159±45. CF CCh: Rp: 41±4, Cp: 23±4, Im: 26±7
The capacitance response to carbachol is absent in mice lacking colonic mucus
To further link the capacitance response to CCh to mucus exocytosis, we proceeded to study the response in the distal colon of WT mice and mice lacking the main component of the colonic mucus layer, the Muc2 mucin. To determine whether there was a dose-dependent relation between the Muc2 genotype and the CCh effect on capacitance, we included both Muc2−/− and Muc2+/− mice in the analysis. The results showed that the Cp response to CCh was decreased by 70 % in the Muc2+/− mice and was absent in the Muc2−/− mice (Fig. 4a). In addition to the electrophysiological measurements, we also studied the effect of CCh on mucus growth in WT and Muc2+/− mice. The results showed that the CCh-induced increase in mucus thickness was reduced by 50 % in the Muc2+/− mice compared to WT (Fig. 4b). Thus, mucus production is required for the CCh-induced increase in capacitance.
Fig. 4.
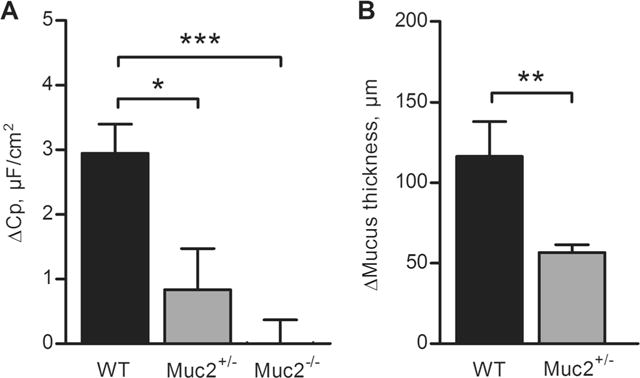
Carbachol (CCh)-induced effect on capacitance and mucus thickness in mouse distal colon. a Delta increase in Cp after stimulation with CCh (1 mM) in WT (n=7), Muc2+/− (n=5) and Muc2−/− (n=6) mice. b Delta increase in mucus thickness after 30 min of stimulation with CCh (1 mM) in WT (n=9) and Muc2+/− (n=6) mice. Data are presented as mean±SEM, *p<0.05; **p<0.01; ***p<0.001. Baseline values: Rp (Ω×cm2), Cp (μF/cm2), Im (μA/cm2). WT: Rp: 40±2, Cp: 19±1, Im: 159±45. Muc2+/−: Rp: 30±2, Cp: 18±3, Im: 120±7. Muc2−/−: Rp: 46±7, Cp: 15±2, Im: 91±10
The carbachol-induced increase in capacitance partially depends on basolateral transport via NKCC1 and K+ channels, but not on a functional Cftr
In addition to inducing mucus secretion, stimulation with CCh induces a rapid increase in Im which reflects electrogenic anion secretion [51]. This process is known to involve a large number of ion transporters such as NKCC1, K+ channels, NBCe1, bestrophin 2 and the Cftr [30, 45, 46, 55, 63]. To determine whether anion secretion is essential for mucus exocytosis, we inhibited various parts of the transport process and studied the CCh effect on Im and Cp. Baseline electrical parameters are presented in Tables 2 and 3. When plotting the maximum CCh effect on Im against the maximum effect on Cp during the different treatments, the results showed a parallel decrease in the Im and Cp responses during inhibition of bicarbonate transport using bicarbonate-free buffers and when basolateral K+-transport was blocked by Ba2+ or the combination of Ba2+ and CTX. However, only the combination of Ba2+ and CTX reduced the Im and Cp responses, as compared to the Ctrl group (Im p<0.001, Cp p<0.05) (Fig. 5). In contrast, interventions with chloride transport (bumetanide to inhibit NKCC1, chloride-free buffer and F508del Cftr mice which lack a functional Cftr channel) decreased the Im response to CCh by approximately 90 % in all three groups (Ctrl vs. bumetanide, Cl− free and F508del Cftr, p<0.001) but had varying effects on the Cp response (Fig. 5). Inhibition of NKCC1 by bumetanide reduced the Cp response by 90 % (p<0.05), whilst the Cp response in the chloride-free buffer group and the F508del Cftr mice (marked with arrow in 5) did not differ from the Ctrl group (Fig. 5). A parallel decrease in the Im and Cp responses was also observed in the Muc2+/− and Muc2−/− mice (Fig. 5). Thus, CCh-induced electrogenic anion secretion is not essential for mucin exocytosis, but a functional NKCC1 and basolateral K+ channel activity appear to be involved in the exocytosis process. To ensure that the inhibitory effect of bumetanide and the combination of Ba2+ and CTX on the Cp response to CCh is not secondary to their inhibitory effect on the Im response to CCh, we repeated the bumetanide and Ba2+ + CTX experiments in the F508del Cftr mice in the presence of apical GlyH-101(50 μM). The results showed that bumetanide and the combination of Ba2+ and CTX inhibited the Cp response to CCh by 63 and 69 %, respectively, in the F508del Cftr (CF) mice. CF ctrl (n=6): Δ4.9±0.9 μF/cm2; CF+bumetanide (n=7): Δ1.8±0.8 μF/cm2, p<0.05 vs. CF ctrl; CF+Ba2+/CTX (n=5): Δ1.5±0.8 μF/cm2, p<0.05 vs. CF ctrl. Thus, the inhibitory effects of bumetanide and Ba2+ and CTX on the Cp response to CCh are independent of their effect on the Im response to CCh.
Table 2.
Membrane current (Im), epithelial capacitance (Cp) and epithelial resistance (Rp) in wild-type mouse distal colon
| Control | Bumetanide (0.1 mM) | Ba2+ (5 mM) | Ba2+ + CTX (5 mM+5 nM) | Cl− (0 mM) | HCO3− (0 mM) | |
|---|---|---|---|---|---|---|
| Im, μA/cm2 | 159±45 | 117±21 | 201±35 | 250±31* | 109±12 | 132±32 |
| Cp, μF/cm2 | 19±1 | 16±1 | 19±2 | 21±2 | 19±1 | 20±2 |
| Rp, Ω×cm2 | 40±2 | 38±5 | 29±3 | 32±3 | 51±5 | 44±5 |
Control conditions or after 30 min incubation with bumetanide, Ba2+ or the combination of Ba2+ and Charybdotoxin (CTX) and in chloride- (Cl−) or bicarbonate (HCO3−)-free buffers. Data are presented as mean±SEM. n=6 in all groups except for control n=7 and Cl− (0 mM), n=4
p<.05 (represents) comparison between control and Ba2+ + CTX
Table 3.
Membrane current (Im), epithelial capacitance (Cp) and epithelial resistance (Rp) in WT, F508del Cftr, Muc2+/− and Muc2−/− mouse distal colon
| Genotype | WT | F508del Cftr | Muc2+/− | Muc2−/− |
|---|---|---|---|---|
| Im, μA/cm2 | 159±45 | 26±7** | 120±7 | 91±10 |
| Cp, μA/cm2 | 19±1 | 23±4 | 18±3 | 15±2 |
| Rp, Ω×cm2 | 40±2 | 41±4 | 30±2 | 46±7 |
Data are presented as mean±SEM. WT n=7, F508del Cftr n=7, Muc2+/− n=5, Muc2−/− n=6
p<0.01 (represents comparison between WT and F508del Cftr)
Fig. 5.
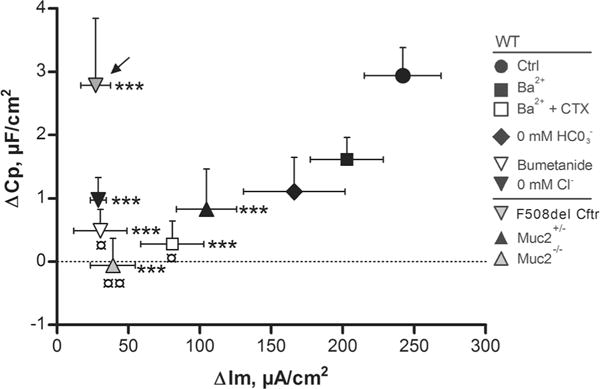
Effect of inhibition of ion transport on the relation between the carbachol-induced increase in Im and Cp in WT, F508del Cftr, Muc2+/− and Muc2−/− mouse distal colon. Delta values represent the maximum Im and Cp response to CCh (1 mM) during 20 min stimulation. Data are presented as mean±SEM. Arrow points towards the F508del Cftr group. ¤p<0.05; ¤¤p<0.01; ***p<0.001. Asterisks represent comparisons between the ΔIm of the WT Ctrl group (CCh alone) and the rest of the groups. Currency sign represent comparisons between the ΔCp of the WT Ctrl group (CCh alone) and the rest of the groups. Baseline values: Rp (Ω×cm2), Cp (μF/cm2), Im (μA/cm2). WT Ctrl: n=7, Rp: 40±2, Cp: 19±1, Im: 159±45. Bumetanide: n=6, Rp: 38±5, Cp: 16±1, Im: 117±21. Ba2+: n=6, Rp: 29±3, Cp: 19±2, Im: 201±35. Ba2++CTX: n=6, Rp: 32±3, Cp: 21±2, Im: 250±31. Cl− (0 mM): n=4, Rp: 51±5, Cp: 19±1, Im: 109±12. HCO3− (0 mM): n=6, Rp: 44±5, Cp: 20±2, Im: 132±32. F508del Cftr: n=7, Rp: 41±4, Cp: 23±4, Im: 26±7. Muc2+/−: n=5, Rp: 30±2, Cp: 18±3, Im: 120±7. Muc2−/−: n=6, Rp: 46±7, Cp: 15±2, Im: 91±10
To determine whether the same principles apply to the Cp response to CCh in the LS513 cells, the cells were pretreated with either the NKCC1 inhibitor bumetanide or the Cftr inhibitor GlyH-101, followed by stimulation with CCh. The results showed that pretreatment with bumetanide reduced the Cp response to CCh (p<0.05), whilst pretreatment with GlyH-101 did not alter the Cp response to CCh (Fig. 6a). However, the magnitude of the Cp response did not differ when comparing the bumetanide and GlyH-101 treated groups, suggesting that GlyH-101 has a partial but not significant effect on the Cp response to CCh in the LS513 cells. In contrast, the Im response to CCh was reduced by bumetanide (p<0.05) and potentiated by GlyH-101 (p<0.001) (Fig. 6b). The efficiency of GlyH-101 was tested on the forskolin-induced Im response which was reduced by 80 % (ctrl: Δ536±87 μA/cm2, GlyH-101: Δ110±21, p<0.001). Furthermore, pretreatment with GlyH-101 reduced the Cp response to forskolin by 97 % (ctrl: Δ6.23±1.70 μF/cm2, GlyH-101 Δ0.16±0.06 μF/cm2, p<0.01). Thus, the CCh-induced increase in Cp in the LS513 cells is dependent on a functional NKCC1 and independent of Cftr, whereas the forskolin-induced increase in Cp is Cftr dependent, similar to what was observed in the mouse distal colon.
Fig. 6.
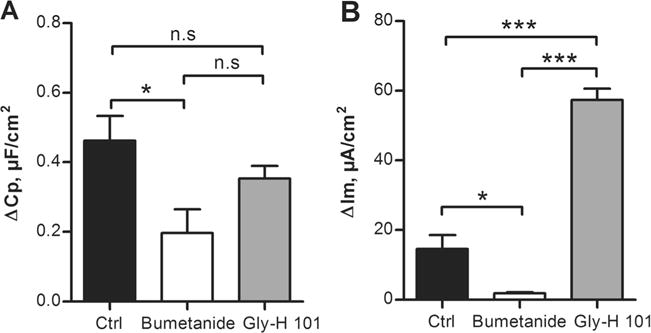
Carbachol (CCh)-induced effect on Cp and Im in LS513 cells. a Cp response to CCh (1 mM) during control conditions (Ctrl) and after 30 min incubation with bumetanide (0.1 mM) or GlyH-101 (50 μM). b Im response to CCh during control conditions and after 30 min incubation with bumetanide or GlyH-101. n=6 in all groups and data are presented as mean±SEM, *p<0.05; ***p<0.001. Baseline values: Rp (Ωxcm2), Cp (μF/cm2), Im (μA/cm2). Ctrl: Rp: 121±13, Cp: 4.6±0.2, Im: 6±2. Bumetanide: Rp: 185±19, Cp: 4.7±0.2, Im: 4±2. GlyH-101: Rp: 178±24, Cp: 4.6±0.3, Im: 10±4
Both NKCC1 and Cftr are required for carbachol-induced mucus growth
Since the electrophysiological data showed that the CCh-induced increase in Cp was dependent on NKCC1-mediated uptake and independent of a functional Cftr channel, we proceeded by testing whether this discrepancy also was reflected in the CCh-induced effect on mucus growth. Specimens from WT and F508del Cftr mouse distal colon were stimulated with CCh (1 mM), and the effect on mucus thickness was followed for 30 min. The results showed that 30 min stimulation with CCh significantly increased the mucus thickness in the WT mouse distal colon (Fig. 7a, b). To confirm that CCh stimulation indeed induced mucus secretion, tissue specimens from control and CCh-treated tissues were stained for glycoproteins using PAS/Alcian blue. The results showed less PAS/Alcian blue staining in the CCh-treated tissues (Fig. 8b) compared to the control tissues (Fig. 8a), which confirms CCh-induced mucin granule exocytosis. Pretreatment with bumetanide (30 min) reduced the CCh-induced increase in mucus thickness in the WT mice (bumetanide+CCh vs. CCh, p<0.05), which correlates with the reduced Cp response to CCh observed in the Ussing chamber experiments (Fig. 7a, b). In contrast, in the F508del Cftr mice, CCh stimulation did not induce a significant increase in mucus thickness, despite an intact Cp response to CCh in the Ussing chamber experiments (Fig. 7a, b). Thus, it appears that the F508del Cftr mice respond to CCh with mucus exocytosis but fail to unfold and expand the secreted mucus. Despite the lack of a CCh-induced increase in mucus thickness, baseline spontaneous mucus growth was similar in the WT control group and the F508del Cftr control group (Fig. 7a, b).
Fig. 7.
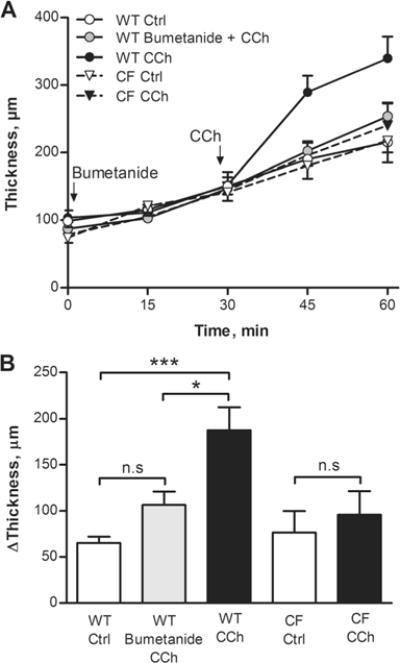
Carbachol (CCh)-induced increase in mucus thickness in WT and F508del Cftr (CF) mouse distal colon. a Mucus thickness measurements over time in WT and CF mice. WT Ctrl (n=9), WT bumetanide + CCh (n=6), WT CCh (n=9), CF Ctrl (n=6) and CF CCh (n=6). Bumetanide (0.1 mM) was added at the start of the experiment. b Delta increase in mucus thickness after 30 min incubation with CCh (1 mM) in WT and CF mice. Data are presented as mean±SEM, *p<0.05; ***p<0.001
Fig. 8.
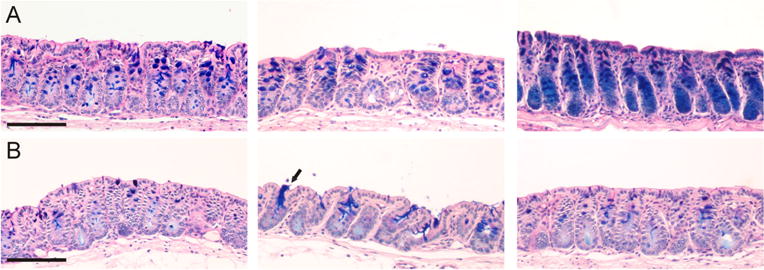
Staining of mucus-containing goblet cells in control and carbachol-treated wild-type distal colon using PAS/Alcian blue. Tissue sections from unstimulated control samples (a) and carbachol (1 mM) stimulated samples (b). Scale bar: 100 μm. Arrow points towards mucus-secreting goblet cells
Discussion
In the colon, epithelial anion and mucus secretion occur simultaneously in response to cholinergic activation, but whether the two processes are causally linked is not known. The main observation of the present study is that CCh-induced mucus exocytosis partially depends on basolateral transport via NKCC1 and K+ channels whereas the CCh-induced increase in mucus thickness requires Cftr-mediated transport. These findings suggest that secretion of ions, fluids and mucus is synchronised and that ion secretion plays an important role in regulation of mucus formation, both by affecting mucin granule exocytosis and expansion and unfolding of the secreted protein.
Capacitance measurements for studies of mucus exocytosis
To enable parallel studies of anion secretion and mucus exocytosis, we used measurements of net membrane current and epithelial capacitance to monitor these processes. Measurements of membrane current have been extensively used for studies of ion transport in epithelial tissue, whereas the use of epithelial capacitance to study exocytosis is less established [14, 34, 44]. Changes in epithelial capacitance have successfully been used to monitor vesicular trafficking of the Cftr channel, insulin release and mucin granule exocytosis [4, 5, 23, 61]. However, due to the limited number of studies using capacitance measurements for studies of mucus exocytosis, we decided to validate the method using both cultured epithelial cells and mice that lack the main component of the intestinal mucus, the Muc2 mucin. To ensure that the capacitance response to CCh does not reflect insertion of Cftr into the apical membrane, we also included the F508del Cftr mice in the study, as these mice are known to have defective trafficking of the Cftr channel [52]. The results confirmed that the CCh- and ATP-induced increase in capacitance was only present in the mucus producing epithelial cell line LS513 and not in the enterocyte cell line Caco-2. Stimulation with CCh induced a 10 % increase in capacitance in the LS513 cells which is lower compared to previous studies in HT29-Cl.16E cells that have shown a 30–40 % increase in capacitance following CCh stimulation [6]. The larger capacitance response observed in the HT29-Cl.16E cells compared to the LS513 cells correlates with the proportion of cells that stain positive for gel-forming mucins that has been shown to be approximately 80 % in the HT29 cells and up to 25 % in the LS513 cells [41, 43]. The capacitance response to CCh and PGE2 was reduced by exocytosis inhibitors, and most importantly, the capacitance response to CCh was absent in mice lacking the Muc2 mucin. These mice exhibit a medium grade of colitis which could affect the magnitude of the capacitance response. However, the finding that that Muc2+/− mice had a 70 % decrease in the capacitance response to CCh suggests that the decreased response is indeed due to the loss of Muc2 expression rather than inflammation since the heterozygous mice do not develop colitis [58]. An alternative interpretation of the capacitance response to CCh is vesicular trafficking of transporters such as Cftr, NKCC1 and NBCe1 which are known to be inserted into the plasma membrane upon CCh stimulation via vesicle fusion [2, 37]. However, an unaltered capacitance response to CCh in the F508del Cftr mice argues at least against Cftr vesicular recirculation being responsible for the CCh-induced increase in capacitance [52]. In contrast to the intact capacitance response to CCh observed in the F508del Cftr mice, the F508del Cftr mice failed to respond to forskolin with a significant increase in capacitance, suggesting that the forskolin response mainly reflects insertion of Cftr into the apical plasma membrane which correlates with previous studies of Cftr trafficking [61]. Thus, when using measurements of epithelial capacitance to study exocytosis in the colonic epithelium, it is important to include experiments that can differentiate between vesicular trafficking and mucin granule exocytosis.
Mucus secretion and mucus expansion
Analysis of the relation between ion transport and mucus physiology requires two considerations: (1) the effect of ion transport on mucin granule exocytosis and (2) the effect of ion transport on mucus expansion. Due to the severe pulmonary mucus pathology in patients with cystic fibrosis, the majority of studies related to ion transport and mucus has been performed in lung tissue or cultured airway epithelial cells. Although the secretory machinery of the airways and the intestines differs in several aspects, the majority of substances that induce mucus secretion in the airways also induce mucus secretion in the intestines, e.g. CCh, VIP and PGE2 [7, 21, 29, 50]. Studies in lung tissue have shown that inhibition of chloride and bicarbonate transport reduces the secretory response to CCh, VIP and histamine, whereas activation of K+ secretion potentiates mucus secretion [8, 9]. These results correlate with our observation that inhibition of NKCC1 reduces carbachol-induced mucus exocytosis, suggesting that accelerated mucus secretion is regulated by similar mechanisms in the airways and the distal large intestine. Our results also showed that inhibition of basolateral K+ channels reduced the capacitance response to CCh. CCh-induced anion secretion is known to induce activation basolateral K+ channels resulting in hyperpolarization of the plasma membrane which enhances the driving force for anion secretion via the Cftr channel. In addition to sustaining the electrochemical gradient for anion secretion, hyperpolarization of the membrane also enhances Ca2+ influx to the epithelial cells, resulting in increased concentration of intracellular Ca2+ which is needed for mucin granule exocytosis [17, 18]. One possible explanation to the inhibitory effect of Ba2+ and charybdotoxin on the capacitance response to carbachol is inhibition of cell membrane hyperpolarisation resulting in decreased Ca2+ influx, decreased effect on intracellular Ca2+ concentrations and decreased mucin granule exocytosis. Our results from the CF mice show that electrogenic anion secretion is not essential for mucin granule exocytosis. In these mice, both NKCC1 activity and K+ transport have been shown to be intact which may explain the intact capacitance response to CCh due to normal membrane hyperpolarization [3, 45].
Additional support for the importance of these transporters in goblet cell functions was recently reported by Jakab et al. showing that the goblet cells of a rat jejunum express three times as much NKCC1 as the neighbouring enterocytes [37, 38]. Furthermore, asthma-induced goblet cell hyperplasia is associated with goblet cell specific upregulation of NKCC1 [15]. Studies have also shown that both the Ba2+-sensitive K+ channel KCNQ1 and the charybdotoxin-sensitive K(Ca) 3.1 channel are expressed in the basolateral membrane of goblet cells [13, 33, 42].
It is well established that bicarbonate transport is required for formation of a normal mucus layer in the small intestine and the cervix [21, 28, 48]. Our present results show that spontaneous mucus growth was independent of Cftr-mediated transport whereas the carbachol-induced increase in mucus thickness required a functional Cftr channel. CCh-induced mucus secretion is restricted to the intestinal crypts whereas baseline secretion mainly occurs from the surface epithelium [39, 54]. This also correlates with Cftr expression which mainly is localised to the colonic crypt cells [37]. Cftr might thus be less important for baseline mucus growth that is mainly provided by the surface goblet cells but seems to be essential for carbachol-induced mucus growth from the crypts [39]. Our results suggest that in the CF mouse colon, mucus is secreted (exocytosed) in response to carbachol, but due to the lack of synchronised Cftr-mediated ion secretion, the secreted mucus fails to expand in volume. We recently showed that Cftr-mediated ion secretion is essential for proper mucus expansion in the ileum, and the same mechanisms may apply to carbachol-induced mucus expansion in the colon [28]. Although we cannot distinguish between chloride and bicarbonate secretion in our experimental systems, it is possible that impaired bicarbonate secretion via the Cftr is responsible for the lack of a carbachol effect on mucus growth in the CF mice. Regarding spontaneous baseline mucus secretion and expansion, it is not known which ion transporters that are involved; however, possible candidates are the goblet cell-specific transporter bestrophin 2 and and/or apical chloride/bicarbonate exchangers [63].
Physiological significance
The interest in the colonic mucus barrier as an important part of epithelial defence has increased rapidly during the last years due to reports showing that the colonic mucus layer forms a physical barrier between the microbiota and the epithelium and that alterations in the mucus barrier are associated with development of colitis [20, 40]. Despite this renewed interest, little is still known about how the properties of the colonic mucus are regulated and maintained. Our results show that the transport function of the colonic epithelium plays an important role in regulation of both mucus exocytosis and expansion. Thus, loss of electrogenic transport which is a common consequence of inflammation [26] will likely have a negative impact on the protective properties of the mucus layer. Furthermore, these results highlight the difference between baseline and secretagogue-induced mucus growth in the murine colon, where baseline mucus growth is Cftr independent and carbachol-induced mucus growth is Cftr dependent. Together, these results give an additional understanding of how ion transport takes part in maintaining gut homeostasis.
Conclusion
In summary, we provide evidence that CCh-stimulated changes in membrane capacitance can be used as a surrogate variable for mucin exocytosis. Blockage of the transport machinery at various levels generally caused parallel inhibitions of the net current response and the capacitance response, with one exception: In the F508del Cftr mice, the net current response was blocked, but the capacitance response remained intact. In contrast, the CCh-induced increase in mucus growth was absent in the F508del Cftr animals whilst baseline mucus growth was normal. To account for these findings, we postulate that a functional Cftr is not required for either spontaneous or induced mucin exocytosis but is essential for of CCh-stimulated mucin expansion, whilst functional NKCC1 co-transport and basolateral K+ channels are involved in secretagogue-induced mucin exocytosis.
Acknowledgments
This work was supported by the Swedish Research Council (nos. 7461, 8288, 21027, 21372, 20693, 2195501, 2009-2420, 521-2011-2370 and 342-2004-4434), The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren’s University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Åke Wibergs Foundation, Torsten and Ragnar Söderbergs Foundations, The Swedish Foundation for Strategic Research-The Mucosal Immunobiology and Vaccine Center (MIVAC) and the Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program. We acknowledge Dr. Anna Velcich for the Muc2−/− animals and Maria Sapnara for the technical assistance.
Footnotes
Ethical standards All experiments were performed according to the ethical guidelines of the University of Gothenburg.
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
Jenny K. Gustafsson, Email: jenny.gustafsson@gu.se, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, Box 440, Gothenburg 405 30, Sweden.
Sara K. Lindén, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, Box 440, Gothenburg 405 30, Sweden
Ala H. Alwan, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, Box 440, Gothenburg 405 30, Sweden
Bob J. Scholte, Department of Cell Biology, Erasmus MC, Rotterdam, The Netherlands
Gunnar C. Hansson, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, Box 440, Gothenburg 405 30, Sweden
Henrik Sjövall, Department of Internal Medicine, University of Gothenburg, Gothenburg, Sweden.
References
- 1.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+−HCO3- cotransport activity in murine colonic crypts in a M3-, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G650–G657. doi: 10.1152/ajpgi.00376.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann O, Wuchner K, Rossmann H, Leipziger J, Osikowska B, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. Expression and regulation of the Na+−K+−2Cl- cotransporter NKCC1 in the normal and CFTR-deficient murine colon. J Physiol. 2003;549(2):525–536. doi: 10.1113/jphysiol.2002.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand CA, Danahay H, Poll CT, Laboisse C, Hopfer U, Bridges RJ. Niflumic acid inhibits ATP-stimulated exocytosis in a mucin-secreting epithelial cell line. Am J Physiol Cell Physiol. 2004;286(2):C247–C255. doi: 10.1152/ajpcell.00593.200200593.2002. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand CA, Durand DM, Saidel GM, Laboisse C, Hopfer U. System for dynamic measurements of membrane capacitance in intact epithelial monolayers. Biophys J. 1998;75(6):2743–2756. doi: 10.1016/S0006-3495(98)77718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand CA, Laboisse CL, Hopfer U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am J Physiol. 1999;276(4 Pt 1):C907–C914. doi: 10.1152/ajpcell.1999.276.4.C907. [DOI] [PubMed] [Google Scholar]
- 7.Cho HJ, Joo NS, Wine JJ. Mucus secretion from individual submucosal glands of the ferret trachea. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00049.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JY, Joo NS, Krouse ME, Wu JV, Robbins RC, Ianowski JP, Hanrahan JW, Wine JJ. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest. 2007;117(10):3118–3127. doi: 10.1172/JCI31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HS, Yang WS, Kim SC, Lee WI, Lee HJ, Choi JY. Functional study of mucus secretion of the eustachian tube in guinea pigs. Otol Neurotol. 2010 doi: 10.1097/MAO.0b013e3181d35e69. [DOI] [PubMed] [Google Scholar]
- 10.Cuffe JE, Bertog M, Velazquez-Rocha S, Dery O, Bunnett N, Korbmacher C. Basolateral PAR-2 receptors mediate KCl secretion and inhibition of Na+ absorption in the mouse distal colon. J Physiol. 2002;539(Pt 1):209–222. doi: 10.1113/jphysiol.2001.013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danahay H, Atherton HC, Jackson AD, Kreindler JL, Poll CT, Bridges RJ. Membrane capacitance and conductance changes parallel mucin secretion in the human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L558–L569. doi: 10.1152/ajplung.00351.2005. [DOI] [PubMed] [Google Scholar]
- 12.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 13.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442(6):896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 14.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolganov GM, Woodruff PG, Novikov AA, Zhang Y, Ferrando RE, Szubin R, Fahy JV. A novel method of gene transcript profiling in airway biopsy homogenates reveals increased expression of a Na+−K+−Cl- cotransporter (NKCC1) in asthmatic subjects. Genome Res. 2001;11(9):1473–1483. doi: 10.1101/gr.191301. [DOI] [PubMed] [Google Scholar]
- 16.Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer H, Illek B, Negulescu PA, Clauss W, Machen TE. Carbachol-activated calcium entry into HT-29 cells is regulated by both membrane potential and cell volume. Proc Natl Acad Sci U S A. 1992;89(4):1438–1442. doi: 10.1073/pnas.89.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer KG, Leipziger J, Rubini-Illes P, Nitschke R, Greger R. Attenuation of stimulated Ca2+ influx in colonic epithelial (HT29) cells by cAMP. Pflugers Arch. 1996;432(4):735–740. doi: 10.1007/s004240050192. [DOI] [PubMed] [Google Scholar]
- 19.French PJ, van Doorninck JH, Peters RH, Verbeek E, Ameen NA, Marino CR, de Jonge HR, Bijman J, Scholte BJ. A delta F508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J Clin Invest. 1996;98(6):1304–1312. doi: 10.1172/JCI118917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel JM, Sferra TJ, Turner JR, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121(4):1657–1666. doi: 10.1172/JCI4553845538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119(9):2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geibel JP. Secretion and absorption by colonic crypts. Annu Rev Physiol. 2005;67:471–490. doi: 10.1146/annurev.physiol.67.031103.153530. [DOI] [PubMed] [Google Scholar]
- 23.Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, Salehi A, Rorsman P. Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans. J Physiol. 2004;556(Pt 3):711–726. doi: 10.1113/jphysiol.2003.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouyer V, Gottrand F, Desseyn JL. The extraordinarily complex but highly structured organization of intestinal mucus-gel unveiled in multicolor images. PLoS One. 2011;6(4):e18761. doi: 10.1371/journal.pone.0018761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greig ER, Boot-Handford RP, Mani V, Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+−ATPase in ulcerative colitis. J Pathol. 2004;204(1):84–92. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- 26.Greig E, Sandle GI. Diarrhea in ulcerative colitis. The role of altered colonic sodium transport. Ann N Y Acad Sci. 2000;915:327–332. doi: 10.1111/j.1749-6632.2000.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 27.Grootjans J, Hundscheid IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK, Dejong CH, von Meyenfeldt MF, Beets GL, Buurman WA. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 2013;62(2):250–258. doi: 10.1136/gutjnl-2011-301956. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209(7):1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson JK, Ermund A, Johansson ME, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2012;302(4):G430–G438. doi: 10.1152/ajpgi.00405.2011.10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafsson JK, Hansson GC, Sjovall H. Ulcerative colitis patients in remission have an altered secretory capacity in the proximal colon despite macroscopically normal mucosa. Neurogastroenterol Motil. 2012 doi: 10.1111/j.1365-2982.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsson JK, Hansson GC, Sjovall H. Ulcerative colitis patients in remission have an altered secretory capacity in the proximal colon despite macroscopically normal mucosa. Neurogastroenterol Motil. 2012;24(8):e381–e391. doi: 10.1111/j.1365-2982.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol. 2000;278(1):C212–C233. doi: 10.1152/ajpcell.2000.278.1.C212. [DOI] [PubMed] [Google Scholar]
- 33.Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl- and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol. 2006;291(4):C636–C648. doi: 10.1152/ajpcell.00557.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hardcastle J, Hardcastle PT, Taylor CJ, Goldhill J. Failure of cholinergic stimulation to induce a secretory response from the rectal mucosa in cystic fibrosis. Gut. 1991;32(9):1035–1039. doi: 10.1136/gut.32.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol. 1999;277(3 Pt 1):C351–C358. doi: 10.1152/ajpcell.1999.277.3.C351. [DOI] [PubMed] [Google Scholar]
- 36.Hemlin M, Jodal M, Lundgren O, Sjovall H, Stage L. The importance of the subepithelial resistance for the electrical properties of the rat jejunum in vitro. Acta Physiol Scand. 1988;134(1):79–88. doi: 10.1111/j.1748-1716.1988.tb08462.x. [DOI] [PubMed] [Google Scholar]
- 37.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G82–G98. doi: 10.1152/ajpgi.00245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci. 2012;57(11):2826–2845. doi: 10.1007/s10620-012-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson ME. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS One. 2012;7(7):e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Natl Acad Sci U S A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laburthe M, Augeron C, Rouyer-Fessard C, Roumagnac I, Maoret JJ, Grasset E, Laboisse C. Functional VIP receptors in the human mucus-secreting colonic epithelial cell line CL.16E. Am J Physiol. 1989;256(3 Pt 1):G443–G450. doi: 10.1152/ajpgi.1989.256.3.G443. [DOI] [PubMed] [Google Scholar]
- 42.Liao T, Wang L, Halm ST, Lu L, Fyffe RE, Halm DR. K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl- secretion. Am J Physiol Cell Physiol. 2005;289(3):C564–C575. doi: 10.1152/ajpcell.00561.2004. [DOI] [PubMed] [Google Scholar]
- 43.Linden SK, Driessen KM, McGuckin MA. Improved in vitro model systems for gastrointestinal infection by choice of cell line, pH, microaerobic conditions, and optimization of culture conditions. Helicobacter. 2007;12(4):341–353. doi: 10.1111/j.1523-5378.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 44.Mall M, Bleich M, Schurlein M, Kuhr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol. 1998;275(6 Pt 1):G1274–G1281. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- 45.Mall M, Wissner A, Seydewitz HH, Kuehr J, Brandis M, Greger R, Kunzelmann K. Defective cholinergic Cl(−) secretion and detection of K(+) secretion in rectal biopsies from cystic fibrosis patients. Am J Physiol Gastrointest Liver Physiol. 2000;278(4):G617–G624. doi: 10.1152/ajpgi.2000.278.4.G617. [DOI] [PubMed] [Google Scholar]
- 46.Matthews JB. Molecular regulation of Na+−K+−2Cl-cotransporter (NKCC1) and epithelial chloride secretion. World J Surg. 2002;26(7):826–830. doi: 10.1007/s00268-002-4059-z. [DOI] [PubMed] [Google Scholar]
- 47.McCormick DA, Horton LW, Mee AS. Mucin depletion in inflammatory bowel disease. J Clin Pathol. 1990;43(2):143–146. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol. 2010;588(13):2329–2342. doi: 10.1113/jphysiol.2010.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navabi N, McGuckin MA, Linden SK. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS One. 2013;8(7):e68761. doi: 10.1371/journal.pone.0068761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neutra MR, O’Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol. 1982;242(4):G380–G387. doi: 10.1152/ajpgi.1982.242.4.G380. [DOI] [PubMed] [Google Scholar]
- 51.Osbak PS, Bindslev N, Poulsen SS, Kaltoft N, Tilotta MC, Hansen MB. Colonic epithelial ion transport is not affected in patients with diverticulosis. BMC Gastroenterol. 2007;7:37. doi: 10.1186/1471-230X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T, Karp PH, Welsh MJ. Processing and function of CFTR-DeltaF508 are species-dependent. Proc Natl Acad Sci U S A. 2007;104(39):15370–15375. doi: 10.1073/pnas.0706974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulzke JD, Fromm M, Hegel U. Epithelial and subepithelial resistance of rat large intestine: segmental differences, effect of stripping, time course, and action of aldosterone. Pflugers Arch. 1986;407(6):632–637. doi: 10.1007/BF00582644. [DOI] [PubMed] [Google Scholar]
- 54.Specian RD, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 1980;85(3):626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabcharani JA, Harris RA, Boucher A, Eng JW, Hanrahan JW. Basolateral K channel activated by carbachol in the epithelial cell line T84. J Membr Biol. 1994;142(2):241–254. doi: 10.1007/BF00234946. [DOI] [PubMed] [Google Scholar]
- 56.Tousson A, Fuller CM, Benos DJ. Apical recruitment of CFTR in T-84 cells is dependent on cAMP and microtubules but not Ca2+ or microfilaments. J Cell Sci. 1996;109(Pt 6):1325–1334. doi: 10.1242/jcs.109.6.1325. [DOI] [PubMed] [Google Scholar]
- 57.TranVan Nhieu G, Clair C, Grompone G, Sansonetti P. Calcium signalling during cell interactions with bacterial pathogens. Biol Cell. 2004;96(1):93–101. doi: 10.1016/j.biolcel.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 59.van Doorninck JH, French PJ, Verbeek E, Peters RH, Morreau H, Bijman J, Scholte BJ. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995;14(18):4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 61.Weber WM, Cuppens H, Cassiman JJ, Clauss W, Van Driessche W. Capacitance measurements reveal different pathways for the activation of CFTR. Pflugers Arch. 1999;438(4):561–569. doi: 10.1007/s004249900086. [DOI] [PubMed] [Google Scholar]
- 62.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10(2):S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 63.Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest. 2010;120(5):1722–1735. doi: 10.1172/JCI41129. [DOI] [PMC free article] [PubMed] [Google Scholar]


