Abstract
Objective
An emerging paradigm for understanding how anesthetics induce altered arousal is relating receptor targeting in specific neural circuits to electroencephalogram (EEG) activity. We have previously found that enhanced gamma amino-butyric acid A (GABAA) inhibitory post-synaptic currents (IPSCs) manifest with large-amplitude slow (0.1–1 Hz) and frontally coherent alpha (8–12 Hz) oscillations. Therefore, we investigated the EEG signatures of modern day derivatives of ether (MDDE) to assess the extent to which we could obtain insights into MDDE anesthetic mechanisms
Methods
We retrospectively studied cases from our database in which patients received isoflurane vs. isoflurane/ketamine (n=10 each) or desflurane vs. desflurane/ketamine (n = 9 each). We analyzed the EEG recordings with spectral power and coherence methods.
Results
Similar to known GABAA circuit level mechanisms, we found that MDDE induced large amplitude slow and frontally coherent alpha oscillations. Additionally, MDDE also induced frontally coherent theta (4–8 Hz) oscillations. Reduction of GABAergic IPSCs with ketamine resulted in beta/gamma (13–40 Hz) oscillations, and significantly reduced MDDE-induced slow, theta and alpha oscillation power.
Conclusions
MDDE are associated with large amplitude slow oscillations and coherent alpha and theta oscillations.
Significance
These observations are consistent with the notion that GABAA circuit-level mechanisms are associated with MDDE-induced unconsciousness.
Keywords: EEG, Isoflurane, Desflurane, Ketamine, Slow oscillations, Alpha oscillations
Introduction
Modern-day derivatives of ether (MDDE; desflurane, isoflurane, sevoflurane) are the principal inhalational anesthetic drugs administered for general anesthesia to enable the safe and humane conduct of traumatic surgical and diagnostic procedures (Campagna et al., 2003). Despite considerable progress in anesthesiology care and research, neural circuit mechanisms to explain MDDE-induced unconsciousness are yet to be defined. However, an emerging paradigm for probing anesthetic mechanisms is to study electroencephalogram (EEG) recordings and relate the findings to the behavioral state and putative neural circuit-level mechanisms (Ching et al., 2010, Cimenser et al., 2011, Supp et al., 2011, Purdon et al., 2013, Vijayan et al., 2013, Akeju et al., 2014a, Akeju et al., 2014b, Blain-Moraes et al., 2014, Akeju et al., 2015, Purdon et al., 2015a, Pavone et al., 2016). This approach is beginning to provide key insights into the mechanisms of anesthetic action.
Molecular studies of MDDE have characterized actions at many receptor targets including gamma amino-butyric acid A (GABAA) receptors (GABAr), acetylcholine receptors, glycine receptors, hyperpolarization-activated cyclic nucleotide-gated channels (HCN), N-methyl-D-aspartate receptors (NMDAr), potassium channels, serotonin receptors, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptors, amongst others (Campagna et al., 2003, Rudolph et al., 2004, Hemmings et al., 2005, Franks, 2006, Alkire et al., 2008, Franks, 2008, Brown et al., 2011). Among these molecular targets, GABAr agonism and NMDAr antagonism are two principal receptor level targets that have been proposed to explain MDDE-induced unconsciousness (Campagna et al., 2003, Rudolph et al., 2004, Hemmings et al., 2005, Franks, 2008). However, despite extensive molecular and in vivo studies, it is not well defined in humans, the extent to which GABAr agonism and NMDAr antagonism mediates MDDE-induced unconsciousness. We have recently found that the EEG oscillations observed during sevoflurane-induced unconsciousness are similar to those observed during propofol-induced unconsciousness (Akeju et al., 2014b).
Propofol binds to the GABAr and potentiates chloride mediated IPSCs (Hales et al., 1991). At surgical anesthetic depth, potentiation of GABAergic IPSCs manifests with large-amplitude slow oscillations (0.1–1Hz) and coherent frontal alpha oscillations (8–12 Hz) (Gugino et al., 2001, Feshchenko et al., 2004, Leslie et al., 2009, Ching et al., 2010, Cimenser et al., 2011, Supp et al., 2011, Lewis et al., 2012, Mhuircheartaigh et al., 2013, Purdon et al., 2013, Vijayan et al., 2013, Akeju et al., 2014a, Akeju et al., 2014b). More recently, biophysical modeling studies have proposed a GABAA mediated thalamocortical mechanism for propofol-induced frontal alpha oscillations (Ching et al., 2010, Cimenser et al., 2011, Purdon et al., 2013, Vijayan et al., 2013). Evidence that GABAergic inhibition may be the primary mechanism of MDDE anesthetic action is suggested by similar oscillations observed in the EEG during general anesthesia maintained with MDDE to those observed with propofol (Akeju et al., 2014b, Brown et al., 2015, Pavone et al., 2016).
Based on our clinical experience with the EEG and a synthesis of prior works, we can readily test GABAergic predictions for MDDE circuit level mechanisms. We predict that MDDE like propofol should produce large amplitude slow and frontal alpha oscillations, which are largely dependent upon inhibition of pyramidal neurons in the cortex (Ching et al., 2010, Purdon et al., 2013, Vijayan et al., 2013, Akeju et al., 2014a, Akeju et al., 2014b). Presynaptic inhibition of these pyramidal neurons is accomplished by cortical interneurons (Homayoun et al., 2007, Seamans, 2008). Since ketamine down-regulates interneuron activity and inhibitory tone, we also predict that ketamine would diminish MDDE-induced alpha and slow oscillations.
Therefore, we retrospectively studied the EEG during isoflurane- and desflurane-induced unconsciousness with and without ketamine co-administration. We report that similar to the putative GABAA mediated circuit level mechanism described for propofol, isoflurane and desflurane are also associated with large amplitude slow oscillations, and coherent frontal alpha oscillations at surgical anesthesia levels. Additionally, isoflurane and desflurane are also associated with frontally coherent theta (4–8 Hz) oscillations. Ketamine significantly diminished the amplitude of these oscillations and induced a beta oscillatory dynamic. These observations are consistent with our predictions that GABAA circuit-level mechanisms are dominant during MDDE-induced unconsciousness.
Materials and Methods
Patient Selection and Data Collection
The Human Research Committee at Massachusetts General Hospital approved this retrospective observational study. We reviewed our database of general anesthesia and simultaneous EEG recordings collected between September 1, 2011 and December 1, 2015 to identify the cases studied in this manuscript.
Isoflurane
We identified 13 cases with baseline pre-induction EEG recordings that received isoflurane as the sole hypnotic agent for the maintenance of unconsciousness (isoflurane cohort). To study the effects of ketamine on isoflurane-induced EEG signatures, we also identified 10 age-matched cases where ketamine (mean±std: 180±34 mg) was administered for the induction of general anesthesia and isoflurane was administered as the sole hypnotic agent for the maintenance of unconsciousness and (isoflurane/ketamine cohort). For data analysis, we chose the first artifact-free consecutive 1-minute EEG segment during the baseline recordings (eyes closed period before the induction of general anesthesia) and during stable isoflurane administration between 15–30 minutes after the induction of general anesthesia and airway instrumentation, but before surgical stimulation.
Desflurane
We identified 9 cases that received desflurane as the sole hypnotic agent for maintenance of unconsciousness (desflurane cohort). To study the effects of ketamine on desflurane-induced EEG signatures, we also identified 9 age-matched cases with ketamine (53.3±10 mg, followed by an infusion at 5 μg/kg/min) administered as an analgesic adjunct (desflurane/ketamine cohort). For data analysis, we chose the first artifact-free consecutive 1-minute EEG segment during stable desflurane administration occurring at least 15 minutes after the induction of general anesthesia and airway instrumentation. The selected epochs occurred after the onset of surgery. We could not identify in our database desflurane cases with baseline EEG recordings.
Frontal EEG data were recorded with the Sedline EEG monitor (Masimo Corporation, Irvine, CA) with a pre-amplifier bandwidth of 0.5 to 92 Hz, and a sampling rate of 250 Hz. The standard Sedline Sedtrace electrode array records from electrodes located approximately at positions Fp1, Fp2, F7, and F8, with ground electrode at Fpz and reference electrode approximately 1 cm above Fpz. Electrode impedance was less than 5kΩ in each channel. We selected EEG data segments with information from both the electronic anesthesia record (Metavision, Dedham, MA) and EEG analysis in the spectral domain. We visually examined the spectrogram to ensure that the EEG dynamics were approximately stable (i.e., not transitioning to burst suppression or emergence). The electronic medical record was used to identify the sole hypnotic agent administered. None of the patients had known neurological or psychiatric abnormalities that may have interfered with the EEG. Tables 1 and 2 summarize the patient characteristics and the co-administered medications, respectively.
Table 1.
Patient characteristics.
| Isoflurane n = 10 | Isoflurane/Ket n = 10 | Desflurane n = 9 | Desflurane/Ket n = 9 | |
|---|---|---|---|---|
| Age (yr), mean (±SD) | 51 (10) | 49 (11) | 43 (17) | 47 (11) |
|
| ||||
| Sex (female), n (%) | 7 (70) | 2 (20) | 6 (66.6) | 6 (66.6) |
|
| ||||
| Weight (kg), mean (±SD) | 83 (21) | 83 (17) | 86 (35) | 128 (29) |
|
| ||||
| BMI (kg/m2), mean (±SD) | 30 (7) | 26 (3)* | 30 (13) | 45 (6)* |
|
| ||||
| Nerve block, n (%) | 5 (50) | 5 (50) | - | - |
|
| ||||
| Surgery type, n (%) | ||||
| General | 5 (50) | 4 (40) | 9 (100) | 9 (100) |
| Urology | 2 (20) | 2 (20) | 0 | 0 |
| Orthopedic | 0 | 4 (40) | 0 | 0 |
| Gynecology | 3 (30) | 0 | 0 | 0 |
missing height for 1 subject.
SD, standard deviation.
Table 2.
Medications.
| Isoflurane | Isoflurane/Ket | Desflurane | Desflurane/Ket | |
|---|---|---|---|---|
| Anesthetic Vapor % | 1 (0.2) n=10 | 1 (0.3) n=10 | 5.3 (0.9) n=9 | 5.7 (0.8) n=9 |
| Ketamine induction (mg), mean (±SD) | - | 180 (34.00) n=10 | - | 53.33 (10) n=9 |
| Ketamine infusion (μg/kg/min) | - | 10 n=1 | - | 5 n=9 |
| Propofol induction bolus (mg), mean (±SD) | 218 (59.78) n=10 | - | 182.22 (26.82) (n=9) | 216.67 (43.30) (n=9) |
| Fentanyl (μg), mean (±SD) | 192.86 (73.19) n=7 | 156.25 (77.63) n=8 | 133.33 (70.71) n=9 | 157.14 (93.22) n=7 |
| Sulfentanil (μg), mean (±SD) | - | 40 n=1 | - | - |
| Remifentanil (μg), mean (±SD) | - | - | 37.02 (32.47) n=3 | - |
| Remifentanil infusion (μg/kg/hr), mean (±SD) | - | - | 0.07 (0.03) (n=3) | - |
| Morphine mean (±SD) | - | 7.5 n=1 | - | - |
| Hydromorphone (mg), mean (±SD) | 0.68 (0. 39) n=4 | 0.4 n=1 | 0.45 (0.07) n=2 | 0.5 n=1 |
| Midazolam (mg), mean (±SD) | 1.89 (0.33) n=9 | 2.31 (1.16) n=8 | 1.8 (0.45) n=5 | 2.33 (0.82) n=6 |
| Neuromuscular blocker, n (%) | 9 (90) | 6 (60) | 9 (100) | 8 (88) |
Ket, Ketamine; SD, standard deviation.
Spectral Analysis
The power spectral density quantifies the frequency distribution of power within a signal, where power is the log base 10 of the EEG signal amplitude squared. The spectrogram is a time-varying version of the power spectral density, estimated on consecutive windows of EEG data. For example, figure 1 shows representative frontal EEG spectrograms of isoflurane and desflurane during surgical depth of general anesthesia. Figure 2a illustrates that changes occur in the EEG spectrogram during surgical depth of general anesthesia that is maintained with MDDDE when ketamine is administered. In these spectrograms, frequencies are arranged along the y-axis and time along the x-axis, and power is indicated by color on a decibel scale. For each patient, we computed the power spectrum and spectrogram with multitaper spectral methods implemented in the Chronux toolbox (Percival et al., 1993). To obtain estimates of power spectra robust to noise and artifacts, we derived an EEG electrode that equally weighted the signals obtained from Fp1, Fp2, F7 and F8 (average of all four channels). The parameters of the multitaper spectral analysis were: window length T = 2s with no overlap, time-bandwidth product TW = 3, number of tapers K = 5, and spectral resolution of 3 Hz. We computed group-level spectrograms by taking the median spectrogram across all patients. We calculated the median spectra and 95% confidence intervals using a bootstrap procedure. Bootstrap samples for the spectrum were drawn from the full sample of data, consisting of 60 non-overlapping 2-second EEG windows for each subject. We computed the median spectrum across subjects and repeated this procedure 10,000 times to obtain bootstrapped spectral estimates of the median.
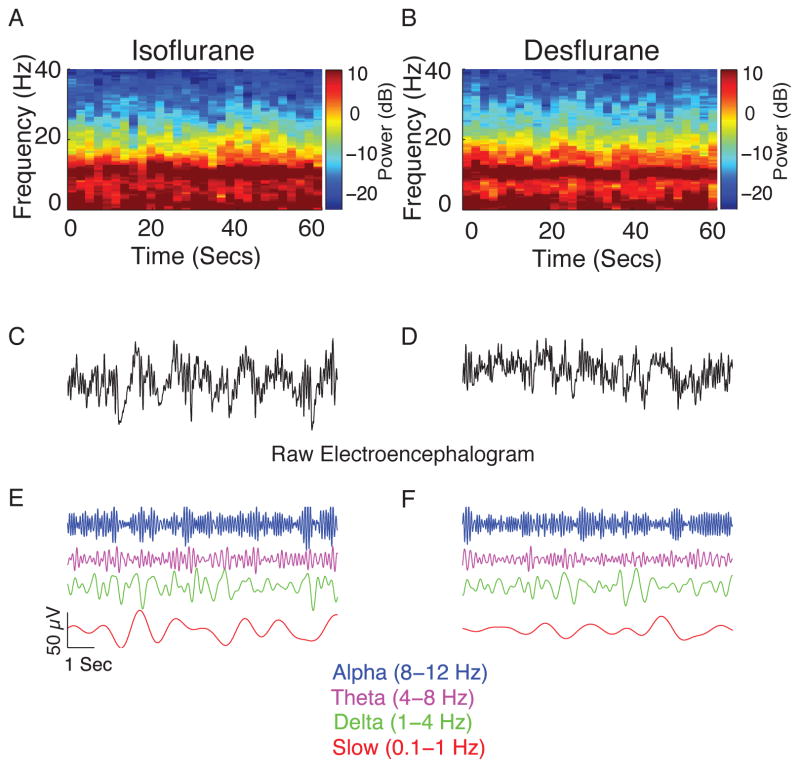
Figure 1.
Representative individual spectrograms and the time domain electroencephalogram data obtained during isoflurane- and desflurane-induced unconsciousness. (A) Spectrogram of a patient who received isoflurane. (B) Spectrogram of a patient who received desflurane. The spectrogram displays the frequency content of signals as they change over time. Frequency is plotted on the y-axis, time is plotted on the x-axis, and the energy or power in the signal is indicated in color. Both spectrograms show power in the slow, theta and alpha frequency bands. (C) Representative 10-s electroencephalogram trace from A. (D) Representative 10-s electroencephalogram trace from B. (E–F) Bandpass-filtered electroencephalogram signals from the raw tracings to more clearly illustrate gross similarities in the electroencephalogram.
dB, decibel; Hz, hertz, Secs, seconds.
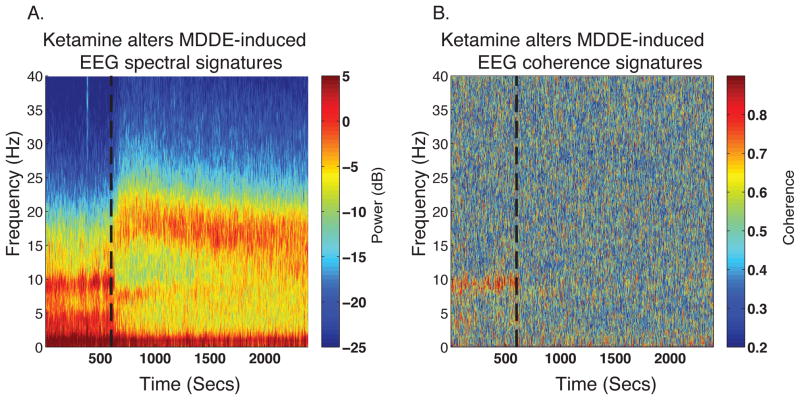
Figure 2.
Representative spectrogram and coherogram of ketamine-induced changes to MDDE-induced EEG signatures. (A) Spectrogram of a patient who received isoflurane GA at a stable concentration of 0.9%. After the administration of an adjunctive ketamine bolus for intraoperative analgesia (50 mg, dashed black line), decreased power can be noted in the slow, theta and alpha frequency bands alongside increased power in beta/gamma frequency bands. (B) Coherogram of data from panel A illustrating that ketamine disrupts the coherence of frontal alpha oscillations. The frontal alpha oscillations have a putative GABAergic circuit level mechanism.
dB, decibel; Hz, hertz; MDDE, modern day derivatives of ether; Secs, seconds, NMDAR; N-methyl-D-Aspartate receptor.
Coherence Analysis
The coherence Cxy(f) function between two signals x and y is defined as
where Sxy(f) is the cross-spectrum between the signals x(t) and y(t), Sxx(f) is the power spectrum of the signal x(t), and Syy(f) is the power spectrum of the signal y(t). The coherence can be interpreted as a frequency-dependent correlation coefficient, and also as a measure of association between two signals at the same frequency. The coherogram is a time-varying version of the coherence, estimated with consecutive windows of EEG data. Figure 2b illustrates that changes occur in the EEG coherogram during surgical depth of general anesthesia that is maintained with MDDDE when ketamine is administered. We estimated the coherence between the two distant frontal EEG electrodes F7 and F8 for each patient with multitaper methods implemented in the Chronux toolbox (Percival et al., 1993). The parameters for the multitaper coherence analysis, and methods for calculating the group-level coherograms and median coherence were the same as those for outlined for spectral analysis.
Statistical Analysis
To assess statistical significance for the difference in spectra and coherence at each frequency, we computed the 95% confidence interval of the median difference between groups by using an empirical bootstrap approach. We resampled spectral and coherence estimates for each non-overlapping window to obtain replicates of the estimates for each subject and took the median value across subjects for each group. We took the difference between two median estimates, repeated this 10,000 times and calculated the 95% confidence interval of the median difference at each frequency. To account for the underlying spectral resolution of the spectral and coherence estimates, we considered differences to be significant only if they are present for contiguous frequencies over a frequency band wider than the spectral resolution, 2W.
Results
Spectral Analysis
Modern day derivatives of ether vs. Modern day derivatives of ether/Ketamine
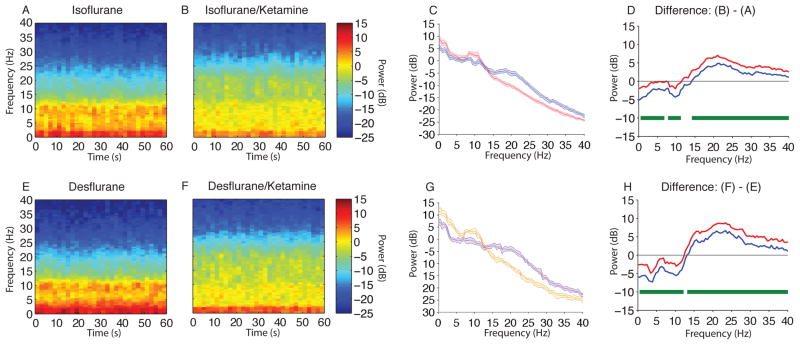
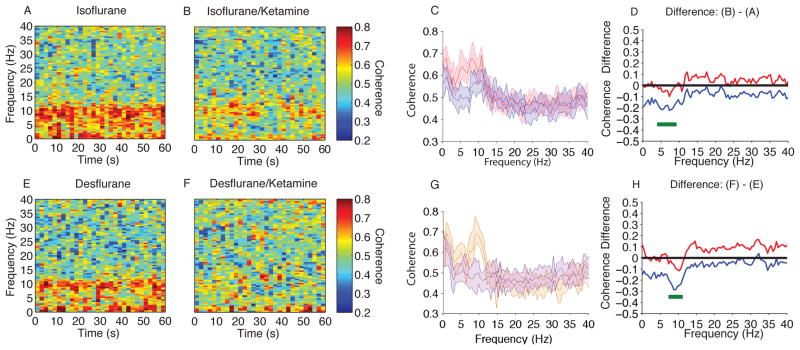
We observed larger amplitude slow-delta and alpha oscillations in the raw EEG and spectrogram during isoflurane-induced unconsciousness when compared to isoflurane/ketamine-induced unconsciousness (Fig. 3A,B). To describe these differences, we compared the spectra between both groups and found significant differences in power (Fig. 3C,D; isoflurane > isoflurane/ketamine, 0.1–6.8 Hz, 7.8–11.23 Hz; isoflurane < isoflurane/ketamine, 14.2–40 Hz). The peak median difference (isoflurane/ketamine – isoflurane) in slow-delta power was (power difference [95% confidence interval]) −3.6 dB [−5.2 dB, −1.9 dB] at 0.1 Hz. The peak median difference in theta power was −1.4 dB [−2.6 dB, −0.3 dB] at 6.8 Hz. The peak median difference in alpha power was −3.2 dB [−4.3 dB, −2.1 dB] at 9.8 Hz. The peak median difference in beta power was 5.9 dB [4.8 dB, 7.5 dB] at 21 Hz. Thus the administration of ketamine during isoflurane-induced unconsciousness resulted in a significant increase in beta band power and significant decreases in slow-delta, theta, and alpha band power.
Figure 3.
Spectral comparison of MDDE vs MDDE/ketamine. (A, B) Median frontal spectrograms during isoflurane- and isoflurane/ketamine-induced unconsciousness at surgical anesthetic depth (n = 10; between subject comparison). (C) Overlay of median isoflurane-induced unconsciousness spectrum (red), and median isoflurane/ketamine-induced unconsciousness spectrum (blue). Bootstrapped median spectra are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median spectrum. (D) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between spectra shown in panel C. During isoflurane-induced unconsciousness, there is significantly increased EEG power between 0.1–6.8 Hz, 7.8–11.23 Hz and decreased EEG power between 14.2–40 Hz. (E, F) Median frontal spectrograms of desflurane and desflurane/ketamine-induced unconsciousness at surgical anesthetic depth (n = 9; between subject comparison). (G) Overlay of median desflurane spectrum (orange), and median desflurane/ketamine-induced unconsciousness spectrum (purple). Bootstrapped median spectra are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median spectrum. (H) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between spectra shown in panel G. During desflurane/ketamine-induced unconsciousness, there is significantly increased EEG power between 0.1–12.21 Hz and decreased EEG power between 13.2–40 Hz.
B, decibel; Hz, hertz; MDDE, modern day derivatives of ether; s, seconds. Horizontal solid green lines represent frequency ranges at which significant difference existed.
We also observed larger amplitude slow-delta and alpha oscillations in the raw EEG and spectrogram during desflurane-induced unconsciousness when compared to desflurane/ketamine-induced unconsciousness (Fig. 3E,F). To better describe these differences, we compared the spectra between both groups and found significant differences in power (Fig. 3G,H; desflurane > desflurane/ketamine, 0.1–12.21 Hz; desflurane < desflurane/ketamine, 13.2–40 Hz). The peak median difference (desflurane/ketamine – desflurane) in slow-delta power was −5.9 dB [−7.2 dB, −4.5 dB] at 3.9 Hz. The peak median difference in theta power was −4.8 dB [−5.8 dB, −3.7 dB] at 4.4 Hz. The peak median difference in alpha power was −4.3 dB [−5.5 dB, −2.9 dB] at 10.3 Hz. The peak median difference in beta power was 7.7 dB [6.7 dB, 8.8 dB] at 23.4 Hz. Thus, the administration of ketamine during desflurane-induced unconsciousness resulted in a significant increase in beta band power and significant decreases in slow-delta, theta, and alpha band power.
Isoflurane and Isoflurane/ketamine vs. Baseline
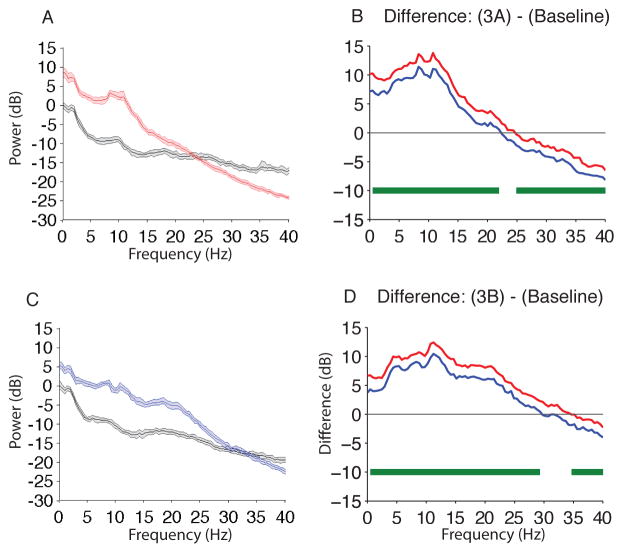
To provide a frame of reference for MDDE-induced EEG signatures, we performed a within subject comparison of the spectra during isoflurane-induced unconsciousness to baseline and found significant differences in power (Fig. 4A,B; isoflurane > baseline, 0.1–23 Hz; isoflurane < baseline, 24.9–40 Hz). The peak median difference (isoflurane – baseline) in slow-delta power was 9.5 dB [8.9 dB, 10 dB] at 3.9 Hz. The peak median difference in theta power was 10.5 dB [10.3 dB, 11.6 dB] at 7.8 Hz. The peak median difference in alpha power was 12.5 dB [11.2 dB, 13.8 dB] at 10.7 Hz. The peak median difference in beta power was 4.9 dB [4.4 dB, 5.4 dB] at 16 Hz. Thus, MDDE-induced unconsciousness resulted in a significant increase in slow-delta, theta, alpha, and beta band power.
Figure 4.
Spectral comparison of Isoflurane and Isoflurane/ketamine vs. Baseline. (A) Overlay of median baseline spectrum (black), and median isoflurane-induced unconsciousness spectrum (red). Bootstrapped median spectra are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median spectrum. (B) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between spectra shown in panel A. During isoflurane-induced unconsciousness, there is significantly increased EEG power between 0.1–23 Hz and decreased EEG power between 24.90 – 40 Hz. (C) Overlay of median baseline spectrum (black), and median isoflurane/ketamine-induced unconsciousness spectrum (blue). Bootstrapped median spectra are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median spectrum. (D) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between spectra shown in panel C. During isoflurane/ketamine-induced unconsciousness, there is significantly increased EEG power between 0.1–29.3 Hz and decreased EEG power between 34.67–40 Hz.
dB, decibel; Hz, hertz; MDDE, modern day derivatives of ether; s, seconds. Horizontal solid green lines represent frequency ranges at which significant difference existed.
To provide a frame of reference for MDDE/ketamine-induced EEG signatures, we also performed a within subject comparison of isoflurane/ketamine-induced unconsciousness to baseline and found significant differences in power (Fig. 4C,D; isoflurane/ketamine > baseline, 0.1–29.3 Hz; isoflurane < baseline, 34.67–40 Hz). The peak median difference (isoflurane/ketamine – baseline) in slow-delta power was 8.1 dB [7.3 dB, 8.9 dB] at 3.9 Hz. The peak median difference in theta power was 9.6 dB [8.8 dB, 10.3 dB] at 7.8 Hz. The peak median difference in alpha power was 11.4 dB [10.5 dB, 12.4 dB] at 11.2 Hz. The peak median difference in beta power was 7.4 dB [6.4 dB, 8.5 dB] at 17.6 Hz. Thus, MDDE/ketamine-induced unconsciousness also resulted in a significant increase in slow-delta, theta, alpha, and beta band power.
Coherence Analysis
Modern day derivatives of ether vs. Modern day derivatives of ether/Ketamine
We observed more coherent alpha oscillations in the coherogram during isoflurane-induced unconsciousness when compared to isoflurane/ketamine-induced unconsciousness (Fig. 5A,B). To better describe these differences, we compared the coherence between these groups and found significant differences in coherence (Fig. 5C,D; isoflurane > isoflurane/ketamine, 3.91–9.28 Hz). The peak median theta coherence difference (isoflurane – isoflurane/ketamine) was −0.16 [−0.22, −0.10] at 7.32 Hz. The peak median alpha coherence difference was −0.12 [−0.20, −0.03] at 8.3 Hz. Thus, the administration of ketamine during isoflurane-induced unconsciousness resulted in significant decreases in theta and alpha band coherence.
Figure 5.
Coherence comparison (F7–F8) of MDDE vs MDDE/Ketamine. (A, B) Median frontal coherograms of isoflurane- and isoflurane/ketamine-induced unconsciousness (n = 10; between subject comparison). (C) Overlay of median isoflurane/ketamine-induced unconsciousness coherence (blue), and median isoflurane-induced unconsciousness coherence (red). Bootstrapped median coherences are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median coherence. (D) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between coherence shown in panel C. During isoflurane-induced unconsciousness, there is significantly increased coherence between 3.91–9.28 Hz. (E, F) Median frontal coherograms of Desflurane- and Deflurane/ketamine-induced unconsciousness (n = 9; between subject comparison). (G) Overlay of median desflurane coherence (orange), and median isoflurane/ketamine-general anesthesia induced spectrogram (purple). Bootstrapped median coherences are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median coherence. (H) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between coherence shown in panel G. During isoflurane/ketamine-induced unconsciousness, there is significantly increased coherence between 7.32 – 11.23 Hz.
B, decibel; Hz, hertz; MDDE, modern day derivatives of ether; s, seconds. Horizontal solid green lines represent frequency ranges at which significant difference existed.
Compared to desflurane/ketamine-induced unconsciousness, we also observed coherent alpha during desflurane unconsciousness (Fig. 5E,F). To describe these differences, we compared the spectra during desflurane/ketamine-induced unconsciousness to baseline and found significant differences in coherence (Fig. 5G,H; desflurane/ketamine > baseline, 7.32 – 11.23 Hz). The peak median theta coherence difference (isoflurane – isoflurane/ketamine) was −0.11 [−0.21, −0.02] at 7.81 Hz. The peak median alpha coherence difference was −0.17 [−0.27, −0.06] at 8.79 Hz. Thus, the administration of ketamine during deflurane-induced unconsciousness resulted in significant decreases in theta and alpha band coherence.
Isoflurane and Isoflurane/ketamine vs. Baseline
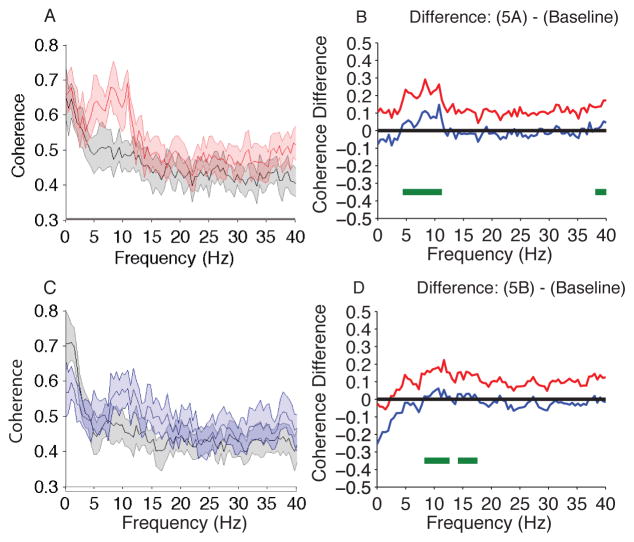
We compared the coherence during isoflurane-induced unconsciousness to baseline and found significant differences in theta and alpha coherence (Fig. 6A,B; isoflurane > baseline, 4.39–11.23 Hz, 38.09–40 Hz). The peak median theta coherence difference (isoflurane – baseline) was 0.17 [0.10, 0.24] at 7.8 Hz. The peak median alpha coherence difference was 0.20 [0.10, 0.29] at 8.3 Hz.
Figure 6.
Coherence comparison (F7–F8) of Isoflurane and Isoflurane/ketamine vs. Baseline. (A) Overlay of median baseline coherence (black), and median isoflurane-induced unconsciousness coherence (red). Bootstrapped median coherences are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median coherence (n = 10; within subject comparison). (B) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between coherence shown in panel A. During isoflurane-induced unconsciousness, there is significantly increased coherence between 4.39–11.23 Hz and decreased coherence between 38.09–40 Hz. (C) Overlay of median baseline coherence (black), and median isoflurane/ketamine-general anesthesia induced spectrogram (blue). Bootstrapped median coherences are presented and the shaded regions represent the 95% confidence interval for the uncertainty around each median coherence (n = 10; within subject comparison). (D) The upper (red) and lower (blue) represent the bootstrapped 95% confidence interval bounds for the difference between spectra shown in panel C. During isoflurane/ketamine-induced unconsciousness, there is significantly increased coherence between 8.30 – 12.70 Hz, 14.16 – 17.58 Hz.
B, decibel; Hz, hertz; MDDE, modern day derivatives of ether; s, seconds. Horizontal solid green lines represent frequency ranges at which significant difference existed.
We also compared the coherence during isoflurane/ketamine-induced unconsciousness to baseline and found significant differences in alpha and beta coherence (Fig. 6C,D; isoflurane/ketamine > baseline, 8.30 – 12.70 Hz, 14.16 – 17.58 Hz). The peak median alpha coherence difference (isoflurane/ketamine – baseline) was 0.14 [0.05, 0.22] at 11.7 Hz. The peak median beta coherence difference was 0.09 [0.03, 0.15] at 16.1 Hz. Thus, there was increased frontal alpha coherence during isoflurane- and isoflurane/ketamine induced unconsciousness. However, compared to isoflurane- induced unconsciousness, alpha band coherence was decreased during isoflurane/ketamine-induced unconsciousness.
Discussion
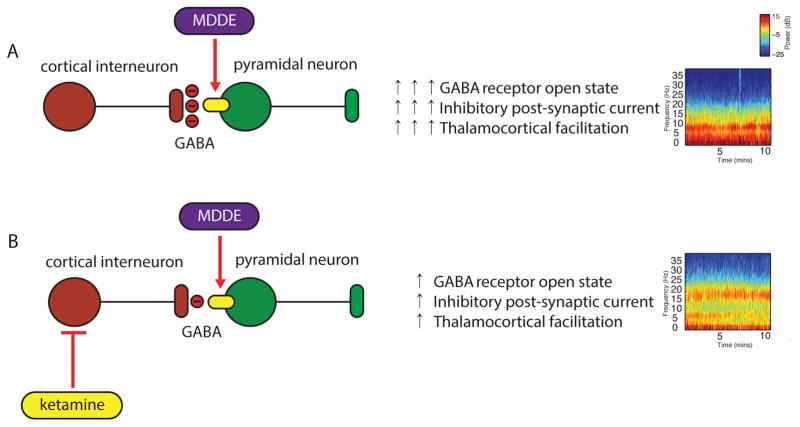
An emerging paradigm for understanding how anesthetics induce altered arousal is relating receptor targeting in specific neural circuits to EEG activity. We have previously found that propofol, an anesthetic that enhances GABAergic IPSCs manifests with large-amplitude slow and frontally coherent alpha oscillations. These oscillations, which lend to dynamical systems modeling, have been related to GABAA circuit level mechanisms. Therefore, we tested the GABAergic predictions that (1) MDDE circuit level mechanisms will manifest with large amplitude slow and frontal alpha oscillations at surgical anesthetic depth and, (2) ketamine will diminish the power of MDDE-induced slow and frontal alpha oscillations (Fig. 7). We briefly summarize our findings that strongly implicate a GABAA neural circuit mechanism for MDDE as follows: (1) MDDE-induced unconsciousness was associated with large amplitude slow-delta oscillations; (2) MDDE-induced unconsciousness was associated with coherent alpha oscillations; (3) MDDE-induced unconsciousness was associated with coherent theta oscillations; and (4) ketamine reduced the amplitude of MDDE-induced slow, theta and alpha oscillations and induced beta oscillations.
Figure 7.
A schematic of how MDDE and MDDE/ketamine may alter neuronal function to induce unconsciousness. (A) Interneurons release GABA to inhibit pyramidal neurons. Pyramidal neurons contain glutamate and act on other pyramidal neurons (not shown) or interneurons (not shown) to excite them through activation of NMDA and non-NMDAr. MDDE at clinical concentrations and in the presence of GABA enhance the amplitude of responses to GABA and prolong the duration of GABA mediated synaptic inhibition. This is manifest on the electroencephalogram as slow oscillations, theta and coherent frontal alpha oscillations. Subcortical mechanisms (not shown) are likely important for the generation and maintenance of these oscillations. (B) Ketamine antagonizes NMDAr on inhibitory interneurons more effectively than those on pyramidal cells. This leads to reduced inhibitory tone, possible interneuron asynchrony, and less effective ability of MDDE to maintain slow oscillations and coherent alpha oscillations. This depiction is meant solely for illustrative purposes and does not represent all the network ensembles that may be essential for these oscillations.
MDDE, modern day derivatives of ether; NMDAr, N-methyl-D-aspartate receptors.
Alpha and Slow Oscillations during MDDE-induced unconsciousness
Barbiturates, which are anesthetics with GABAA mechanisms (Macdonald et al., 1978, Nicoll, 1978, Leeb-Lundberg et al., 1980), produce sustained oscillations in the alpha frequency range in cat thalamic neurons (Steriade et al., 1993a). These oscillations exhibit thalamic and cortical coherence (Steriade et al., 1993a), which require corticothalamic projections (Contreras et al., 1996). Since alpha oscillations are characterized by prolonged IPSPs in thalamocortical cells (Steriade et al., 1993b, a), as suggested for propofol, they may contribute to MDDE-induced unconsciousness by disrupting the normal thalamic and cortical activity necessary for information integration (Ching et al., 2010, Cimenser et al., 2011, Purdon et al., 2013).
Neither the isolated cortex nor the isolated thalamus can produce slow waves that are identical to those observed in vivo (Crunelli et al., 2015). It is now widely accepted that generation of slow waves requires an intact thalamocortical network (David et al., 2013, David et al., 2014, Lemieux et al., 2014, Crunelli et al., 2015). The origin of GABAA induced cortical slow oscillations may result from diminished cortical inputs from subcortical and brainstem arousal centers (Brown et al., 2011, Pavone et al., 2016). However, further research is needed to fully decipher the precise contributions of these different brain regions to slow wave generation and propagation. A known circuit implicated in the cortical slow rhythm involves pyramidal neurons and inhibitory cells (Steriade et al., 1993b, a). These cortical slow oscillations drive cells in the reticular nucleus to trigger alpha oscillations (Steriade et al., 1993b, a). Thus, the thalamus and the cortex may sufficiently converge to generate alpha and slow oscillations (Ching et al., 2010, Purdon et al., 2013, Vijayan et al., 2013). Therefore, it is not altogether surprising that coherent alpha oscillations are modulated by slow oscillations (Purdon et al., 2013), and that ketamine significantly reduces the power of MDDE-induced slow and alpha oscillations.
Ketamine is typically associated with gamma oscillations on the EEG (Purdon et al., 2015b). These oscillations arise because ketamine blocks excitatory NMDAr on fast-spiking cortical interneurons more effectively than on pyramidal neurons, resulting in decreased GABA release at the interneuron-pyramidal neuron synapse, and greatly facilitated pyramidal neuron activity (Homayoun et al., 2007, Seamans, 2008). During MDDE/ketamine-induced unconsciousness, the gamma oscillations typically associated with ketamine are replaced by beta oscillations. This is likely because MDDE in the presence of ketamine results in slightly increased IPSCs, leading to a reduction in pyramidal neuron facilitation. Similarly, during MDDE/ketamine-induced unconsciousness, the slow and alpha oscillations that are typically associated with GABAergic mechanisms are also reduced in power (MDDE IPSCs are larger that the MDDE/ketamine IPSCs). Importantly, at clinical concentrations MDDE require GABA at the synapse to potentiate IPSC (Garcia et al., 2010).
Beta oscillations have been described when low doses of drugs that modulate the GABAA receptor are administered (Gibbs et al., 1937). This dynamic is postulated to be a consequence of an interaction between the synaptic GABAA current and an intrinsic membrane slow-potassium current that leaves the postsynaptic neuron in a more excited state over a portion of its interspike interval (McCarthy et al., 2008). A consequence of this membrane level interaction is the generation of beta frequency asynchrony between reciprocally connected interneurons to which pyramidal neurons also pattern their spiking behavior (McCarthy et al., 2008). Furthermore, only neurons with intrinsic spiking frequencies below beta1 (12–22 Hz) are excited to higher frequency spiking (McCarthy et al., 2008). This correlates with our finding that ketamine resulted in decreased MDDE-induced EEG power in the lower frequencies and increased power in the beta bands. Thus, the beta oscillations we describe in this manuscript may result from interneuron asynchrony.
Hayashi et al. examined the effect of ketamine on propofol-induced alpha oscillations and found that ketamine increased the peak frequencies of alpha oscillations from ~10Hz to ~15 Hz (Hayashi et al., 2007). Our present results for MDDE are in line with this finding, and suggests that ketamine modulates the mechanisms necessary for MDDE-induced alpha oscillations to produce beta oscillations. While it is tempting to think that ketamine antagonizes GABAergic mechanisms to place patients in an anesthetized brain state that is easier to rouse to consciousness, a behavioral study during general anesthesia maintained with propofol/ketamine suggests that the ketamine/GABAA brain state reflects a deeper state of unconsciousness (Sakai et al., 1999). This is because hypnotic endpoints were achieved at lower doses of propofol when ketamine was administered as an adjunct (Sakai et al., 1999). We note that subcortical mechanisms that are not readily apparent on the surface EEG and the spatiotemporal characteristics of the beta oscillations that are produced with MDDE/ketamine may lend further insights into this brain state of unconsciousness.
Theta Oscillations, Ethers Anesthetics and Ketamine
In addition to large amplitude slow oscillations and coherent alpha oscillations, our results are in concordance with previous reports that have described theta oscillations when high concentrations of MDDE are administered (Leslie et al., 2009, Akeju et al., 2014b). Theta oscillations have also been described for subanesthetic doses of ketamine (Corssen et al., 1966, Engelhardt et al., 1994, Kochs et al., 1996). This suggests that these oscillations are a manifestation of either ketamine-induced NMDA or HCN antagonism. However, since propofol also modulates HCN receptors and does not result in theta oscillations on the EEG (Feshchenko et al., 2004, Ching et al., 2010, Cimenser et al., 2011, Supp et al., 2011, Mhuircheartaigh et al., 2013, Purdon et al., 2013, Akeju et al., 2014a), we suggest that the ketamine-induced theta oscillations may result from NMDA antagonism. Thus, NMDA circuit level mechanisms may account for both MDDE- and ketamine-induced coherent theta oscillations. A prediction of a similar mechanism for MDDE-induced theta oscillations and ketamine-induced theta oscillations is that the MDDE induced theta oscillations should be potentiated by ketamine. However, we found that ketamine did not potentiate MDDE-induced theta oscillations. Rather, ketamine decreased the power and coherence of these oscillations. Therefore, our results suggest that different mechanisms are responsible for MDDE-induced theta oscillations and ketamine-induced theta oscillations. This distinct EEG dynamic may be studied further to provide additional insights into the molecular and neural circuit mechanisms of the human theta rhythm that is observed during the anesthesia-induced brain states.
Reconciling Findings with other Human Studies of Anesthetic Action
At surgical levels of unconsciousness, sevoflurane (Akeju et al., 2014b) and now isoflurane and desflurane EEG signatures are similar to the GABAA mediated signatures that have been described for propofol (Gugino et al., 2001, Feshchenko et al., 2004, Leslie et al., 2009, Ching et al., 2010, Cimenser et al., 2011, Supp et al., 2011, Lewis et al., 2012, Mhuircheartaigh et al., 2013, Purdon et al., 2013, Vijayan et al., 2013, Akeju et al., 2014a, Akeju et al., 2014b, Brown et al., 2015). Therefore, the mechanism of MDDE-induced unconsciousness is likely closer to propofol and the other GABAergic anesthetics than previously appreciated. A recent investigation of sevoflurane unconsciousness (Blain-Moraes et al., 2014) failed to demonstrate the GABAA mediated EEG signatures that we describe in this manuscript, suggesting that a non-GABA mediated circuit-level mechanism of action accounts for MDDE-induced unconsciousness. However, the authors studied concentrations of sevoflurane only sufficient to induce a sedative and not surgical unconscious state (Blain-Moraes et al., 2014). This sedative brain state is characterized on the spectrogram by increased frontal beta oscillation power (Blain-Moraes et al., 2014). This increase is consistent with the increase in frontal beta oscillation power that has also been reported for sedative brain state induced by propofol (Purdon et al., 2013). Consequently the results reported by Blain-Moraes et al. do not indicate a non-GABAergic mechanism for MDDE unconsciousness.
Kaskinoro et al. describe results that were similar to Blain-Moraes et al. at the sedative state (Blain-Moraes et al., 2014, Kaskinoro et al., 2015). However, a slightly increased sevoflurane concentration resulted in slow-delta and alpha-beta oscillations (Kaskinoro et al., 2015). This increase in slow-delta and alpha-beta oscillation amplitude is also similar to “trough-max” (sedative to general anesthesia brain-state transition) that has been described for propofol (Purdon et al., 2013, Akeju et al., 2014a). Thus, even though different circuit level alterations are present during the GABAA sedative and unconscious brain-states, a GABAA neural circuit mechanism is likely responsible for both brain states.
Limitations and Future Directions
The EEG channels analyzed in this study precluded us from accurately describing the spatiotemporal dynamics of the oscillations we describe across the entire scalp. However, anesthesia induced oscillations most readily observed in frontal EEG recordings. Because this is a retrospective clinical study of frontal EEG electrodes obtained during routine clinical care, anesthetics were not administered in a controlled fashion and the EEG was not recorded at the highest sampling rates and resolution that is currently feasible. Furthermore, the scalp EEG represents local field potentials produced in various layers of the cortex and subcortical regions. Therefore, future volunteer clinical studies with high-density EEG recordings are warranted. Such studies could incorporate EEG source localization techniques that will better inform us on where the oscillations we describe originate. However, the EEG dynamics we describe are robust and unlikely to be spurious because our findings were consistent for isoflurane and desflurane in the presence and absence of surgical stimulus, respectively.
Conclusions
GABAr are established receptor level targets of MDDE. The close similarities between the neurophysiological signatures of the MDDE and propofol during general anesthesia suggest that enhanced GABAergic IPSCs are most likely the primary mechanism through which the ethers induce unconsciousness. Future studies such as multi-site intracranial recordings obtained from laboratory models with preserved neural circuitry are necessary. These studies are poised to provide deeper insights into the mechanisms of how MDDE induce unconsciousness and improved methods of targeting these mechanisms for the therapeutic benefit of patients.
Highlights.
Large amplitude slow oscillations, frontally coherent theta oscillations and frontally coherent alpha oscillations occur with the administration of modern day derivatives of ether.
Reduction of GABAergic inhibitory post-synaptic potentials with ketamine resulted in beta/gamma (13–40 Hz) oscillations and significantly reduced MDDE-induced slow, theta and alpha oscillation power.
GABAA circuit-level mechanisms are associated with MDDE-induced unconsciousness.
Acknowledgments
Funding
DP2-OD006454 (to PLP); DP1-OD003646, TR01-GM104948 (to ENB), and Ruth L. Kirchstein National Research Service Award to (AH) from the National Institutes of Health, Bethesda, Maryland; Foundation of Anesthesia Education and Research, Rochester, Minnesota (to OA); Massachusetts General Hospital Faculty Development Award, Boston, MA (to OA); Funds from the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Footnotes
Disclosures
The authors Oluwaseun Akeju, Emery N. Brown, and Patrick L. Purdon have submitted a provisional patent application describing the use of the EEG measures described in this manuscript for monitoring sedation and general anesthesia. All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akeju O, Pavone KJ, Thum JA, Firth PG, Westover MB, Puglia M, et al. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth. 2015;115(Suppl 1):i66–i76. doi: 10.1093/bja/aev114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014a;121:978–89. doi: 10.1097/ALN.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014b;121:990–8. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain-Moraes S, Lee U, Ku S, Noh G, Mashour GA. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci. 2014;8:114. doi: 10.3389/fnsys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Solt K, Purdon PL, Akeju O. Monitoring the Depth of Anesthesia. In: Miller RD, editor. Miller’s Anesthesia. 8. Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–70. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimenser A, Purdon PL, Pierce ET, Walsh JL, Salazar-Gomez AF, Harrell PG, et al. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci U S A. 2011;108:8832–7. doi: 10.1073/pnas.1017041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–4. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45:29–40. [PubMed] [Google Scholar]
- Crunelli V, David F, Lorincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol. 2015;31:72–80. doi: 10.1016/j.conb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- David F, Schmiedt JT. Thalamus and cortex: inseparable partners in shaping sleep slow waves? J Neurosci. 2014;34:11517–8. doi: 10.1523/JNEUROSCI.2226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, et al. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33:19599–610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt W, Stahl K, Marouche A, Hartung E, Dierks T. Ketamine racemate versus S-(+)-ketamine with or without antagonism with physostigmine. A quantitative EEG study on volunteers [Article in German] Anaesthesist. 1994;43(Suppl 2):S76–82. [PubMed] [Google Scholar]
- Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–66. doi: 10.1159/000079981. [DOI] [PubMed] [Google Scholar]
- Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol. 2010;8:2–9. doi: 10.2174/157015910790909502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs FA, Gibbs LE, Lennox WG. Effects on the electroencephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:154–66. [Google Scholar]
- Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–8. doi: 10.1093/bja/87.3.421. [DOI] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104:619–28. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Tsuda N, Sawa T, Hagihira S. Ketamine increases the frequency of electroencephalographic bicoherence peak on the alpha spindle area induced with propofol. Br J Anaesth. 2007;99:389–95. doi: 10.1093/bja/aem175. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskinoro K, Maksimow A, Georgiadis S, Langsjo J, Scheinin H, Karjalainen P, et al. Electroencephalogram reactivity to verbal command after dexmedetomidine, propofol and sevoflurane-induced unresponsiveness. Anaesthesia. 2015;70:190–204. doi: 10.1111/anae.12868. [DOI] [PubMed] [Google Scholar]
- Kochs E, Scharein E, Mollenberg O, Bromm B, Schulte am Esch J. Analgesic efficacy of low-dose ketamine. Somatosensory-evoked responses in relation to subjective pain ratings. Anesthesiology. 1996;85:304–14. doi: 10.1097/00000542-199608000-00012. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg F, Snowman A, Olsen RW. Barbiturate receptor sites are coupled to benzodiazepine receptors. Proc Natl Acad Sci U S A. 1980;77:7468–72. doi: 10.1073/pnas.77.12.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, Chen JY, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 2014;34:5689–703. doi: 10.1523/JNEUROSCI.1156-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K, Sleigh J, Paech MJ, Voss L, Lim CW, Sleigh C. Dreaming and electroencephalographic changes during anesthesia maintained with propofol or desflurane. Anesthesiology. 2009;111:547–55. doi: 10.1097/ALN.0b013e3181adf768. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–86. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Barker JL. Different actions of anticonvulsant and anesthetic barbiturates revealed by use of cultured mammalian neurons. Science. 1978;200:775–7. doi: 10.1126/science.205953. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008;28:13488–504. doi: 10.1523/JNEUROSCI.3536-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh R, Warnaby C, Rogers R, Jbabdi S, Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5:208ra148. doi: 10.1126/scitranslmed.3006007. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Pentobarbital: differential postsynaptic actions on sympathetic ganglion cells. Science. 1978;199:451–2. doi: 10.1126/science.202032. [DOI] [PubMed] [Google Scholar]
- Pavone KJ, Akeju O, Sampson AL, Ling K, Purdon PL, Brown EN. Nitrous oxide-induced slow and delta oscillations. Clin Neurophysiol. 2016;127:556–64. doi: 10.1016/j.clinph.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral Analysis for Physical Applications. Cambridge, United Kingdom: Cambridge University Press; 1993. Multitaper Spectral Estimation. [Google Scholar]
- Purdon PL, Pavone KJ, Akeju O, Smith AC, Sampson AL, Lee J, et al. The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015a;115(Suppl 1):i46–i57. doi: 10.1093/bja/aev213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–51. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology. 2015b;123:937–60. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Sakai T, Singh H, Mi WD, Kudo T, Matsuki A. The effect of ketamine on clinical endpoints of hypnosis and EEG variables during propofol infusion. Acta Anaesthesiol Scand. 1999;43:212–6. doi: 10.1034/j.1399-6576.1999.430216.x. [DOI] [PubMed] [Google Scholar]
- Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4:91–3. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13:3266–83. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13:3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp GG, Siegel M, Hipp JF, Engel AK. Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Curr Biol. 2011;21:1988–93. doi: 10.1016/j.cub.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Vijayan S, Ching S, Purdon PL, Brown EN, Kopell NJ. Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. J Neurosci. 2013;33:11070–5. doi: 10.1523/JNEUROSCI.5670-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]