Introduction
Atrial Fibrillation (AF) is the most frequently observed arrhythmia in the clinic, affects ~2-to-6 million people in the US, and is known to increase the risk of stroke, heart failure and sudden death.1 Epidemiological studies have identified multiple factors that enhance risk for susceptibility to AF, including age, coronary artery disease, heart failure, hypertension, and more recently obesity.1 The increase in the incidence of obesity is at alarming proportions; more than a third of the adult population in the US is classified as obese, an observation that is expected to impact on AF incidence.2 Several mechanisms have been proposed to underlie the observed association between obesity and AF, including increased left atrial dimensions, impaired diastolic function, and inflammatory processes, as well as enhanced fibrosis and fatty infiltration of the myocardium and associated co-morbidities such as obstructive sleep apnea. All such events lead to electrophysiological and structural remodeling of the atrium and predispose to AF induction and maintenance.3 In this brief viewpoint article, we will focus on the emerging evidence showing the importance of adipose tissue in increasing the risk of AF in obesity,3 and outline possible molecular/ionic mechanism(s) involved.
Myocardial adipose tissue and atrial fibrillation
The fat in the atria is located either (i) on the outside of the visceral pericardium and on the external surface of the parietal pericardium (pericardial fat), or (ii) between the myocardium and the visceral pericardium (epicardial fat).4 The embryonic origin of these fat depots and their composition is different in nature. Epicardial fat in particular has been the focus of adipose tissue studies in the atrium because of its contiguity with the myocardium and a shared vasculature (important for paracrine effects), and because it is a better predictor of metabolic syndrome.4The epicardial adipose tissue (EAT) is thought to influence cardiac metabolism by the release and uptake of free fatty acids (FFAs), and also provide thermal protection to the myocardium against a drop in temperature during ischemia.4 Clinical studies have reported the association between atrial fat depots and AF using transthoracic echocardiogram, as well as imaging techniques such computer tomography (CT) and magnetic resonance imaging (MRI).5, 6 Unfortunately, it has not always been possible to distinguish clearly between epicardial and pericardial fat, with the result that these two have been lumped together, often as pericardial fat.3 Analyses of data obtained from patients in the Framingham study showed that pericardial adipose tissue volumes assessed by CT were associated with the prevalence of AF, whereas others reported that left atrial epicardial fat thickness measured by CT was associated with AF, and that this fat was also thicker in patients with persistent compared to paroxysmal AF.3 Al Chekakie et al reported greater pericardial fat volumes (using CT) in AF patients versus those in sinus rhythm (and also persistent AF > paroxysmal AF).5 Wong et al reported (using MRI) that not only was pericardial fat associated with AF as in earlier studies, but that it was also able to predict AF recurrence after catheter ablation.6
Epicardial Adipose Tissue and atrial remodeling
A number of studies have provided evidence linking EAT to structural and electrical remodeling of the myocardium, which have major implications on wave propagation. These studies for example have shown extensive atrial electrical remodeling, including changes in effective refractory period (ERP, usually shortened), as well as alterations in impulse propagation (slowing), both of which allow for high frequency rotors to sustain AF in both patients and animal models.1 One way to quantify rotors is to examine their rate of activation in the frequency domain, and characterize the dominant frequency (DF).1 Nagashima and colleagues investigated the correlation between EAT and AF electrical signals in patients with paroxysmal and persistent AF, and found that high DF sites were located adjacent to EAT sites in the atrium; in contrast, there was poor correlation between complex fractionated electrograms (CFAEs) and EAT locations.7 The authors speculated that EAT was involved in the establishment of high DF sites, either through locally released cytokines leading to inflammation, or by modulating the function of ganglionated plexi in the fat depot, both ultimately affecting the atrial electrical properties.7
There is increasing interest to determine the precise origin of the (fibro) fatty infiltrates in the myocardium. In healthy lean individuals, EAT is approximately 1% of the mass of the organ, a percentage that dramatically increases with obesity.4 An increase in EAT is expected to result in more extensive fatty infiltration in the myocardium. Moreover, presence of the ubiquitous mesenchymal stromal cells (MSCs), with a potential to undergo adipogenesis also requires consideration as MSCs have been reported to play a role in arrhythmogenic cardiomyopathy. The possibility exists, for example, that oxidative stress attendant to rapid electrical pacing or to excess adiposity may enhance adipogenesis in such cells, thereby also contributing to myocardial adiposis. Recent studies have started to explore the mechanisms of electrical remodeling due to EAT. In a sheep model of obesity obtained by high calorie diet feeding, it was found that along with an increased epicardial fat infiltration in the left atrium (LA), the conduction velocity was reduced, conduction heterogeneity was increased and voltage heterogeneity was increased, but there was no change in the ERP compared to controls.8 However, the underlying ionic mechanisms were not investigated. As EAT is a source of FFAs, O’Connell and colleagues investigated their effects on the sheep atrial electrophysiological properties.9 The results indicated that when sheep left atrial myocytes were incubated in stearic acid (SA) for 24 hours, the action potential duration (APD) was shortened, and was attributable to reduced densities of both the L-type Ca2+ current, and the Ca2+-independent transient outward K+ current, Ito.9 Further, SA was shown to disrupt the t-tubule structure.9 Those findings recapitulated in part the phenotype of atrial cells isolated from persistent AF patients,1 although the precise relationship between how EAT secretes these FFAs in obesity, and how that can lead to chronicity of AF remains unclear.
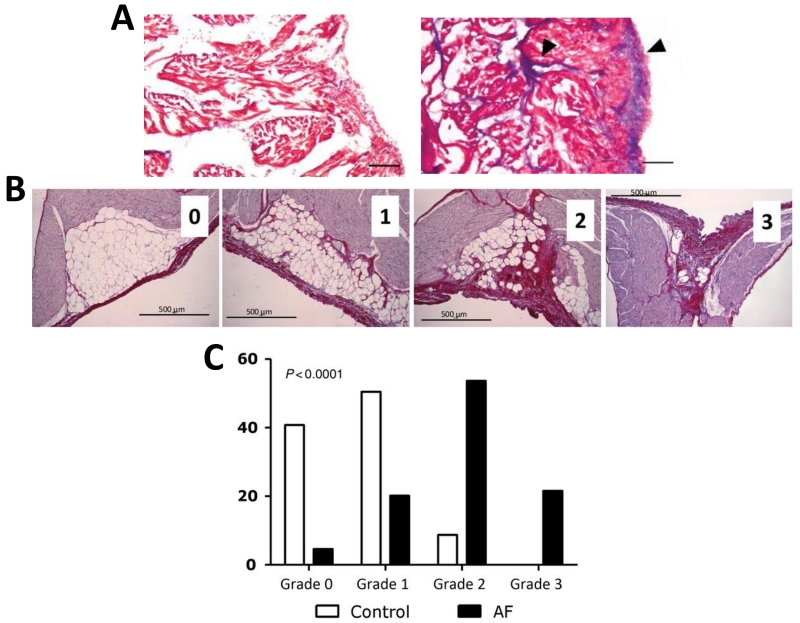
The sheep experimental model of obesity also showed an increase in interstitial fibrosis and enhanced levels of pro-fibrotic TGF-β expression, along with infiltration of the LA by EAT.8 These structural modifications could have directly contributed to the reduced conduction velocities in the LA of obese sheep by creating barriers for impulse propagation. Moreover, recent studies have now actively begun to explore the link between EAT and fibrosis. Venteclef et al obtained samples of EAT from patients undergoing coronary bypass surgery, and exposed an organo-culture model of adult rat atria to a conditioned media of EAT-derived adipose-tissue maintained in culture.10 The results indicated that when exposed to EAT (but not sub-cutaneous adipose tissue) conditioned media, enhanced fibrosis was seen in the rat atrial organo-culture model (Fig. 1A).10 Fibrosis induction was blocked when the secretion of adipo-fibrokine, Activin A (a member of the TGF-β superfamily), was blocked with neutralizing antibodies.10 Human EAT releases cytokines such matrix metalloproteinases (MMP 8), which may also contribute to extracellular matrix remodeling in the atrium.10 However, the possibility of other adipokines such as omentin being involved cannot be ruled out, since a recent genomic analyses found that 2728 genes are differentially upregulated in EAT versus sub-cutaneous fat, and many of these are related to extracellular matrix remodeling and inflammation.11 The properties of the EAT also appear to be non-static, i.e. they change in response to rapid atrial pacing and AF. In a pig model of rapid atrial tachypacing, using microarrays and qt-PCR, it was found that atrial adipocyte/adipositas-related gene expression was substantially altered; however, how this could lead to either fat infiltration and fibrosis remained unclear.12 Further evidence of a close relationship between fat and fibrosis was obtained in a recent study that obtained atrial samples from patients who underwent cardiac surgery, and from a sheep model of atrial tachypacing that induced persistent AF.13 The study showed an inverse correlation between fibrotic remodeling and amount of Sub-epicardial adipose tissue, suggesting conversion of adipose tissue to fibrotic tissue. A similar fibro-fatty infiltration was seen in the sheep left atrium of persistent AF model. The sub-epicardial fat infiltrations were digitized, and classified into 4 different grades, which are shown in Fig 1B and quantified in Fig. 1C.13 Compared to healthy controls, AF caused a shift to higher grades of infiltration. It was suggested that an increase in cytotoxic T lymphocytes and adipocyte cell death might be involved in the observed fibro-fatty accumulation.13 Thus these emerging data point toward a close relationship between EAT and the formation of fibrotic tissue, which may accelerate progression to persistent AF.
Figure 1. Effect of EAT on atrial remodeling.
(A) Masson’ trichrome staining of fibrosis in rat atria after exposure to control media (left) or EAT conditioned media (10 × magnification, scale bar = 100 μm,, arrows point to fibrotic area). (B) Scoring of subepicardial infiltration in sheep LA.13 Grade 0 indicated limited fibrosis without infiltration and large adipocytes; Grade 1 indicated increased fibrosis and no or limited infiltration; Grade 2 indicated increased fibrosis with infiltration and small adipocytes, and Grade 3 indicated extensive scarring and limited or no adipocytes. (C) Distribution pattern of fibro-fatty infiltration in AF sheep. Modified from Venteclef et al,10 by permission.
Molecular/Ionic mechanisms
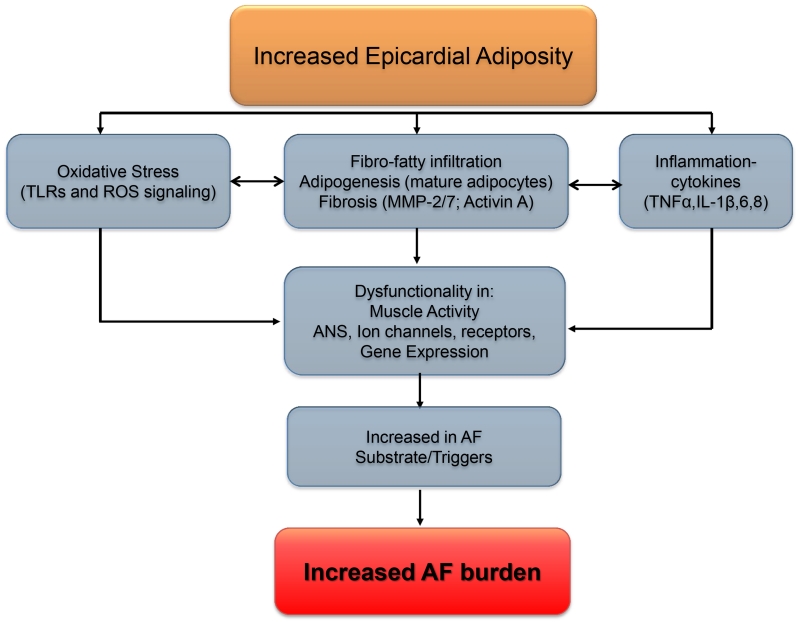
The molecular mechanisms underlying EAT-induced electrical and structural remodeling via paracrine signaling mechanisms are ill understood. There is increasing evidence implicating reactive oxygen species (ROS) and Toll-like receptors (TLRs) (Fig. 2). ROS are known to be upregulated in AF,1 and may represent a possible link between cytokines/adipokines secreted by EAT and electro-structural remodeling. ROS is known to affect the electrical properties of the atria by modulating ion channel properties either directly or indirectly,1 and may also influence fibroblast proliferation.1 A recent study using human atrial tissue reported that EAT secreted adipokine influenced myocardial NADPH oxidase activity via endocrine or paracrine effects.14 Further, this modulation was mutual, in that it was found that oxidation products (such as 4-hydroxynoneal) also regulated the expression of adiponectin in EAT.14 However the role of other sources of ROS that are important in AF perpetuation e.g., from mitochondria, and how these are related to adipokine production remains to be elucidated. It is now well understood that membrane ion channels, as well as intracellular Ca2+ homeostasis are remodeled in both paroxysmal and persistent AF, and govern both the electrical properties atrial myocytes and fibroblasts, and also cell proliferation in the latter.1Similarly, there is evidence pointing to the presence of ion channels in the adipocytes as well. For example, voltage-gated K+ channels have been identified in isolated cultured brown fat cells from neonatal rats, and more recently the transient receptor potential vanilloid 3 channel, TRPV3, has been shown to suppress adipocyte differentiation.15 However the expression of such ion channels in EAT from the atrium, their similarity to ion channels found in atrial myocytes and fibroblasts, and finally their function and role in influencing atrial remodeling and susceptibility to AF remains unexplored.
Figure 2.
Schematic of mechanisms underlying increased AF risk in obesity.
Finally, from a variety of studies including observational, autopsy, in vivo and in vitro, it is abundantly clear that excess of EAT is associated with arrhythmogenesis. However, a number of challenges preclude an adequate understanding of the precise mechanisms by which EAT enhances arrhythmogenicity. One significant challenge is the availability of imaging modalities and development of signal processing techniques capable of adequately resolving details of EAT infiltrations in the myocardium.3 Data from the quantification of EAT will need to be correlated with optical mapping data on ex vivo intact hearts in order to fully appreciate the role fatty infiltrations in wave dynamics. Moreover, a comparative biochemical analysis, including proteomics, of myocardial adipose tissue biofactors from normal weight and obese individuals must be conducted to understand underlying signaling mechanisms, as well as post-translational modifications, such as via O-GlcNAcylation. An overall schema of mechanisms underlying increased AF propensity in obesity is shown in Figure 2.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the NHLBI: R01HL122352-02 (J.J.) and R01HL124319 (JA), and by a grant from the UMHS-PUHSC Joint Institute (J.J.)
REFERENCES
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, Members AATF 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: Executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatem SN, Redheuil A, Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: The perils of adiposity. Cardiovasc Res. 2016;109:502–509. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 5.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 6.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, Wittert GA, Abhayaratna WP, Worthley SG, Sanders P. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–1751. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune M, Mano H, Sonoda K, Hiro T, Nikaido M, Hirayama A. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol. 2012;5:676–683. doi: 10.1161/CIRCEP.112.971200. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell RP, Musa H, Gomez MS, Avula UM, Herron TJ, Kalifa J, Anumonwo JM. Free fatty acid effects on the atrial myocardium: Membrane ionic currents are remodeled by the disruption of t-tubular architecture. PLoS One. 2015;10:e0133052. doi: 10.1371/journal.pone.0133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European heart journal. 2015;36:795–805a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 11.Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clement K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res. 2015;108:62–73. doi: 10.1093/cvr/cvv208. [DOI] [PubMed] [Google Scholar]
- 12.Chilukoti RK, Giese A, Malenke W, Homuth G, Bukowska A, Goette A, Felix SB, Kanaan J, Wollert HG, Evert K, Verheule S, Jais P, Hatem SN, Lendeckel U, Wolke C. Atrial fibrillation and rapid acute pacing regulate adipocyte/adipositas-related gene expression in the atria. International journal of cardiology. 2015;187:604–613. doi: 10.1016/j.ijcard.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, Claus P, LePrince P, Nicoletti A, Jalife J, Wolke C, Lendeckel U, Jais P, Willems R, Hatem SN. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2015 Nov 26; doi: 10.1093/eurheartj/ehv625. pii: ehv625. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah HM, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM, Antoniades C. Mutual regulation of epicardial adipose tissue and myocardial redox state by ppar-gamma/adiponectin signaling. Circ Res. 2016;118:842–855. doi: 10.1161/CIRCRESAHA.115.307856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung SY, Huang Y, Kwan HY, Chung HY, Yao X. Activation of transient receptor potential vanilloid 3 channel suppresses adipogenesis. Endocrinology. 2015;156:2074–2086. doi: 10.1210/en.2014-1831. [DOI] [PubMed] [Google Scholar]