Abstract
The aging population is increasing in developed countries. Since the incidence of cardiac disease increases dramatically with age, it is important to understand the molecular mechanisms through which the heart becomes either more or less susceptible to stress. Cardiac aging is characterized by the presence of hypertrophy, fibrosis, and accumulation of misfolded proteins and dysfunctional mitochondria. Macroautophagy (hereafter referred to as “autophagy”) is a lysosome-dependent bulk degradation mechanism that is essential for intracellular protein and organelle quality control. Autophagy and autophagic flux are generally decreased in aging hearts, and murine autophagy loss-of-function models develop exacerbated cardiac dysfunction that is accompanied by accumulation of misfolded proteins and dysfunctional organelles. On the other hand, stimulation of autophagy generally improves cardiac function in mouse models of protein aggregation by removing accumulated misfolded proteins, dysfunctional mitochondria, and damaged DNA, thereby improving the overall cellular environment and alleviating aging-associated pathology in the heart. Increasing lines of evidence suggest that autophagy is required for many mechanisms that mediate lifespan extension, such as caloric restriction, in various organisms. These results raise the exciting possibility that autophagy may play an important role in combating the adverse effects of aging in the heart. In this review, we discuss the role of autophagy in the heart during aging, how autophagy alleviates age-dependent changes in the heart, and how the level of autophagy in the aging heart can be restored.
Keywords: Aging, autophagy, oxidative stress, mitochondria, mitophagy, protein acetylation, mitochondrial unfolded protein response, NAD+
Subject Terms: Aging, Cell Signaling/Signal Transduction, Myocardial Biology, Metabolism
The incidence of cardiovascular disease progressively increases with age in both men (from 10 per 1000 people at 45–54 years of age to 74 per 1000 people at 85–94 years of age) and women (from 4 to 65 per 1000 for the comparable age groups) 1. Despite recent progress in cardiovascular treatment, the total prevalence of age-related diseases, including cardiovascular diseases (CVD), is still increasing 2. High blood pressure, obesity, and metabolic syndrome increase with age, and these conditions facilitate the development of heart disease, which is a major cause of chronic disability, morbidity and mortality in the elderly 3, 4.
Examination of the cell biology of the aging process has revealed that it is often associated with dysfunctional organelles, which trigger oxidative stress, protein misfolding, and cell death, and induce precipitous declines in the maintenance of cellular quality control mechanisms 5–7. In particular, an important pathological feature of aging is the development of mitochondrial abnormalities, where reactive oxygen species (ROS) progressively accumulate during aging as a result of damage to mitochondrial proteins, an imbalance between oxidative stress and anti-oxidant mechanisms, and increases in electron leakage from dysfunctional electron transport chains 8–12. Accumulation of ROS induces mutations in mitochondrial DNA (mtDNA) and impedes the tricarboxylic acid cycle and electron transport chain complexes, thereby attenuating the bioenergetic function of mitochondria. This causes a vicious cycle that progressively aggravates oxidative stress and mitochondrial dysfunction 10–12. Prevention of these aging-associated catastrophes requires properly functioning quality control mechanisms that allow elimination and well-coordinated replenishment of damaged proteins and organelles. Cardiomyocytes in the adult heart appear to retain the ability to divide in response to stress, but their proliferation is very inefficient. The adult heart is thus greatly reliant on the cellular quality control mechanisms, since damaged proteins and organelles cannot be easily diluted through cell division 13, 14. Autophagy is an evolutionarily conserved mechanism that targets damaged or long-lived proteins and organelles for degradation through lysosomes 15, 16. Autophagy can be either non-selective or cargo-specific, and mitochondrial-specific autophagy is referred to as mitophagy. Accumulating lines of evidence support a crucial role for autophagy and mitophagy in the regulation of cardiac homeostasis at baseline and in response to stress 15, 17, 18, and their downregulation appears to contribute to the aging process 16, 19, 20 in many organs. This review will summarize the current understanding of the role of autophagy in the regulation of aging in the heart. Readers are also referred to recent reviews on similar topics with distinct focuses, such as longevity and metabolism, in order to better understand the broader functions of autophagy 16, 21.
2. Manifestation of aging in the heart
Aging hearts exhibit unique histological and biochemical features 22–25. Increases in apoptosis and necrosis, proliferation of myocyte nuclei, increased myocyte volume, and connective tissue accumulation are frequently observed in the myocardium of old animals 26–28. Senescence-associated ectopic β-galactosidase activity and dense bodies with autofluorescence, consisting of damaged proteins and lipid accumulates, called lipofuscin 29, are also increased. In addition, aging affects the abundance of some molecules, including the tumor suppressors p16INK4 and p19ARF 30, 31. How do these changes affect cardiac function and susceptibility to heart failure in the elderly? Aging cardiomyocytes are less capable of undergoing compensatory hypertrophy and proliferation in response to increased workload 22, 32–35. As a result, aging increases wall stress that is not normalized by ventricular remodeling 36. Furthermore, induction of cell protective mechanisms, such as expression of anti-oxidant and heat shock proteins, in response to pathologic insults is attenuated in aging hearts 33, 37, 38. Optimal therapeutic interventions to alleviate the adverse effects of aging should prevent cell death and accumulation of senescent myocytes 22. For example, A2E, a major component of toxic lipofuscin, upregulates inflammatory cytokines and chemokines and contributes to the inflammatory response in aging tissues, but activation of autophagy appears to protect the tissue by eliminating both toxic aggregates and activation of inflammation 39. As we discuss later, however, autophagy and autophagic flux are generally downregulated in the aging heart.
3. Molecular mechanisms of aging in the heart
Since aging increases the risk of diseases and reduces organ function, elucidation of the mechanisms that serve to counteract the adverse effects of aging has significant clinical implications 40. Evolutionarily conserved defined molecular mechanisms are involved in the regulation of lifespan in animals 40–43, and activation of these mechanisms may affect the aging of individual organs and the cells therein. Aging is a multifunctional process, with many mechanisms contributing to functional decline in organs and tissues. Accumulation of damaged proteins and mitochondria is commonly observed in aged cells. In addition, production of ROS in various organs is progressively enhanced over the years and oxidative stress accumulates during the aging process 10, 12. Likewise, increased ROS and accumulation of damaged proteins and organelles in the heart also occur in the presence of high blood pressure and metabolic abnormality, which facilitate senescence in the heart 44, 45.
ROS progressively accumulate during aging, due to both electron leakage from mitochondria resulting from impaired mitochondrial oxidative phosphorylation and an imbalance between the expression of ROS-producing enzymes and anti-oxidant proteins 10, 46. The accumulated ROS further promote the mtDNA mutations and deletions that accumulate over time, leading to a progressive reduction in mtDNA content 47. ROS can also impair the enzymes involved in oxidative phosphorylation, particularly those containing an iron-sulfur cluster 48. Finally, oxidative stress is associated with mitochondrial permeability transition pore opening, which promotes necrosis and cell death in mammalian cells 49. Mitochondrial dysfunction is a common feature of the aging process 50, and systemic mitochondrial overexpression of catalase extends lifespan and delays the aging process in mice 51. These observations suggest that accumulation of ROS in mitochondria contributes to the aging process in mammals 52.
In addition to electron leakage from the electron transport chain, mitochondrial expression of Nox4, a major intracellular isoform of NADPH oxidase in the heart, is upregulated in the aged heart, contributing to ROS production and the development of cardiac abnormalities in aging mice 53. Nox4 is upregulated by pressure overload through an NF-κB-dependent mechanism 54 but the mechanism by which Nox4 is upregulated in the aging heart is currently unknown. It would be interesting to test whether the increased susceptibility of aging hearts to stress is alleviated in cardiac-specific Nox4 knock-out mice 55. However, it should be noted that endogenous Nox4 also possesses physiological functions, such as induction of angiogenesis 56 and autophagy in response to glucose deprivation 57 in cardiomyocytes. Thus, further investigation is required to fully elucidate the role of ROS in the overall responses of the heart during aging.
Mitochondrial stress triggers a series of events, including damage to proteins 58 and mtDNA 59, activation of mitochondrial mechanisms of degradation of both individual proteins 60 and partial or whole organelles 61, and mitochondrial biogenesis 62, thereby causing a condition in which misfolded proteins accumulate or there is a mismatch between mtDNA-encoded proteins and nuclear-encoded proteins. This condition activates a set of adaptive responses collectively termed the mitochondrial unfolded protein response (UPRmt) 63, including activation of chaperones and other mitochondrial quality control mechanisms such as mitophagy 64. The UPRmt is essential for long-term maintenance of mitochondrial quality and affects many mechanisms known to influence either lifespan or aging 60. Although the UPRmt is evolutionarily conserved and has been studied extensively in Caenorhabditis elegans, the detailed signaling mechanisms found in mammalian cells are poorly understood. Elucidating the molecular mechanisms by which the UPRmt maintains mitochondrial homeostasis and whether the UPRmt acts through autophagy and/or mitophagy in mammalian cardiomyocytes would be interesting and should provide clues for the development of novel interventions to achieve anti-senescence therapy in the heart. It should be noted that endoplasmic reticulum (ER) stress also induces autophagy and a UPR to eliminate misfolded proteins and defective organelles 65. However, the connection between ER stress and aging is, to date, less well defined than that between the UPRmt and aging.
Increases in oxidative stress induce DNA damage, which, in turn, either induces a series of DNA repair mechanisms or activates either cell death or cellular senescence mechanisms. Increases in DNA damage and defective DNA repair have been shown to inhibit autophagy 66, 67. Somatic mtDNA mutation inhibits autophagy, thereby allowing mutated mtDNA to escape degradation and promote mitochondrial dysfunction. When DNA damage induces senescence, it is accompanied by a unique phenotype, called the “senescence-associated secretory phenotype” (SASP) 68, that mediates chronic inflammation, thereby further promoting DNA damage and senescence. DNA damage-induced cellular senescence stabilizes GATA4, thereby inducing SASP through activation of NF-κB. Interestingly, p62-mediated selective autophagy degrades GATA4, whereas GATA4 is stabilized in human fetal lung fibroblasts undergoing senescence due to suppression of autophagy 69. These observations suggest that autophagy may inhibit the adverse effects of aging by negatively regulating SASP and aging-associated inflammation.
Telomeres are regions of repetitive nucleotide sequences located at the ends of chromosomes that protect the chromosomal end structures from damage. Each time cells divide, the telomere ends become shorter. Since telomere shortening causes senescence through activation of the p53 and Rb pathways, the molecular mechanism controlling telomere length is intimately connected to aging 70. Although cardiomyocytes in the adult heart rarely proliferate, telomere damage is also caused by oxidative stress, which, in turn, activates signaling mechanisms leading to senescence 70. Importantly, the health of the heart is dependent upon basal turnover of cardiomyocytes generated from cardiac progenitor cells. Telomere attrition and DNA damage induce senescence in cardiac progenitors, resulting in a loss of regenerative capacity and promotion of cardiac senescence 71. It has recently been shown that quiescent satellite cells in skeletal muscles are capable of maintaining their stemness by activating autophagy 72. However, autophagy is attenuated in aged satellite cells, leading to stimulation of senescence through accumulation of oxidative stress and damaged proteins and organelles 72.
Another important mechanism regulating senescence is protein acetylation, which is regulated by a balance between protein acetylases and deacetylases. Spermidine, an acetyltransferase inhibitor, prolongs the lifespan of yeast, flies, and nematodes in an autophagy-dependent fashion, by inhibiting protein acetylation of cytoplasmic and nuclear proteins 73. In addition, the sirtuin family of protein deacetylases is particularly relevant in the regulation of lifespan and aging. Sirtuins are a family of seven NAD+-dependent protein deacetylases known to be involved throughout the evolutionary tree in lifespan extension in response to caloric restriction (CR) and resveratrol, a naturally occurring polyphenol that stimulates Sirt1. The Sirt1 isoform acts in both the nucleus and the cytoplasm 74, and its expression increases in the aging heart, most likely as a compensatory mechanism 75, 76. Sirt1 can retard senescence and confer resistance to oxidative stress in the heart 75, and promotes autophagy through deacetylation and stimulation of FoxO1 in cardiomyocytes 77. Sirt1 negatively regulates p66shc expression in human umbilical vein endothelial cells 78. p66shc is a multifunctional adapter protein and its deletion prolongs lifespan in mice 79. p66shc positively regulates oxidative stress by stimulating mitochondrial oxidative phosphorylation and downregulating FoxO-mediated protection against oxidative stress 80–82. Sirt3, a mitochondrial sirtuin isoform, can stimulate oxidative phosphorylation by directly deacetylating the electron transport chain complexes 83. Sirt3 also inhibits pathological cardiac hypertrophy by deacetylating cyclophilin D, a protein that regulates mitochondrial permeability transition pore opening and prevents the adverse effects of aging in the heart 84, 85.
Mammalian target of rapamycin (mTOR), a serine threonine kinase that regulates protein synthesis, transcription of mitochondrial proteins and autophagy, has also been implicated in the regulation of lifespan and aging 86. mTOR suppresses autophagy through phosphorylation of Ulk1, primarily through activation of mTOR complex 1 (mTORC1) 87. mTOR has drawn particular attention in the area of aging because the mTOR inhibitor rapamycin has been shown to increase lifespan in mammals 88. A recent report showed that the activity of mTOR does not increase with age in C57BL/6J mice 89. Although these findings suggest that endogenous mTOR may not be directly involved in the aging process, they nonetheless do not exclude the possibility that the beneficial effects of rapamycin treatment on lifespan are mediated through suppression of endogenous mTOR.
A recent study found that serum levels of GDF11, a member of the TGF-β superfamily of cytokines, decrease with age in mice and that restoring the level of GDF11 decreases aging-associated cardiac phenotypes, including cardiac hypertrophy 90. Although the age-dependent decrease in GDF11 and its effect upon aging-induced cardiac hypertrophy could not be reproduced in another report 91, the anti-senescence effect of GDF11 was also observed in skeletal muscles 92. Furthermore, systemic supplementation of GDF11 was shown to increase the LC3-II/LC3-I ratio in skeletal muscle. However, whether GDF11 increases autophagic flux or whether autophagy plays a causative role in mediating the anti-senescence effect of GDF11 was not tested in this study.
Taken together, these studies show that the list of potential mechanisms controlling senescence of the organism as a whole and of individual organs such as the heart is growing. According to the hormesis hypothesis, the accumulation of molecular mechanisms conferring stress resistance may prevent senescence of organisms. Thus, it would be interesting to investigate whether the aforementioned mechanisms controlling senescence protect the heart against stress and inhibit accumulation of damage in the heart. Interestingly, as described below, these mechanisms generally affect autophagy.
4. The role of autophagy during aging of the heart
Damage to proteins, DNA and cellular organelles plays an important role in aging 93. Accumulation of damaged proteins and organelles leads to the age-associated malfunction of many biological processes. Thus, reduced degradation of proteins and organelles may contribute to the aging process 29. In cardiomyocytes, the accumulation of dysfunctional organelles and toxic proteins results in global cardiac dysfunction. Since autophagy plays a crucial role in the degradation of long-lived proteins and organelles, it is an essential mechanism for maintenance of tissue homeostasis in the heart during the aging process 15, 16, 94. Three types of autophagy have been identified: microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy. In microautophagy, cytoplasmic cargo is directly trapped and engulfed through membrane invagination by lysosomes. Although it is believed to be a random process, recent evidence suggests that parts of mitochondria are also degraded through this mechanism 95. CMA, on the other hand, specifically degrades cytosolic proteins with a KFERQ motif, by directly transferring them to lysosomes. In macroautophagy, hereafter referred to as autophagy, small vesicular sacs, called isolation membranes or phagophores, are initially formed. The phagophores enclose cytosolic long-lived proteins and organelles, resulting in the formation of double-membraned structures called autophagosomes. The autophagosomes then fuse with lysosomes, which leads to the degradation of the sequestered cellular contents through digestion of the cargo by lysosomal hydrolases 15, 16, 94. Essentially nothing is known regarding the role of microautophagy and CMA during aging in the heart. Thus, we focus on macroautophagy in the discussion below.
4.1 Autophagy is downregulated during the course of aging
Autophagy is downregulated in the heart during the course of aging 96. The general mechanisms of aging described above may also contribute to this downregulation of autophagy (Figure 1). Seemingly paradoxically, although ROS and misfolded proteins can stimulate autophagy, suppression of autophagy is commonly observed in aging hearts with increased oxidative stress, misfolded proteins, and dysfunctional mitochondria. One possible explanation for this apparent paradox is that continuous activation of autophagy due to higher oxidative stress and protein misfolding may lead to exhaustion of the autophagic machinery, eventually causing suppression of autophagy. A similar phenomenon, termed “autophagy exhaustion”, was reported to have been observed following HIV-1 infection 97. Aging is also characterized by attenuation of stress-induced adaptations, which may result, in part, from the suppression of autophagy. In particular, activation of autophagy is required to potentiate the protective effect of ischemic preconditioning 98, raising the possibility that autophagy exhaustion may contribute to the increased susceptibility of elderly patients to myocardial ischemia. Whether autophagy exhaustion actually takes place in aging hearts remains to be tested.
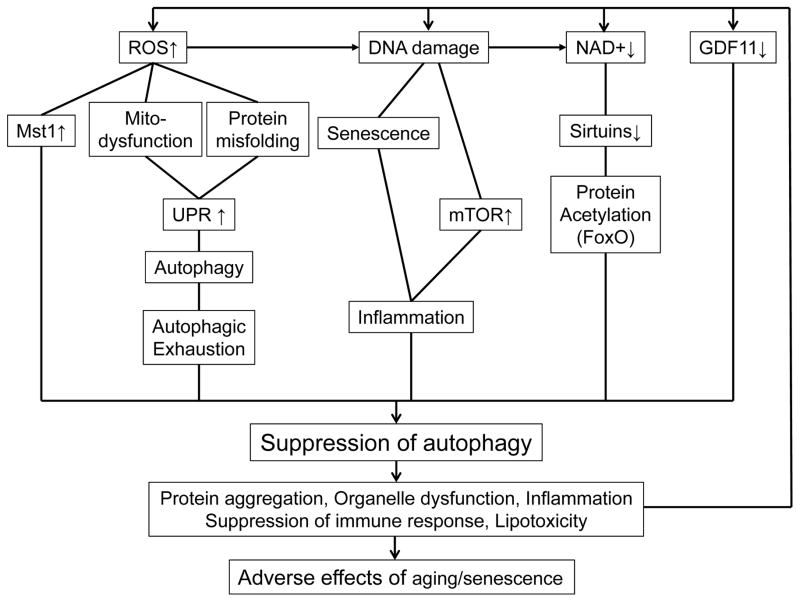
Figure 1. Regulation of cardiac aging by autophagy.
Aging inhibits autophagy in cardiomyocytes through multiple mechanisms. Aging-induced suppression of autophagy induces accumulation of misfolded proteins and dysfunctional organelles, sterile infection caused by undigested mtDNA, inflammation, and lipotoxicity, thereby leading to a metabolically unhealthier environment, precipitous mitochondrial dysfunction and eventual cell death.
Activation of major suppressors of autophagy, such as Mst1 99 and mTOR 100, or suppression of major activators of autophagy, such as Sirt1 77, may also cause downregulation of autophagy in the heart. Further experimentation is required to test these hypotheses.
In addition, a variety of other mechanisms have been shown to contribute to the age-dependent downregulation of autophagy. The genes involved in general and mitochondria-specific autophagy are regulated by transcription factors, including FoxO, HIF-1, p53, E2F1, NF-κB, KLF4, TFEB, and ZKSCAN3 77,101,102. The transcriptional activity of FoxO is negatively regulated by Akt-mediated phosphorylation and by lysine acetylation resulting from decreases in NAD+ and consequent inactivation of Sirt1 in the heart. This, in turn, may contribute to decreased expression of autophagy-related genes in the aged heart. Aging has also been shown to upregulate miR-216a, which, in turn, downregulates Beclin1, thereby inhibiting ox-LDL-induced stimulation of autophagy in endothelial cells 103. In contrast, atrogin-1, an E3 uibiquitin ligase known to be activated in response to muscle atrophy, induces autophagy through degradation of charged multivesicular body protein 2B, a component of the endosomal sorting protein complex that is essential for autophagy 104. However, although the activity of the ubiquitin proteasome system (UPS) declines with aging, whether atrogin-1 is downregulated in aging hearts is unknown. Finally, saturated and unsaturated fatty acids induce metabolomic profiles that differentially affect aging through activation of distinct forms of autophagy in the liver: the former activates PIK3C3- and Beclin1-dependent autophagy by depleting autophagy-inhibitory amino acids, whereas the latter activates PIK3C3- and Beclin1-independent autophagy by increasing the level of NAD+. Whereas the effects of saturated fatty acids on health are detrimental, those of unsaturated fatty acids are beneficial 105. However, how the metabolic perturbation observed in age-associated heart disease affects metabolomic profiles and autophagy remains to be elucidated.
4.2 Autophagy is essential for preventing aging in the heart
Increasing lines of evidence suggest that autophagy is intimately involved in the regulation of lifespan and aging (Reviewed in 21). Loss-of-function mutations in Atg1, Atg7, Atg18 and Beclin1, key autophagy genes, decrease the lifespan of the nematode C. elegans 106. Similarly, in a genetic screen of budding yeast, several short-lived mutants had autophagy defects, including 10 ATG mutations found among a total of 117 short-lived mutants 107. Deficient expression of Atg1 facilitates accumulation of ROS and muscle degeneration, mimicking the aging phenotype, in the fruit fly Drosophila melanogaster 108. Mutations in Atg8 also induce accumulation of insoluble proteins, increase sensitivity to ROS and shorten lifespan in Drosophila, whereas increased expression of Atg8 in the nervous system of Drosophila extends its lifespan 109. Autophagy-related genes have also been shown to promote survival in worms and flies exposed to prolonged starvation 110, by alleviating age-associated pathologies, including mitochondrial and cardiac dysfunction 108.
In mammals, tissue-specific knockout of ATG genes induces multiple age-associated symptoms, including accumulation of intracellular inclusion bodies containing ubiquitinylated proteins, accumulation of lysosomes containing lipofuscin, disorganized mitochondria, and protein oxidation 111–115. In contrast, adenovirus-directed overexpression of Atg7 that corrected a hepatic autophagic defect was shown to diminish ER stress and counteract insulin resistance 115. Stimulation of autophagy reduces oncogenesis, maintains neuronal function 116, improves immune responses 117, reduces inflammation 118, and improves lipid mobilization 119 in the organism as a whole, thereby improving overall fitness during aging 16, 21. However, more studies may be needed to clarify whether the lifespan of mice can be prolonged by selectively correcting aging-associated defects in autophagy.
Autophagy may be especially important in non-proliferating cells since there is no dilution of toxic materials accumulated during aging through cell division. In terms of cardiac aging, mice in which the Atg5 protein is cardiac-specifically deleted through constitutive αMHC-Cre expression develop dilated cardiomyopathy with severe systolic dysfunction during aging, accompanied by sarcomeric disarray and accumulation of dysfunctional and abnormal mitochondria 96. Cardiac-specific deletion of GSK-3α also promoted the development of cardiac aging, and this was accompanied by suppression of autophagy 120. One cautionary note is that the use of αMHC-Cre, originally generated by Dr. Michael Schneider’s laboratory for use in aging studies, may potentially be problematic since cardiac-specific Cre alone induces significant time-dependent cardiac dysfunction 121. Nevertheless, these results strongly suggest that autophagy is required for cardiac homeostasis during aging and that downregulation of autophagy contributes to cardiac pathology during aging. Indeed, a genome-wide association study of aging identified a SNP near the Atg4c gene as being associated with a higher risk of death, suggesting that autophagy may be intimately involved in the risk of heart disease in elderly patients 122. Furthermore, autophagy also alleviates accumulation of advanced glycation end products (AGEs) 123 and preamyloid oligomers 124. However, determining whether or not downregulation of autophagy mechanistically contributes to the development of cardiac aging awaits further experimentation to test whether restoring the level of autophagy rescues the aging phenotype.
The lysosome-associated membrane protein 2a (LAMP2a), a protein required for CMA, is also downregulated during the aging process in the mouse liver 125. Restoration of LAMP2a prevents aging-associated defects in CMA, and decreases the abundance of oxidized proteins, polyubiquitinated protein aggregates, and apoptotic cells in the liver 125. The role of CMA in cardiac aging remains to be elucidated.
5. The role of mitophagy
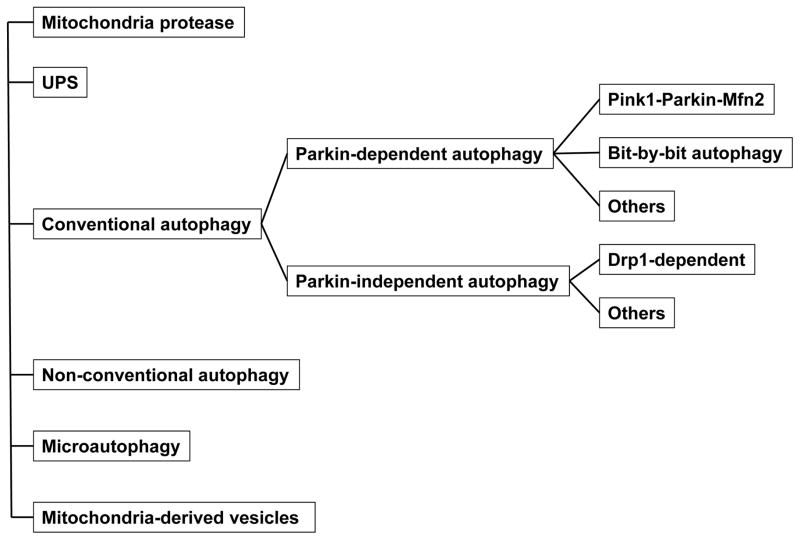
Damaged mitochondria can be eliminated through a specialized form of autophagy termed “mitophagy” or “mitochondrial autophagy”. Autophagosomes containing only mitochondria have been observed in electron microscopic analyses of the hearts of adult mice, providing evidence for the existence of mitophagy in the heart 126. The molecular mechanisms mediating mitochondrial autophagy and its functional significance in the heart have been reviewed recently 127. In perhaps the most well-characterized mechanism of mitochondrial autophagy, depolarized mitochondria are marked by a PINK1-Parkin-Mfn2-dependent mechanism and engulfed by autophagosomes through an LC3-receptor-dependent mechanism 128. A recent report showed that PINK1-induced phosphorylation of ubiquitin recruits LC3 receptor proteins, including NDP52 and optineurin, which in turn recruit the autophagy factors, including Ulk1, DFCP1 and WIPI1, to mitochondria 129. However, more investigation is needed to fully elucidate how depolarized mitochondria are marked and recognized by LC3 and the involvement of Parkin-Mfn2 in this process. On the other hand, increasing lines of evidence suggest that mitochondrial autophagy can take place in a Parkin-independent manner 130 or even in the absence of Atg5/7 131, suggesting that there are multiple mechanisms by which mitochondria may be degraded, perhaps in a stimulus-dependent fashion. In addition, mitochondria can be degraded by microautophagy and mitochondria-derived vesicles 61. Given that mitochondrial dysfunction can develop with aging and that mitochondria are the major source of ROS in aging hearts, it is critically important to understand how mitochondrial autophagy is regulated in aging hearts (Figure 2).
Figure 2. Mechanisms of mitochondrial autophagy in the heart.
Molecular mechanisms mediating mitochondrial degradation are summarized. The contribution of each mechanism to the regulation of cardiac senescence at baseline and under stress remains to be elucidated.
It should be noted that there have been no studies quantitatively evaluating the changes in mitophagy during cardiac aging thus far. Since mitochondrial dysfunction is a common feature of cardiac aging, in theory, more mitophagy and mitochondrial autophagy should be observed, at least in the early phase of aging. In C. elegans, age-associated stress such as oxidative stress stimulates both mitochondrial biogenesis and mitophagy through SKN-1, a homolog of Nrf2, thereby coordinating mitochondrial turnover 132. As noted above, thus far, most studies of mitophagy in the heart have focused on PINK1-Parkin-mediated mitophagy. One study showed that, although young Parkin knockout (KO) mice exhibit a normal cardiac phenotype, abnormal mitochondria accumulate in cardiomyocytes with age in these mice 133. This suggests that endogenous Parkin may mediate mitophagy in aging hearts. It is unclear, however, how Parkin expression is regulated during aging. Furthermore, considering the fact that Parkin has multiple functions besides mitophagy 134, whether or not downregulation of Parkin directly promotes accumulation of abnormal mitochondria through a defect in mitophagy remains to be tested. Damaged mitochondria can be eliminated by multiple mechanisms, including mitochondrial proteases, ubiquitin proteasome-dependent mechanisms, Parkin-independent macroautophagy, a non-conventional form of autophagy, microautophagy and mitochondria-derived vesicles (reviewed in 61). Currently, how these mechanisms are affected by cardiac aging is unknown. In erythroid cells in which mtDNA mutation is stimulated, mitochondrial autophagy is downregulated by aging 67. Accumulation of damaged mtDNA activates mTOR, which inhibits autophagy, thereby preventing elimination of mitochondria containing damaged mtDNA and initiating a feed-forward mechanism that causes rapid accumulation of dysfunctional mitochondria and cell death. It will be interesting to test whether similar mechanisms exist in aging hearts.
Mito-Timer is a time-sensitive fluorescent protein targeted to the mitochondrial matrix that can be used to evaluate mitochondrial age by quantifying the proportionate integration of young (green) and old (red) Mito-Timer protein 135. Transgenic mice with cardiac-specific expression of Mito-Timer have recently been developed 136. These mice should be useful for evaluating age-dependent changes in mitochondrial turnover. Age-associated deterioration in the mitochondrial quality control mechanisms, including decreases in mitophagy, may be indicated by a slower mitochondrial turnover rate in this animal model.
6. Relevant intracellular signaling mechanisms controlling autophagy during aging
6.1 Sirtuins
Yeast silent information regulator 2 (Sir2) is an evolutionarily conserved molecule that mediates lifespan extension in yeast and Drosophila in response to CR137–139. Sir2 is an NAD+-dependent protein deacetylase, and functions in a wide array of cellular processes, including gene silencing, rDNA recombination, lifespan extension, and DNA damage repair (reviewed in 140, 141). Overexpression of Sir2 increases the lifespan of many organisms, including yeast, C. elegans and Drosophila 138, 142. Recently, the concept that lifespan extension in terminally differentiated cells might depend upon mechanisms similar to those that regulate chronological lifespan in yeast has been challenged 143. Nonetheless, we have shown that Sirt1, a mammalian ortholog of Sir2, is able to retard aging of the heart, an organ whose major component is terminally differentiated cardiomyocytes 140. Sirt1 deacetylates p53 and the FoxO family transcription factors, thereby inhibiting apoptotic cell death in mice and humans 144–149. Mice deficient in Sirt1exhibit developmental abnormality in the heart and only infrequently survive postnatally 150. Sirt1 is upregulated by CR, and regulates fat metabolism by inhibiting fat cell differentiation and fat accumulation through regulation of PPAR-γ151. Three- to six-fold overexpression of Sirt1 attenuates aging-induced cardiac pathology, including hypertrophy, fibrosis, apoptosis, and upregulation of senescence markers, and protects the heart against oxidative stress 75. Furthermore, endogenous Sirt1 protects the heart against ischemia/reperfusion injury, in part through deacetylation of FoxO1 and consequent upregulation of anti-oxidants 152. Sirt1 also mediates fasting-induced deacetylation of FoxO1 in the heart, thereby stimulating autophagosome formation and autophagosome-lysosome fusion through transcriptional upregulation of Rab7 in cardiomyocytes 77. Sirt3 mediates the effect of CR upon age-related hearing loss and oxidative stress in mice 153, 154. Given that their functions favor longevity and cell survival, and that they are coupled with the metabolic state of cells and with autophagy, both Sirt1 and Sirt3 are attractive candidates for critically regulating cell survival in cardiomyocytes in response to stresses such as energy starvation.
6.2 NAD+ and protein acetylation
NAD+ is an electron acceptor in the mitochondrial electron transport chain and also acts as an essential substrate for NAD+-dependent enzymes, including sirtuins and PARP, a DNA repair enzyme. Cellular levels of NAD+ are regulated by the balance between synthesis through the de novo and salvage pathways and consumption through sirtuins and PARP. NAD+ in turn regulates the level of protein acetylation through regulation of sirtuins 155. We have shown previously that nicotinamide phosphoribosyl transferase (Nampt), a key enzyme in the salvage pathway of NAD+ synthesis in cardiomyocytes, is downregulated in the heart in response to prolonged ischemia, which leads to decreases in the level of NAD+ in the heart, inhibition of autophagic flux, and consequent increases in cell death 156. However, restoration of the NAD+ level by overexpressing Nampt restores the level of autophagy during prolonged ischemia and reduces the extent of myocardial infarction 156. Autophagic flux is negatively affected by decreases in NAD+ during ischemia due to resultant decreases in lysosomal acidification. We hypothesize that maintaining the NAD+ level is essential for maintaining the activity of the H+ pump on the lysosomal membrane 157. Decreases in NAD+ also increase protein acetylation through suppression of sirtuins, which in turn suppresses autophagy.
The level of NAD+ decreases with age in many organs, due to downregulation of Nampt, defective circadian rhythm, oxidative stress, and accumulation of DNA damage 155. Although the level of Nampt in the mouse heart is upregulated at 1 year of age, whether or not it is downregulated thereafter has not been shown. We speculate that, as in the case of prolonged ischemia, aging-induced decreases in NAD+ may induce lysosomal dysfunction, which in turn contributes to age-dependent increases in the susceptibility of the heart to ischemic injury. In the rate-limiting step of the salvage pathway of NAD+ synthesis, Nampt produces nicotinamide mononucleotide (NMN), which is then converted to NAD+. Exogenous supplementation of NMN increases NAD+ content in cardiomyocytes, stimulating Sirt1 and inducing deacetylation of cellular proteins, including FoxO, in the heart, which, in turn, protects the heart from ischemia/reperfusion injury 158. This suggests that supplementation of NAD+ via its precursors, including NMN and nicotinamide riboside, may allow suppression of cardiac aging through activation of sirtuins, including Sirt1 and Sirt3, increases in protein deacetylation in the nucleus and mitochondria, and activation of autophagy.
6.3 IGF-I signaling
In lower organisms, inhibition of insulin-like growth factor (IGF-I) signaling, such as by Daf2 mutation in C. elegans, causes lifespan extension 159. This is mediated by a loss of suppression of Daf16 or FoxO transcription factors, which regulate expression of anti-oxidants (MnSOD) and DNA damage repair enzymes (GADD45) 160–163. In mammals, Ames and Snell dwarf mice lacking growth hormone/IGF-I signaling and IGF-I receptor heterozygous KO mice have longer lifespans 164, 165. Systemic overexpression of klotho, a hormone known to inhibit insulin/IGF-I signaling also extends lifespan in mice 166. It should be noted, however, that whether or not inhibiting IGF-I signaling (and thus stimulating FoxOs) positively affects senescence and aging-related diseases in mammals without affecting normal function is not fully understood 167. IGF-I may only produce a trade-off between current benefits to reproduction and later costs in senescence, like the relationship between inotropic agents and exacerbation of heart failure 168. Interestingly, activation of Akt weakens cardiac adaptation to stress 169 and exacerbates the aging phenotype in the heart, effects which are accompanied by suppression of autophagy 170. However, the specific role of Akt in cardiac aging and how autophagy affects this process are not yet known. Considering the apparent cell survival-promoting effects of the IGF-I-Akt axis, this issue appears to represent a paradox in longevity research, and will require clarification in each organ 165.
6.4 GSK-3, AMPK and mTOR
mTOR inhibits autophagy through phosphorylation of Ulk1, a mammalian ortholog of Atg1, at Ser 757 87. Conversely, suppression of mTORC1 by rapamycin stimulates autophagy in cardiomyocytes 100, 171. Although inhibition of mTORC1 by rapamycin has been shown to attenuate the adverse effects of aging in the heart 172, whether the effect of mTORC1 suppression is mediated through stimulation of autophagy awaits further investigation using more specific interventions to control autophagy, since mTORC1 has a wide variety of cellular functions besides suppression of autophagy 86. The activity of mTOR is regulated by multiple mechanisms 86. However, negative regulation by GSK-3β and AMPK are particularly relevant with regards to aging and autophagy.
We have shown that GSK-3β stimulates autophagy through suppression of mTOR in cardiomyocytes 173. In addition, cardiac-specific GSK-3α KO mice show significantly shorter lifespans and exacerbated cardiac aging due to suppression of autophagy 174. Although it is currently unclear how the activities of GSK-3α and GSK-3β are regulated during the course of aging, they have been shown to be intimately involved in the growth and death of cardiomyocytes, mitochondrial permeability transition pore opening, and regulation of mTOR signaling 175. Thus, further investigation is required to elucidate the roles of GSK-3α and GSK-3β in regulating cardiac aging and autophagy signaling.
Likewise, AMPK stimulates autophagy through suppression of mTORC1 and phosphorylation of Ulk1 87, 176. Metformin, an activator of AMPK, has been shown to increase lifespan in mice 177. In addition, metformin also attenuates aging-induced cardiomyocyte contractile defects 178. It would be interesting to investigate to what extent stimulation of autophagy contributes to the salutary effect of metformin.
6.5 Oxidative stress
Oxidative stress can either activate or inactivate autophagy in cardiomyocytes in a context-dependent manner (reviewed in 179). We have shown previously that oxidative stress during ischemia/reperfusion in the heart stimulates autophagy by upregulating Beclin1 180. In addition, glucose deprivation and hypoxia activate autophagy through upregulation of Nox4 in the ER and consequent activation of PERK-dependent mechanisms 181. Although increases in oxidative stress induce tissue damage, mitochondrial dysfunction and cell death, whether age-dependent increases in oxidative stress suppress autophagy in the heart has not yet been clearly demonstrated.
7. How to stimulate autophagy
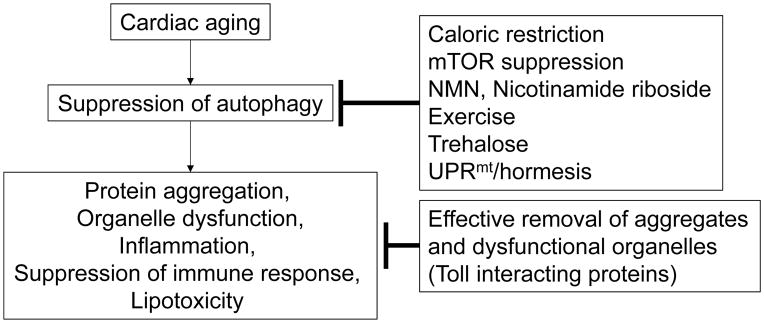
Since autophagy is downregulated with age and downregulation of autophagy promotes senescence of the heart, interventions to increase the level of autophagy may prevent or slow the progression of aging in the heart. Furthermore, considering the fact that cardiac aging is accompanied by accumulation of insoluble polymeric materials, such as lipofuscin, and damaged organelles, it would appear to be advantageous to have a degradation mechanism with a large capacity (Figure 3).
Figure 3. Possible interventions to normalize autophagy during cardiac aging.
Aging inhibits autophagy and mitophagy in cardiomyocytes. Interventions to normalize autophagy and mitophagy and to alleviate protein aggregates may attenuate aging of the heart and increase the resistance of the heart stress.
Interestingly, both CR182,139, 183, 184,185 and suppression of mTOR 88, 186, 187, interventions that alleviate the adverse effects of aging and increase lifespan, promote autophagy in many cell types and organs, even when autophagy is suppressed by aging. Importantly, CR has been shown to reduce age-related pathologies and diseases in animals 188–190. However, whether or not the beneficial effects of these interventions are mediated primarily through activation of autophagy in the heart requires further study.
Voluntary exercise activates autophagy by stimulating phosphorylation of Bcl-2 and its dissociation from Beclin1 191. Exercise leads to a reduction in protein aggregates in the heart, which is accompanied by increases in autophagic flux, but not in the UPS or UPR 192. We speculate that the anti-senescence effect of exercise upon the heart 192 may be in part mediated through activation of autophagy and consequent amelioration of protein aggregate formation.
Toll interacting proteins interact with LC3 and ubiquitin, thereby binding to ubiquitin conjugates more tightly than p62 and effectively clearing highly aggregation-prone proteins such as huntington poly-glutamine protein 193. Thus, an intervention that upregulates them in vivo might be effective in clearing protein aggregates associated with aging, such as preamyloid bodies and lipofuscin. A recent report shows, however, that Toll interacting protein promotes inflammation and apoptosis in the heart after myocardial infarction 194. Thus, further investigation is needed to clarify the function of this protein in the heart.
Trehalose is a naturally occurring disaccharide that is induced in response to dehydration in plants and promotes their survival by inducing autophagy, thus fulfilling the definition of a hormetic response 195. We have shown recently that trehalose treatment increases autophagy in the mouse heart and the cardiomyocytes therein 196. It will be interesting to test whether trehalose also inhibits the adverse effects of aging in the heart by stimulating autophagy.
It should be noted that the interventions discussed above have multiple effects in the heart besides autophagy. Furthermore, their effects may be mediated through non-cardiac cells. For example, growth factors, miRNA and exosomes excreted from distal organs may mediate autophagy and inhibit senescence in the heart after reaching the heart through the systemic circulation 197. Thus, where such is likely to be the case for a particular anti-senescence intervention, further investigation is needed to identify the responsible cell type and the mechanism of transmission.
8. Concluding remarks
Accumulating lines of evidence suggest that the ability of cardiomyocytes to maintain appropriate levels of autophagy may decline during aging of the heart. However, many questions remain unanswered. First, it is unclear when the level of autophagy becomes significantly altered during the course of aging in the human heart. Currently, evaluating the level of autophagy and autophagic flux is challenging in the human heart in vivo. Developing convenient and reliable methods to accurately evaluate cardiac autophagy is essential. Second, more investigation is needed to elucidate the molecular mechanism by which autophagy or mitochondrial autophagy is regulated during the course of aging in the heart. Autophagy is regulated not only at the level of autophagosome formation but also at the levels of autophagosome-lysosome fusion and lysosomal degradation. In particular, how the function of lysosomes is affected by aging requires further investigation. Third, more investigation is needed to clarify the functional role of autophagy or mitophagy during cardiac aging. Currently, none of the available molecular interventions allow purely specific modification of either autophagy or mitophagy in mammalian cells. Whether or not protein aggregates, such as accumulated lipofuscin, are causatively involved in cardiac aging has not been formally addressed. Development of a more selective intervention or improvement of the selectivity by combining multiple interventions appears essential. Fourth, it is important to clarify the molecular mechanisms by which autophagy or mitophagy regulates cardiac aging. Although autophagy is generally thought to be a non-specific mechanism of protein degradation, there is increasing evidence that some proteins may be specifically degraded through their association with LC3 receptor proteins 198. Identification of specific targets of autophagy during aging may allow a better understanding of the molecular mechanisms of cardiac aging. Fifth, most of the investigations reported thus far focused only on autophagy in cardiomyocytes during aging. It is important to determine whether autophagy in other cell types, such as inflammatory cells, also affects cardiac aging and, if so, how these cells communicate with cardiomyocytes, such as through secretion of paracrine factors, circulating miRNA, or exosomes. Likewise, a better understanding of the function of autophagy in cardiac progenitor cells is also highly relevant for aging research in the heart. Finally, it is essential to develop more convenient and specific interventions to normalize the level of autophagy in the heart during aging.
Autophagy mediates many lifespan-extending and anti-senescence mechanisms 21. In particular, considering the emerging importance of the UPRmt as a fundamental mechanism of longevity enhancement, elucidating the connection between the UPRmt and autophagy/mitophagy is of great interest 199. Together with the recent advancement in understanding the molecular mechanisms of autophagy, investigating the role of autophagy/mitophagy during cardiac aging should eventually lead to the development of more efficient and specific interventions to slow senescence and increase stress resistance in the heart.
Supplementary Material
Acknowledgments
The authors thank Christopher D. Brady for critical reading of the manuscript.
Sources of funding
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330 and AG23039 and by the Leducq Foundation Transatlantic Network of Excellence (J.S.). A.S. has been supported by a Postdoctoral Fellowship from the Uehara Memorial Foundation.
Nonstandard Abbreviations and Acronyms
- Atg
autophagy related genes
- CR
caloric restriction
- CVD
cardiovascular diseases
- ER
endoplasmic reticulum
- FoxO
Forkhead box O
- HDAC
histone deacetylase
- IGF-I
insulin-like growth factor-I
- KO
knockout
- Lamp2
lysosome-associated membrane protein 2
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- NAD+
nicotinamide adenine dinucleotide
- Nampt
nicotinamide phosphoribosyl transferase
- NMN
nicotinamide mononucleotide
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SIRT
silent information regulator
- UPS
ubiquitin proteasome system
- UPR
unfolded protein response
- UPRmt
mitochondrial unfolded protein response
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Arnold AM, Naydeck BL, Fried LP, Burke GL, Enright P, Gottdiener J, Hirsch C, O’Leary D, Tracy R Cardiovascular Health Study Research G. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 5.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156:1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 8.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 9.Harmon HJ, Nank S, Floyd RA. Age-dependent changes in rat brain mitochondria of synaptic and non-synaptic origins. Mech Ageing Dev. 1987;38:167–177. doi: 10.1016/0047-6374(87)90076-5. [DOI] [PubMed] [Google Scholar]
- 10.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 11.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy. 2011;7:297–300. doi: 10.4161/auto.7.3.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Pharmacological modulation of autophagy during cardiac stress. J Cardiovasc Pharmacol. 2012;60:235–241. doi: 10.1097/FJC.0b013e3182575f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- 23.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 26.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 27.Swynghedauw B, Besse S, Assayag P, Carre F, Chevalier B, Charlemagne D, Delcayre C, Hardouin S, Heymes C, Moalic JM. Molecular and cellular biology of the senescent hypertrophied and failing heart. Am J Cardiol. 1995;76:2D–7D. doi: 10.1016/s0002-9149(99)80484-6. [DOI] [PubMed] [Google Scholar]
- 28.Olivetti G, Melissari M, Balbi T, Quaini F, Sonnenblick EH, Anversa P. Myocyte nuclear and possible cellular hyperplasia contribute to ventricular remodeling in the hypertrophic senescent heart in humans. J Am Coll Cardiol. 1994;24:140–149. doi: 10.1016/0735-1097(94)90554-1. [DOI] [PubMed] [Google Scholar]
- 29.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satyanarayana A, Rudolph KL. p16 and ARF: activation of teenage proteins in old age. J Clin Invest. 2004;114:1237–1240. doi: 10.1172/JCI23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajstura J, Pertoldi B, Leri A, Beltrami CA, Deptala A, Darzynkiewicz Z, Anversa P. Telomere shortening is an in vivo marker of myocyte replication and aging. Am J Pathol. 2000;156:813–819. doi: 10.1016/S0002-9440(10)64949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Schunkert H, Isoyama S, Wei JY, Nadal-Ginard B, Grossman W, Izumo S. Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J Clin Invest. 1992;89:939–946. doi: 10.1172/JCI115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 35.Levine TB, Levine AB, Bolenbaugh J, Green PR. Reversal of heart failure remodeling with age. Am J Geriatr Cardiol. 2002;11:299–304. doi: 10.1111/j.1076-7460.2002.01362.x. [DOI] [PubMed] [Google Scholar]
- 36.Capasso JM, Palackal T, Olivetti G, Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am J Physiol. 1990;259:H1086–1096. doi: 10.1152/ajpheart.1990.259.4.H1086. [DOI] [PubMed] [Google Scholar]
- 37.Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 38.Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA. Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci. 2004;1019:85–95. doi: 10.1196/annals.1297.017. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Bai Y, Huang L, Qi Y, Zhang Q, Li S, Wu Y, Li X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: implications for age-related macular degeneration. Cell death & disease. 2015;6:e1972. doi: 10.1038/cddis.2015.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- 41.Martin FM, Friedman JS. Ticking fast or ticking slow, through Shc must you go? Sci Aging Knowledge Environ. 2004;2004:pe32. doi: 10.1126/sageke.2004.32.pe32. [DOI] [PubMed] [Google Scholar]
- 42.Tissenbaum HA, Guarente L. Model organisms as a guide to mammalian aging. Dev Cell. 2002;2:9–19. doi: 10.1016/s1534-5807(01)00098-3. [DOI] [PubMed] [Google Scholar]
- 43.Quarrie JK, Riabowol KT. Murine models of life span extension. Sci Aging Knowledge Environ. 2004;2004:re5. doi: 10.1126/sageke.2004.31.re5. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Takatsu M, Hattori T, Murase T, Ohura S, Takeshita Y, Watanabe S, Murohara T, Nagata K. Premature cardiac senescence in DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Nagoya journal of medical science. 2014;76:35–49. [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd AC, Eshoo S, Richards DA, Thomas L. Hypertension accelerates the ‘normal’ aging process with a premature increase in left atrial volume. Journal of the American Society of Hypertension : JASH. 2013;7:149–156. doi: 10.1016/j.jash.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed SA, Hanke T, Erasmi AW, Bechtel MJ, Scharfschwerdt M, Meissner C, Sievers HH, Gosslau A. Mitochondrial DNA deletions and the aging heart. Experimental gerontology. 2006;41:508–517. doi: 10.1016/j.exger.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009;137:1247–1258. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 52.Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochimica et biophysica acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Activation of Nox4 promotes cardiomyocyte autophagy and survival during nutrient starvation. Circulation. 2012;126:A18056. [Google Scholar]
- 58.Ago T, Sadoshima J. Thioredoxin1 as a negative regulator of cardiac hypertrophy. Antioxid Redox Signal. 2007;9:679–687. doi: 10.1089/ars.2007.1529. [DOI] [PubMed] [Google Scholar]
- 59.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 60.Quiros PM, Langer T, Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol. 2015;16:345–359. doi: 10.1038/nrm3984. [DOI] [PubMed] [Google Scholar]
- 61.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–2156. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott I, Webster BR, Chan CK, Okonkwo JU, Han K, Sack MN. GCN5-like protein 1 (GCN5L1) controls mitochondrial content through coordinated regulation of mitochondrial biogenesis and mitophagy. J Biol Chem. 2014;289:2864–2872. doi: 10.1074/jbc.M113.521641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20:214–225. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang ZV, Hill JA. Protein quality control and metabolism: bidirectional control in the heart. Cell Metab. 2015;21:215–226. doi: 10.1016/j.cmet.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aumailley L, Garand C, Dubois MJ, Johnson FB, Marette A, Lebel M. Metabolic and Phenotypic Differences between Mice Producing a Werner Syndrome Helicase Mutant Protein and Wrn Null Mice. PLoS One. 2015;10:e0140292. doi: 10.1371/journal.pone.0140292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li-Harms X, Milasta S, Lynch J, Wright C, Joshi A, Iyengar R, Neale G, Wang X, Wang YD, Prolla TA, Thompson JE, Opferman JT, Green DR, Schuetz J, Kundu M. Mito-protective autophagy is impaired in erythroid cells of aged mtDNA-mutator mice. Blood. 2015;125:162–174. doi: 10.1182/blood-2014-07-586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Valdes I, Hidalgo I, Bujarrabal A, Lara-Pezzi E, Padron-Barthe L, Garcia-Pavia P, Gomez-del Arco P, Redondo JM, Ruiz-Cabello JM, Jimenez-Borreguero LJ, Enriquez JA, de la Pompa JL, Hidalgo A, Gonzalez S. Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence. Nature communications. 2015;6:6473. doi: 10.1038/ncomms7473. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res. 2012;110:1226–1237. doi: 10.1161/CIRCRESAHA.111.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hosoda T, Kajstura J, Leri A, Anversa P. Mechanisms of myocardial regeneration. Circ J. 2010;74:13–17. doi: 10.1253/circj.cj-09-0665. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Munoz-Canoves P. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 73.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 74.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annual review of pathology. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 76.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circulation research. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- 77.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, Liang CC. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 79.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 80.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 81.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- 83.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sack MN. The role of SIRT3 in mitochondrial homeostasis and cardiac adaptation to hypertrophy and aging. J Mol Cell Cardiol. 2012;52:520–525. doi: 10.1016/j.yjmcc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baar EL, Carbajal KA, Ong IM, Lamming DW. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell. 2015 doi: 10.1111/acel.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. GDF11 Does Not Rescue Aging-Related Pathological Hypertrophy. Circ Res. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stadtman ER. Protein oxidation in aging and age-related diseases. Annals of the New York Academy of Sciences. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 94.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015;116:456–467. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hwang S, Disatnik MH, Mochly-Rosen D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO molecular medicine. 2015 doi: 10.15252/emmm.201505256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 97.Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, Schwartz O, Deretic V, Piguet V. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, Jr, Gottlieb RA. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 2010;3:365–373. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting Beclin1-Bcl-2 interaction. Nature Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a Critical Regulator of Autophagy during Myocardial Ischemia: Pathophysiological Implications in Obesity and Metabolic Syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao X, Zhang R, Lu Y, Prosdocimo DA, Sangwung P, Zhang L, Zhou G, Anand P, Lai L, Leone TC, Fujioka H, Ye F, Rosca MG, Hoppel CL, Schulze PC, Abel ED, Stamler JS, Kelly DP, Jain MK. Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis. J Clin Invest. 2015;125:3461–3476. doi: 10.1172/JCI79964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chauhan S, Goodwin JG, Chauhan S, Manyam G, Wang J, Kamat AM, Boyd DD. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol Cell. 2013;50:16–28. doi: 10.1016/j.molcel.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A, Ippoliti A, Martelli F, Lauro R, Federici M. MiR-216a: a link between endothelial dysfunction and autophagy. Cell death & disease. 2014;5:e1029. doi: 10.1038/cddis.2013.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, Sarais C, Catalucci D, Kruger M, Mongillo M, Sandri M. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest. 2014;124:2410–2424. doi: 10.1172/JCI66339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enot DP, Niso-Santano M, Durand S, Chery A, Pietrocola F, Vacchelli E, Madeo F, Galluzzi L, Kroemer G. Metabolomic analyses reveal that anti-aging metabolites are depleted by palmitate but increased by oleate in vivo. Cell Cycle. 2015;14:2399–2407. doi: 10.1080/15384101.2015.1064206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 107.Matecic M, Smith DL, Pan X, Maqani N, Bekiranov S, Boeke JD, Smith JS. A microarray-based genetic screen for yeast chronological aging factors. PLoS genetics. 2010;6:e1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 110.Vellai T. Autophagy genes and ageing. Cell death and differentiation. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- 111.Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 113.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KS, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nature neuroscience. 2013;16:1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 117.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou J, Force T. Focusing the spotlight on GSK-3 in aging. Aging (Albany NY) 2013;5:388–389. doi: 10.18632/aging.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walter S, Atzmon G, Demerath EW, Garcia ME, Kaplan RC, Kumari M, Lunetta KL, Milaneschi Y, Tanaka T, Tranah GJ, Volker U, Yu L, Arnold A, Benjamin EJ, Biffar R, Buchman AS, Boerwinkle E, Couper D, De Jager PL, Evans DA, Harris TB, Hoffmann W, Hofman A, Karasik D, Kiel DP, Kocher T, Kuningas M, Launer LJ, Lohman KK, Lutsey PL, Mackenbach J, Marciante K, Psaty BM, Reiman EM, Rotter JI, Seshadri S, Shardell MD, Smith AV, van Duijn C, Walston J, Zillikens MC, Bandinelli S, Baumeister SE, Bennett DA, Ferrucci L, Gudnason V, Kivimaki M, Liu Y, Murabito JM, Newman AB, Tiemeier H, Franceschini N. A genome-wide association study of aging. Neurobiology of aging. 2011;32:2109, e2115–2128. doi: 10.1016/j.neurobiolaging.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, Handa JT, Brownlee M, Nagaraj R, Taylor A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics) Aging Cell. 2012;11:1–13. doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nature medicine. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 127.Saito T, Sadoshima J. The molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116:1477–1490. doi: 10.1161/CIRCRESAHA.116.303790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hirota Y, Yamashita S, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, Kanki T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy. 2015;11:332–343. doi: 10.1080/15548627.2015.1023047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 133.Kubli DA, Quinsay MN, Gustafsson AB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Communicative & integrative biology. 2013;6:e24511. doi: 10.4161/cib.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, Suen DF, Youle RJ, Amar M, Remaley AT, Sack MN. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferree AW, Trudeau K, Zik E, Benador IY, Twig G, Gottlieb RA, Shirihai OS. MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy. 2013;9:1887–1896. doi: 10.4161/auto.26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stotland A, Gottlieb RA. alpha-MHC MitoTimer mouse: In vivo mitochondrial turnover model reveals remarkable mitochondrial heterogeneity in the heart. J Mol Cell Cardiol. 2015;90:53–58. doi: 10.1016/j.yjmcc.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 138.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 140.Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309:H1375–1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamoto T, Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc Med. 2011;21:27–32. doi: 10.1016/j.tcm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 143.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 144.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 145.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 146.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 147.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 148.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith J. Human Sir2 and the ‘silencing’ of p53 activity. Trends Cell Biol. 2002;12:404–406. doi: 10.1016/s0962-8924(02)02342-5. [DOI] [PubMed] [Google Scholar]
- 150.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 154.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Verdin E. NAD+ in aging, metabolism, and Neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 156.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hsu CP, Hariharan N, Alcendor RR, Oka S, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through autophagy in cardiomyocytes. Autophagy. 2009;5:1229–1231. doi: 10.4161/auto.5.8.10275. [DOI] [PubMed] [Google Scholar]
- 158.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 160.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 161.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. Faseb J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 162.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 163.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 164.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 165.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 166.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Crow MT. Sirviving cardiac stress: cardioprotection mediated by a longevity gene. Circ Res. 2004;95:953–956. doi: 10.1161/01.RES.0000148666.20729.1c. [DOI] [PubMed] [Google Scholar]
- 168.Anversa P. Aging and longevity: the IGF-1 enigma. Circ Res. 2005;97:411–414. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- 169.Cattin ME, Wang J, Weldrick JJ, Roeske CL, Mak E, Thorn SL, DaSilva JN, Wang Y, Lusis AJ, Burgon PG. Deletion of MLIP (Muscle-enriched A-type Lamin-interacting Protein) Leads to Cardiac Hyperactivation of Akt/Mammalian Target of Rapamycin (mTOR) and Impaired Cardiac Adaptation. J Biol Chem. 2015;290:26699–26714. doi: 10.1074/jbc.M115.678433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 171.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct Roles of Autophagy in the Heart During Ischemia and Reperfusion. Roles of AMP-Activated Protein Kinase and Beclin 1 in Mediating Autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 172.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lal H, Ahmad F, Woodgett J, Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116:138–149. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, Sadoshima J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxidative medicine and cellular longevity. 2014;2014:210934. doi: 10.1155/2014/210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hariharan N, Zhai P, Sadoshima J. Oxidative Stress Stimulates Autophagic Flux during Ischemia/Reperfusion. Antioxid Redox Signal. 2011;14:2179–2190. doi: 10.1089/ars.2010.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ Res. 2013;113:1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 183.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 184.Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin SJ, Sinclair DA. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–1864. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- 185.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 187.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Science translational medicine. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:B379–389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- 189.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 190.Tocchi A, Quarles EK, Basisty N, Gitari L, Rabinovitch PS. Mitochondrial dysfunction in cardiac aging. Biochim Biophys Acta. 2015;1847:1424–1433. doi: 10.1016/j.bbabio.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu K, Psakhye I, Jentsch S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell. 2014;158:549–563. doi: 10.1016/j.cell.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 194.Wan N, Liu X, Zhang XJ, Zhao Y, Hu G, Wan F, Zhang R, Zhu X, Xia H, Li H. Toll-interacting protein contributes to mortality following myocardial infarction through promoting inflammation and apoptosis. Br J Pharmacol. 2015;172:3383–3396. doi: 10.1111/bph.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams B, Njaci I, Moghaddam L, Long H, Dickman MB, Zhang X, Mundree S. Trehalose Accumulation Triggers Autophagy during Plant Desiccation. PLoS Genet. 2015;11:e1005705. doi: 10.1371/journal.pgen.1005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sciarretta S, Zhai P, Volpe M, Sadoshima J. Trehalose, a natural dissachride, reduces cardiac remodeling after myocardial infarction through autophagy activation. Circulation. 2012;126:A18096. [Google Scholar]
- 197.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 198.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, Dong Z, Apte U, Pallet N, Johnson RL, Terzi F, Kwiatkowski DJ, Scoazec JY, Martignoni G, Pende M. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.