Abstract
Chronic distal symmetrical sensory peripheral neuropathy is a common neurological complication of cancer chemotherapy, HIV treatment and diabetes. Although aetiology-specific differences in presentation are evident, the clinical signs and symptoms of these neuropathies are clearly similar. Data from animal models of neuropathic pain suggest that the similarities have a common cause: mitochondrial dysfunction in primary afferent sensory neurons. Mitochondrial dysfunction is caused by mitotoxic effects of cancer chemotherapeutic drugs of several chemical classes, HIV-associated viral proteins, and nucleoside reverse transcriptase inhibitor treatment, as well as the (possibly both direct and indirect) effects of excess glucose. The mitochondrial injury results in a chronic neuronal energy deficit, which gives rise to spontaneous nerve impulses and a compartmental neuronal degeneration that is first apparent in the terminal receptor arbor—that is, intraepidermal nerve fibres—of cutaneous afferent neurons. Preliminary data suggest that drugs that prevent mitochondrial injury or improve mitochondrial function could be useful in the treatment of these conditions.
Introduction
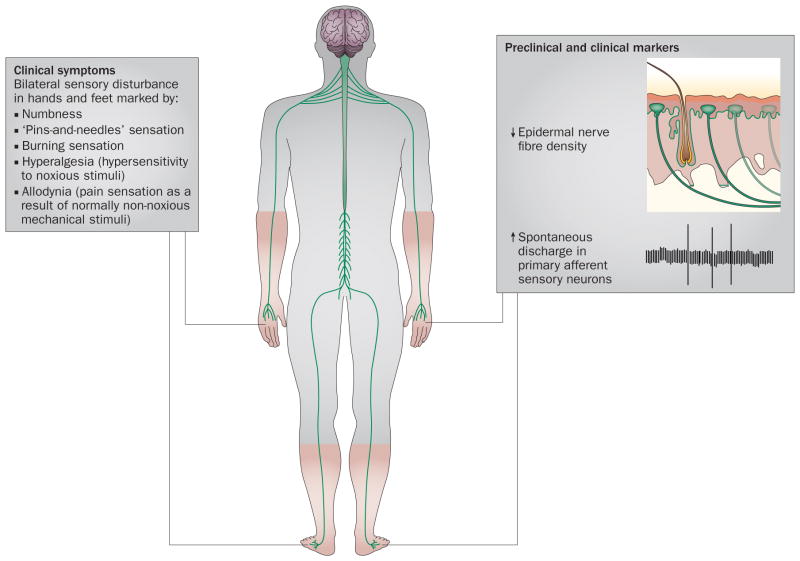
The most common chronic distal symmetrical sensory peripheral neuropathies are associated with diabetes, cancer chemotherapy, and pharmacological therapy for HIV. Although the neuropathies linked to these conditions differ in their details, they share general similarities; for example, they all begin with sensory symptoms in the feet. Typically, the patient reports numbness and dysaesthesias (tingling sensations) in affected regions. Despite the numbness, a large percentage of patients also report neuropathic pain, which is generally described as burning, pricking, or a sensation of ‘pins and needles’. Patients with pain also display hypersensitivity to cold and tactile stimuli, including both hyperalgesia and allodynia. Motor function in the same areas as the sensory symptoms is usually unaffected, except for symptoms referable to impaired proprioception. Sensory nerve conduction can seem either normal or abnormal (slowed latency and/or reduced amplitude of the potential), and nerve biopsies might reveal axonal degeneration in some patients. Staining of a skin punch biopsy with the pan-neuronal marker PGP9.5 often reveals a loss of intraepidermal nerve fibres (IENFs), which are the sensory terminal arbors of somatosensory afferent neurons (Figure 1).1–5
Figure 1.
Clinical symptoms and biomarkers of chronic distal symmetrical sensory peripheral neuropathy. Patients with distal symmetrical sensory peripheral neuropathy due to cancer chemotherapeutic treatment, HIV and nucleoside analogue reverse transcriptase inhibitor therapy, or diabetes complain of similar sensory disturbances: numbness combined with a dysaesthetic sensation of pins-and-needles and/or burning pain, along with allodynia and hyperalgesia. These sensory abnormalities appear in both of the feet, or in both the feet and the hands. Skin punch biopsy often reveals a significant loss of sensory axons from the epidermis (intraepidermal nerve fibres), even in cases where electrodiagnostic studies are normal.
In this Review, we discuss the evidence for the hypothesis that mitotoxic effects on somatosensory afferent neurons are the fundamental cause of the chronic distal symmetrical sensory peripheral neuropathies associated with cancer chemotherapy, HIV therapy and diabetes. In addition, we explore the implications of this hypothesis for the treatment and prevention of distal symmetrical sensory peripheral neuropathies.
The mitotoxicity hypothesis
Initial evidence from CIPN
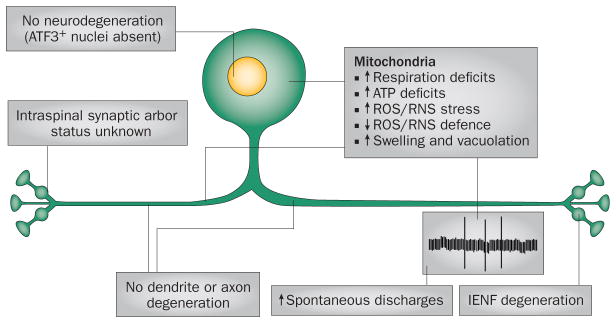
Studies of cancer chemotherapy-induced peripheral neuropathy (CIPN) in experimental animal models gave rise to the mitotoxicity hypothesis, which posits that the fundamental cause of this form of chronic peripheral neuropathy is a toxic effect on mitochondria in primary afferent somatosensory neurons.6–9 According to the mitotoxicity hypothesis, the mitochondrial insult leads to a chronic energy deficit in the neurons that causes abnormal spontaneous discharges and compartmental degeneration in somatosensory primary afferent neurons (Figure 2).
Figure 2.
Underlying mechanisms in the development of distal symmetrical sensory peripheral neuropathy. Distal neuropathy does not result from degeneration of the somatosensory primary afferent neuron, as indicated by a lack of ATF3+ nuclear signalling (a marker of axonal injury) and preservation of peripheral nerve axons. Instead, both preclinical and clinical stages of distal neuropathy are associated with mitochondrial dysfunction that leads to IENF degeneration and emergence of spontaneous discharges. Abbreviations: IENF, intraepidermal nerve fibre; RNS, reactive nitrogen species; ROS, reactive oxygen species.
The mitotoxicity hypothesis does not specify the exact mechanisms whereby different chemotherapeutic agents cause mitochondrial injury; the mechanisms are probably agent-specific. Instead, the hypothesis posits that mitochondrial injury is a final common pathway that accounts for the similar clinical presentations of CIPN associated with chemotherapeutic agents of diverse chemical classes (such as taxanes, vinca alkaloids, platinum-complex agents and proteasome inhibitors). The mitotoxicity hypothesis resolves a striking paradox: similar neurological symptoms are observed despite diverse anticancer mechanisms of action, for example, binding to β–tubulin for the taxanes and vinca alkaloids, formation of platinum adducts between DNA bases or bases and proteins for the platinum-complex agents, and inhibition of protein processing for the proteasome inhibitors. Moreover, these anticancer mechanisms target rapidly dividing cells, which do not include primary afferent sensory neurons. An obvious extension of this idea is that mitochondrial injury is also the final common pathway that accounts for the general similarity of all distal symmetrical sensory peripheral neuropathies.
It is important to note that some chemotherapeutic agents produce multiple kinds of peripheral neuropathy. For example, oxaliplatin produces dysaesthesias in the face and sometimes hands (but not in the feet); this effect has a very rapid onset (sometimes beginning during the initial drug infusion) and resolves within days.10 Moreover, paclitaxel produces a syndrome of aching pain that is usually localized to proximal joints; these symptoms also occur very early in the course of treatment and abate within days.11 The dysaesthesias associated with oxaliplatin treatment are probably due to an action of the oxalate salt on axonal sodium channels,10 but the cause of the pain associated with paclitaxel treatment is unknown. These acute syndromes are distinctly different from the chronic distal symmetrical conditions considered here.
Key points.
Experiments in animal models of chronic distal symmetrical sensory peripheral neuropathies have demonstrated mitochondrial dysfunction in primary afferent sensory neurons
The mitochondrial dysfunction manifests as a cellular energy deficit, and has been linked to the emergence of spontaneous discharges and compartment-specific degeneration beginning with the terminal receptor arbor of the afferent neurons
Although the mitotoxic mechanisms differ according to aetiology, the consequences of the energy deficit are consistent and account for the similarity of symptoms across conditions
According to the mitotoxicity hypothesis, drugs that protect or restore mitochondrial function could aid the prevention and treatment of chronic distal symmetrical sensory peripheral neuropathies; preliminary data support this prediction
Spontaneous neuronal discharges
Abnormal spontaneous discharges in primary afferent neurons have been documented in patients with various types of peripheral neuropathy.12 Single-fibre recordings from sensory axons in both animals and humans show that discharges are usually minimal or absent in the absence of stimulation. However, in animals with painful peripheral neuropathies induced by paclitaxel, vincristine or oxaliplatin, about 15% of sensory A fibres and 20–35% of sensory C fibres display spontaneous discharges that have an irregular pattern and a low frequency (around 1.0 Hz).13,14 Spontaneous discharges in A fibres and C fibres are likely to evoke spontaneous sensations, which will be painful if the fibres are nociceptors, and dysaesthesic if the fibres are low-threshold mechanoreceptors. The site of origin of the spontaneous discharges has not been established, but they are most likely to originate in axons affected by IENF degeneration.
Spontaneous discharges might be a consequence of mitotoxicity-induced energy deficiency. Sodium ions continuously flow into neurons, and the Na+/K+ pump must, therefore, operate continuously to maintain membrane polarization; this process accounts for about 50% of neuronal energy consumption.15 Our research group has proposed that a spontaneous discharge arises when localized ion-pumping insufficiency leads to depolarization that crosses the threshold for impulse initiation.16 Depolarizations would consequently arise at loci corresponding to aggregations of functionally impaired mitochondria. Mitochondrial positioning within the axon is in constant flux, so that discharges would be initiated sporadically and at variable locations. This process would lead to the observed irregular discharge pattern.
Somatosensory neurons with damaged axons have altered expression of voltage-gated sodium channel subtypes, which could also contribute to the generation of spontaneous discharges. Emerging evidence indicates that polymorphisms and mutations in sodium channel subtype genes may be predisposing factors for spontaneous discharges and pain.17,18
Axonal degeneration
IENF loss has been found in patients with forms of distal symmetrical peripheral neuropathy associated with chemotherapy, diabetes, idiopathic small fibre neuropathy with and without glucose intolerance, and HIV treatment.19–23 IENF loss in these patients can be present despite normal nerve conduction21 and peripheral nerve axon counts.24 These observations suggest that IENF loss is the earliest sign of axonal pathology. A simple relationship between the extent of IENF loss and measures of sensory deficit and pain might be expected; however, the real situation is highly complex.25
IENFs originate from subepidermal nerve fibre bundles. Individual axons leave the bundle, traverse the epidermal basal lamina, and branch within the epidermis, where they show axonal and terminal varicosities that comprise the receptor organs.26 Electron microscopy studies in paclitaxel-treated rats with peripheral neuropathy have shown that degeneration is restricted to IENFs; no degeneration occurs in the parent axons immediately below the epidermal basal lamina. Similarly, no activation of ATF3, a nuclear protein that is a standard marker of axonal injury, has been observed in the sensory neuron cell bodies in the dorsal root ganglia (DRG).26 These observations suggest that these forms of peripheral neuropathy represent compartment-specific degeneration, wherein the IENFs are particularly vulnerable to mitochondrial dysfunction.
Different neuronal compartments (cell bodies, axons, synaptic boutons) have independent mechanisms of degeneration and different thresholds for response to toxins. The IENF compartment is likely to have a distinctly low threshold for mitotoxic insult. Mitochondria are concentrated in locations with high energy demands, and IENF varicosities are packed with mitochondria. Moreover, the epidermis is continuously renewing itself, and the birth of new keratinocytes and the subsequent upwards migration and flattening of pre-existing keratinocytes results in a constant remodelling of the intercellular spaces through which the IENFs must travel. IENFs accommodate these changes by an ongoing process of remodelling involving fibre retraction (degeneration), followed by advancement (regeneration). This process is energetically costly, which is a probable reason for the uniquely high vulnerability of IENFs to energy deficiency.26 It is important to note that if parent axons in the peripheral nerve are intact, even extensive IENF degeneration cannot be detected by nerve conduction studies, because both stimulation and recording are done at loci proximal to the IENF.
In patients receiving chemotherapy, the exposure to mitotoxic effects is relatively limited because the emergence of neuropathy prompts dose reduction or discontinuation. However, situations can be envisaged in which prolonged exposure to a mitotoxin (for example, in patients receiving HIV therapy or in those with diabetes, discussed below) results in crossing of the degeneration threshold for the next most susceptible compartment (the parent axon in the peripheral nerve), with the consequence that electrodiagnostic tests are positive and axonal degeneration is apparent in nerve biopsy.
Evidence for mitochondrial dysfunction in CIPN
An abnormal incidence of swollen and vacuolated mitochondria in peripheral nerve sensory axons was first noted in rats with paclitaxel-induced painful peripheral neuropathy;6 the same feature was subsequently demonstrated in peripheral nerves of rats with painful peripheral neuropathy induced by vincristine, oxaliplatin or bortezomib,7,9,14,27–29 and in the lumbar DRG of paclitaxel-treated rats.30 The effect is exclusively axonal: no mitochondrial structural abnormalities are present in either the myelinating Schwann cells that surround A fibres or the nonmyelinated Schwann cells that surround C fibres. Moreover, a paclitaxel-induced increase in the incidence of swollen and vacuolated mitochondria is found only in the sensory axons in the dorsal roots, not in the motor axons of the ventral roots, which is consistent with the presence of sensory pathology and absence of motor pathology.14
Mitochondrial energy production depends on the maintenance of a proton gradient across the mitochondrial inner membrane. This inner membrane is disrupted when the mitochondria swell and vacuolate—a process that has been linked to functional impairment. Experiments in ex vivo preparations of sciatic nerve axons from rats with painful peripheral neuropathy induced by paclitaxel, oxaliplatin or bortezomib show significant deficits in maximally stimulated complex I-mediated and complex II-mediated mitochondrial respiration, and corresponding ATP production deficits.8,9,29 These deficits can be detected before the emergence of pain and IENF degeneration, and are chronic, persisting for at least 3 weeks after the last drug exposure. The respiration deficits are not corrected by the addition of cytochrome c, suggesting that the respiration deficit is not caused by the release of mitochondrial cytochrome c, which is a hallmark of apoptosis. Moreover, the deficits are not accompanied by a decrease in levels of the mitochondrion-specific enzyme citrate synthase, suggesting that the mitotoxicity damages but does not kill mitochondria. In paclitaxel-treated rats, the deficits in mitochondrial function are found in dorsal root sensory axons, but not in ventral root motor axons, which is again consistent with the presence of a sensory neuropathy without a motor component.14 The deficits are present by 7 days after the initiation of drug treatment (before the development of statistically significant hypersensitivity to painful stimuli and IENF loss) and persist for 3–4 weeks after cessation of chemotherapy, which is the time of peak hypersensitivity to painful stimuli and IENF loss.8,9,26
If the neuropathic pain is a consequence of mitochondrial dysfunction, then increasing dysfunction should increase the pain. Indeed, with mitotoxin dosing protocols that have no effect on pain sensitivity in control animals, mitotoxin treatment significantly worsens mechano-allodynia and mechano-hyperalgesia in paclitaxel and oxaliplatin models of CIPN. Administration of oligomycin—a mitotoxin that inhibits ATP synthase—at the same dose observed to worsen pain rapidly increases the frequency of abnormal spontaneous discharges in both A fibres and C fibres, indicating a close link between mitochondrial dysfunction, spontaneous discharges, and pain hypersensitivity.7 However, contrary to the mitotoxicity hypothesis, intradermal injections of inhibitors of mitochondrial respiratory complexes and ATP synthase reduce mechano-hypersensitivity in rats with vincristine-induced peripheral neuropathy.31 The reason for this discrepancy is unknown.
HIV-related peripheral neuropathy
HIV-related peripheral neuropathy has two well-established causes—exposure to viral proteins and nucleoside analogue reverse transcriptase inhibitors (NRTIs)4—the effects of which are independent, but undoubtedly interact. Viral proteins have been shown to preferentially damage large Aβ-fibre myelinated primary afferent axons that convey touch and proprioceptive information, whereas NRTIs preferentially damage small myelinated Aδ fibres and nonmyelinated C fibres that convey temperature and pain information.32
Electron micrographs of sensory (sural) nerve biopsies from zalcitabine-treated patients with HIV with distal symmetrical painful peripheral neuropathy (before the era of highly active antiretroviral therapy [HAART]) revealed a large increase in the incidence of swollen and vacuolated mitochondria in myelinated and nonmyelinated axons, as well as in Schwann cells. The incidence of abnormal mitochondria was greater in untreated patients with HIV than in uninfected controls, and was accompanied by a 20-fold decrease in mitochondrial gene copy number.33 The researchers who conducted this study were the first to recognize that mitotoxicity might be the cause of distal symmetrical sensory peripheral neuropathies in general.
When compared with HAART-treated HIV patients who do not have neuropathy, HAART-treated HIV patients with neuropathy have a significant increase in the incidence of a common mitochondrial DNA mutation (mtDNA4977, which represents a deletion of 4,977 bp) that results in impaired mitochondrial function.34 This difference is present in the distal (ankle) sural nerve but not in the lumbar DRG. A significant decrease in sural nerve levels of the mtDNA-encoded enzyme subunit, cytochrome c1, is also found. Assuming that mitochondria are made in the cell body and transported down the axon, mitochondria in the distal nerve would be older, have greater mtDNA4977 accumulation and, thus, be more vulnerable to HIV-related mitotoxic factors than are mitochondria in the proximal nerve and lumbar DRG. This hypothesis is attractive, because it might also account for the peculiar fact that peripheral neuropathies are generally more common in tall people.34 The reader should, however, note that we currently do not know whether mitochondria in distal nerves really are constructed in the DRG and then transported down the axon. The presence of ribosomes in the axon35 supports the possibility that mitochondria might divide and then grow in situ via local protein synthesis guided by mRNA transported from the cell body.
HAART-treated monkeys infected with simian immunodeficiency virus have decreased levels of mtDNA-encoded COX-1—a catalytic subunit of mitochondrial respiratory complex IV—and increased levels of oxidized and nitrated proteins in the distal sural nerve, but not in the proximal sciatic nerve. Moreover, mitochondria isolated from the sural nerve of these animals had increased sensitivity to Ca2+-evoked swelling.34 The relative contributions of virus-related and treatment-related factors to the observations reported in this study are, however, unknown. The HAART protocol used to treat these monkeys included the nucleotide analogue reverse transcriptase inhibitor tenofovir, which (unlike NRTIs) is not known to be neurotoxic or mitotoxic.
Viral proteins
Three HIV proteins, gp120, Tat and Vpr, are known to be neurotoxic, and possibly mitotoxic.36 These proteins have very diverse direct and indirect effects on neuronal function. Acute exposure to gp120, applied directly to a nerve, produces a painful peripheral neuropathy in rats.37 This neuropathy is not accompanied by demyelination or degeneration of axons at the application site, but IENF degeneration does occur.38 In addition, gp120 evokes the release of inflammatory cytokines from immune cells, causing secondary neuronal damage.39 In vitro experiments show that gp120 causes axonal degeneration via a direct effect independent of its action in the cell body, and this degeneration follows activation of the mitochondrial caspase-dependent apoptotic pathway.40 It should be noted that although mitochondria orchestrate the apoptotic pathway, apoptosis is distinct from the mitochondrial dysfunction (and the resulting bioenergetic deficit) proposed by the mitotoxicity hypothesis. We are not aware of any evidence that gp120 has a direct mitotoxic effect in neurons.
Injection of Tat into mouse cortex results in swelling and vacuolation of neuronal mitochondria,41 and cultured CNS neurons briefly exposed to Tat have several mitochondrial abnormalities.41,42 The mitotoxic effects of Vpr are also well established in several cell types. This protein binds to adenine nucleotide translocator and voltage-dependent anion channels, which induces mitochondrial swelling, loss of the mitochondrial membrane potential, and release of cytochrome c.43 Vpr also decreases expression of several mitochondrial proteins,44 reduces ATP production, and increases oxidative stress.45 We are not aware of any studies that have examined the effects of Tat or Vpr on somatosensory primary afferent neurons.
NRTIs
NRTIs prevent viral gene replication by blocking viral DNA polymerase. However, replication of the 37 genes that constitute the mitochondrial genome requires the mitochondria-specific enzyme DNA polymerase γ, which is also blocked by NRTIs.46 Expression and replication of mitochondrial genes are essential to oxidative phosphorylation and ATP synthesis; thus, chronic interference with DNA polymerase γ will substantially impair energy production.
Only certain NRTIs, most notably didanosine, stavudin and zalcitabine, have mitotoxic effects on primary afferent sensory neurons. Other NRTIs have mitotoxic effects in different cell types; for example, zidovudine has little or no effect on neurons but has a clinically significant mitotoxic effect on myocytes. Non-nucleoside reverse transcriptase inhibitors (such as efavirenz and rilipivirine) and the nucleotide analogue reverse transcriptase inhibitor tenofovir block viral DNA polymerase but are associated with little or no clinically detectable neurotoxicity. The reason for these differences is unknown.
Addition of NRTIs to cell cultures of neonatal rat DRG causes a loss of mitochondrial membrane potential and cell death, without an effect on Schwann cells.47 Cell death is not prevented by cyclosporin, indicating that the mitochondrial permeability transition pore is not involved. NRTI exposure also induces mitochondrial structural abnormalities in cultured human neonatal DRG nociceptor cells.48 These effects are seen with exposures of 3–72 h, raising doubts as to whether an effect on mitochondrial DNA is involved. The rapidity of onset of these in vitro effects is also inconsistent with the 4–6 week interval between initiation of treatment and the clinical onset of NRTI-evoked peripheral neuropathy.
In vivo studies of NRTI-evoked peripheral neuropathy have also found evidence of mitotoxicity in somatosensory primary afferent neurons. Swollen and vacuolated mitochondria are present in rat DRG neurons after zalcitabine treatment, with the most pronounced effects in small to medium-sized neurons.49 Decreased levels of subunits of mitochondrial respiratory complex I and ATP synthase are found in the rat sural nerve after a stavudine treatment protocol that also induces pain and IENF loss without degeneration of peripheral nerve axons.50 Decreased levels of COX-1 are found in cat DRG neurons after exposure to didanosine.51 Loss of IENFs and selective loss of peripheral nerve C fibres is evident in distal plantar nerves, but not in the proximal sciatic nerve, after administration of neurotoxic NRTIs to mice engineered to express viral protein gp120.47 This evidence of mitochondrial dysfunction suggests that additional compromise of mitochondrial function should worsen NRTI-evoked symptoms. However, in rats with painful peripheral neuropathy due to zalcitabine (as in the vincristine-treated rats with CIPN, mentioned above), subcutaneous injections of inhibitors of complex I–IV and ATP synthase decrease neuropathic pain at doses that have no effect on pain sensitivity in control animals.31 The reason for this discrepancy is unknown.
Diabetic peripheral neuropathy
CIPN-induced and NRTI-induced peripheral neuropathies appear within weeks of exposure to a clearly identified toxin. Diabetic peripheral neuropathy is different, and potentially much more complex. First, although type 1 and type 2 diabetes are generally accepted to produce similar or identical distal symmetrical sensory peripheral neuropathies, emerging observations suggest that their underlying mechanisms might be distinct.52 This point is noteworthy because most of the experimental data were obtained in animals with streptozocin-induced type 1 diabetes. Second, for type 2 diabetes especially, the onset of neuropathy is insidious, and might perhaps occur even before the onset of frank diabetes.53 The progression of diabetic peripheral neuropathy occurs over several years3 and has a pattern characteristic of dying-back disease, with symptoms in the feet developing months to years before they appear in the hands. By marked contrast, in CIPN and NRTI-induced peripheral neuropathy, symptoms in the feet and hands sometimes appear simultaneously or—albeit rarely—in the hands before the feet.26
Neuronal mitotoxicity in diabetic neuropathy
We hypothesize, as have others, that a mitotoxic effect of excess glucose in somatosensory primary afferent neurons is the fundamental cause of diabetic peripheral neuropathy.54,55 High levels of glucose are directly neurotoxic.56 In addition, long-term exposure to excess glucose produces many effects that might impair mitochondrial function: hypoxia secondary to intraneural microvascular disease, accumulation of advanced glycation end products, exposure to elevated levels of methylglyoxal, polyol pathway hyperactivity, disruption of metabolic support from Schwann cells, and abnormal calcium homeostasis.57–60 A full discussion of this complex picture is outside the scope of this Review, but we propose that a toxic effect of excess glucose on axonal mitochondria may be the fundamental precipitating factor, and that the other phenomena are contributors to an evolving pathological process.
Evidence for mitochondrial dysfunction in diabetic neuropathy comes mostly from studies in cultured DRG neurons and isolated mitochondrial preparations from these cells, obtained from rats and mice with streptozocin-evoked diabetes.55,58 These models show decreased expression of various subunits of mitochondrial respiratory complexes I and IV, and impairment of maximally stimulated respiration. Furthermore, respiratory capacity and the proportion of ATP generated by the electron transport system are decreased. Abnormalities of regulation of mitochondrial membrane potential and depletion of mitochondrial cytochrome c have also been observed.61 Oddly, although the presence of such abnormalities might be expected to lead to an increase in the production of reactive oxygen species (ROS), levels of ROS have been reported to be unchanged62 or even decreased.63
Neuronal energy deficit
Cell energy requirements are sensed, in part, by circuits involving AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor co-activator-1α (PGC-1α), which in turn regulate mitochondrial ATP production. The expression of AMPK and PGC-1α is significantly decreased in sensory neurons from rats with streptozocin-induced diabetes and in sensory neurons from diabetic mice carrying the obesity-inducing Leprdb/db gene mutation.61 In rats with streptozocin-induced diabetes, resveratrol treatment (which would be expected to improve AMPK signalling) reversed the animals’ hypoalgesia to heat stimuli and partially restored the diabetes-induced loss of IENFs.61
Impaired energy production leading to neuronal degeneration might be expected to engage a response from heat shock proteins (Hsp). KU-32, an inhibitor of Hsp90, reverses sensory deficits, improves slowed nerve conduction, and reverses the IENF loss seen in diabetic mice. Moreover, KU-32 treatment reverses the decrease in mitochondrial respiration in DRG neurons from these mice.64 Prophylactic treatment with ciliary neurotrophic factor protects mice against streptozocin-induced diabetic neuropathy, and such treatment also ameliorates the mitochondrial respiration deficit seen in DRG neurons harvested from animals with streptozocin-induced diabetes.65 In Leprdb/db diabetic mice, the number of mitochondria increase in DRG neurons and dorsal root sensory axons, but not in ventral root motor axons; this observation might represent compensation for a decrease in mitochondrial energy production.66
In summary, the data suggest that in diabetes, sensory neurons are unable to produce enough energy to meet the demand. The results of this energy deficiency would be expected to be the same as those described above for CIPN: spontaneous sensory afferent discharges due to membrane depolarization secondary to inadequate Na+/K+ pumping, and degeneration that first appears in the regions of neurons with the highest energy demands, namely, IENFs. Whether mitochondrial dysfunction is primarily due to direct or indirect glucose-induced injury to the mitochondria is unclear. Resolution of this question will be very difficult for many reasons, including doubts about the clinical relevance of streptozocin-induced models of diabetes. However, we can hope that investigations of neuropathology and mitochondrial function in genetic models of type 2 diabetes67 will be able to address the mitotoxicity hypothesis directly.
Implications for therapy
The mitotoxicity hypothesis predicts that drugs that protect or restore mitochondrial energy production (Figure 3) should be effective for the treatment and prevention of pain and degeneration in distal symmetrical sensory peripheral neuropathies. Several existing drugs might work via mitochondrial mechanisms, and new drugs with mitoprotective actions are under development. Currently, the most promising therapeutic approaches for combating neuropathic pain act by supporting the high metabolic demand associated with mitochondrial respiration and/or by protecting cells from the damage caused by ROS and reactive nitrogen species (RNS).
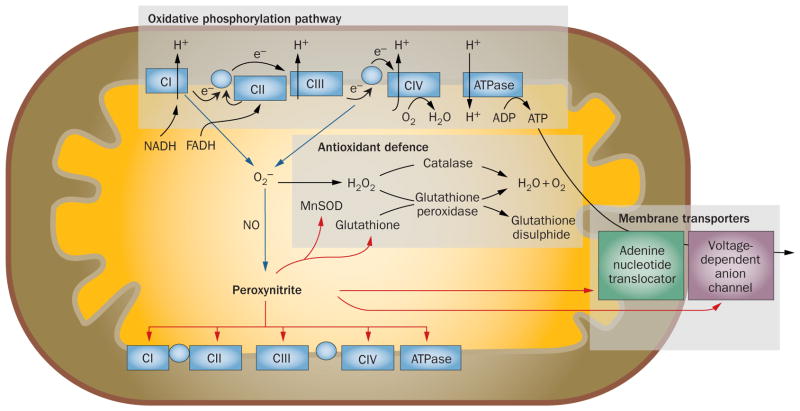
Figure 3.
Contribution of nitro-oxidative stress to mitochondrial dysfunction. During mitochondrial respiration, the oxidative phosphorylation pathway produces the proton gradient required for ATP production. Electrons leaking from the electron transport chain react with molecular oxygen to produce potentially damaging O2−, which has the potential to generate other reactive oxygen species and—via interaction with NO—peroxynitrite. Under normal conditions, the antioxidant defence system (including MnSOD, catalase, glutathione and glutathione peroxidase) adequately reduces superoxide to water and molecular oxygen;81 however, if these mechanisms are overwhelmed, formation of peroxynitrite increases.89 In turn, peroxynitrite induces a bioenergetic deficit by disrupting the activities of metabolic enzymes, mitochondrial electron transport chain proteins, ATP synthase, and membrane transport proteins.80,81 Notably peroxynitrite-induced damage to MnSOD creates a feed-forward mechanism for enhancing its own production, thus facilitating the hazardous accumulation of superoxide.80,81 Blue lines indicate pathways for the formation of reactive oxygen and reactive nitrogen species, red lines indicate potential targets of peroxynitrite-induced mitochondrial dysfunction. Abbreviations: C, complex; MnSOD, manganese superoxide dismutase; NO, nitric oxide; O2−, superoxide ion.
α-Lipoic acid
α-Lipoic acid is an endogenous metabolite with multiple effects on mitochondrial function. This compound is an essential cofactor for key enzymes of the Krebs cycle, and it restores levels of the endogenous antioxidant defence enzyme glutathione peroxidase. In addition, both oxidized α-lipoic acid and its reduced form, dihydrolipoic acid, are themselves potent antioxidants.68
In animals with CIPN or streptozocin-evoked diabetic neuropathy, α-lipoic acid treatment reverses neuropathic pain and normalizes markers of impaired peripheral nerve mitochondrial energy production.69,70 In patients with diabetic peripheral neuropathy, α-lipoic acid treatment has a long and controversial history.71 We are not aware of any reports on the effect of α-lipoic acid in patients with HIV or NRTI-evoked neuropathy, but preliminary studies suggest that α-lipoic acid treatment might be effective in CIPN. In rats, α-lipoic acid prevents and reverses the neuropathic pain induced by bortezomib and oxaliplatin,72 and a pilot study indicated that α-lipoic acid treatment was associated with improvements in a subset of patients with oxaliplatin-induced neuropathy.73
Acetyl-L-carnitine
Acetyl-L-carnitine is another endogenous molecule that contributes to mitochondrial energy production. Its metabolite, carnitine, is used to transport fatty acids into the mitochondria for β-oxidation, and acetyl-L-carnitine regulates the activities of several Krebs cycle enzymes, contributes to the regulation of mitochondrial membrane lipid composition, enhances glutathione levels, and is itself an antioxidant.74
In rats with streptozocin-induced diabetic neuropathy, acetyl-L-carnitine ameliorates pain and nerve conduction slowing, and enhances nerve regeneration.75 In paclitaxel-treated rats, acetyl-L-carnitine reverses established pain, and prophylactic treatment prevents the pain.6,28 Prophylactic treatment with acetyl-L-carnitine also prevents paclitaxel-induced swelling and vacuolation of mitochondria in primary afferent C fibres (but not in A fibres); however, it does not protect against IENF loss.28 In rats, a prophylactic dosing protocol that prevents pain induced by paclitaxel, oxaliplatin and bortezomib prevents dysfunction of mitochondrial respiration complex I and II and the associated deficits in ATP production.8,9
In patients with diabetic neuropathy, acetyl-L-carnitine treatment reverses pain and loss of vibratory sense, and promotes nerve regeneration.75 Acetyl-L-carnitine also reverses pain and IENF loss in patients with HIV76 and improves pain scores and sensory nerve conduction in patients with CIPN.77 Inexplicably, however, acetyl-L-carnitine treatment worsened CIPN in a large double-blind, placebo-controlled study in women receiving taxane treatment for breast cancer.78
Olesoxime (TRO19622)
The only known mechanisms of action of olesoxime involve binding to two mitochondrial transport proteins, the voltage-dependent anion channel and the 18 kDa translocator protein (also known as the peripheral benzodiazepine receptor).
In rats, olesoxime reverses established paclitaxel-induced pain, and prophylactic treatment significantly reduces pain and IENF loss; prophylactic dosing also significantly reduces oxaliplatin-induced pain. Moreover, olesoxime reverses established pain in animals with vincristine-induced neuropathy or streptozocin-induced diabetes.7,79 The results of the initial clinical trials of olesoxime in patients with CIPN (NCT00876538) and diabetic neuropathy (NCT00496457) have not been announced.
Targeting reactive oxygen and nitrogen species
Under normal conditions of mitochondrial respiration, a small percentage of electrons escape from the electron transport system and react with oxygen to form superoxide anions, which in turn generate other ROS (hydrogen peroxide and hydroxyl radicals), as well as the RNS peroxynitrite (Figure 3). Mitochondrial dysfunction is expected to lead to an increase in the escape of free electrons and, thus, an increase in both ROS and RNS.80,81 Importantly, superoxide anions are created within the mitochondrial matrix, and the mitochondrial inner membrane is relatively impermeable to them; consequently, the respiratory complex proteins and ATP synthase within the mitochondrial matrix are exposed to especially high ROS and RNS concentrations.80,81 In addition, these reactants can diffuse through the membrane and produce fast and direct damage to proteins in all cellular compartments.82
The mitochondrial matrix contains several enzymes that defend against nitro-oxidative damage.80,81 Importantly, in the presence of high RNS levels, these enzymes are themselves vulnerable to incapacitating nitro-oxidative damage.80,81 The result is a positive feedback loop that intensifies injury to the energy production system. In this sense, nitro-oxidative stress can be considered to be both a consequence and a cause of CIPN-induced mitotoxicity.
Reactive oxygen species
Increased production of hydrogen peroxide and decreased synthesis of the mitochondrial antioxidant enzyme glutathione peroxidase have been found in the primary afferent cell body in rats treated with paclitaxel.30 Paclitaxel-induced allodynia and hyperalgesia are reversed by α-phenyl-tert-N-butyl nitrone (PBN), a nonselective ROS blocker, and by 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, a nonselective superoxide anion scavenger.83 Moreover, administration of PBN during paclitaxel exposure prevents the development of hypersensitivity to pain.83 SS-31, a novel antioxidant that localizes within mitochondria, prevents and treats oxaliplatin-induced neuropathy.84 Treatment with N-acetylcysteine, itself a potent lipophilic antioxidant and a precursor to the endogenous antioxidant, glutathione, prevents pain in rats with streptozocin-induced diabetes and protects the activity of endogenous antioxidant enzymes in animal models of diabetic peripheral neuropathy.85 Treatment with N-acetylcysteine86 and glutathione87 decreases the incidence and severity of neuropathy in patients treated with platinum-complex chemotherapeutic agents.
Reactive nitrogen species
Peripheral nerves harvested from rats with painful peripheral neuropathy induced by paclitaxel, oxaliplatin or bortezomib contain significantly elevated levels of nitrated manganese superoxide dismutase (MnSOD), a key mitochondrion-specific antioxidant enzyme.29 As a result, MnSOD activity is greatly reduced. Levels of CuZn superoxide dismutase, the cytosol-specific isoform of the enzyme, are not affected.29 Treatment with the manganese porphyrin MnTE-2-PyP5+, a superoxide dismutase mimetic and peroxynitrite decomposition catalyst, prevents and reverses chemotherapy-induced pain symptoms, nitration of MnSOD, the decrease in MnSOD activity, and the chemotherapy-induced deficit in ATP production.29,88
Peroxynitrite is generated as a result of interactions between superoxide and nitric oxide;89 thus, reduction of nitric oxide levels via inhibition of nitric oxide synthase ought to be an effective treatment for CIPN. The nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (l-NAME) prevents paclitaxel-induced neuropathic pain.88 However, l-NAME blocks all nitric oxide synthase isoforms, and selective inhibition of inducible nitric oxide synthase, the isoform associated with pathology, is preferable. Selective inhibitors of inducible nitric oxide synthase have analgesic effects in animal models of post-traumatic neuropathy and are also expected to be effective in distal symmetrical sensory peripheral neuropathies.90
Peroxynitrite decomposition catalysts, molecules that decompose peroxynitrite as soon as it is formed, are under development as analgesic agents (Figure 4). Two highly selective peroxynitrite decomposition catalysts can prevent the development of pain in rats treated with paclitaxel, oxaliplatin or bortezomib.88
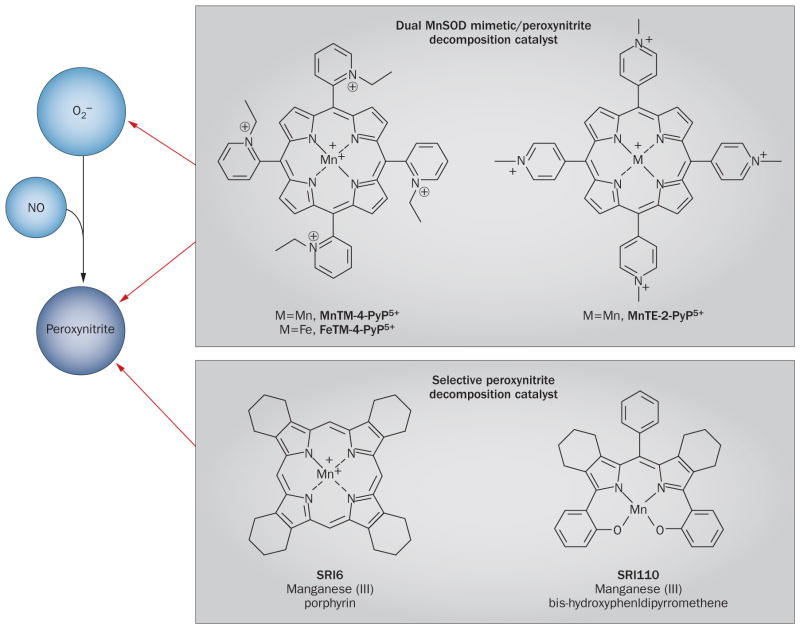
Figure 4.
Peroxynitrite decomposition catalysts. These porphyrin-based agents, typified by MnTM-4-PyP5+, FeTM-4-PyP5+ and MnTE-2-PyP5+, act as dual MnSOD mimetics and peroxynitrite decomposition catalysts to eliminate peroxynitrite as well as potentially beneficial superoxide. The newer selective agents, SRI6 and SRI110, eliminate peroxynitrite while sparing superoxide. The charged metal centre of SRI6 is shielded by β-fused cyclohexenyl substituents, rendering it membrane-permeable and orally bioavailable.92 Moreover, the charged electron-withdrawing functionality of other agents such as FeTMPyP5+ is absent from SRI6, which reduces its MnSOD mimetic activity.92 SRI110 is also orally bioavailable owing to its electroneutrality.93 Through a two-electron reduction mechanism, SRI110 reacts with peroxynitrite to produce a manganese (V)–oxo intermediate with concomitant reduction of peroxynitrite to nitrite.93 The manganese (III) catalyst is then regenerated by endogenous reductants, completing a reductase-type cycle.93 Abbreviations: MnSOD, manganese superoxide dismutase; NO, nitric oxide; O2−, superoxide ion.
Summary and conclusions
Extensive evidence, mostly from work in experimental animal models, supports the hypothesis that mitochondrial dysfunction in primary afferent sensory neurons is the fundamental causative factor in the chronic distal symmetrical sensory peripheral neuropathies seen in patients receiving cancer chemotherapeutic drugs. Strong evidence also supports mitotoxicity as the cause of the peripheral neuropathy seen in NRTI-treated patients with HIV; however, the evidence for a role for mitotoxicity in diabetic peripheral neuropathy is less clear.
The mechanisms whereby mitochondria are damaged probably differ for CIPN, HIV and diabetes, but the result is the same—a bioenergetic deficit that causes degeneration of IENFs and abnormal spontaneous discharges—which accounts for the similarity of the symptoms. Abnormal spontaneous discharges in C-fibre nociceptors result in increased release of neurotransmitters, including glutamate, in neurons involved in nociception, which evokes central sensitization—a state of hyperexcitability in spinal cord neurons.91 Of note, mitotoxicity-evoked degeneration is a compartmental phenomenon, and neurons are well known to have compartment-specific mechanisms of degeneration that are engaged at different threshold levels of exposure to toxins.26 In distal symmetrical peripheral neuropathies, IENFs are the first compartment to succumb, but we have no reason to doubt that increased or prolonged energy deficits will cause degeneration of axons in the peripheral nerve, and perhaps degeneration of the afferent fibres’ soma in the DRG. Moreover, the fact that exposure to a mitotoxic chemical can increase pain and spontaneous neuronal discharges within minutes is worthy of note.7 Such an effect cannot be caused by IENF degeneration, but clearly indicates a link between these discharges and the axonal energy supply.
The mitotoxicity hypothesis predicts that drugs that protect or improve mitochondrial function will be useful treatments for the chronic distal symmetrical sensory peripheral neuropathies themselves, as well as for the accompanying neuropathic pain. Preliminary data support this prediction, but whether these favourable findings can be translated to the clinic remains to be seen.
Review criteria.
We searched the PubMed database using the following search terms: “neuron AND mitochondria”, “chemotherapy neuropathy AND mitochondria”, “HIV neuropathy AND mitochondria”, and “diabetic neuropathy AND mitochondria”. This search was supplemented by inspection of the reference lists of relevant papers to identify further articles.
Acknowledgments
We regret that space limitations prevented us from giving proper acknowledgement in the reference list of the many investigators who have contributed to this field. To compensate, we have attempted to cite recent articles that do reference their contributions.
Footnotes
Competing interests
D.S. and the Saint Louis University School of Medicine have patents concerning the use of superoxide dismutase mimetics and peroxynitrite decomposition catalysts for the treatment and prevention of neuropathic pain (WO 2012033916 A1 20120315; US 20080318917 A1 20081225; WO 2005060437 A2 20050707; US 6214817 B1 20010410; WO 9858636 A1 19981230). G.J.B. and T.D. declare no competing interests.
Author contributions
All three authors provided substantial contributions to discussions of the content of the manuscript, researched the literature, and participated in writing, editing and revision of the manuscript.
References
- 1.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 2.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593–1599. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centner CM, Bateman KJ, Heckmann JM. Manifestations of HIV infection in the peripheral nervous system. Lancet Neurol. 2013;12:295–309. doi: 10.1016/S1474-4422(13)70002-4. [DOI] [PubMed] [Google Scholar]
- 5.Lauria G, Merkies IS, Faber CG. Small fibre neuropathy. Curr Opin Neurol. 2012;25:542–549. doi: 10.1097/WCO.0b013e32835804c5. [DOI] [PubMed] [Google Scholar]
- 6.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153:704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011;232:154–161. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol. 2012;238:225–234. doi: 10.1016/j.expneurol.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol. 2005;146:1027–1039. doi: 10.1038/sj.bjp.0706407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loprinzi CL, et al. The Paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechanism. Cancer J. 2007;13:399–403. doi: 10.1097/PPO.0b013e31815a999b. [DOI] [PubMed] [Google Scholar]
- 12.Kleggetveit IP, et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153:2040–2047. doi: 10.1016/j.pain.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135:262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao WH, et al. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience. 2011;199:461–469. doi: 10.1016/j.neuroscience.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han C, et al. The G1662S NaV1.8 mutation in small fibre neuropathy: impaired inactivation underlying DRG neuron hyperexcitability. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2013-306095. http://dx.doi.org/10.1136/jnnp-2013-306095. [DOI] [PubMed]
- 18.Argyriou AA, et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: results from a prospective multicenter study. Cancer. 2013;119:3570–3577. doi: 10.1002/cncr.28234. [DOI] [PubMed] [Google Scholar]
- 19.Boyette-Davis JA, et al. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017–1024. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koskinen MJ, et al. Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 2011;31:4413–4416. [PubMed] [Google Scholar]
- 21.Loseth S, Stalberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255:1197–1202. doi: 10.1007/s00415-008-0872-0. [DOI] [PubMed] [Google Scholar]
- 22.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 23.Polydefkis M, et al. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–119. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 25.Nebuchennykh M, Loseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol. 2009;256:1067–1075. doi: 10.1007/s00415-009-5065-y. [DOI] [PubMed] [Google Scholar]
- 26.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration—a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33:1667–1676. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janes K, et al. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain. 2013;154:2432–2440. doi: 10.1016/j.pain.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barriere DA, et al. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 2012;153:553–561. doi: 10.1016/j.pain.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121:105–114. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Kokotis P, et al. Polyneuropathy induced by HIV disease and antiretroviral therapy. Clin Neurophysiol. 2013;124:176–182. doi: 10.1016/j.clinph.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC) Lab Invest. 2001;81:1537–1544. doi: 10.1038/labinvest.3780367. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann HC, Chen W, Borzan J, Mankowski JL, Höke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69:100–110. doi: 10.1002/ana.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campenot RB, Eng H. Protein synthesis in axons and its possible functions. J Neurocytol. 2000;29:793–798. doi: 10.1023/a:1010939307434. [DOI] [PubMed] [Google Scholar]
- 36.Mocchetti I, Bachis A, Avdoshina V. Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotox Res. 2012;21:79–89. doi: 10.1007/s12640-011-9279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 38.Wallace VC, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130:2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamerman PR, et al. Pathogenesis of HIV-associated sensory neuropathy: evidence from in vivo and in vitro experimental models. J Peripher Nerv Syst. 2012;17:19–31. doi: 10.1111/j.1529-8027.2012.00373.x. [DOI] [PubMed] [Google Scholar]
- 40.Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- 41.Norman JP, et al. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS ONE. 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecoeur H, et al. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis. 2012;3:e282. doi: 10.1038/cddis.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deniaud A, Brenner C, Kroemer G. Mitochondrial membrane permeabilization by HIV-1 Vpr. Mitochondrion. 2004;4:223–233. doi: 10.1016/j.mito.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Huang CY, Chiang SF, Lin TY, Chiou SH, Chow KC. HIV-1 Vpr triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction. PLoS ONE. 2012;7:e33657. doi: 10.1371/journal.pone.0033657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitayama H, et al. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J Virol. 2008;82:2528–2542. doi: 10.1128/JVI.02094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung GP. Iatrogenic mitochondriopathies: a recent lesson from nucleoside/nucleotide reverse transcriptase inhibitors. Adv Exp Med Biol. 2012;942:347–369. doi: 10.1007/978-94-007-2869-1_16. [DOI] [PubMed] [Google Scholar]
- 47.Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson B, Li Z, Nath A. Nucleoside reverse transcriptase inhibitors and human immunodeficiency virus proteins cause axonal injury in human dorsal root ganglia cultures. J Neurovirol. 2007;13:160–167. doi: 10.1080/13550280701200102. [DOI] [PubMed] [Google Scholar]
- 49.Van Steenwinckel J, et al. Role of spinal serotonin 5-HT2A receptor in 2′,3′-dideoxycytidine-induced neuropathic pain in the rat and the mouse. Pain. 2008;137:66–80. doi: 10.1016/j.pain.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Huang W, et al. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain. 2013;154:560–575. doi: 10.1016/j.pain.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression. Brain. 2007;130:2011–2023. doi: 10.1093/brain/awm148. [DOI] [PubMed] [Google Scholar]
- 52.Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol. 2012;25:536–541. doi: 10.1097/WCO.0b013e328357a797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 54.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury SK, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56–65. doi: 10.1016/j.nbd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 57.Hinder LM, Vincent AM, Burant CF, Pennathur S, Feldman EL. Bioenergetics in diabetic neuropathy: what we need to know. J Peripher Nerv Syst. 2012;17(Suppl 2):10–14. doi: 10.1111/j.1529-8027.2012.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36:439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Eberhardt MJ, et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J Biol Chem. 2012;287:28291–28306. doi: 10.1074/jbc.M111.328674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernyhough P, Roy Chowdhury SK, Schmidt RE. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev Endocrinol Metab. 2010;5:39–49. doi: 10.1586/eem.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy Chowdhury SK, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–1766. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowdhury SK, et al. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes. 2010;59:1082–1091. doi: 10.2337/db09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akude E, et al. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes. 2011;60:288–297. doi: 10.2337/db10-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban MJ, et al. Modulating molecular chaperones improves sensory fiber recovery and mitochondrial function in diabetic peripheral neuropathy. Exp Neurol. 2012;235:388–396. doi: 10.1016/j.expneurol.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleh A, et al. Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology. 2013;65:65–73. doi: 10.1016/j.neuropharm.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent AM, et al. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120:477–489. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011;2:69. doi: 10.3389/fphar.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-α-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 70.Melli G, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214:276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Boulton AJ, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29:327–333. doi: 10.1002/dmrr.2397. [DOI] [PubMed] [Google Scholar]
- 72.Trevisan G, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73:3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- 73.Gedlicka C, Scheithauer W, Schull B, Kornek GV. Effective treatment of oxaliplatin-induced cumulative polyneuropathy with alpha-lipoic acid. J Clin Oncol. 2002;20:3359–3361. doi: 10.1200/JCO.2002.99.502. [DOI] [PubMed] [Google Scholar]
- 74.Chiechio S, Copani A, Nicoletti F, Gereau RW. 4th. L-acetylcarnitine: a proposed therapeutic agent for painful peripheral neuropathies. Curr Neuropharmacol. 2006;4:233–237. doi: 10.2174/157015906778019509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sima AA, Calvani M, Mehra M, Amato A Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- 76.Youle M, Osio M ALCAR Study Group. A double-blind, parallel-group, placebo-controlled, multicentre study of acetyl L-carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV-1 infection. HIV Med. 2007;8:241–250. doi: 10.1111/j.1468-1293.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 77.Bianchi G, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41:1746–1750. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 78.Hershman DL, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31:2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147:202–209. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 81.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 82.Drose S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 83.Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE. 2011;6:e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toyama S, et al. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology. 2014;120:459–473. doi: 10.1097/01.anes.0000435634.34709.65. [DOI] [PubMed] [Google Scholar]
- 85.Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem. 2010;112:77–91. doi: 10.1111/j.1471-4159.2009.06435.x. [DOI] [PubMed] [Google Scholar]
- 86.Lin PC, et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer. 2006;14:484–487. doi: 10.1007/s00520-006-0018-9. [DOI] [PubMed] [Google Scholar]
- 87.Cascinu S, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002;20:3478–3483. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 88.Doyle T, et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci. 2012;32:6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Payne JE, et al. Discovery of dual inducible/neuronal nitric oxide synthase (iNOS/nNOS) inhibitor development candidate 4-((2-cyclobutyl-1H-imidazo[4,5-b]pyrazin-1-yl)methyl)-7, 8-difluoroquinolin-2(1H)-one (KD7332) Part 2: identification of a novel, potent, and selective series of benzimidazole-quinolinone iNOS/nNOS dimerization inhibitors that are orally active in pain models. J Med Chem. 2010;53:7739–7755. doi: 10.1021/jm100828n. [DOI] [PubMed] [Google Scholar]
- 91.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rausaria S, et al. Retooling manganese(III) porphyrin-based peroxynitrite decomposition catalysts for selectivity and oral activity: a potential new strategy for treating chronic pain. J Med Chem. 2011;54:8658–8669. doi: 10.1021/jm201233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rausaria S, et al. Manganese(III) complexes of bis(hydroxyphenyl)dipyrromethenes are potent orally active peroxynitrite scavengers. J Am Chem Soc. 2011;133:4200–4203. doi: 10.1021/ja110427e. [DOI] [PMC free article] [PubMed] [Google Scholar]