Abstract
Purpose
Common variable immunodeficiency (CVID) is the most frequent form of primary symptomatic hypogammaglobulinemia. CVID patients display a number of abnormalities in lymphocyte subpopulations including chronic T-cell activation and decreased numbers of circulating CD4+ T cells and NK cells. We and others have recently shown that CVID is associated with increased concentration of soluble CD14 (sCD14) and other factors indicating limited microbial translocation.
Methods
To address the mechanisms of chronic immune activation in CVID, we performed a detailed analysis of cytokine serum levels in 36 patients with CVID, 52 patients with selective IgA deficiency (IgAD), and 56 healthy volunteers.
Results
We show that CVID is associated with elevated serum levels of CXCL-10/IP-10, IL-1R antagonist, TNF-α, IL-10, IL-12( p40), CCL-2/MCP-1, G-CSF, and CCL-11/eotaxin. The detected cytokine signature is consistent with an ongoing activation of cells of myeloid lineage. In contrast, the levels of cytokines typically produced by CD4+ T helper cells of Th1 (IFN-γ, IL-2), Th2 (IL-9, IL-13), and Th17 (IL-17) subtypes were suppressed in CVID patients compared to healthy donors.
Conclusions
Presented data suggest that the altered cytokine profile observed in patients with CVID may be attributed to the activation of monocyte-macrophage and granulocyte lineages, possibly driven by the translocation of bacterial components across the gastrointestinal or respiratory tracts mucosal barrier.
Keywords: Common variable immunodeficiency, IgA deficiency, cytokine, lymphocyte activation
Introduction
The most frequent form of primary symptomatic hypogammaglobulinemia, common variable immunodeficiency (CVID), is a group of conditions characterized by the disturbed specific antibody response, decreased serum IgG and IgA levels in the presence of variable levels of IgM. CVID patients suffer from clinically significant immunodeficiency that manifests by frequent and severe respiratory tract infections, diarrhea and also autoimmune disorders [1]. Selective IgA deficiency (IgAD) is defined by serum IgA levels below 0.07 g/l in an absence of other disturbances in immunoglobulin production [2]. IgAD patients display higher frequency of autoimmune diseases; however, only a minority of these patients display immunodeficiency symptoms. Mutations in genes coding for CD19, CD20, CD21, CD81, inducible T-cell costimulator (ICOS), B-cell activating factor-receptor (BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) were observed in CVID [3], but, with the exception of TACI, not in IgAD. However; frequent familiar occurrence of both IgAD and CVID and repeatedly reported progression IgAD to CVID suggest a common genetic trait [4].
Studies by us and others have previously demonstrated a number of abnormalities in lymphocyte subpopulations in CVID patients including chronic T-cell activation indicated by an increased expression of activation markers HLA-DR and CD38 and decreased numbers of CD4+ T- cells and NK cells in the systemic circulation [5–10]. The mechanisms underlying systemic T-cell activation in CVID patients are not well understood. We have recently demonstrated that CVID is associated with increased concentration of soluble cluster of differentiation (sCD)14, C-reactive protein (CRP), and other factors consistent with limited microbial translocation[5]. Similar results were recently reported by Barbosa et al. who showed that in addition to elevated sCD14, CVID patients display chronic monocytic activation [11].
Microbial translocation is a process of transfer of commensal microbial products from the intestinal lumen into systemic circulation in an absence of overt bacteraemia. Low level of microbial translocation occurs in healthy individuals; however, its extent dramatically increases in various pathological conditions including inflammatory bowel disease, coeliac disease, visceral leishmaniasis, dengue virus infection, HIV infection, hepatic cirrhosis caused by alcohol abuse or hepatitis B and C infections [12]. Translocation of bacterial and fungal products result in an activation of both innate and acquired immune response mechanisms [12]. Although intestinal symptoms are frequent in CVID [13], the extent of potential damage to gut epithelial barrier in CVID patients is currently unknown.
In this report we sought to determine whether chronic immune activation in CVID and IgAD is associated with significantly altered serum levels of cytokines and chemokines.
Methods
Study population
The study includes 36 patients with CVID (age range 19–78 years, median 45 years, 24 females, 12 males), 52 patients with IgAD (age range 18–63 years, median 32.5 years, 35 females, 17 males) and 56 healthy volunteers without any known immunopathological condition (age range 18–71 years, median 31 years, 33 females, 23 males). All CVID and IgAD patients fulfilled ESID/PAGID diagnostic criteria [14]. Of 36 CVID patients, 28 were on intravenous immunoglobulin (IVIG) in a dose 170 to 440 mg/kg/3–4 weeks (trough IgG levels ranging 3.1–8.3 g/l), 5 on subcutaneous immunoglobulin in a dose 60 – 123 mg /kg/week (IgG levels ranging 5.3–7.7 g/l ), 1 on intramuscular immunoglobulin replacement treatment (40 mg/kg/week, IgG level 3,0 g/l), and 2 were not on immunoglobulin replacement treatment (IgG levels 3.5 and 2.4 g/l). Three patients were on regular antibiotic prophylaxis treatment at the time of blood collection. In the case of patients on IVIG treatment, blood samples were collected before the IVIG infusion. In one patient p. C104R mutation of the TNFRSF13B gene (coding for TACI) was observed; no mutations in ICOS (performed in 20 patients), BAFF-R (performed in 4 patients) genes were recorded.
Twenty CVID patients suffered from bronchiectasis (as determined by the high resolution computed tomography - HRCT), 26 patients displayed splenomegaly defined as spleen length more than 11 cm as determined by sonography, 12 suffered of chronic diarrhea, granuloma formation was detected in 8 subjects. In 5 patients a hepatopathy determined as an increase of aspartate aminotrasnferase (AST) and/or alanine aminotransferase (ALT) above the local reference value was documented. Seven patients suffered from autoimmune diseases (3 atrophic gastritis, 2 hypothyroidism, 1 vitiligo + atrophic gastritis, 1 hypothyroidism + atrophic gastritis). Using the EURO-CLASS classification [15], 21 patients belonged to group smB-21lo; 6 patients to group smB-21norm; 5 patient to group smB+21lo; 1 patients to smB+21norm. In 3 patients the number of B-cells was < 1% of peripheral blood mononuclear cells. Nine patients displayed absolute CD4+ count <400 106/l. None of the patients suffered from opportunistic infections typical for late-onset combined immunodeficiency (LOCID)[16]. One patient was treated by steroids (methyprednisolone 4mg every other day) for lymphocytic interstitial pneumonia. No patient was under cytostatic treatment at the time or prior to the study
All study subjects included in the study were Caucasians of Moravian origin (eastern part of the Czech Republic). All samples were collected during apparent acute infection-free period defined as worsening cough, rhinitis, or presence of new symptoms suspicious of respiratory, urinary or gastrointestinal tract infections or significant increase in CRP above the levels typically observed in the given patient. The study was approved by the St Annés University Hospital Ethic Committee (protocol number 12G/2009); all patients gave informed consent before inclusion into the study, the study was performed according to the Declaration of Helsinki.
MILLIPLEX cytokine/chemokine assay
Concentrations of cytokines and chemokines were determined using the 39-plex kit of MILLIPLEX Human Cytokine/Chemokine Panel (Millipore) and samples were analyzed undiluted on a Bioplex 100 system with Bioplex Manager Software 5.0 (Biorad, Hercules, CA). The cytokines/chemokines detected in this kit includes: interleukin (IL)-1α, IL-1β, IL-1receptor antagonist (RA), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7-IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, interferon (IFN)γ, IFNα 2, granulocyte-macrophage (GM) colony stimulating factor (CSF), granulocyte (G)-CSF, tumor necrosing factor (TNF) α, TNF β, transforming growth factor (TGF) α, soluble (s)CD40 ligand (L), vascular endothelial growth factor (VEGF), C-C motif chemokine ligand (CCL)-3/ macrophage inflammatory protein (MIP)-1 α, CCL-4/ MIP-1 β, CCL-22/macrophage-derived chemokine (MDC), CCL-2/monocyte chemotactic protein (MCP)-1, CCL7/MCP-3, CXC Motif Chemokine Ligand (CXCL)-10/ interferon gamma-induced protein (IP)-10, CXCL-1/ growth regulated oncogene (GRO), CX3CL-1/ fractalkine, CCL-11/ eotaxin, Fms-like tyrosine kinase(Flt)-3 ligand, epidermal growth factor (EGF), EGF-2, and sCD 25 (sIL-2 receptor).
Determination of lymphocyte populations and expression of activation markers
The frequency of lymphocyte subsets and expression of activation markers was determined by five-color flow cytometry on FC 500 MCL cytometer (Beckman Coulter, Inc Miami, FL) using standard procedures for direct immunofluorescence. For the purpose of this study the following monoclonal antibodies-fluorochrome combinations were used: phycoerythrin-cyanine 5 (PC5) labeled CD3, phycoerythrin-cyanine 7 (PC7)-labeled anti-CD4 and CD8, phycoerythrin-texas red labeled CD45RO (all from Beckman Coulter) and fluorescein isothiocyanate (FITC) labeled HLA-DR, (BD Bioscences, San Jose, CA ).
Data analysis
Data were analyzed using Mann-Whitney rank sum and Spearman rank order correlation tests. The adjustments for multiple comparisons of cytokine expression analysis was not applied as it would result in an increase in the frequency of the null hypothesis being valid (Type II error) when the association in the data is not a result of chance [17]. The correction for the Type I error applies only to the universal null hypothesis, that is that the two groups are identical on all variables analyzed [18]. Altered levels of cytokine expression in CVID patients have been reported previously [19–21]; therefore a valid association was not null. Furthermore, there is no empirical justification for a hypothesis that all the associations observed are unpredictable manifestations of random processes [17]. CVID causes systemic interdependent effects on cytokine and chemokine expression and is likely to simultaneously upregulate and inhibit the production of multiple factors. Therefore, the null hypothesis does not apply and an application of Bonferroni adjustment would result in amplification of the Type II error [17]. Statistical packages GraphPad Prism 5, SigmaStat 3.1, and STATISTICA were used. A standard level of statistical significance α =0.05 was used.
Results
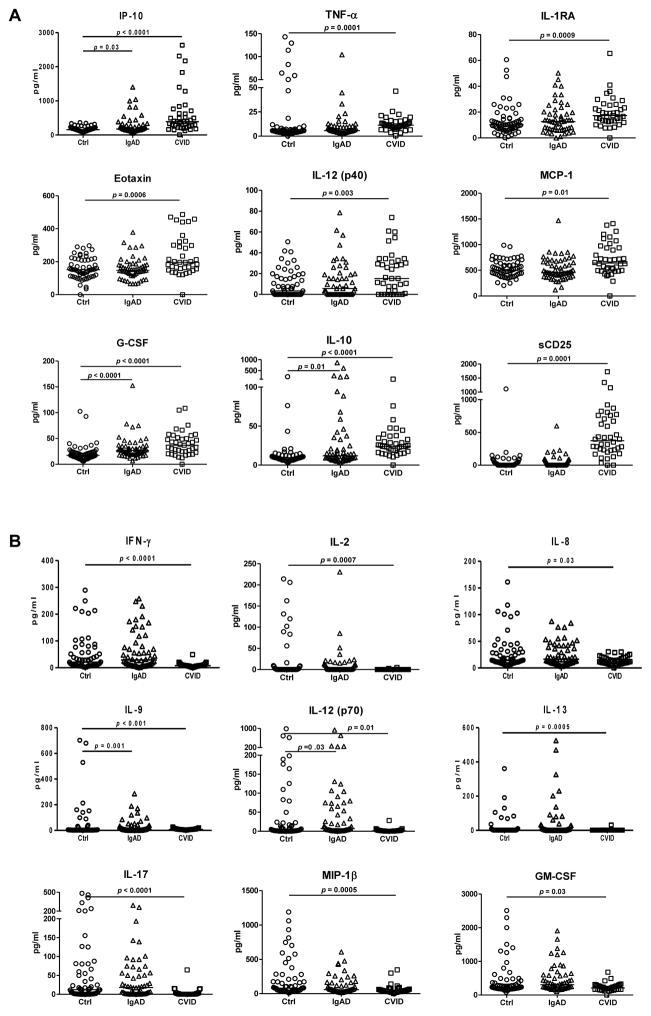
To address the mechanisms of chronic immune activation in CVID, we performed a detailed analysis of cytokine serum levels in patients with CVID and IgAD, and healthy volunteers. Of 39 cytokines and chemokines analyzed, 9 factors were significantly upregulated and 9 downregulated in the serum of CVID patients (Fig. 1). The spectrum of cytokines up-regulated in CVID patients was consistent with an ongoing activation of cells of myeloid lineage including monocytes, macrophages and neutrophils (CXCL-10/IP-10, IL-1R antagonist, TNF-α, IL-10, IL-12 p40, CCL-2/MCP-1, G-CSF, CCL-11/eotaxin). In contrast, the levels of cytokines typically produced by T helper cells of Th1 (IFN-γ, IL-2), Th2 (IL-9, IL-13) and Th17 (IL-17) subtypes were present at lower quantities in CVID patients compared to healthy donors. The results of sCD25 determination in the patients and control groups partially overlapped set of data published elsewhere [5]. Importantly, a 10-fold increase in plasma concentration of sCD25 was observed in plasma of CVID but not IgAD patients. In individuals with IgAD, serum levels of CXCL-10/IP-10, IL-10, and G-CSF were significantly higher compared to healthy donors (Fig. 1).
Figure 1. Analysis of cytokine profile in the serum of CVID and IgA patients.
Serum concentration of factors that are upregulated (A) or downregulated (B) in CVID and IgA patients are presented. Horizontal line indicates median; Mann-Whitney rank sum test was used for statistical evaluation.
Since hepatocytes have been reported to produce both CXCL-10/IP-10 [22] and IL-1R antagonist [23, 24], we have correlated the levels of those mediators to serum AST and ALT levels. A significant direct correlation between CXCL-10/IP-10 and serum AST levels was detected (R= 0.387, p=0.03, Spearman rank order correlation test), no other significant correlations were observed (data not presented).
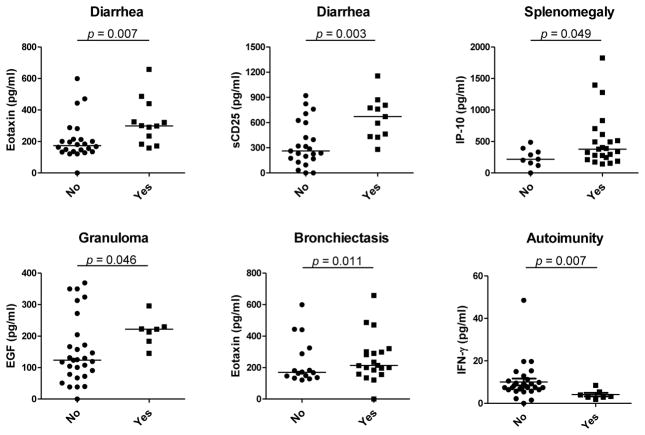
Serum cytokine levels in CVID patients were analyzed in relation to key clinical characteristics including the presence of bronchiectasis, splenomegaly, chronic diarrhea, granuloma, hepatopathy, and autoimmunity. Significant differences are shown on Fig. 2. There was an increase in CCL-11/eotaxin and sCD25 levels in patients with diarrhea, elevated CXCL-10/IP-10 levels in patients with splenomegaly, increase in EGF in patients with granuloma, increase in CCL-11/eotaxin levels in patients with bronchiectasis, and decrease in IFN- γ levels in patients with autoimmunity compared to patients without these clinical complications; no other significant difference was detected.
Figure 2. Significant differences in serum levels of determined cytokines/chemokines in patients with or without diarrhea, splenomegaly, granuloma, bronchiectasis, and autoimmunity.
There were no differences comparing patients with and without hepatopathy. Horizontal line indicates median, Mann-Whitney rank sum test was used for statistical evaluation.
The correlations between serum cytokine levels and presence of activation markers (expression of HLA-DR and CD45RO) on CD8+ and CD4+ T cells are presented in Table 1. In general, the expression of cytokines that are primarily produced by activated cells of myeloid lineage including TNF-α, G-CSF, CXCL-10/IP-10, Flt-3L, and IL-10 directly correlated with the expression of lymphocyte activation markers. Furthermore, the levels of sCD25 inversely correlated with absolute CD4+ count (R= −0.571; p= 0.03).
Table 1. Correlations between determined serum cytokine levels and the expression of activation markers (HLA-DR and CD45R0) on peripheral blood CD4+ and CD8+ lymphocytes of CVID patients.
No significant correlations were observed for IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-7, IL-8, IL-9, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IFNα2, GM-CSF, TNFβ, TGFα, sCD40-L, VEGF, CCL-3/MIP-1α, CCL-4/MIP-1β, CCL-22/MDC, CCL-2/MCP-1, CCL-7/MCP-3, GRO, fractalkine, EGF, EGF-2, and CCL-11/eotaxin. Spearman rank order correlation was used for the analysis, Spearman coefficient R is indicated; results with p<0.05 are in bold letters.
| CD4+HLA-DR+/CD4+ | CD8+HLA-DR+/CD8+ | CD4+CD45RO+/CD4+ | CD8+CD45RO+/CD8+ | |||||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | |
| Flt3Ligand | 0.38 | 0.02 | 0.27 | 0.11 | 0.45 | 0.01 | 0.01 | 0.94 |
| G-CSF | 0.37 | 0.03 | 0.26 | 0.13 | 0.42 | 0.01 | 0.25 | 0.15 |
| IFNγ | 0.21 | 0.23 | 0.35 | 0.04 | 0.09 | 0.58 | −0.01 | 0.97 |
| IL-1RA | 0.35 | 0.04 | 0.26 | 0.14 | 0.20 | 0.24 | 0.39 | 0.02 |
| IL-5 | 0.33 | 0.05 | 0.35 | 0.04 | 0.23 | 0.18 | −0.07 | 0.67 |
| IL-6 | 0.37 | 0.03 | 0.37 | 0.03 | 0.34 | 0.05 | 0.13 | 0.45 |
| IL-10 | 0.37 | 0.03 | 0.06 | 0.72 | 0.44 | 0.01 | 0.06 | 0.74 |
| IP-10 | 0.60 | <0.01 | 0.42 | 0.02 | 0.51 | <0.01 | 0.36 | 0.04 |
| sCD25 | 0.35 | 0.04 | 0.19 | 0.28 | 0.20 | 0.25 | 0.37 | 0.03 |
| TNFα | 0.39 | 0.02 | 0.40 | 0.02 | 0.29 | 0.09 | −0.01 | 0.96 |
The differences in cytokine levels in relation to the classification of patients according to EUROclass criteria [15] were analyzed using Kruskal-Wallis analysis. Statistically significant differences were observed for IL-12 (p40) (p=0.02): increase in group smB+21lo compared to groups smB-21lo (p=0.002,), smB-21norm (p=0.01), and low B-cells (p=0.02), and sIL-2R (p=0.01): increase in group smB-21lo compared to groups smB-21norm (p=0.05) and smB+21lo (p=0.01).
Discussion
The studies presented here have demonstrated significantly increased serum levels of cytokines and chemokines TNF-α, CXCL-10/IP-10, IL-1RA, IL-10, IL-12 (p40), CCL-2/MCP-1, G-CSF, sCD25, and CCL-11/eotaxin in CVID patients compared to healthy donors. These factors are either directly produced by or are associated with a recruitment and activation of cells of myeloid lineage including monocytes, macrophages, and neutrophils. Activation of monocytes and other myeloid cells is an important contributing factor in the pathogenesis of multiple inflammatory conditions [25]. Chronic monocyte activation represents a characteristic feature of CVID [11]. We and others have recently suggested that systemic immune activation in CVID is induced by a local damage to the gastrointestinal tract barrier and ensuing microbial translocation as evidenced by increased serum levels of sCD14 and CRP [5, 11]. It is plausible that in hypogammaglobulinemic patients microbial components are translocated across the epithelial barrier of other organs including lung, bronchi, and sinuses. Interestingly, microbial translocation caused by the damage to intestinal epithelial barrier represents a driving mechanism of monocyte activation in acquired immunodeficiency syndrome (AIDS) [26, 27] and possibly other pathological conditions [12]. Increased frequency of activated monocytes was also reported in patients with X-linked agammaglobulinemia [28]. In summary, the accumulated data are consistent with the hypothesis that microbial translocation may be the driving force of chronic myeloid and, subsequently, lymphoid activation in CVID.
Altered levels of cytokine production in CVID have been previously reported. Rezaei et al. observed in 24 CVID patients increased serum IL-4 and IL-10 but not IL-2 and IFN-γ levels, suggesting Th2 predominance in CVID patients [20]. Kasztalska et al. documented increased IL-2 and IL-10 plasma levels [21]. In another study, serum levels of IL-6, IL-8, IL-1RA and TNF- α, but not of IL-2 and IL-1 were increased in 29 CVID patients compared to healthy controls [19]. Aukrust et al. observed increased serum levels of IL-4 and IL-6 but not of TNF α and IL-1 in 25 patients with CVID [29]. Increased plasma level of IL-7 and impaired response to this cytokine was observed in a subset of CVID patients [30].
Our observation of increased levels of IL-10, IL-RA, TNF-α in CVID patients is consistent with some of the previously published observations. However, in contrast to the study of Rezai et al. [20], we do not attribute the abnormalities in cytokine levels to Th2 predominance but rather to activation of monocyte-macrophage population in CVID, as IL- 10 is not produced exclusively by TH2 cells, but also by monocytes and macrophages that are considered to be an important producer population of this cytokine [31]. This is further supported by previous observations of increase in serum CCL-19/MIP-3b and CCL-21 in CVID, the increase in CCL-19 being higher in patients with bronchiectasis compared to patients without bronchiectasis [32].
Serum levels of IL-12 (measured p40 and p70 together, but not IL-12p70 measured separately) were previously reported to be increased in CVID [33]. The data presented here are consistent with a report by Cambronero et al. showing that monocytes from patients with CVID express elevated levels of IL-12 p40 [34]. IL-12 p40 subunit, a protein shared by IL-12 and IL-23, is produced primarily by macrophages. The p40 homodimer is a potent inhibitor of the effect of IL-12 and IL-23 heterodimers [35, 36]. In conjunction with IL-6 and TGF-β1, IL-23 stimulates naive CD4+ T cells to differentiate into Th17 cells. Antagonistic effect of IL-23 p40 homodimer may be partially responsible for the inhibition of IL-17 production in CVID patients by interference with IL-23 signaling pathway. Consistent with this data, Barbosa et al. have recently reported that patients with CVID and congenital agammaglobulinemia display severe reduction of frequency of circulating Th17 T cells [37]. However, more detailed studies are needed to understand the role of IL-23 p40 homodimer and Th17 cells in CVID.
The levels of CXCL-10/IP-10, originally identified as a cytokine produced by monocytes in response to IFN-γ, were shown to be increased in hepatitis C virus (HCV) infection where they correlate with HCV viral load, hepatic inflammation and fibrosis, and have been used for prediction of HCV virus relapse following HCV therapy in HCV-HIV-1 co-infected patients [38, 39]. CCL-11/eotaxin was shown to be produced primarily by pro-inflammatory macrophages in multiple models where it mediates eosinophilic inflammation [40, 41].
IL-1RA is a member of the interleukin-1 cytokine family inhibiting the activities of IL-1α and β by binding to their cognate receptor and preventing the signal pathway. Soluble form of IL-1RA has been shown to be produced by hepatocytes and HepG2 cells and regulated by IL-1β and IL-6 in a manner similar to acute phase proteins [23, 24]. CRP and other acute phase proteins enhance the production of IL-1RA by human monocytes in synergy with low levels of LPS [42]. IL-1RA is also produced by IFN-γ-induced neutrophils[43]. We have previously demonstrated elevated levels of CRP in individuals with IgAD and CVID [5]. Altered levels of CRP, CXCL-10/IP-10, and IL-1RA may indicate an activation of hepatocytes in CVID patients, as shown also by the documented correlation of AST with CXCL-10/IP-10.
sCD25 (sIL-2Rα) is produced by activated T cells. It is released by a proteolytic cleavage of the surface molecule driven by a family of enzymes collectively referred to as “sheddases’ [44, 45]. sCD25 competes with its cell surface analog for binding of IL-2; however, the ramifications of this process are not fully understood. The sCD25/IL-2 complex enhanced IL-2-mediated phosphorylation of STAT-5 in CD4+ T cells, upregulated FoxP3, and promoted differentiation of T cells to Treg rather than Th1 or Th17 cells [46]. Cabrera et al. have reported that elevated levels of sCD25 in sera of patients with hepatocellular carcinoma (HCC) correlated with blunted effector T cell responses [47]. HCC serum and sCD25 suppressed T effector responses in a dose-dependent fashion [47]. We observed that sCD25 correlates positively with plasma concentration of sCD14 and negatively with the frequency of CD4+ CD25+ T cells [5]. It is feasible that high concentrations of sCD25 observed in CVID patients contribute to the suppression of T cell function.
sCD25 is used in several diagnostic purposes as a marker of immune system activation. Elevated sCD25 concentrations are associated with activation of lymphocytes in inflammation of infectious and non-infectious origin, in autoimmune diseases, and in cancer [48, 49]. We [5] and others [50] have shown markedly increased levels of sCD25 in CVID patients. sCD25 thus represents a promising marker of immune dysregulation in CVID; its clinical value in diagnosing disease stage and progression warrants further investigation.
Although there were some similarities between the abnormalities in cytokine/chemokine levels in patients with IgAD compared to CVID patients [increase of CXCL10/IP-10, IL-10, and G-CSF and decrease of IL-9 and IL-12(p70)], the observed abnormalities were less extensive in IgAD subjects compared to those present in CVID patients. This is consistent with our previous observation demonstrating an increased level of sCD14, a marker of bacterial translocation, in CVID but not IgAD patients. Accumulated evidence suggests that the deficiency of secretory IgA plays a minor, if any, role in the activation of immune system in CVID and that the absence of secretory IgA alone most likely does not result in an increased microbial translocation across the mucosal barrier. Loss of IgA is partially compensated by increased IgM in the secretions in IgAD [2]; protective effect of IgM was documented on bacterial nontypeable Haemophilus influenzae colonization of the respiratory tract of patients with hyper. IgM syndrome [51]. However, this mechanism has not yet been documented in CVID patients.
In conclusion, our observations suggest that altered cytokine profile observed in patients with CVID may be attributed to the activation of monocyte-macrophage and granulocyte lineages, possibly driven by the translocation of bacterial components across the mucosal barrier of the gastrointestinal or respiratory tracts. The alteration of levels of most cytokines is not observed in IgAD patients suggesting that the defect in IgA production does not exert a significant effect on these mechanisms.
Acknowledgments
Supported by grants awarded by the Czech Ministry of Health, Czech Republic (11414-5; J.L., M.V.) and National Institutes of Health, USA (AI074438; Z.H.). UAB Center for AIDS Research (CFAR; funded by NIH grant P30 AI027767) Flow Cytometry Core was instrumental in the analysis of samples by the MILLIPLEX MAP assay. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145:709–27. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–6. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdani R, Hakemi MG, Sherkat R, Homayouni V, Farahani R. Genetic defects and the role of helper T-cells in the pathogenesis of common variable immunodeficiency. Adv Biomed Res. 2014;3:2. doi: 10.4103/2277-9175.124627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammarström L, Vorechovsky I, Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120:225–31. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litzman J, Nechvatalova J, Xu J, Ticha O, Vlkova M, Hel Z. Chronic immune activation in common variable immunodeficiency (CVID) is associated with elevated serum levels of soluble CD14 and CD25 but not endotoxaemia. Clin Exp Immunol. 2012;170:321–32. doi: 10.1111/j.1365-2249.2012.04655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guazzi V, Aiuti F, Mezzaroma I, Mazzetta F, Andolfi G, Mortellaro A, et al. Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles. Clin Exp Immunol. 2002;129:346–53. doi: 10.1046/j.1365-2249.2002.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlková M, Thon V, Sárfyová M, Bláha L, Svobodník A, Lokaj J, Litzman J. Age dependency and mutual relations in T and B lymphocyte abnormalities in common variable immunodeficiency patients. Clin Exp Immunol. 2006;143:373–9. doi: 10.1111/j.1365-2249.2006.02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspalter RM, Sewell WA, Dolman K, Farrant J, Webster AD. Deficiency in circulating natural killer (NK) cell subsets in common variable immunodeficiency and X-linked agammaglobulinaemia. Clin Exp Immunol. 2000;121:506–14. doi: 10.1046/j.1365-2249.2000.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nechvatalova J, Pikulova Z, Stikarovska D, Pesak S, Vlkova M, Litzman J. B-lymphocyte Subpopulations in Patients with Selective IgA Deficiency. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9655-6. [DOI] [PubMed] [Google Scholar]
- 10.Litzman J, Vlková M, Pikulová Z, Stikarovská D, Lokaj J. T and B lymphocyte subpopulations and activation/differentiation markers in patients with selective IgA deficiency. Clin Exp Immunol. 2007;147:249–54. doi: 10.1111/j.1365-2249.2006.03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa RR, Silva SP, Silva SL, Tendeiro R, Melo AC, Pedro E, et al. Monocyte activation is a feature of common variable immunodeficiency irrespective of plasma lipopolysaccharide levels. Clin Exp Immunol. 2012;169:263–72. doi: 10.1111/j.1365-2249.2012.04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Mayer L. Gastrointestinal manifestations in primary immune disorders. Inflamm Bowel Dis. 2010;16:703–11. doi: 10.1002/ibd.21040. [DOI] [PubMed] [Google Scholar]
- 14.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 15.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 16.Malphettes M, Gérard L, Carmagnat M, Mouillot G, Vince N, Boutboul D, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis. 2009;49:1329–38. doi: 10.1086/606059. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 18.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez C, Sune P, Fierro A, Rodriguez S, Lopez M, Alvarez A, et al. Modulating Effects of intravenous immunoglobulins on serum cytokine levels in patients with primary hypogammaglobulinemia. Biodrugs. 2005;19:59–65. doi: 10.2165/00063030-200519010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Rezaei N, Aghamohammadi A, Kardar GA, Nourizadeh M, Pourpak Z. T- helper 1 and 2 cytokine assay in patients with common variable immunodeficiency. J Investig Allergol Clin Immunol. 2008;18:449–53. [PubMed] [Google Scholar]
- 21.Kasztalska K, Ciebiada M, Cebula-Obrzut B, Górski P. Intravenous immunoglobulin replacement therapy in the treatment of patients with common variable immunodeficiency disease: an open-label prospective study. Clin Drug Investig. 2011;31:299–307. doi: 10.1007/BF03256928. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666–75. doi: 10.1002/hep.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–40. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabay C, Porter B, Guenette D, Billir B, Arend WP. Interleukin-4 (IL-4) and IL-13 enhance the effect of IL-1beta on production of IL-1 receptor antagonist by human primary hepatocytes and hepatoma HepG2 cells: differential effect on C-reactive protein production. Blood. 1999;93:1299–307. [PubMed] [Google Scholar]
- 25.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Wang B, Han N, Zhao Y, Song C, Feng X, et al. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009;52:553–9. doi: 10.1097/qai.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
- 27.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amoras AL, da Silva MT, Zollner RL, Kanegane H, Miyawaki T, Vilela MM. Expression of Fc gamma and complement receptors in monocytes of X-linked agammaglobulinaemia and common variable immunodeficiency patients. Clin Exp Immunol. 2007;150:422–8. doi: 10.1111/j.1365-2249.2007.03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aukrust P, Müller F, Frøland SS. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency (CVI) are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin Immunol Immunopathol. 1994;70:217–24. doi: 10.1006/clin.1994.1032. [DOI] [PubMed] [Google Scholar]
- 30.Holm AM, Aukrust P, Damas JK, Muller F, Halvorsen B, Froland SS. Abnormal interleukin-7 function in common variable immunodeficiency. Blood. 2005;105:2887–90. doi: 10.1182/blood-2004-06-2423. [DOI] [PubMed] [Google Scholar]
- 31.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Fevang B, Yndestad A, Damås JK, Halvorsen B, Holm AM, Beiske K, et al. Chemokines and common variable immunodeficiency; possible contribution of CCL19, CCL21 and CCR7 to immune dysregulation. Clin Exp Immunol. 2009;158:237–45. doi: 10.1111/j.1365-2249.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Pomar N, Raga S, Ferrer J, Pons J, Munoz-Saa I, Julia MR, et al. Elevated serum interleukin (IL)-12p40 levels in common variable immunodeficiency disease and decreased peripheral blood dendritic cells: analysis of IL-12p40 and interferon-gamma gene. Clin Exp Immunol. 2006;144:233–8. doi: 10.1111/j.1365-2249.2006.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cambronero R, Sewell WA, North ME, Webster AD, Farrant J. Up-regulation of IL-12 in monocytes: a fundamental defect in common variable immunodeficiency. J Immunol. 2000;164:488–94. doi: 10.4049/jimmunol.164.1.488. [DOI] [PubMed] [Google Scholar]
- 35.Shimozato O, Ugai S, Chiyo M, Takenobu H, Nagakawa H, Wada A, et al. The secreted form of the p40 subunit of interleukin (IL)-12 inhibits IL-23 functions and abrogates IL-23-mediated antitumour effects. Immunology. 2006;117:22–8. doi: 10.1111/j.1365-2567.2005.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt DA, Ullrich SE. Exposure to ultraviolet radiation causes dendritic cells/macrophages to secrete immune-suppressive IL-12p40 homodimers. J Immunol. 2000;165:3162–7. doi: 10.4049/jimmunol.165.6.3162. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa RR, Silva SP, Silva SL, Melo AC, Pedro E, Barbosa MP, et al. Primary B-cell deficiencies reveal a link between human IL-17-producing CD4 T-cell homeostasis and B-cell differentiation. PLoS One. 2011;6:e22848. doi: 10.1371/journal.pone.0022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, Gangl A, et al. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther. 2008;13:969–76. [PubMed] [Google Scholar]
- 39.Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 40.Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, Hogan SP. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993–6003. doi: 10.4049/jimmunol.1003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, et al. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES) J Immunol. 1999;163:3976–84. [PubMed] [Google Scholar]
- 42.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–36. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald PP, Gasperini S, Calzetti F, Cassatella MA. Modulation by interferon-gamma of the production and gene expression of IL-1 receptor antagonist in human neutrophils. Cell Immunol. 1998;184:45–50. doi: 10.1006/cimm.1998.1255. [DOI] [PubMed] [Google Scholar]
- 44.Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz HJ, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytokine Res. 1999;19:1277–87. doi: 10.1089/107999099312957. [DOI] [PubMed] [Google Scholar]
- 45.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–42. [PubMed] [Google Scholar]
- 46.Yang ZZ, Grote DM, Ziesmer SC, Manske MK, Witzig TE, Novak AJ, Ansell SM. Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. 2011;118:2809–20. doi: 10.1182/blood-2011-03-340885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, et al. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010;72:293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008;13:1–26. doi: 10.1080/13547500701674063. [DOI] [PubMed] [Google Scholar]
- 49.Downes K, Marcovecchio ML, Clarke P, Cooper JD, Ferreira RC, Howson JM, et al. Plasma concentrations of soluble IL-2 receptor α (CD25) are increased in type 1 diabetes and associated with reduced C-peptide levels in young patients. Diabetologia. 2014;57:366–72. doi: 10.1007/s00125-013-3113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.North ME, Spickett GP, Webster AD, Farrant J. Raised serum levels of CD8, CD25 and beta 2-microglobulin in common variable immunodeficiency. Clin Exp Immunol. 1991;86:252–5. doi: 10.1111/j.1365-2249.1991.tb05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Micol R, Kayal S, Mahlaoui N, Beauté J, Brosselin P, Dudoit Y, et al. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129:770–7. doi: 10.1016/j.jaci.2011.09.047. [DOI] [PubMed] [Google Scholar]