Abstract
Millions of patients are admitted each year to intensive care units (ICUs) in the United States. A significant fraction of ICU survivors develop life-long cognitive impairment, incurring tremendous financial and societal costs. Delirium, a state of impaired awareness, attention and cognition that frequently develops during ICU care, is a major risk factor for post-ICU cognitive impairment. Recent studies suggest that patients experiencing electroencephalogram (EEG) burst suppression have higher rates of mortality and are more likely to develop delirium than patients who do not experience burst suppression. Burst suppression is typically associated with coma and deep levels of anesthesia or hypothermia, and is defined clinically as an alternating pattern of high-amplitude “burst” periods interrupted by sustained low-amplitude “suppression” periods. Here we describe a clustering method to analyze EEG spectra during burst and suppression periods. We used this method to identify a set of distinct spectral patterns in the EEG during burst and suppression periods in critically ill patients. These patterns correlate with level of patient sedation, quantified in terms of sedative infusion rates and clinical sedation scores. This analysis suggests that EEG burst suppression in critically ill patients may not be a single state, but instead may reflect a plurality of states whose specific dynamics relate to a patient’s underlying brain function.
I. Introduction
Millions of patients each year are admitted to intensive care units (ICUs) in the United States for post-surgical care and treatment of serious medical conditions such as respiratory failure and sepsis. Typically, ICU patients are mechanically ventilated and administered sedative as well as analgesic drugs (e.g. opioids) over a period of days to weeks until their conditions improve. The survival rate of ICU patients has improved dramatically over the past several decades. [1,2] However, up to 50% of ICU survivors go on to develop life-altering long-term cognitive impairments [3]. Delirium, an acute neuropsychiatric syndrome characterized by fluctuations in arousal and decreased awareness, attention, and cognition, is a major risk factor for post-ICU cognitive impairment, and may be prevalent in up to 75% of critically ill patients [3]. These ICU-related cognitive dysfunction incurs persistent financial and social costs [4].
The brain mechanisms underlying ICU-related delirium and cognitive impairment remain poorly understood. Recent studies have shown that ICU patients exhibiting an electroencephalogram (EEG) pattern referred to as burst suppression have higher mortality [5] and delirium rates [6] than patients who do not experience burst suppression. Burst suppression is typically associated with coma, deep levels of anesthesia, or significant hypothermia, and is defined clinically as an alternating pattern of high-amplitude “burst” periods interrupted by sustained low-amplitude “suppression” periods. Patients may enter a state of burst suppression for a variety of reasons, including over-sedation or underlying brain dysfunction, both of which are thought to contribute to post-ICU delirium and cognitive impairment. The amplitude and dynamics of burst suppression signals have recently been linked to the metabolic and functional properties of underlying brain circuits [7–9]. An analysis of the EEG dynamics of burst suppression in ICU patients might therefore provide insights into the mechanisms and risk factors for ICU-related delirium and cognitive impairment. In this paper, we describe a method for identifying characteristic EEG components during burst suppression in sedated ICU patients, and analyze the relationship between these EEG components and patient sedation and arousal levels.
II. Methods
A. Subject Selection
Subjects were recruited from three ICUs at the Massachusetts General Hospital after obtaining approval from the local Institutional Review Board. In order to be considered eligible for the study, patients were required to be sedated and receiving mechanical ventilation at the start of monitoring, with no known focal neurological deficits. Sixty-five subjects were recruited in total. All subjects were over 18 years of age (ranging 25–90, median of 57, IQR = 46–68). 18 subjects were female. 78 percent of the patients were sedated with propofol as the primary sedative agent, with median of 72 hours of sedation per patient (IQR = 39.8–147.9). Propofol was not administered as the primary sedative in 10 patients during the times in which EEG was recorded.
B. Data Collection
EEG data were collected using a Masimo (Irvine, CA, USA) SedLine 4-channel forehead EEG monitor, recorded at a sampling frequency of 250 Hz. Recording was initiated when eligible patients were identified and continued until the patients were liberated from mechanical ventilation. Patients were continuously monitored for 7060.6 patient-hours in total (median of 70.3 hours per patient, IQR = 22.9–149.4). Sedative infusion and weight data was collected from 24 hours prior to the start of EEG monitoring, continuing until the patient was extubated.
The Richmond Agitation and Sedation Scale (RASS) is used by clinicians in the ICU to assess level of arousal, and ranges from −5 (completely unresponsive) to +4 (violently agitated) with 0 as being classified as alert and calm. RASS scores performed by ICU nurses and trained clinical research staff in approximately 2-hour increments were collected from the medical charts of each individual patient and recorded.
C. Artifact Rejection
Artifacts were rejected using standard methods [10]. Data were first bandpass filtered to 0.5–35hz. Using a 1 second moving window, segments were rejected if they contained any the following 3 possible signs of artifact: (1) abnormally high signal amplitude (>1000 uV), typically caused by movement or amplifier saturation; (2) 60 Hz activity above 20 uV (measured spectrographically); (3) evidence of a loose electrode, defined as a segment for which the mean amplitude of the sum of signals is less than half the mean amplitude of the first channel.
D. Burst Suppression Probability
All EEG data were segmented into a binary sequence of burst and suppression epochs using a previously validated automated suppression detection algorithm [11]. The resulting binary signal was then filtered to produce a continuous measure of burst suppression depth, the BSP (‘burst suppression probability’), which quantifies the instantaneous the probability of being in the suppressed state [12]. Burst suppression probability was used to confirm accurate selection of burst and suppression features.
E. Selection of Burst and Suppression Features
Within the binary results, we identified all 2.5-second segments that were classified as either bursts or suppressions in their entirety and free of artifacts. For each patient, a maximum of 500 randomly selected burst and suppression segments were collected for analysis, up to 1000 segments per patient. A total of 325,000 burst segments and 19,128 suppression segments were identified. We use these segments to produce power spectral estimates using multi-taper spectral analysis on the time-domain EEG activity averaged across the 4 electrodes. These estimates were used to create features for the burst and suppression data with which to cluster. All spectra were downsampled to 25 percent of their original frequency resolution and converted to decibel units. The mean and variance at each frequency were computed for the burst and suppression features sets, respectively. The burst and suppression spectra were then z-transformed at each frequency using the appropriate mean and variance estimate. The resulting features were used for cluster analysis. After completing the clustering analysis, spectra were reconstructed from the resulting clusters by inverting the z-transformation.
F. Clustering Analysis
We utilized k-means clustering of the feature set in order to identify putative distinct burst and suppression states. To find the best seed value k, we performed tenfold cross-validation on the data set using the average within-cluster Euclidean distance as the goal function. The standard deviation of goal function metric values among folds was used as the criterion for selecting k. We selected k = 7 to be the number of clusters, since at this value the standard deviation was below 0.03 percent of its original value at k = 2. We then initialized clustering with 7 individual seeds over the entirety of the data set. Fifty replicates of clustering were performed, and the replicate with the smallest sum of Euclidean distances was selected. Throughout all of the clustering performed, seeds were initialized among the existing data. A maximum of 500 iterations were performed per trial until convergence of the sum of Euclidean distances, and clusters that did not find features were excluded. Scripts and algorithms were implemented in MATLAB (The Mathworks Inc., Natick, Massachusetts, USA), using built-in k-means clustering functions.
G. Clinical Data
EEG segments were identified with available RASS scores and weight-normalized propofol doses (in milligrams/kilogram/hour, mg/kg/hr) of the patients from which they were taken at the time in which the RASS score was assessed or the drug was given. Only the closest RASS score within one hour were used for a given EEG segment. Only the infusion beginning before and ending after the time of a given segment was likewise used. Only segments that could be identified with a RASS score and infusion rate under these rules were included in the analysis of cluster relationship to clinical states, and other segments were excluded. A total of 8000 burst segments and 3450 suppression segments were excluded from analysis of RASS and propofol infusion relationships.
III. Results
A. Cluster Characteristics
Fig. 1 shows the reconstructed spectra retrieved from the centroids of the clustered burst and suppression states. The centroid spectra of burst states tend to show higher power than those of suppression states, with range of power greater in burst states in excess of ~10 dB in low frequency components less than 4 Hz. Within each classification, states vary widely in terms of power, with states A, B, C, H, and I separating themselves in terms of power from the rest of states in both classification.
Figure 1.
Reconstructed spectra representing cluster centroids produced by k-means clustering of z-normalized burst and suppression feature sets using k = 7. For all clusters, n > 500. Mean standard deviation of power at each frequency was 3.33 dB among all clusters.
Fig. 2 shows exemplar time-domain EEG activity from distinct burst and suppression clusters. Both pairs of burst and suppression exemplar activity show variation in their spectral composition. In the bursts. high beta (~20 Hz) oscillations in burst cluster A are visible in the peaks and troughs of the time-domain exemplar chosen from a segment belonging to that cluster. These oscillations are absent from the exemplar time series of another burst state G. These clusters confirm clinical observations that the spectral characteristics of burst and suppression activity are heterogeneous, likely borne of distinct brain states. For instance, the greatest variation between clusters within both burst and suppression categories exists in the slow and delta frequency ranges, while other clusters diverge in the theta and alpha frequency ranges.
Figure 2.
Exemplar time-domain EEG activity from segments categorized into four burst and suppression clusters. Segments were Chebyshev filtered between 0.5 and 35 Hz for illustration purposes.
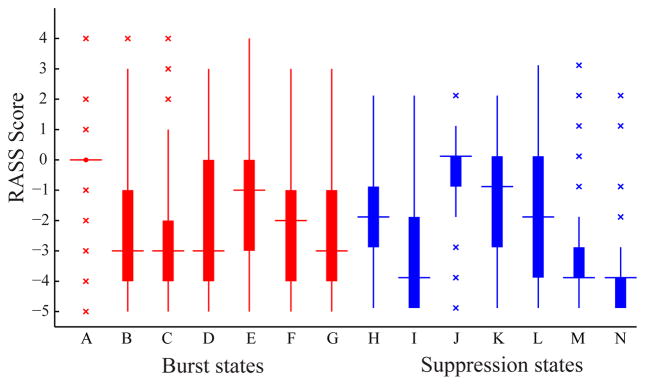
B. Relation to Clinical States
The RASS and propofol dose distributions pertaining to each clustered segment are heterogeneous between clusters of the same burst / suppression category. The clusters, therefore, reveal different clinical and brain state conditions of the patient at the times from which they originate. Fig. 3 and 4 show RASS score and propofol infusion rate distributions of segments clustered into each of the seven clusters in the burst and suppression categories. By the Mann-Whitney U test, each of the pairwise distributions within each burst and suppression category are distinct with p < 0.01, with the exception of B–D, B–G, D–G, and B–C burst cluster pairs in the RASS distributions, and E–D and B–F burst cluster pairs, and H–L and J–K suppression cluster pairs in the propofol rate distributions.
Figure 3.
RASS score distributions of burst and suppression segments in each cluster. For all distributions, n ≥ 470.
Figure 4.
Propofol drug infusion rate distributions during burst and suppression segments in each cluster. For all distributions, n ≥ 470.
Among all states, RASS score and propofol infusion rate were inversely related, especially among suppression states J through N, among burst states A through C, and between suppression states H and I. The continuity of the RASS-drug rate inverse relationship among these three subsets of states reveals possible partitions among both burst and suppression classifications of states supported by their connection with both the ongoing clinical environment and the behavioral consequences of the sedated brain.
Burst state A, which has the highest power across all frequencies and states, was linked to RASS distributions with the highest median score of 0, suggesting that burst state A corresponds to higher levels of consciousness approximating an alert conscious state. Burst A was also linked to the distribution with the lowest median propofol infusion rates among bursts. The state’s RASS and infusion rate distributions both possessed the lowest variance in its distribution, demonstrating a highly distinct brain state marked by a high beta oscillation.
Suppression states I, M, and N were all linked to RASS distributions with a median score of −4, denoting a state of impaired consciousness where patients can move in response to tactile stimuli, but do not respond to verbal commands. Suppression states M, and N show the lowest power among burst and suppression states at all frequencies, suggesting that reduced power in this band corresponds to a deeply sedated or otherwise impaired state of consciousness. Suppression states I, M, and N also corresponded to the highest median propofol infusion rates.
The RASS distributions of states H, J, K, and L were all centered at their median between 0 and −2, denoting states are either alert or drowsy but conscious, able to be aroused in response to voice stimuli. H shows the highest power among all suppressions, while J, K, and L show higher power among the partition of low-power suppression states at or above 8 Hz, corresponding to the EEG alpha and beta bands. Suppression states H, J, and K also corresponded to among the lowest propofol infusion rates.
IV. Discussion
These analyses show that burst suppression in critically ill patients is not a monolithic state, but rather a plurality of states showing a range of spectral features. The ability to partition the clusters into to high-power and low-power groups within burst and suppression classifications reveals potential brain dynamics not captured by the binary classification of burst suppression alone.
The burst and suppression states showed a consistent within-partition relationship between RASS scores and propofol infusion rates, with certain burst and suppression states reflecting high RASS scores and low propofol infusion rates, and others corresponding to low RASS scores and higher propofol infusion rates. Surprisingly, a subset of suppression states (J, K, and L), which would conventionally be interpreted as isoelectric, deeply comatose states, in fact corresponded to states centered between RASS 0 and −2, all states that are either spontaneously awake or able to be aroused by voice. One of these suppression states (state L), exhibited among the lowest power among all states studied, implying that patients can remain conscious despite a deeply “suppressed” EEG.
The focus of this paper is to describe how clustering analysis can be applied to EEG burst suppression data to analyze spectral structure within burst and suppression periods. As such, this preliminary analysis utilizes a limited subset of total available clinical data, excluding drug administrations of other sedative, analgesic, and antipsychotic drugs known to have effects on both neuropsychiatric and EEG measurements. In addition, we have not analyzed other neuropsychiatric assessments such as the Confusion Assessment Method in the ICU (CAM-ICU) and the Delirium Rating Scale (DRS), among others, which supply additional detailed metrics of delirium and post-cognitive impairment not captured by a single-score measure of arousal such as RASS. Instead, we use the fluctuating level of arousal as an indicator of cognitive deficit that may lead to cognitive impairment caused by continuous sedative infusion. These preliminary analyses are also limited to a relatively small patient cohort in a single institution.
This analysis suggests that conventional clinical definitions of burst suppression centered on the amplitude of the EEG signal over a length of time may obscure oscillatory structure within the EEG that may be functionally significant, and suggests that in some cases, clinically-defined “burst suppression” may be deeply misleading with regard to a patient’s state. Overall, this work illustrates how clustering can be applied to EEG spectra recorded in critically ill patients to assess dynamic brain states and suggests that this method can be used to identify a plurality of distinct brain states hidden within the generic clinical definition of “burst suppression.”
Acknowledgments
This work was supported by National Institutes of Health grants K23-NS090900 (Westover) and DP2-OD006454 (Purdon).
Contributor Information
David W. Zhou, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114.
M. Brandon Westover, Email: mwestover@mgh.harvard.edu, Department of Neurology, Massachusetts General Hospital, Boston, MA 02114.
Lauren M. McClain, Department of Neurology, Massachusetts General Hospital, Boston, MA 02114
Sunil B. Nagaraj, Department of Neurology, Massachusetts General Hospital, Boston, MA 02114
Sadeq A. Quraishi, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114
Oluwaseun Akeju, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114.
J. Perren Cobb, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114.
Patrick L. Purdon, Email: patrickp@nmr.mgh.harvard.edu, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114.
References
- 1.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014 Mar;42:625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014 Apr 2;311:1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014 Jan 9;370:185–6. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Brown M, Merry H, Sketris I, Fisk JH Vascular Cognitive Impairment Investigators of the Canadian Study of, et al. Societal costs of vascular cognitive impairment in older adults. Stroke. 2002 Jun;33:1605–9. doi: 10.1161/01.str.0000017878.85274.44. [DOI] [PubMed] [Google Scholar]
- 5.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008 Dec;36:3171–7. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014 Oct;42:2244–51. doi: 10.1097/CCM.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proceedings of the National Academy of Sciences of the United States of America. 2012 Feb 21;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis LD, Ching S, Weiner VS, Peterfreund RA, Eskandar EN, Cash SS, et al. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain. 2013 Sep;136:2727–37. doi: 10.1093/brain/awt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westover MB, Ching S, Kumaraswamy VM, Akeju O, Pierce E, Cash SS, et al. The human burst suppression electroencephalogram of deep hypothermia. Clinical Neurophysiology. doi: 10.1016/j.clinph.2014.12.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol. 2005 Feb;116:427–42. doi: 10.1016/j.clinph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Westover MB, Ching S, Shafi MM, Cash SS, Brown EN. Real-time segmentation and tracking of brain metabolic state in ICU EEG recordings of burst suppression. Conf Proc IEEE Eng Med Biol Soc; 2013; 2013. pp. 7108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemali J, Ching S, Purdon PL, Solt K, Brown EN. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J Neural Eng. 2013 Oct;10:056017. doi: 10.1088/1741-2560/10/5/056017. [DOI] [PMC free article] [PubMed] [Google Scholar]