Summary
Objectives
Because early etiologic identification is critical to select appropriate specific status epilepticus (SE) management, we aim to validate a clinical tool we developed that uses history and readily available investigations to guide prompt etiologic assessment.
Methods
This prospective multicenter study included all adult patients treated for SE of all but anoxic causes from four academic centers. The proposed tool is designed as a checklist covering frequent precipitating factors for SE. The study team completed the checklist at the time the patient was identified by electroencephalography (EEG) request. Only information available in the emergency department or at the time of in-hospital SE identification was used. Concordance between the etiology indicated by the tool and the determined etiology at hospital discharge was analyzed, together with interrater agreement.
Results
Two hundred twelve patients were included. Concordance between the etiology hypothesis generated using the tool and the finally determined etiology was 88.7% (95% confidence interval (CI) 86.4–89.8) (κ = 0.88). Interrater agreement was 83.3% (95% CI 80.4–96) (κ = 0.81).
Significance
This tool is valid and reliable for identification early the etiology of an SE. Physicians managing patients in SE may benefit from using it to identify promptly the underlying etiology, thus facilitating selection of the appropriate treatment.
Keywords: Epilepsy, Diagnostic test assessment, Critical care, Coma, Neurologic emergency
With an annual incidence of 10–40 per 100,000 person-years and a mortality between 7 and 33%,1–3 status epilepticus (SE) is one of the most frequent neurologic emergencies. Several independent predictors of poor outcome have been identified, including advanced age, de novo presentation, impairment of consciousness before treatment, and seizure type, but the most critical factor by far is the underlying etiology.4–7 Although much attention has been paid to seizure cessation with administration of antiseizure drugs (ASDs),8,9 it is far more critical to rapidly identify and target a treatable underlying etiology.9 Indeed, some etiologies such as cerebrovascular events, severe metabolic disturbances, alcohol withdrawal or intoxication, brain tumor–related events, and infections need emergent and specific treatments beyond ASDs. Earlier identification of the SE etiology would enhance rapid and more focused treatment, and potentially improve outcome.
Because of the diversity of possible causes,10 finding the underlying etiology might be a puzzling process in acute and emergent situation for a clinician unfamiliar with SE, particularly outside of a tertiary care facility. Clinical decision supporting tools may help clinicians gather important data for the decision-making process, and guide medical management more effectively, thus reducing practice errors and costs.11 These tools are widely available in many other clinical settings, and notably for other acute conditions for which rapid identification of the underlying etiology is fundamental, such as chest pain12 and acute headache.13
To assist clinicians in rapidly identifying an underlying etiology, we developed a user-friendly tool called Status Epilepticus Etiology Identification Tool (SEEIT), which utilizes elements of the clinical history and routinely available laboratory investigations that can be used at the bedside in the emergency department (ED) or the intensive care unit (ICU) to guide the evaluation into etiology. We performed a multicenter prospective observational study to determine the validity and reliability of this tool.
Methods
Primary research question
The primary research question was to evaluate the validity and reliability of the SEEIT by assessing its propensity to identify the correct etiology and its interrater agreement.
Standard protocol approvals, registrations, and patient consents
The institutional review boards of each center approved this study. Because this observational study involved no risk for patients and focused on the acute phase of critically ill patients, consent was waived.
Cohort and SE definition
In this observational study, we prospectively identified every consecutive adult patient (age >16 years) with SE admitted to four university hospitals, from February 1, 2013 at the Lausanne University Hospital (CHUV), Lausanne, Switzerland; from June 1st 2013 at the Brigham and Women’s Hospital (BWH) and the Massachusetts General Hospital (MGH), Boston, U.S.A.; and from November 1, 2013 at the Beth Israel Deaconess Medical Center (BIDMC), Boston, U.S.A. The inclusion period ended on February 28, 2014. All patients with suspected SE at each institution have electroencephalography (EEG) studies within 24 h, so subjects were screened through review of all EEG studies ordered during that period. SE was defined as the occurrence of ongoing epileptic or repeated epileptic seizures without full recovery lasting >5 min.9 EEG diagnosis was required for nonconvulsive SE, as recently described.14 This cohort includes patients admitted for SE and also patients developing SE during the hospital stay, but patients with postanoxic SE were excluded.
Definition of variables
Demographic data recorded included the following: (1) age; (2) gender; (3) worst seizure type categorized as focal seizures without impairment of consciousness, focal seizures with impairment of consciousness, generalized convulsions, absence seizures, myoclonic seizures,15 and nonconvulsive SE in coma (NCSEC); and (4) level of consciousness before treatment was categorized as follows: alert, confused, somnolent (arousable with clear contact), stuporous (arousable without contact), and comatose. The Status Epilepticus Severity Score (STESS) was calculated for every patient using age, seizure type, level of consciousness, and history of previous seizures.16 The timing of onset of the SE was determined as precisely as possible using pre-hospital chart and emergency department summaries. For SE episodes without clear onsets (unwitnessed, subtle non-convulsive SE), we considered the last observed time of good health as the beginning of the SE. Each ASD treatment was recorded prospectively, but treatments modified or initiated after control of seizures were not evaluated. Refractory SE was defined as failure to respond to an adequate dose of an initial benzodiazepine followed by a second line of a nonsedating ASD.9 The end of the SE episode was defined by the last clinical or electrical seizure without recurrence for at least 48 h off sedation.
The etiology of each SE episode was described in free text based on medical charts and then assigned to the 19 categories listed in Table 1.
Table 1. List of diagnostic categories and their frequencies as definitive SE etiology.
| Underlying etiology after complete workup (n = 212) | n | % |
|---|---|---|
| Total, n = 212 | ||
| ASD-related (nonadherence, recent change or low levels) | 34 | 16.04 |
| Brain tumor without acute change (no change or increase in tumor load) |
28 | 13.21 |
| Acute hemorrhagic cerebrovascular event | 21 | 9.91 |
| Known epilepsy (non structural) without provocative factors (breakthrough seizures) |
16 | 7.55 |
| Remote ischemic cerebrovascular event | 14 | 6.6 |
| Unclassifieda | 13 | 6.13 |
| CNS infection (meningitis or encephalitis) | 12 | 5.66 |
| Unknown origin | 11 | 5.19 |
| Toxic-metabolic | 10 | 4.72 |
| Systemic infection/sepsis | 10 | 4.72 |
| Remote hemorrhagic cerebrovascular event | 8 | 3.77 |
| Acute TBI | 7 | 3.3 |
| Acute ischemic cerebrovascular event | 5 | 2.36 |
| Remote TBI | 6 | 2.83 |
| Alcohol related (withdrawal or intoxication) | 6 | 2.83 |
| Brain tumor with acute change (bleeding, recent biopsy/surgery or rapid increase in edema) |
5 | 2.36 |
| Benzodiazepine withdrawal | 4 | 1.89 |
| Neurodegenerative disease | 2 | 0.94 |
| Other drugs known to reduce seizure threshold | 0 | 0 |
ASD, antiseizure drug; CNS, central nervous system; TBI, traumatic brain injury.
Unclassified includes: three multiple sclerosis, two confirmed and one possible posterior reversible encephalopathy syndrome (PRES), two tumoral meningitis, one NMDA encephalitis, one neurosarcoidosis, one eclampsia, one arteriovenous malformation without bleeding, and one case of microangiopathic hemolytic anemia.
Outcome at discharge was categorized as return to premorbid baseline, new morbidity, or death.
Status Epilepticus Etiology Identification Tool (SEEIT): description and evaluation
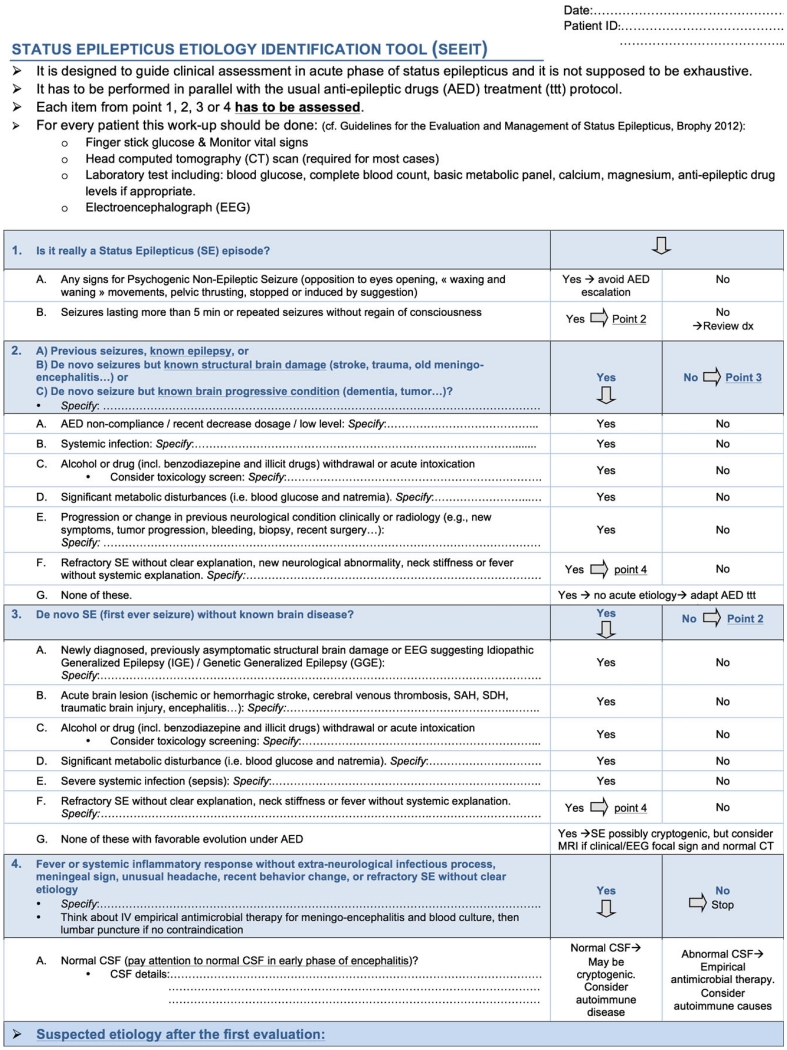
The proposed tool, shown in Figure 1, was developed by two of the authors (VA and AOR) based on the list of the potential underlying etiologies included in the current SE guidelines9 and adapted based on their clinical experience. After its completion, it was reviewed by two others authors, who are experts in the field (JWL and FWD). Hypertensive encephalopathy was not included in the tool; because hypertension is frequently seen secondary to the acute brain injury, too much emphasis on hypertension in the acute setting could be misleading. Moreover, hypertensive encephalopathy is not a frequent cause of SE.10,17
Figure 1.
The Status Epilepticus Etiology Identification Tool (SEEIT). The SEEIT tool has been designed to guide SE etiology assessment. It has to be used along with antiseizure drug protocol. Each point has to be assessed.
The tool is designed as a checklist including four main parts and several subsequent questions. The first part aims to confirm the diagnosis of SE (fulfilling the operational definition)18 and also raises the question of psychogenic nonepileptic status epilepticus (PNESE), which can be mistaken for refractory SE.19 The tool then discriminates between SE in the setting of known epilepsy or a structural brain disorder versus occurring without any known brain pathology. For each of these parts, the tool includes questions about common treatable etiologies. Finally, the fourth part emphasizes signs suggestive of a central nervous system (CNS) infection and includes cerebrospinal fluid (CSF) findings if a lumbar puncture is performed. At the end of the assessment, the rater is invited to record the suspected etiology as free text based on the assessment directed by the SEEIT. The tool also includes the list of investigations required by current guidelines for SE evaluation.9 The etiology is eventually placed into one of the 19 categories (see Table 1) to enable evaluating concordance with the definitive etiology determined at the end of the hospital stay. Of note, for the concordance evaluation, when an acute precipitating factor occurred in the context of a remote brain injury, the “acute” condition was considered predominant, as the tool aims to identify acute treatable conditions.
The SEEIT was completed for every patient at the time of identification by the study team—based only on the information available in the ED or at the time of in-hospital SE identification and before discharge summary diagnosis was available. The first author (VA) completed the SEEIT for the three centers involved in Boston, U.S.A. (BWH, MGH, BIDMC) and the EEG attending filled the assessment under the same conditions for the patients in the CHUV, Lausanne, Switzerland.
Because the SEEIT was designed to be used by nonspecialist physicians and was also completed by neurologists with specialty training in epilepsy, an interrater evaluation between one of the investigators (VA) and an emergency physician (fourth-year emergency resident at BWH) (DC) was performed for the first 30 cases of SE treated at BWH. To reflect the “real-life” use of the tool, the ED physician did not receive any training in use of the SEEIT.
Statistical analysis
Interrater evaluation between VA and DC, and concordance between the etiologies generated by the SEEIT and the etiology finally determined during the hospitalization, were evaluated with Cohen’s kappa coefficient. To identify any misleading factors for correct early etiology identification, patients with correct and incorrect etiologies generated using the SEEIT were compared using chi-square, analysis of variance (ANOVA), and Wilcoxon rank-sum test, as required. Significance was assumed with p < 0.05. Data were analyzed using Stata 11.1 (StataCorp, College Station, TX, U.S.A.).
Results
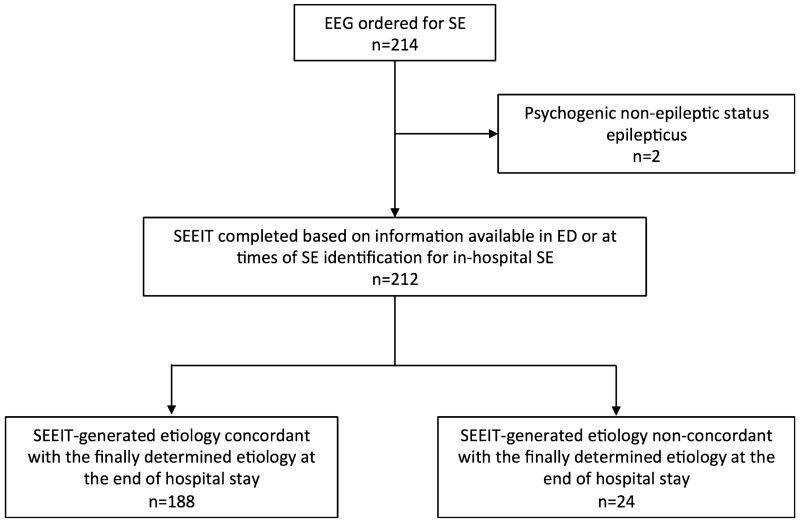
Figure 2 outlines the study profile. A total of 212 consecutive patients were included in the study. Demographics and SE characteristics are summarized in Table 2. Gender was evenly distributed; the median age was 60 years (range 18–93). Premorbid seizures occurred in 49.1% patients. About half of the subjects had generalized convulsive seizures, followed by 28.9% with focal seizures with consciousness impairment, 15% with focal seizures without impairment of consciousness, and 8% with NCSEC. Absence and myoclonic status were infrequent: 1.42% and 0.5%, respectively. Consciousness was impaired in most, with 17% of patients presenting as “comatose” and 41.5% as “stuporous.” The mean STESS was 2.64 (standard deviation [SD] 1.63) and around half of patients had refractory SE. A median of three ASDs (range 0–13) was used and 11.3% underwent intubation as part of a SE treatment protocol. The mortality rate was 12.8%, and 45.3% of patients returned to their premorbid clinical baseline at discharge.
Figure 2.
Study profile. EEG, electroencephalography; SE, status epilepticus; SEEIT, Status Epilepticus Etiology Identification Tool.
Table 2. Cohort description.
| Patients (n = 212) | ||
|---|---|---|
| Demographics | ||
| Age (median, range) | 60 | 18–93 |
| Male (n,%) | 106 | 50 |
| History of previous seizures (n,%) | 104 | 49.1 |
| Center (n,%) | ||
| CHUV | 104 | 49.1 |
| BWH | 65 | 30.7 |
| MGH | 30 | 14.2 |
| BIDMC | 13 | 6.1 |
| SE characteristics | ||
| Worst seizure type (n,%) | ||
| Focal without consciousness impairment | 32 | 15.1 |
| Focal with consciousness impairment | 57 | 28.9 |
| Absence | 3 | 1.42 |
| Myoclonic | 1 | 0.5 |
| Generalized convulsive | 102 | 48.1 |
| Nonconvulsive SE in coma | 17 | 8 |
| Level of consciousness before treatment (n,%) | ||
| Alert | 24 | 11.3 |
| Confused | 51 | 24.1 |
| Somnolent | 13 | 6.1 |
| Stuporous | 88 | 41.5 |
| Comatose | 36 | 17 |
| STESS (mean, SD) | 2.64 | 1.63 |
| Refractory SE (n,%) | 119 | 56.12 |
| Number of different ASD used (median, range) | 3 | 0–13 |
| Coma induction for SE control (n,%) | 24 | 11.3 |
| Outcome at discharge (n,%) | ||
| Return to clinical premorbid baseline | 96 | 45.3 |
| New morbidity | 89 | 42 |
| Death | 27 | 12.8 |
ASD, antiseizure drug; BWH, Brigham and Women’s Hospital; BIDMC, Beth Israel Deaconess Medical Center; CHUV, Lausanne University Hospital; MGH, Massachusetts General Hospital; STESS, Status Epilepticus Severity Score.
In addition to the 212 patients in SE, two had EEG request for suspected SE but were eventually found to have PNESE. Both were treated acutely as refractory SE. One was intubated for “convulsion control.” Of note, in the patients’ charts, there were descriptions of the events including features such as “waxing and waning” symptoms “stopped by suggestion” for the first patient; and “waxing and waning” and “pelvic thrusting movements” for the second. The SEEIT-generated etiology was correct for these two events.
The definitive etiologies at hospital discharge are listed in Table 1. ASD-related causes (non-adherence, iatrogenic withdrawal, and subtherapeutic level) were the most frequent, occurring in 16.3%, followed by brain tumor (without acute change in the tumor) in 13.2%. The “unclassified” category included three cases of multiple sclerosis, two confirmed and one possible posterior reversible encephalopathy syndrome (PRES), two neoplastic meningitis, and single cases of N-methyl-d-aspartate (NMDA) encephalitis, neurosarcoidosis, eclampsia, arteriovenous malformation without bleeding, and microangiopathic hemolytic anemia. A need for specific etiologic treatment in addition to ASDs was considered necessary in 90 of 212 patients (42.45%).
The etiology identified early using the SEEIT was correct in 188 patients (88.7%) (95% confidence interval [CI] 86.4–89.8) with a kappa coefficient of 0.88. There was interrater agreement in 83.3% (95% CI 80.4–96) of cases between VA and the DC, with a kappa coefficient of 0.81.
A further analysis comparing features of patients with a correct SEEIT-generated etiology versus an incorrect one did not show any significant differences regarding age (p = 0.95), gender (p = 0.08), participating center (p = 0.81), type of seizure (p = 0.81), level of consciousness (p = 0.94), time to treatment (p = 0.36), or refractory SE (p = 0.50). Only the absence of previously known seizures was associated with a higher risk of incorrect early etiology identification. A total of 103 of the 188 patients with an etiology correctly determined by the SEEIT had a history of earlier seizures (54.8%), whereas this was the case for only 5 of 24 patients with an incorrectly SEEIT-determined etiology (20.8%) (p = 0.002, χ2).
Table 3 provides a detailed description of the 24 cases in which the etiology generated using the tool was incorrect. Seven (29.2%) were misdiagnosed due to information missed on early imaging, five (20.8%) due to CSF misinterpretation, and three (12.5%) to incomplete history, and in three (12.5%) presentations were probably too complex to be diagnosed accurately in the ED setting (one NMDAencephalitis, one with microangiopathic hemolytic anemia, and one with toxoplasmosis). In two patients (8.4%), known remote conditions were incorrectly assumed to be the etiology when others factors were actually responsible. One misdiagnosis (4.2%) was caused by misinterpretation of a systemic inflammatory response syndrome (SIRS). Finally, three (12.5%) were misdiagnosed because of disagreement on causality judgment of minor precipitants between the tool rater and the hospital discharge summary.
Table 3. Details of patients for which the early suspected etiology using the SEEIT was incorrect.
| Pt | Age | Gender | Previous seizures |
Etiology generated using the SEEIT |
Final etiology | Case description | Explanation |
|---|---|---|---|---|---|---|---|
| 1 | 54 | F | No | Cryptogenic | Brain glioma | Small temporal glioma was missed in the CT performed in ED, but seen on MRI later. Of note, because seizures were focal, the tool advised an MRI |
Etiology missed on CT |
| 2 | 76 | M | No | Cryptogenic/ encephalitis? |
Brain glioma | Because of new-onset refractory epilepsy with normal CT and normal CSF analysis, SEEIT evoked a cryptogenic SE or encephalitis in early phase/autoimmune process. The later MRI revealed a glioma |
Etiology missed on CT |
| 3 | 40 | F | Yes | Drug related (ciprofloxacin) |
Known epilepsy without provocative factors |
Patient with known epilepsy experienced SE in the context of ciprofloxacin prescribed for UTI without systemic involvement. The discharge summary did not retain ciprofloxacin as provocative factor |
Disagreement on causality judgment of minor precipitants |
| 4 | 57 | F | No | Meningoencephalitis (infectious) |
Carcinomatous leptomeningitis |
SE after lumbar surgery for vertebral metastasis (breast cancer). CSF showed a pleocytosis (115 whitecells/ mm3). Infectious meningitis was proposed by the SEEIT. Further CSF analysis revealed metastatic cells |
CSF data misinterpreted |
| 5 | 21 | F | No | Meningoencephalitis (infectious) |
NMDA encephalitis |
Presented with refractory SE and mild CSF pleocytosis. Possible CNS infectious was retained using the SEEIT. Further analysis didnot find any infectious agent and revealed NMDA antibodies |
Failure to identify a complex disease in the emergency setting |
| 6 | 72 | M | No | Remote ischemic stroke |
Lymphomatous meningitis |
Known for Waldenstrom disease. Initial imaging showed an old previously asymptomatic stroke retained as responsible using the SEEIT. LP done because of unexpected evolution revealed lymphomatous meningitis |
Remote brain pathology incorrectly retained |
| 7 | 19 | F | Yes | Known epilepsy without provocative factors |
Cryptogenic | History revealed a couple febrile seizures during childhood and no other explanation. Because of the long time before recurrence of seizure, she was not considered as having epilepsy before the SE episode and thus considered as cryptogenic |
Disagreement on causality judgment of minor precipitants |
| 8 | 71 | F | No | Drug related (clozapine) |
Posterior reversible encephalopathy syndrome (PRES) |
In the context of severe anxiety for 3 days, clozapine was prescribed and increased. Then the patient presented with altered mental status and visual hallucinations. Focal SE was diagnosed after EEG. Initial imaging was nonconclusive. The etiology retained using the SEEIT was related to the clozapine. Later MRI revealed a PRES |
Etiology missed on CT |
| 9 | 67 | F | No | Meningoencephalitis (infectious) |
Cryptogenic | Refractory SE and fever at the presentation. Despite a mild pleocytosis, the CSF remained sterile. The pleocytosis was attributed to seizures |
CSF data misinterpreted |
| 10 | 75 | M | No | Cryptogenic | HSV-1 encephalitis |
Because of fever and new onset SE, the SEEIT suggested a CSF analysis, which was normal (four white cells). Later, PCR came back positive for HSV-1. LP was performed early (ca. 36 h after onset), so the SEEIT warned against “false” normal CSF in early phase of an encephalitis |
CSF data misinterpreted |
| 11 | 46 | F | Yes | Sepsis | Possible posterior reversible encephalopathy syndrome (PRES) |
SE in the context of sepsis (pulmonary origin) and known epilepsy. So, using the SEEIT, sepsis was considered as a provocative factor. Later MRI was consistent with a PRES. However, it was not excluded for certain that the MRI changes were due to seizures |
Etiology missed on CT |
| 12 | 40 | F | Yes | Sepsis | Known epilepsy without provocative factors |
SE in the context of fever, systemic inflammatory response syndrome (SIRS) and known epilepsy. So, using the SEEIT, sepsis was considered as a provocative factor. The complete evaluation did not find any infectious source. The SIRS was attributed to the SE itself |
SIRS incorrectly suspected |
| 13 | 54 | F | No | Acute ischemic stroke |
Brain abscess due to Bacillus cereus endocarditis |
Patient known for acute myeloid leukemia. Initial CT showed a probable new ischemic stroke. Subsequent MRI revealed an abscess. Endocarditis was subsequently found |
Etiology missed on CT |
| 14 | 60 | M | No | Cryptogenic | Alcohol withdrawal |
Alcohol withdrawal was denied during initial assessment |
Incomplete history information |
| 15 | 79 | M | No | Dementia | Chronic lymphocytic leukemia with CNS infiltration |
Known for advanced dementia and chronic lymphocytic leukemia. Initial imaging was nonconclusive. MRI was performed 4 days later and showed focal lesions likely due to infiltrative lymphoma |
Remote brain pathology incorrectly retained |
| 16 | 69 | F | No | Toxic-metabolic (in the context of a known CNS B lymphoma) |
Microangiopathic hemolytic anemia |
Initial laboratory testing showed renal and liver impairments of unknown origin. The extensive evaluation revealed a microangiopathic hemolytic anemia |
Failure to identify a complex disease in the emergency setting |
| 17 | 71 | M | No | Meningoencephalitis (infectious) |
Diffuse large B-cell lymphoma with CNS infiltration |
Presented with SE preceded by rapid cognitive decline. CSF showed pleocytosis (728 white cells/mm3). CNS infection was suspected. Extensive evaluation did not find any etiology. A malignant edema leaded to herniation. Autopsy showed a diffuse CNS infiltration by large B-cell lymphoma |
CSF data misinterpreted |
| 18 | 36 | M | No | Brain lesion of unclear origin |
Cerebral toxoplasmosis |
Known for HIV. The evaluation in the emergency department identified a newly diagnosed mass without clear precision. The complete evaluation revealed a cerebral toxoplasmosis |
Failure to identify a complex disease in the emergency setting |
| 19 | 76 | M | No | Cryptogenic | Remote subarachnoid hemorrhage |
The previous history of subarachnoid hemorrhage was unknown at initial presentation |
Incomplete history information |
| 20 | 68 | F | No | Toxic-metabolic | Acute ischemic stroke |
Presented with several mild metabolic disturbances and the initial CT was considered as normal. Subsequent MRI advised by the SEEIT because of focality in the clinical manifestation, revealed an acute stroke |
Etiology missed on CT |
| 21 | 83 | F | No | Cryptogenic | Acute ischemic stroke |
Initial imaging was considered as normal. Subsequent MRI advised by the SEEIT because of focality in the clinical manifestation, revealed an acute ischemic stroke |
Etiology missed on CT |
| 22 | 79 | F | No | Drugs intoxication | Dementia | Patient had mild increase in antipsychotic treatment in setting of dementia and very mild hypernatremia. However, the features identified by the SEEIT were not considered as sufficient to provoke SE |
Disagreement on causality judgment of minor precipitants |
| 23 | 49 | F | Yes | Known epilepsy without provocative factors |
ASD related | Patient known for epilepsy treated with LEV, VPA, and LCM. There was no evidence of nonadherence in initial evaluation. Later, low level of VPA level became available and pointed out nonadherence |
Incomplete history information |
| 24 | 27 | F | No | CNS infection | Cryptogenic (NORSE) |
Presented with flu-like symptoms a week before entering a prolonged refractory nonconvulsive SE in coma. The CSF in early phase showed a mild lymphocytosis (15 white bloodcells/mm3). Despite a very broad evaluation including wide infectious and autoimmune panels, no etiology was found. She left the hospital 74 days later with significant cognitive problems |
CSF data misinterpreted |
CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; ED, emergency department; F, female; HIV, human immunodeficiency virus; HSV, herpes simplex virus; LCM, lacosamide; LEV, levetiracetam; LP, lumbar puncture; M, male; MRI, magnetic resonance imaging; NMDA, N-methyl-d-aspartate; NORSE, new onset refractory status epilepticus; PCR, polymerase chain reaction; SE, status epilepticus; SEEIT, Status Epilepticus Etiology Identification Tool; UTI, urinary tract infection; VPA, valproic acid; WC, white cells.
Discussion
The principal finding of this study is that early identification of the underlying etiology for SE is possible using a tool designed to guide differential diagnosis assessment. The SEEIT appears valid, with concordance in 88.7% of cases between the etiology hypothesis generated using SEEIT and the definitive etiology determined at hospital discharge. It is also reliable, with a high interrater agreement between physicians of different subspecialties and levels (ED resident and trained neurologist). Consequently, the SEEIT may be of assistance to nonspecialist physicians in guiding their identification of the etiology of SE promptly and expeditiously.
This early identification of SE etiology is important, as in this cohort nearly half of patients warranted a specific treatment of the illness causing their SE, along with ASD treatment. Furthermore, because etiology is one the most important determinants of SE outcome,4,5,10,20 an etiology-tailored treatment should be initiated as early as possible, particularly in conditions such as CNS infection, sepsis, metabolic disturbances, or acute cerebrovascular illnesses. This tool may be valuable in prompting clinicians to think earlier about etiology-guided treatment. Trying to improve ASD protocols and refining them may have a limited impact on SE outcome. Indeed, protocol adherence21 and newer ASDs do not appear to affect prognosis,22 whereas intramuscular treatment23 and prehospital protocols24 already allow rapid ASD administrations. Therefore, alternatives to ASD trials should be explored to improve outcomes in patients with SE. Efforts aimed at identifying and targeting the underlying biologic background could be one option.10,25
A further relevant finding is that two patients presenting with PNESE signs noted in the first part of the SEEIT were treated as having refractory SE, possibly because of lack of awareness of PNESE symptoms in the ED; one was even intubated. Indeed, these episodes are frequently misdiagnosed as “refractory SE,”19 and poor outcome due to overtreatment has been reported.26 By highlighting some clinical features of PNESE, the SEEIT may help avoid unnecessary, and potentially harmful, treatment in these occasions. Of note, the rate of PNESE mistaken for SE is low in this cohort. This is likely explained by the tertiary care setting and the 24/7 availability of neurology consultants in the four centers involved in this study.
We were unable to demonstrate any significant factors that interfered with correct etiology identification using our tool, other than presence of prior seizures. This may reflect the fact that medication nonadherence or recent treatment adjustments are common SE causes and are easy to recognize. This reinforces the principle that all patients with SE should be evaluated carefully to identify the underlying etiology, independent of age, seizure type, or SE severity.
The detailed description of misdiagnosed cases (Table 3) shows that brain magnetic resonance imaging (MRI) is crucial if history and computerized tomography (CT) scan fail to identify the etiology; in another smaller study, MRI improved the diagnosis by 32% in a cohort of 34 patients.27 CSF data may be misleading. Some cases of SE were incorrectly labeled as due to infectious processes because of the CSF pleocytosis, which turned out to be noninfectious (due to neoplastic or autoimmune conditions) or caused by the SE itself in one case of mild pleocytosis, which can be seen in 10% of SE occurring in the setting of a known epilepsy.28 Nevertheless, because the exact cause of CSF pleocytosis may take several days to be clarified, and in view of the potential poor outcome associated with CNS infections, it is still reasonable to consider all SE with pleocytosis as infectious until proven otherwise. This study also included a 75-year-old man with new-onset refractory SE associated with fever and a normal CSF study (four white cells) performed 36 h after symptom onset; his CSF polymerase chain reaction showed herpes simplex virus type 1 (HSV-1) encephalitis. CSF is abnormal in 95% of HSV-1 encephalitis,29 but can be normal early in the illness,30 as illustrated by this case. This particular pitfall is pointed out in the SEEIT tool.
As reported earlier,31,32 subtherapeutic ASD levels due to nonadherence or treatment adjustment are among the most frequent causes of SE. This should be addressed carefully by a thorough history, and ASD levels should be obtained when appropriate. Because some newer ASD levels cannot be measured quickly, detecting nonadherence based on this feature alone can be difficult. A careful history with relatives is thus very important in such cases. The relatively high incidence of SE due to brain tumors in this cohort, as opposed to previous studies,31,33 likely results from referral bias, as the four institutions in this study have, or are closely associated with, large neurooncology clinics. Similarly, although alcohol withdrawal was a frequent precipitant in other series, ranging from 13%32 to 17%,31 it was infrequent in ours (2.8%), also probably explained by a referral bias.
The strength of this study is the large number of patients from four international sites and the prospective evaluation implying a good potential for generalization and good data quality. The main limitation is that the SEEIT was completed by the study investigator familiar with it (a neurologist) and not by the treating physician. This could help to explain the high concordance coefficient between the SEEIT and the etiology determined after a comprehensive evaluation. Still, the interrater agreement evaluation between the study investigator and an emergency physician was high, and there was no difference in the agreement rate among the four centers involved. Another limitation is that the SEEIT relies on history for some items and sometimes there are neither relatives nor witnesses. A comprehensive history is a key component in the management of many conditions, including SE, and unfortunately, our tool cannot fill the lack of information in these situations. Moreover, as patients were screened by using the EEG request (and not in the ED), we could not exclude the possibility that some information available in the EEG laboratory influenced the investigator completing the tool, but only information available during the ED stay was used for the early etiology assessment. In addition, we cannot exclude that because of the EEG screening process, some brief or unrecognized SE episodes were missed. Indeed, in these situations, treating physicians might not have requested an EEG. In addition, the yield of each item in the SEEIT was not evaluated, but in clinical practice, a diagnosis is made after a global assessment and not based on one particular feature alone. Another shortcoming is that the SEEIT failed to identify definite etiology correctly because sometimes history, imaging, or some data were not available. The results would perhaps have been different if all information were available in each case. However, in that case, this would probably have increased the performance of SEEIT. The tertiary hospital setting may also confer a selection bias. Indeed, this may have resulted in the inclusion of more patients with severe SE. We do not believe that this should influence the validity of the SEEIT. Moreover, fewer patients were enrolled at the MGH than at the BWH. We cannot exclude the possibility of undersampling at the MGH and do not expect this to have influenced our findings. Finally, we used broad inclusion criteria: all types of SE, and an operational definition,9 as opposed to more rigorous inclusion criteria focusing on generalized convulsive SE lasting >30 min. Because the SEEIT is designed to be used in daily practice, these inclusion criteria may better reflect “actual clinical practice.”
This study shows that the SEEIT correctly identifies the cause of an SE in 88.7%. It also demonstrates that it is possible to identify the etiology of an episode of SE early with a valid and reliable clinical tool to guide differential diagnosis, used by physicians from different subspecialties. Further studies are needed to evaluate whether the SEEIT will improve decision making process in SE management, avoiding unnecessary investigations or treatments, influencing the length of stay, or impacting on clinical outcome.
Acknowledgments
The investigators would like to thank Christine Staeli, RN (CHUV), Christine Scott, R Tech (MGH), and the EEG physicians/fellows from the EEG laboratories at the BWH (Swapna Putta and Hong Yu), BIDMC (Andrew Schomer and Stephen VanHaerents), CHUV (Ian Novy, Muriel Tschirren, Spyridoula Tsetsou, Myriam Guidon, and Anita Barbey), and MGH (Kheder Amar, Yuan Fan, Arash Hadipour Niktarash, Lidia Moura, Marcus Ng, Deirdre O’Rourke, and Sandipan Pati) for their help in patients identification and data collection. In addition, the authors would like to thank the Epilepsy Research Group at the Brigham and Women’s Hospital for their valuable advice and insights during manuscript preparation.
Biography

Dr. Vincent Alvarez is a neurologist and epileptologist at the Valais Hospital and a visiting scientist at the Brigham and Women’s Hospital.
Footnotes
Disclosure or Conflict of Interest
Dr. Alvarez in funded by the Swiss National Science Foundation, grant: P2GEP3_148510 and the Gottfried und Julia Bangerter-Rhyner Foundation. Dr. M Brandon Westover has received research funding from the American Brain Foundation. Dr. Frank W. Drislane has received royalties form UpToDate and from Lippincott Williams & Wilkins. Dr. Barbara A. Dworetzky has received research funding from the FDA, the Epilepsy Foundation, and the American Epilepsy Society. She is a consultant for SleepMed and Best Doctors. Dr. David Curley has nothing to disclose. Dr. Jong Woo Lee has received research funding from UCB, Inc, the Duke Clinical Research Institute, and Sunovion, Inc. He is a consultant for SleepMed. Dr. Andrea O. Rossetti received research support from Sage, UCB, and Eisai. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Coeytaux A, Jallon P, Galobardes B. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR) Neurology. 2000;55:693–697. doi: 10.1212/wnl.55.5.693. [DOI] [PubMed] [Google Scholar]
- 2.Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42:714–718. doi: 10.1046/j.1528-1157.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 3.Vignatelli L, Tonon C, D’Alessandro R. Incidence and short-term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia. 2003;44:964–968. doi: 10.1046/j.1528-1157.2003.63702.x. [DOI] [PubMed] [Google Scholar]
- 4.Towne AR, Pellock JM, Ko D, et al. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 5.Logroscino G, Hesdorffer DC, Cascino G, et al. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997;38:1344–1349. doi: 10.1111/j.1528-1157.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti AO, Hurwitz S, Logroscino G, et al. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J NeurolNeurosurg Psychiatry. 2006;77:611–615. doi: 10.1136/jnnp.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neligan A, Shorvon SD. Prognostic factors, morbidity and mortality in tonic-clonic status epilepticus: a review. Epilepsy Res. 2011;93:1–10. doi: 10.1016/j.eplepsyres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 9.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 10.Trinka E, Höfler J, Zerbs A. Causes of status epilepticus. Epilepsia. 2012;53(Suppl. 4):127–138. doi: 10.1111/j.1528-1167.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 11.Peleg M, Tu S. Decision support, knowledge representation and management in medicine. In: Haux R, Kulikowski C, editors. IMIA yearbook of medical informatics 2006. Schattauer GmbH; Stuttgart: 2006. pp. 72–80. [PubMed] [Google Scholar]
- 12.Fesmire FM, Hughes AD, Fody EP, et al. The Erlanger chest pain evaluation protocol: a one-year experience with serial 12-lead ECG monitoring, two-hour delta serum marker measurements, and selective nuclear stress testing to identify and exclude acute coronary syndromes. Ann Emerg Med. 2002;40:584–594. doi: 10.1067/mem.2002.129506. [DOI] [PubMed] [Google Scholar]
- 13.Cortelli P, Cevoli S, Nonino F, et al. Evidence-based diagnosis of nontraumatic headache in the emergency department: a consensus statement on four clinical scenarios. Headache. 2004;44:587–595. doi: 10.1111/j.1526-4610.2004.446007.x. [DOI] [PubMed] [Google Scholar]
- 14.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(Suppl. 6):28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 15.Berg A, Berkovic SF, Brodie M, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 16.Rossetti AO, Logroscino G, Milligan TA, et al. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255:1561–1566. doi: 10.1007/s00415-008-0989-1. [DOI] [PubMed] [Google Scholar]
- 17.Tan RYL, Neligan A, Shorvon SD. The uncommon causes of status epilepticus: a systematic review. Epilepsy Res. 2010;91:111–122. doi: 10.1016/j.eplepsyres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 19.Holtkamp M, Othman J, Buchheim K, et al. Diagnosis of psychogenic nonepileptic status epilepticus in the emergency setting. Neurology. 2006;66:1727–1729. doi: 10.1212/01.wnl.0000218299.15988.9d. [DOI] [PubMed] [Google Scholar]
- 20.Holtkamp M, Othman J, Buchheim K, et al. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J NeurolNeurosurg Psychiatry. 2005;76:534–539. doi: 10.1136/jnnp.2004.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossetti AO, Alvarez V, Burnand B, et al. Treatment deviating from guidelines does not influence status epilepticus prognosis. J Neurol. 2013;260:421–428. doi: 10.1007/s00415-012-6644-x. [DOI] [PubMed] [Google Scholar]
- 22.Jaques L, Rossetti AO. Newer antiepileptic drugs in the treatment of status epilepticus: impact on prognosis. Epilepsy Behav. 2012;24:70–73. doi: 10.1016/j.yebeh.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranda A, Foucart G, Ducassé JL, et al. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51:2159–2167. doi: 10.1111/j.1528-1167.2010.02688.x. [DOI] [PubMed] [Google Scholar]
- 25.Sutter R, Marsch S, Fuhr P, et al. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;82:656–664. doi: 10.1212/WNL.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuber M, Baker GA, Gill R, et al. Failure to recognize psychogenic nonepileptic seizures may cause death. Neurology. 2004;62:834–835. doi: 10.1212/01.wnl.0000113755.11398.90. [DOI] [PubMed] [Google Scholar]
- 27.Goyal MK, Sinha S, Ravishankar S, et al. Role of MR imaging in the evaluation of etiology of status epilepticus. J NeurolSci. 2008;272:143–150. doi: 10.1016/j.jns.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Barry E, Hauser WA. Pleocytosis after status epilepticus. Arch Neurol. 1994;51:190–193. doi: 10.1001/archneur.1994.00540140100019. [DOI] [PubMed] [Google Scholar]
- 29.Steiner I, Kennedy PGE, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 30.Richard J, Soong S, Linneman C, et al. Herpes simplex encephalitis: clinical assessment. JAMA. 1982;247:317–320. [PubMed] [Google Scholar]
- 31.Aminoff MJ, Simon RP. Status epilepticus. Causes, Clinical features and consequences in 98 patients. Am J Med. 1980;69:657–666. doi: 10.1016/0002-9343(80)90415-5. [DOI] [PubMed] [Google Scholar]
- 32.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 33.Wu YW, Shek DW, Garcia PA, et al. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58:1070–1076. doi: 10.1212/wnl.58.7.1070. [DOI] [PubMed] [Google Scholar]