Abstract
Background
Tacrolimus (Tac) and Cyclosporine A (CyA) calcineurin inhibitors (CNIs) are 2 effective immunosuppressants which are essential to prevent allograft rejection. Calcineurin inhibitors are known to be nephrotoxic. However, the precise mechanism of nephrotoxicity is not fully understood. In this study, we investigated the in vivo effects of CNIs on the local renal renin-angiotensin system in the collecting duct (CD).
Methods
Three-week-old mice were treated with either vehicle, CyA (2 mg/kg per day), Tac (0.075 mg/kg per day), CyA + Aliskiren (25 mg/kg per day), or Tac + Aliskiren for 3 weeks. Serum creatinine was measured. Renin and vascular endothelial growth factor (VEGF) contents in CD were evaluated with flow cytometry and multiphoton microscopy. The diameter of vessels was assessed with multiphoton microscopy, and the amount of renal collagen was determined by real-time polymerase chain reaction and Masson staining.
Results
The elevated level of serum creatinine in CNI groups was abolished by Aliskiren. Flow cytometric analysis found elevated renin content in principal cells, which was prevented by Aliskiren. This result was further confirmed with multiphoton microscopy. The VEGF content in CD correlated with reduced capillary diameter and with the formation of fibrotic islands.
Conclusions
Calcineurin inhibitors induce production of renin in the CD that may contribute to decreased renal blood flow. In turn, CD responds with increased VEGF production, resulting in disproportional vessel growth, further worsening the local hypoxia and striped fibrosis surrounding the CDs. Aliskiren, a direct renin inhibitor blocks these effects and improves CNI-induced nephropathy by decreasing renin production in the CDs. Our data suggest that Aliskiren may be used for the prevention of CNI nephrotoxicity.
The causes of renal allograft loss have changed with the introduction of new immunosuppressive agents. The rate of 1-year renal allograft survival has remarkably improved due to better therapeutic regimens. However, in the long-run, chronic allograft damage is still a significant problem which is often responsible for the loss of grafts.1,2 Among the alloantigen-independent factors, calcineurin inhibitor (CNI)–induced nephrotoxicity is a prominent and potentially modifiable contributor to its pathogenesis.1,2 However, the underlying mechanism of this nephrotoxicity is not fully understood yet. Calcineurin inhibitors are known to cause a remarkable reduction in renal blood flow (BF) due to afferent arteriolar vasoconstriction, and activation of the intrarenal renin-angiotensin system (RAS), increased release of endothelin-1, dysregulation of nitrogen-monoxide and nitrogen-monoxide-synthase, upregulation of transforming growth factor-β1, inappropriate apoptosis, stimulation of innate immunity, and autophagy have all been implicated in the pathogenesis of CNI nephropathy3-5 Consequently, characteristic histological changes of tubular injury, interstitial fibrosis, and arteriolopathy develop accompanied by the worsening of kidney function.6-9
In CNI nephropathy, many regulatory systems are highly activated including the RAS. The key regulator of RAS, the renin beyond the regulation of sodium, extracellular fluid volume, and blood pressure homeostasis10 may also play an important role in tissue fibrosis.11 To date, the juxtaglomerular apparatus (JGA) is considered as the classic anatomical site of highly regulated renin synthesis and release. However, in certain pathophysiological conditions, further cell types can also regain the capability to produce renin.12 One of the recent exciting topics in RAS research is the discovery of renin production in the renal collecting ducts (CDs).13-17 Kang et al14 described the angiotensin II (ANGII)–dependent regulation of CD renin and the hyperplasia of renin-producing CD in diabetes, emphasizing its potential effects as a disease mediator. Compared with its negative feedback on JGA renin, ANGII stimulates CD renin, contributing to the increased intrarenal ANGII levels15 as well as distal nephron sodium reabsorption in ANGII-dependent hypertension16 and in 2-kidney-1-clip model of renovascular hypertension.17
The role of renin in the initiation and maintenance of CNI nephropathy has been investigated by several groups. It has been described that hypertrophy of the JGA occurs in rats treated with CNI.18 In addition, juxtaglomerular cells are known to express calcineurin isoforms, and due to their inhibition Cyclosporine A (CyA) stimulates renin release from cultured granular cells.19 Furthermore, the recruitment of renin in afferent arterioles was observed in vivo in kidneys of CyA-treated rats resulting in major hemodynamic changes in the renal microvasculature.20 Similar observations have been found related to Tacrolimus (Tac) as its administration highly increased plasma renin activity.21 However, all these previous studies have focused on JGA renin, but other locations of intrarenal renin production have not been investigated.
The aim of our present studies was to investigate the role of the CD segment in the development of CNI nephrotoxicity and tissue fibrosis via local renin production.
MATERIALS AND METHODS
Model of CNI Nephrotoxicity
Three-week-old, male C57Bl6 mice (n = 25) were divided into 5 groups. Mice were treated with either vehicle (control), 0.075 mg/kg Tac per day, 2 mg/kg CyA per day or CNIs at the same concentration in combination with the direct renin inhibitor Aliskiren (Alisk) at a dose of 25 mg/kg per day (Tac + Alisk or CyA + Alisk, respectively), intraperitoneally. We aimed the CNIs to reach a trough level comparable with the target range used in renal transplant patients, thus recreating the same adverse effect profile. The dose of Aliskiren was selected and adopted from previous studies on C57Bl6 mice where effective blockade of renin was observed without leading to changes of systemic blood pressure.22
After 3 weeks of drug administration, animals underwent intravital multiphoton microscopy followed by euthanasia and tissue harvest. Samples were stored for further measurements at −80°C. All in vivo experiments were performed according to the Committee on the Care and Use of Laboratory Animals of the Council on Animal Care at the Semmelweis University (22.1/3491/003/2008).
CNI's Trough Level
Whole-blood CNI concentrations were measured using high-performance liquid chromatography (HPLC). Samples were collected 12 hours after the last administration after 3 weeks of treatment. One hundered microliters of blood was mixed with 300 μL acetonitrile. After vortexing and centrifugation, 50 μL of supernatant was transferred to 200 μL vials before injection into the liquid chromatography-mass spectrometry system. The HPLC was performed using a Perkin Elmer Series 200 micro LC system (Perkin Elmer, Waltham, MA) which included a Series 200 Autosampler. For the MS/MS analysis, a QTrap 6500 system with a TurboIon-Spray source (AB Sciex, Dublin, CA) was used. The calibration range was 10 to 250 ng/mL for CyA and 0.1 to 5 ng/mL for Tac. The limit of detection was 5 ng/mL and 0.05 ng/mL, respectively.
Renal Parameters
Serum creatinine was determined by HPLC-mass spectrometry. After the preparation of screening paper samples, for separation LiChroCART 55-2 Purospher STAR RP-18 end capped 3 μm column was used under isocractic circumstances (85% of water, 15% of acetonitrile eluent). The time of creatinine and creatinine d3 retention was 0.9 minutes. The mass spectrometry measurement is done by electrospray source in multiple reaction monitoring mode.
Urinary albumin level was measured with commercially available kits on a Beckmann Coulter AU480 photometric chemistry analyzer.
Measurement of Blood Pressure
Arterial blood pressure (systolic, diastolic, mean arterial pressure) in mice was determined via the tail-cuff method with CODA Standard monitor system using a Volume Pressure Recording sensor (KENT Scientific Corporation, Torrington, CT). Three measurement cycles were averaged in each animal.
Renin and Collagen I Messenger RNA Expression
Total RNA was isolated from whole kidney tissue, renal medulla, and cortex by total RNA Mini Kit (Geneaid Biotech, Bade City, Taiwan). To avoid the contamination of JGA renin to CD renin, kidneys were dissected into medulla and cortex, as it was previously described.14,15 RNA was reverse-transcribed using Maxime First Standard cDNA Synthesis Kit for real-time (RT) quantitative polymerase chain reaction (PCR) (Thermo Scientific, Waltham, MA) to generate first-strand cDNA. The messenger RNA (mRNA) expression of renin, collagen I, and glyceraldehyde-3-phosphate dehydrogenase were determined on a Light Cycler 480 system (Roche Diagnostics, Mannheim, Germany). The reaction mix contained Luminaris Color HiGreen Fluorescein quantitative PCR Master Mix (Thermo Scientific, Waltham, MA), each PCR primers (Table 1) and cDNA sample according to the instructions of the manufacturer. The conditions of the RT-PCR were as follows: 1 cycle at 95°C for 10 minutes, followed by cycles at 95°C for 15 seconds, 60°C for 30 seconds and 72°C for 30 seconds. Quantification was performed with the second-derivative method by monitoring the cycle number at which the fluorescent sign could be distinguished from the background (crossing point). The mRNA expression of renin and collagen I was determined by comparison with glyceraldehyde-3-phosphate dehydrogenase as internal control from the same samples. Results were analyzed with Light-Cycler software, version 1.5.0.39 (Roche Diagnostics, Mannheim, Germany).
TABLE 1.
Nucleotide sequences of the different primers applied for the detection of renin, collagen I and GAPDH by real-time RT-PCR
| Gene | Primer pairs | Product length, bp |
|---|---|---|
| Renin | forward: 5′-TGC CCA CCC TCC CCG ACA TT-3′ | 167 |
| reverse: 5′-GGC ACC CAG GAC CCA GAC AGG-3′ | ||
| Collagen I | forward: 5′-CTG CCC CGG CGC CGA AGT C-3′ | 96 |
| reverse: 5′-CCC TCG ACG CCG GTG GTT TCT TG-3′ | ||
| GAPDH | forward: 5′-ATC TGA CGT GCC GCC TGG AGA AAC-3′ | 164 |
| reverse: 5′-CCC GGC ATC GAA GGT GGA AGA GT-3′ |
GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
Renin Activity
Blood was collected into ethylenediaminetetraacetic acid pretreated tubes from the heart of mice immediately after induction of ketamine/xylazine anesthesia. Plasma was separated by centrifugation and frozen at −20°C until used for renin determinations. Plasma renin activity was measured by angiotensin I radioimmunoassay kit (Beckman Coulter Inc., Brea, CA) on a Riamat 280 analyzator (DiaSorin, Stillwater, MN) according to the protocol described by the manufacturer. Values were expressed as ng Ang 1/mL per hour.
Flow Cytometric Analysis
After 3 weeks of drug administration, kidneys were harvested, and the tissue was homogenized by collagenase II (Sigma-Aldrich, St. Louis, MO). After washing with phosphate-buffered saline, fluorescence-activated cell sorting lysing solution (BD Biosciences, San Jose, CA) was added and after removal, samples were permeabilized with FACS permeabilizing solution 2 (BD Pharmingen, San Diego, CA). After the permeabilization cells were stained for 30 minutes with 2 μL rabbit polyclonal antirenin antibody (200 μg/mL; Anaspec, Fremont, CA), 2 μL goat polyclonal antiaquaporin 2 antibody (AQP2), and 2 μL rabbit polyclonal antivascular endothelial growth factor-A antibody (200 μg/mL, both from Santa Cruz Biotechnology Inc., Dallas, TX). After washing, cells were incubated for 30 minutes with the appropriate secondary antibodies: 1 μL Alexa 488-conjugated chicken antirabbit antibody and 1 μL Alexa 647-conjugated donkey antigoat antibody (Life Technologies, Carlsbad, CA). Negative controls were incubated only with secondary antibody. Thereafter, cells were washed, centrifuged (2 000 g, 5 minutes, RT) and resuspended in phosphate-buffered saline. The cytometric analyses were carried out using a fluorescence-activated cell sorting Aria flow cytometer (BD Biosciences). According to the forward and side scatter, we identified an intact cell gate (without debris). Results were analyzed with BD CellQuest Pro software (BD Biosciences).
Multiphoton Excitation Fluorescence Microscopy
Multiphoton excitation fluorescence microscopy is a state-of-the-art imaging technique ideal for deep optical sectioning of living tissues.23,24 Animals were anesthetized with Ketamine and Xylazine given intraperitoneally (100 mg/kg and 8 mg/kg, respectively). The trachea and carotid artery were cannulated to provide stable respiration and dye administration. Then, the left kidney was gently exteriorized on the dorsal side and put on the stage. The kidney was continuously bathed in warm 0.9% saline during imaging. Fluorescence images were acquired using Femto 2D inverted microscope setup (Femtonics Inc, Budapest, Hungary) incorporating a commercially available Olympus microscope (IX81). Observations were made using an Olympus 60× glycerin immersion objective (UPLSAPO 60×, N. A. = 1.35). Fluorescence excitation was provided by a MaiTai tunable ultrafast laser equipped with dispersion compensation (MaiTai Deep Sea Laser, Spectra-Physics Inc., Irvine, CA). An excitation wavelength of 820 nm was used. Images and data volumes were processed using MES, a separate MATLAB-based modular measurement control and analysis software package (Femtonics Inc.) and ImageJ software (US National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). Seventy-kDa rhodamine dextran (Life Technologies) was used to label the vasculature (in red) and quinacrine dihydrochloride (Sigma-Aldrich) to identify the acidic granules, among which based on their size, the renin containing ones were easily recognizable (in green) as it has been previously described.25 Hochest 33342 (Sigma-Aldrich) was applied to visualize the intact nuclei (in green). To analyze vessel diameters, 3 different images were taken per animal in a blinded fashion, and 50 different measurements were made on each picture as described previously.26
Histological Analysis
Paraffin sections of the excised kidneys were stained with Masson trichrome reagent which shows the nuclei in black, the cytoplasm and erythrocytes in red, and the collagen in blue. Samples were coded and semiquantitatively evaluated for interstitial collagen in a blinded fashion by light microscopy. An average of 12 images per kidney was acquired with a 20× objective. Images from cortex and medulla were obtained by taking consecutive samples avoiding glomeruli and large vessels.
Statistical Analysis
Data were analyzed by STATISTICA.6 software and tested for normal distribution with Kolmogorov-Smirnov test. Comparisons were evaluated using ANOVA (for parametric data) followed by Fisher correction. A P value less than 0.05 was considered to be significant. Data are presented as means ± SEM.
RESULTS
Kidney Function, Body Weight, Blood Pressure
Renal Function Parameters
To evaluate the beneficial effects of Alisk in CNI nephropathy, we measured serum creatinine levels by applying mass spectrometry. Controls showed physiologic range of creatinine, whereas after CNI administration these values doubled, suggesting a significant deterioration of kidney function (C: mean, 19.5 ± 2.6 μmol/:; median, 21.27 μmol/L; range, 10.26-26.59 μmol/l vs CyA: mean, 31.7 ± 3.6 μmol/L; median, 29.56 μmol/L; range, 23.59-51.09 μmol/L; Tac: mean, 33.6 ± 5.4 μmol/L; median, 28.17 μmol/L; range, 21.17-55.53 μmol/L; P ≤ 0.05). Coadministration of CNI with Alisk resulted in a remarkable amelioration of the decline of kidney function (C: mean, 19.5 ± 2.6 μmol/L; median, 21.27 μmol/L; range, 10.26-26.59 μmol/L vs CyA + Alisk: mean, 26 ± 1.3 μmol/L; median, 24.25 μmol/L; range, 23.72-29.68 μmol/L; Tac + Alisk: mean, 23.4 ± 1.7 μmol/L; median, 22.87 μmol/L; range, 20.00-27.76 μmol/L; P = NS). The body weight of the mice in different groups did not differ significantly (Table 2) thus did not influence the measurement of creatinine. In all groups, the urinary protein excretion was found within the range of microalbuminuria (Table 2), demonstrating the absence of glomerular damage.
TABLE 2.
Descriptive parameters
| Control | CyA | Tac | CyA + Alisk | Tac + Alisk | |
|---|---|---|---|---|---|
| Body weight, g | 19.2 ± 0.5 | 19.6 ± 0.7 | 19.2 ± 0.5 | 18.6 ± 0.3 | 18.4 ± 0.3 |
| CyA, ng/mL | — | 42.52 ± 1.2 | — | 20.43 ± 1.1 | — |
| Tac, ng/mL | — | — | 2.14 ± 0.2 | — | 1.73 ± 0.2 |
| Serum creatinine, μmol/L | 19.5 ± 2.6 | 31.7 ± 3.6* | 33.6 ± 5.4* | 26 ± 1.3 | 23.4 ± 1.7 |
| Urinary protein, g/L | 0.30 ± 0.10 | 0.35 ± 0.05 | 0.25 ± 0.06 | 0.30 ± 0.0 | 0.30 ± 0.04 |
| Blood pressure | |||||
| Systolic, mm Hg | 104.6 ± 2.8 | 109.4 ± 3.7 | 107.5 ± 3.3 | 108.1 ± 3.5 | 102.0 ± 4.1 |
| Dyastolic, mm Hg | 84.3 ± 2.6 | 85.9 ± 4.3 | 83.5 ± 3.4 | 85.6 ± 3.2 | 80.6 ± 2.6 |
| MAP, mm Hg | 91.0 ± 2.6 | 93.7 ± 4.3 | 89.2 ± 2.5 | 93.1 ± 3.3 | 85.0 ± 4.3 |
The body weight does not differ significantly between the controls and the treated groups. The trough level of the CNIs was set low enough to avoid a severe nephrotoxicity. Based on the serum creatinine there was already after 3 weeks of CNI treatment a significant deterioration of kidney function, which was abolished by cotreatment with direct renin inhibitor Aliskiren. In all groups, the urinary protein excretion was found under or within the range of microalbuminuria. The tail-cuff method did not reveal any significant difference in blood pressure (systolic, diastolic, and MAP) between the controls and treated groups.
P ≥ 0.05 vs. control
MAP indicates mean arterial pressure
Blood Pressure
To exclude that the CNI's result in damage to the kidney via influencing the blood pressure, we measured the blood pressure and did not find any significant difference between the controls and treated groups (Table 2).
CNI's Trough Level
To evaluate our treatment, we measured the trough level of CyA as well as Tac. Compared with the controls, we have seen a blood trough levels comparable to human target range (CyA: 42.52 ± 1.2 ng/mL; CyA + Alisk: 20.43 ± 1.1 ng/mL; Tac: 2.14 ± 0.2 ng/mL; Tac + Alisk: 1.73 ± 0.2 ng/mL).
Renin mRNA Expression in Renal Medulla and Cortex
To confirm the in situ mRNA expression of renin in cells of the CD, RT-PCR was performed. In control mice, the renal medulla (avoiding contaminants from the cortical JGA) expressed comparable amounts of renin mRNA to the cortex (predominantly JGA containing part of the kidney) (renin/ glyceraldehyde-3-phosphate dehydrogenase in cortex vs medulla: 1.00 ± 0.35 vs 0.74 ± 0.43 relative unit, respectively, P = NS).
Increased Renin Content in the CD and Its Systemic Effect
Flow Cytometry
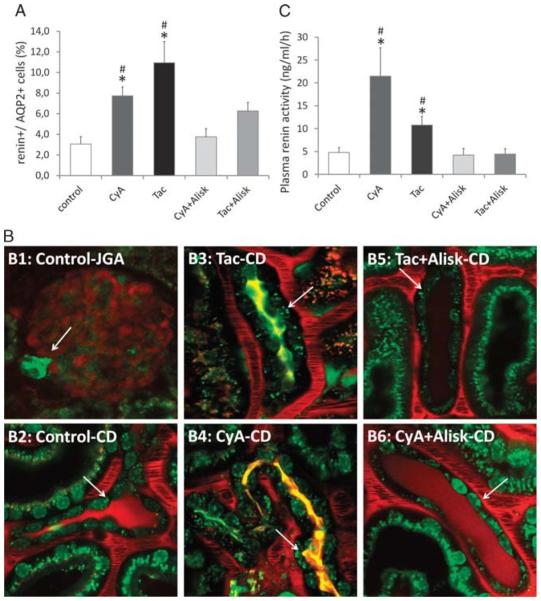
Applying flow cytometric analysis, we sorted the AQP2+ principal cells from the kidney, in which we further evaluated the renin-positive population (Figure 1A). In vehicle-treated animals, the percentage of renin-positive cells was only 3.1 ± 0.7% of all the principal cells, which more than doubled in CyA-treated mice and almost tripled in Tac-treated mice (C: 3.1 ± 0.7% vs CyA: 7.7 ± 0.9%; Tac: 11 ± 2.0%; P ≤ 0.05). Noteworthy, this effect was abolished when CNIs were given in combination with Alisk (C: 3.1 ± 0.7% vs CyA + Alisk: 3.8 ± 0.8%; Tac + Alisk: 6.3 ± 0.8%; P = NS).
FIGURE 1.
Increased renin content in the collecting duct and its systemic effect. A, Renin content in principal (AQP2+) cells: approximately 3% of AQP2+ principal cells were positive for renin in control mice. After 3 weeks of CNI treatment, we have seen a significant increase in renin content, which diminished when the CNI treatment was combined with the direct renin inhibitor Aliskiren. B, Renin content in the collecting ducts: the classical renin-producing granular cells in the juxtaglomerular apparatus (B1: control-JGA) stained with Quinacrine in green (arrows). In the collecting duct, the control group (B2: control-CD) shows renin granules in trace (arrows), whereas the 2 CNI-treated groups presented robust expression of renin (B3: Tac-CD, B4: CyA-CD), which was almost completely abolished by the direct renin inhibitor Aliskiren (B5: Tac + Alisk-CD, B6: CyA + Alisk-CD). C, Serum renin activity: the control mice showed serum renin activity 4.8 ng/mL per hour. After 3 weeks of CNI treatment, we have seen a significant increase in renin activity, which diminished when the CNI treatment was combined with the direct renin inhibitor Aliskiren. (n = 4-6/group) *P ≤ 0.05 versus control, #P ≤ 0.05 versus Alisk; green: quinacrine (renin-positive granules), Hoechst 33342 (nuclei), and autofluorescence; red: 70 kDa rhodamine dextran (vasculature).
Multiphoton Microscopy
Multiphoton microscopy was performed to visualize the changes in renin content and its localization during CNI treatment. Quinacrine (dye for acidic granules) was confirmed to have the applicability to stain the 2 to 4 μm large renin granules in the granular cells of JGA (Figure 1B, B1: Control-JGA). In the CD, predominantly in principal cells, quinacrine-positive granules were seen in the same size like the renin granules in the JGA (Figure 1B, B2: Control-CD). As a second set of multiphoton microscopy experiments, renin content in the CD was analyzed in vivo. In mice treated with vehicle, hardly any renin granulation was present (Figure 1B, B2: Control-CD). However, a different pattern was observed after 3 weeks of CNI administration (Figure 1B, B3: Tac-CD and B4: CyA-CD). Granules 2 to 4 μm in diameter densely populated the principal cells close to the apical and basal membrane of these cells. However, in case of combined treatment of CNI with Alisk, the renin content remarkably decreased in this localization, almost to the level of controls (Figure 1B, B5: Tac + Alisk-CD and B6: CyA + Alisk-CD).
Serum Renin Activity
We measured the serum renin activity to determine whether CNI treatment results in only a local or as well as systemic increase in renin activity (Figure 1C). In vehicle-treated animals the renin activity was 4.8 ng/mL per hour, which increased 5 times in CyA-treated mice and doubled in Tac-treated mice (C: 4.8 ± 1.1 ng/mL per hour vs CyA: 21.5 ± 6.2 ng/mL per hour; Tac: 10.8 ± 1.9 ng/mL per hour; P ≤ 0.05).
According to the mechanism of action, this increase was abolished when CNIs were given in combination with Alisk (C: 4.8 ± 1.1 ng/mL per hour vs CyA + Alisk: 4.2 ± 1.4; Tac + Alisk: 4.4 ± 1.2; P = NS).
Disproportional Vessel Diameter and a Possible Underlying Pathomechanism
Multiphoton Microscopy and Flow Cytometry
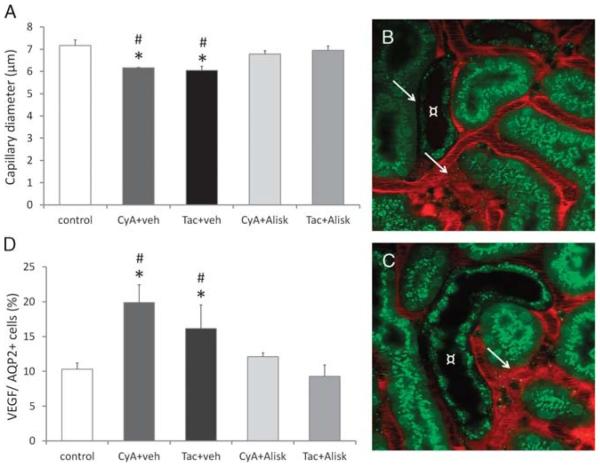
Using multiphoton microscopy, the diameter of peritubular capillaries were measured in vivo (Figure 2A). In control animals, the average diameter was 7.2 ± 0.3 μm, whereas after administration of CNIs, a significantly reduced capillary diameter was seen (C: 7.2 ± 0.3 μm vs CyA: 6.2 ± 0.0 μm; Tac: 6.1 ± 0.2 μm; P ≤ 0.05). Noteworthy, Alisk prevented the remarkable decrease of vessel diameter in both CNI-treated groups (C: 7.2 ± 0.3 μm vs CyA _ Alisk: 6.9 ± 0.2 μm; Tac + Alisk: 7 ± 0.2 μm; P = NS). Looking at the change in vessel diameter closely, one can recognize not only a reduced diameter but also a very disproportional vascular growth in the CNI-treated groups. There were areas where remarkable reduced capillary diameter was present, in other cases, highly dilated vessel assemblies with turbulent BF were observed by multiphoton microscopy. Interestingly, these vessel malformations were seen mostly in the proximity of CDs (Figures 2B, C). To gain a closer insight into the underlying pathomechanism, vascular endothelial growth factor (VEGF) content in the AQP2+ principal cells was investigated by flow cytometry (Figure 2D). In controls, 10% of principal cells showed capacity for VEGF production which increased significantly after administration of CNIs (C: 10.3 ± 0.9% vs CyA: 19.9 ± 2.6%; Tac: 16.1 ± 3.4%; P ≤ 0.05). Although Alisk prevented the remarkable increase in VEGF-positive cells in both CNI-treated groups (C: 10.3 ± 0.9% vs CyA + Alisk: 12.1 ± 0.6%; Tac + Alsik: 9.3 ± 1.7%; P = NS).
FIGURE 2.
Disproportional vessel diameter and a possible underlying pathomechanism. A, Capillary diameter measured with multiphoton microscopy: Control mice had an average capillary diameter of 7.2 ± 0.3 μm. After 3 weeks of CNI treatment, the vessels showed significantly reduced diameter, which was abolished by cotreatment with direct renin inhibitor Aliskiren. B and C, Disproportional vessel diameter revealed with multiphoton microscopy: in groups treated with CNIs, remarkable narrow capillaries as well as dilatation with turbulent blood flow (arrows) were seen in the proximity of collecting ducts (¤). D, Flow cytometry of VEGF in principal cell: VEGF positivity was measured in AQP2+ principal cells. After 3 weeks of CNI treatment, there was a significant elevation in VEGF production in principal cells, which was abolished by cotreatment with direct renin inhibitor Aliskiren (n = 4-6/group). *P ≤ 0.05 versus control, #P ≤ 0.05 versus Alisk, AQP2: collecting duct. Green: Quinacrine (renin-positive granules), Hoechst 33342 (nuclei), and autofluorescence; Red: 70 kDa rhodamine dextran (vasculature).
Development of Fibrosis
mRNA Expression of Renal Collagen I
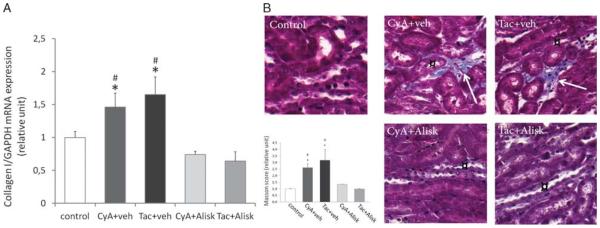
To evaluate the profibrotic effect of CNI treatment and the beneficial effect of Alisk on the progression of CNI nephropathy, we measured the mRNA expression of collagen I in whole kidney (Figure 3A). There was a significant increase in collagen I mRNA expression in both CNI-treated groups compared to controls (C: 1 vs CyA: 1.5; Tac: 1.7 arbitrary units; P ≤ 0.05). In contrast, Alisk treatment prevented the increased collagen I synthesis in the kidney (CyA: 1.5; Tac: 1.7 vs CyA + Alisk: 0.7; Tac + Alsik: 0.6 arbitrary units; P ≤ 0.05).
FIGURE 3.
Development of fibrosis. A, mRNA expression of collagen I in the kidney: the mRNA expression of collagen I significantly increased in both CNI-treated groups. In contrast, Aliskiren treatment prevented the increased collagen production. Panel B, localization of striped fibrotic islands in the kidney: Masson trichrome staining revealed only a weak collagen staining around the arterioles in controls, but hardly any interstitial staining was detected. However, 3-week CNI treatment resulted in significant elevation of collagen amount (arrows) referring to fibrosis in both groups, mostly in the close proximity of collecting ducts (¤). Administering direct renin inhibitor Aliskiren the amount of collagen reduced to the level of controls. (n = 3-5/group) *P ≤ 0.05 versus control, #P ≤ 0.05 versus Alisk. Black: nuclei; red: cytoplasm and erythrocytes; blue: collagen.
Masson Staining
Masson trichrome staining was performed to evaluate the extent and the exact location of interstitial fibrosis as one of the main histological hallmarks of CNI nephrotoxicity (Figure 3B). In controls, there was a weak collagen staining around the arterioles, but we could hardly detect any interstitial staining. However, 3-week CNI treatment resulted in significantly elevated amount of collagens referring to an interstitial fibrosis in both CNI-treated groups (C: 1 ± 0.1 vs CyA: 2.6 ± 0.5; Tac: 3.2 ± 1.5 arbitrary units; P ≤ 0.05). Noteworthy, the rays of interstitial fibrotic plaques appeared to develop and localize around the CDs. In accordance with the data on vessel diameter, the increased rate of collagen deposition was abolished when CNIs were administrated in combination with Alisk (C: 1 ± 0.1× vs CyA + Alisk: 1.3 ± 0.1×; Tac + Alsik: 1 ± 0.3× arbitrary units; P = NS).
DISCUSSION
Our studies demonstrated that the direct renin inhibitor Alisk improves CNI-induced nephropathy. Renin is known to reduce renal BF via increased RAS activity and ANGII production. However, so far, its production was investigated only in the JGA. Our studies showed, for the first time, that CNIs increase renin content in the CD segment as well. Other results suggested that principal cells respond to the reduced blood supply with significant VEGF production. Accordingly, disproportional vessel growth and striped fibrosis were observed which developed mostly in the interstitium surrounding the CDs. Treatment with Alisk prevented the detrimental increase in VEGF, consequent pathological vessel growth, and fibrosis.
The experiments were performed in animals without solid organ transplants, but receiving equivalent doses of immunosuppressive drugs than used in kidney transplantation. Thus, we did not completely recapitulate the situation of an organ transplantation, but exposed the kidney to CNI without confounding factors introduced by the transplantation surgery. Therefore, we think our model system is a reasonable compromise to study CNI nephrotoxicity.
The stimulation of JGA renin by CNIs is well known. Granular cells express calcineurin isoforms, thus they are highly susceptible for CNIs. In vitro, they show significant renin release after CyA treatment.19 Similar observations have been found in vivo; hypertrophy of renin containing granular cells as well as increase in plasma renin activity are widely observed and proved in rats treated with CNIs.18,21,27,28 Moreover, even patients treated with CNIs, but without kidney disease (cardiac and liver transplants), have been reported to have elevated plasma renin activity.29 However, all these previous studies focused on the direct effect of CNIs on renin-producing cells only in the JGA. None of them addressed other potential anatomical sources of the elevated serum renin, like the CD, although, the effect of CNIs on CD segment is known.30,31
In our studies, we used an in vivo imaging approach combined with RT-PCR and flow cytometry to describe the exact intrarenal localization and amount of renin. Thus, we revealed a further tubular segment in CNI nephropathy, the CD where renin mRNA and renin granules were also densely present. It is in line with some recent findings, which showed that CD is able to regain renin-producing capacity in pathophysiological states, including high-salt diet, diabetic, or hypertensive nephropathy.13-17,32 Important to note that this segment does not only contain a larger renin-producing cell population compared to the JGA, but it has high capability for further proliferation.14 In our studies, we observed a 3- to 4-fold increase in renin content already after 3 weeks of CNI treatment, and because we proved the local expression of renin in the CD and could not detect any glomerulopathy, it is highly unlikely that renin is filtered through the glomerulus and then taken up in the CD. Furthermore, we showed that renin granules were localized in the proximity of both the apical and basal cell membrane, raising the possibility of local effects, such as the activation of the ANGII receptor 1 (ATR1)33 and the epithelial Na(+) channel34 as well as for systemic hemodynamical changes.10 The latter one, we could confirm by measuring increased serum renin activity in CNI-treated groups. However, CNIs in this dosage and after 3 weeks of administration has not significantly influenced the blood pressure yet.
Calcineurin inhibitors cause reduction in renal BF which further triggers renin secretion leading to the progression of CNI nephropathy. To date, increased levels of vasoconstrictors, including endothelin and thromboxane, the activation of the RAS, and the decreased release of vasodilator factors have been proposed to lead to reduced BF.5,21,35-37 Beyond the investigation of these paracrin factors and signaling path-ways, there is only one report, in which micropuncture technique was used to provide direct data of increased afferent and efferent arteriolar resistance after acute CyA infusion.38 However, the invasive nature of the micropuncture technique raises concerns regarding the integrity of glomerular hemo-dynamics. Therefore, we used a minimally invasive in vivo imaging approach, which allowed us to visualize the intrarenal renin content and vascular functions in the intact living kidney. We observed significant vasoconstriction in vivo in intact kidney after 3 weeks of treatment. Applying further calculation, the Hagen-Poiseuille equation can show that CNI treatment decreased the flow velocity in capillaries by 50% (if we assumed equal mean arterial and central venous pressures in all groups). Beyond the reduced vessel diameters, we observed the remarkable dilatation of some capillaries with turbulent flow. In this disproportional vessel growth, we suspected the key role of VEGF. Consistent with this, a significantly increased number of VEGF-positive principal cells was detected in the CD after 3 weeks of CNI treatment. The VEGF is known to be expressed by glomerular epithelial and CD cells,39,40 and it stimulates vasculogenesis and angiogenesis. However, its role in CNI-induced nephrotoxicity has not been fully understood. There are reports that monotherapy with CyA or Tac does not modify the mRNA expression of VEGF in salt-depleted rats,41 whereas others showed that in vitro CyA markedly induces VEGF transcriptional activation through protein kinase C.42 Immunohistochemical staining of VEGF was more intense after 8 weeks of Tac treatment,43 whereas other reports showed an initial intense increase, then a progressive decrease in VEGF expression in human protocol biopsies after renal transplantation.44 In our studies after 3 weeks of CNI treatment, we have observed increased content of VEGF in the principal cells of the CD. Supposedly, after secretion of the increased renin content from the principal cells, the level of intratubular ANGII was raised which induces VEGF production of the principal cells in an ATR1-mediated manner. We speculate that as a result of the pathological overproduction of VEGF in the CD, this tubular segment became densely surrounded by disproportional peritubular capillaries, which further worsened the already reduced BF and the severe local hypoxia. In hypoxic environment, the increased amount of hypoxia-inducible factor-2α interacts with transforming growth factor-β signaling to promote fibrogenesis.45 In our studies, we found significantly increased mRNA expression of collagen I and elevated Masson scores in mice treated with CNIs compared to controls. Interestingly, the striped interstitial fibrotic plaques were mostly localized around the CDs, further suggesting the mechanism of increased local VEGF-local hypoxia-fibrosis pathological axis.
Previously, the opposite regulation of renin synthesis by ANGII has been shown in the CD compared to the JGA; ANGII feedback inhibits JGA renin,46 while it stimulates CD renin production.15 Other studies have proved that ATR1 blockade reduced CD renin to control levels.14 Our data are in agreement with the beneficial effects of RAS inhibition in the CD. The direct renin inhibitor Alisk decreased the local renin production in CD. It has been published that radiolabeled Alisk is taken up and persists in the distal nephron segment (distal tubules and CD),47 thus, it can exert a local inhibitory effect on renin. Therefore, in our model, the activation of ANGII not only systemically, but also in the lumen of the tubulus is highly decreased resulting in a negative feedback on CD renin production.14 In the literature, data are scarce addressing the beneficial effect of Alisk in CNI nephrotoxicity. One of the two related studies has investigated only blood and urine samples in CyA-treated hypertensive mice,48 the other report focusing on Tac-induced nephropathy has examined whole kidney samples to prove attenuation of oxidative stress.49 However, none of them focused on the source of renin or explained the subsequent vascular changes. In our present studies, we addressed the issue of localization of renin production and found that Alisk decreased the amount of renin in the entire population of CD principal cells. We uncovered that the significantly increased serum renin activity and reduced BF induced by CNIs is also ameliorated by Alisk. Furthermore, we showed that Alisk treatment resulted in a decreased number of VEGF-positive principal cells and a more physiological capillary network. We know that ANGII via ATR1 increases VEGF production with high preference in the CD.50,51 Thus, we suppose, that in our setting, this is the main pathway, which is inhibited by Alisk. In Alisk-treated mice, the significantly less collagen deposition and better renal function further confirmed the crucial role of renin-VEGF-fibrosis axis in the pathogenesis of CNI nephropathy.
In vivo multiphoton microscopy was an optimal tool to visualize blood vessels and renin granules in the cortex of a living animal.24,52 Although, because of the limited penetration depth, the renal medulla remained hidden from the objective of the microscope, revealing the cortex still resembled the clinical situation more closely than most traditional research methods. Further limitation of our studies was the non-specificity of Quinacrine for renin. However, it has been a useful label for renin granular content and the dynamics of renin release in vivo in the rat and mouse, because of the large size of granules (in the 2- to 3-μm range), as opposed to other acidic vesicular organelles, including lysosomes, that are much smaller (in the nanometer range). Colocalization of quinacrine fluorescence with JGA renin by immunohisto-chemistry validated further the use of this dye.25
In summary, we demonstrated that direct renin inhibitor Alisk improves CNI-induced nephropathy. In this disease model, our studies revealed the crucial role of renin production in the CD. The high amount of renin may contribute to decreased renal blood flow. In turn, the CD responds with significant VEGF production, resulting in disproportional vessel growth, which further worsens the local hypoxia and results in striped fibrosis in the interstitium surrounding the CDs. Aliskiren was able to prevent these effects, suggesting that, as a new therapeutic strategy, it may be used for the prevention of CNI nephropathy.
Acknowledgments
This work was supported by grants OTKA-108688, -PD105361, KMR_12-1-2012-0074 and Lendulet Research Grant LP008/2015.
Footnotes
The authors declare no conflicts of interest.
Á.P. and R.Cs. participated in research design, in the performance of experiments, data analysis and writing of the article. L.B.H., D. P., B.M., K.K.P. participated in the performance of experiments and data analysis. E.Sz. and Sz.B. participated in the performance of experiments and writing of the article. Á.V. and A.F. participated in research design. J.P.P., A.J.Sz. participated in research design, data analysis. and writing of the article.
REFERENCES
- 1.Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16:3015–3026. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- 2.Renders L, Heemann U. Chronic renal allograft damage after transplantation: what are the reasons, what can we do? Curr Opin Organ Transplant. 2012;17:634–639. doi: 10.1097/MOT.0b013e32835a4bfa. [DOI] [PubMed] [Google Scholar]
- 3.Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001;7:384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lipták P, Kemény E, Morvay Z, et al. Peritubular capillary damage in acute humoral rejection: an ultrastructural study on human renal allografts. Am J Transplant. 2005;5:2870–2876. doi: 10.1111/j.1600-6143.2005.01102.x. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 6.Burdmann EA, Andoh TF, Rosen S, et al. Experimental nephrotoxicity, hepatotoxicity and pharmacokinetics of cyclosporin G versus cyclosporin A. Kidney Int. 1994;45:684–691. doi: 10.1038/ki.1994.92. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Yang CW, Kim WY, et al. Reversibility of chronic cyclosporine nephropathy in rats after withdrawal of cyclosporine. Am J Physiol Renal Physiol. 2003;284:F389–F398. doi: 10.1152/ajprenal.00277.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031–3041. doi: 10.1681/ASN.2007040394. [DOI] [PubMed] [Google Scholar]
- 9.Haynes R, Baigent C, Harden P, et al. Campath, calcineurin inhibitor reduction and chronic allograft nephropathy (3C) study: background, rationale, and study protocol. Transplant Res. 2013;2:7. doi: 10.1186/2047-1440-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz A. Renin release: sites, mechanisms, and control. Annu Rev Physiol. 2011;73:377–399. doi: 10.1146/annurev-physiol-012110-142238. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara A, Sakoda M, Kurauchi-Mito A, et al. Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens. 2008;2:332–340. doi: 10.1016/j.jash.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Gomez RA, Pupilli C, Everett AD. Molecular and cellular aspects of renin during kidney ontogeny. Pediatr Nephrol. 1991;5:80–87. doi: 10.1007/BF00852854. [DOI] [PubMed] [Google Scholar]
- 13.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 14.Kang JJ, Toma I, Sipos A, et al. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto-Carrasquero MC, Kobori H, Ozawa Y, et al. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryffel B, Weber E, Mihatsch MJ. Nephrotoxicity of immunosuppressants in rats: comparison of macrolides with cyclosporin. Exp Nephrol. 1994;2:324–333. [PubMed] [Google Scholar]
- 19.Madsen K, Friis UG, Gooch JL, et al. Inhibition of calcineurin phosphatase promotes exocytosis of renin from juxtaglomerular cells. Kidney Int. 2010;77:110–117. doi: 10.1038/ki.2009.418. [DOI] [PubMed] [Google Scholar]
- 20.Norling LL, Tufro-McReddie A, Ariel Gomez R, et al. Accumulation of acidic renin isoforms in kidneys of cyclosporine-A–treated rats. J Am Soc Nephrol. 1996;7:331–337. doi: 10.1681/ASN.V72331. [DOI] [PubMed] [Google Scholar]
- 21.Andoh TF, Bennett WM. Chronic cyclosporine nephrotoxicity. Curr Opin Nephrol Hypertens. 1998;7:265–270. doi: 10.1097/00041552-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Westermann D, Riad A, Lettau O, et al. Renin inhibition improves cardiac function and remodeling after myocardial infarction independent of blood pressure. Hypertension. 2008;52:1068–1075. doi: 10.1161/HYPERTENSIONAHA.108.116350. [DOI] [PubMed] [Google Scholar]
- 23.Peti-Peterdi J, Toma I, Sipos A, et al. Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 2009;24:88–96. doi: 10.1152/physiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peti-Peterdi J, Burford JL, Hackl MJ. The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol. 2012;302:F227–F233. doi: 10.1152/ajprenal.00561.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peti-Peterdi J, Fintha A, Fuson AL, et al. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 2004;287:F329–F335. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 26.Linkermann A, Heller JO, Prókai A, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young BA, Burdmann EA, Johnson RJ, et al. Cyclosporine A induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int. 1995;48:431–438. doi: 10.1038/ki.1995.311. [DOI] [PubMed] [Google Scholar]
- 28.Lee DB. Cyclosporine and the renin-angiotensin axis. Kidney Int. 1997;52:248–260. doi: 10.1038/ki.1997.328. [DOI] [PubMed] [Google Scholar]
- 29.Julien J, Farge D, Kreft-Jais C, et al. Cyclosporine-induced stimulation of the renin-angiotensin system after liver and heart transplantation. Transplantation. 1993;56:885–891. doi: 10.1097/00007890-199310000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Rinschen MM, Klokkers J, Pavenstädt H, et al. Different effects of CsA and FK506 on aquaporin-2 abundance in rat primary cultured collecting duct cells. Pflugers Arch. 2011;462:611–622. doi: 10.1007/s00424-011-0994-6. [DOI] [PubMed] [Google Scholar]
- 31.Gooch JL, Guler RL, Barnes JL, et al. Loss of calcineurin alpha results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci. 2006;119:2468–2476. doi: 10.1242/jcs.02971. Pt 12. [DOI] [PubMed] [Google Scholar]
- 32.Csohány R, Prókai A, Kosik A, et al. The cortical collecting duct plays a pivotal role in the kidney's local renin-angiotensin system. Orv Hetil. 2013;154:643–649. doi: 10.1556/OH.2013.29597. [DOI] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Botros FT, Kobori H, et al. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 35.Murray BM, Paller MS, Ferris TF. Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney Int. 1985;28:767–774. doi: 10.1038/ki.1985.196. [DOI] [PubMed] [Google Scholar]
- 36.Myers BD, Sibley R, Newton L, et al. The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int. 1988;33:590–600. doi: 10.1038/ki.1988.38. [DOI] [PubMed] [Google Scholar]
- 37.Hansen JM, Fogh-Andersen N, Christensen NJ, et al. Cyclosporine-induced hypertension and decline in renal function in healthy volunteers. J Hypertens. 1997;15:319–326. doi: 10.1097/00004872-199715030-00014. [DOI] [PubMed] [Google Scholar]
- 38.Barros EJ, Boim MA, Ajzen H, et al. Glomerular hemodynamics and hormonal participation on cyclosporine nephrotoxicity. Kidney Int. 1987;32:19–25. doi: 10.1038/ki.1987.166. [DOI] [PubMed] [Google Scholar]
- 39.Simon M, Gröne HJ, Jöhren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268(2):F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. Pt 2. [DOI] [PubMed] [Google Scholar]
- 40.Lai L, Pen A, Hu Y, et al. Aldosterone upregulates vascular endothelial growth factor expression in mouse cortical collecting duct epithelial cells through classic mineralocorticoid receptor. Life Sci. 2007;81:570–576. doi: 10.1016/j.lfs.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Lloberas N, Torras J, Alperovich G, et al. Different renal toxicity profiles in the association of cyclosporine and tacrolimus with sirolimus in rats. Nephrol Dial Transplant. 2008;23:3111–3119. doi: 10.1093/ndt/gfn223. [DOI] [PubMed] [Google Scholar]
- 42.Basu A, Contreras AG, Datta D, et al. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008;68:5689–5698. doi: 10.1158/0008-5472.CAN-07-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oğütmen B, Tuğlular S, Cakalağaoğlu F, et al. Transforming growth factor-beta1, vascular endothelial growth factor, and bone morphogenic protein-7 expression in tacrolimus-induced nephrotoxicity in rats. Transplant Proc. 2006;38:487–489. doi: 10.1016/j.transproceed.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 44.Sharma A, Jain S, Gupta R, et al. Ultrastructural alterations in endothelial mitochondria are associated with enhanced nitrotyrosine accumulation and progressive reduction of VEGF expression in sequential protocol renal allograft biopsies with calcineurin inhibitor toxicity. Transpl Int. 2010;23:407–416. doi: 10.1111/j.1432-2277.2009.00988.x. [DOI] [PubMed] [Google Scholar]
- 45.Hanna C, Hubchak SC, Liang X, et al. Hypoxia-inducible factor-2α and TGF-β signaling interact to promote normoxic glomerular fibrogenesis. Am J Physiol Renal Physiol. 2013;305:F1323–F1331. doi: 10.1152/ajprenal.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Alpern RJ, Hebert SC, editors. The Kidney Physiology and Pathophysiology. Vol. 1. Elsevier Academic Press; Burlington-San Diego-London: 2008. pp. 589–626. [Google Scholar]
- 47.Feldman DL, Jin L, Xuan H, et al. The direct renin inhibitor aliskiren localizes and persists in rat kidneys. Am J Physiol Renal Physiol. 2013;305:F1593–F1602. doi: 10.1152/ajprenal.00655.2012. [DOI] [PubMed] [Google Scholar]
- 48.Saraswat MS, Addepalli V, Jain M, et al. Renoprotective activity of aliskiren, a renin inhibitor in cyclosporine A induced hypertensive nephropathy in dTG mice. Pharmacol Rep. 2014;66:62–67. doi: 10.1016/j.pharep.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Al-Harbi NO, Imam F, Al-Harbi MM, et al. Treatment with aliskiren ameliorates tacrolimus-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2014 doi: 10.1177/1470320314530178. [DOI] [PubMed] [Google Scholar]
- 50.Gruden G, Thomas S, Burt D, et al. Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol. 1999;10:730–737. doi: 10.1681/ASN.V104730. [DOI] [PubMed] [Google Scholar]
- 51.Madsen K, Marcussen N, Pedersen M, et al. Angiotensin II promotes development of the renal microcirculation through AT1 receptors. J Am Soc Nephrol. 2010;21:448–459. doi: 10.1681/ASN.2009010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang JJ, Toma I, Sipos A, et al. From in vitro to in vivo: imaging from the single cell to the whole organism. Curr Protoc Cytom. 2008 doi: 10.1002/0471142956.cy1212s44. Chapter 12: Unit 12.12. [DOI] [PubMed] [Google Scholar]