Abstract
Genetic variants of matrix metalloproteases (MMPs)-1, -3, and 9, together with clinical variables, might predict the growth rate (GR) of abdominal aortic aneurysm (AAA). Genotyping of MMP-1 (−1,607 G+/G−), MMP-3 (− 1,171 6A/5A), and MMP-9 microsatellite (13–26 cytosine–adenosine repeats around -90) from peripheral blood was performed in 137 AAA patients with two AAA diameter measurements (at least 3 months to 1 year apart). When the same technique (either ultrasound or computed tomography) was used for the two measurements, yearly GR was estimated and compared with MMP genotype and clinical features by linear and binary logistic regression. Collectively, 36 patients provided 94 observations, with a median GR of 3 mm/year (interquartile range, 0–5.8); GRs in carriers of MMP-1 polymorphism G−/G−, G−/G+, and G+/G+ genotype were 0.3, 3.5, and 4.7mm/year, respectively (p = 0.008). In linear logistic regression, the main determinant of GR was growth arrest (GA, i.e., GR = 0, occurring in 32 observations, 34%). In turn, GA occurred mainly in G−/G− MMP-1 genotype (odds ratio, 3.9; 95% confidence interval, 1.6–9.7; p = 0.002), while variables accounting for GR > 0 were MMP-1 G + /G+ genotype, intake of any antihypertensive drug, and MMP-3 6A/6A genotype. Carriers of none, one, or two/three of these conditions accounted for a GR of 3, 4, and 9 mm/year, respectively (p = 0.001). MMP-1 (−1,607 G+/−) variant is associated to differential GR in AAA: homozygous G deletion variant shows higher GA prevalence and lower GR, while carriers of G + /G+ MMP-1 genotype, together with intake of antihypertensive drugs, and 6A/6A in MMP-3 present cumulative GR increase.
Keywords: abdominal aortic aneurysm, growth rate, single nucleotide polymorphism, matrix metalloproteases, collagenase, MMP-1
Abdominal aortic aneurysm (AAA) is a degenerative vascular disease present in 5% of men older than 60 years, characterized by inflammation and progressive thinning of the vessel wall, with expansion and eventual rupture of the infrarenal aorta. Rupture risk can be estimated by the aortic diameter, but also a sudden increase in size can be detrimental and calls for immediate repair,1 indicating growth rate (GR) as a major determinant of aneurysm outcome.
In different studies, GR ranges from 1 to 4 mm/year, with an average value a little more than 2 mm/year.2 GRs within each study, in turn, are scattered in a wide array of values,3 and are related, at least in part, with the size of the aneurysm, with plasminogen activator inhibitor (PAI) 5G/5G genotype,4 with lipid lowering drug treatment,3 and negatively, with diabetes mellitus or critical limb ischemia.5
As a result of a slow GR, only a part of patients will require aneurysmal repair, the majority eventually dying because of conditions not related to the AAA. Moreover, GR is not consistent over time, with intervals of growth arrest (GA), that is, no progression of expansion (also called staccato growth).6 These episodes have no obvious explanation, but their mechanism(s) could have a therapeutic potential.
Clinical research and experimental animal models on AAA7 has pinpointed the key role of some member of the matrix metalloproteases (MMPs) family, a class of around 26 neutral endoproteases able to degrade all the components of extracellular matrix. Clinical research demonstrates high concentrations of some of these MMPs within the AAA tissue,8 and genetically engineered mice show a pathogenic role of some MMPs in an animal model of AAA.9
Investigation on functional polymorphisms of MMPs (i.e., genetic variants accounting for differential gene expression) in humans, as factors prompting the development of AAA, has been generally frustrating, with similar allelic frequencies in AAA patients and controls. Among the possible explanations, a discrepancy is that some MMPs just boost AAA dilation, but are not genes causing or modifying the disease. If so, the polymorphisms of MMPs might just modulate the positive remodeling of the aorta, that is, GR, and not the occurrence of the disease. Among the around 26 MMPs detected, some have been identified within AAA10 and their gene expression is regulated through functional genetic polymorphisms. In particular, guanosine insertion 1,607 bases upstream of MMP-1 gene (−1,607 G+ for insertion and G− for deletion, also named rs1799750) creates an E-26 virus transcription site (ets) binding site, which increases the MMP gene expression by around eight times.11 Six adenosine at −1,171 of the MMP-3 promoter (6A, rs35068180) bind an inhibitor of transcription, which reduces its expression by around 50% compared with the 5A variant,12 13 and microsatellites longer than 21 cytosine–adenosine (CA) repeats in MMP-9 promoter (13–26 CA repeats around -90, rs2234681) have about doubled expression compared with those with 21 or less repeats.14 15
Aims
The present study addresses the hypothesis that functional genetic polymorphisms of these MMPs already identified in AAA, namely MMP-1 (insertion/deletion of G at −1,607), -3 (5A/6A at −1,171), and -9 (13–26 CA repeats around -90), are associated to differential GR in a group of patients before AAA repair.
Materials and Methods
Study Population
Patients with documented AAA admitted or attending as outpatients at the Division of Vascular Surgery of the Teaching Hospital of the University of Trieste during the period July 15, 2010, to June 15, 2014, were considered for the study. After explaining the aims of the study and having obtained a written informed consent, patients were asked to provide a blood sample for genotyping and information on their clinical status (risk factors, clinical conditions, drug therapy). In tandem, all the past evaluation of AAA size, acquired with either ultrasound (US) or computed tomography (CT) scans, from diagnosis until present were gathered. GR was estimated when two observations of AAA diameter were obtained with the same technique (either US or CT) in time period of minimum 2 months. More observations could be retrieved from the same patient. For patients under preintervention follow-up, records have been collected until June 15, 2014.
Medical personnel reviewed the records unaware of the genetic status of the patients. All diagnostic and therapy procedures followed the institutional guidelines and the study did not interfere with patients' treatment. The Institutional Review Board Ethical Committee approved the study protocol and each patient signed a written informed consent. The study complies with the Helsinki declaration and did not interfere with the good clinical practice in force in the hospital.
DNA Extraction, Polymorphism Analysis, and Sequencing
The details of the procedure have been previously published.16 Briefly, DNA was extracted from a venous blood sample, the regions of interest amplified according to the methods already described11 13 15 with fluoresceinated forward and normal reverse primers, and the size of such amplified genetic region encompassing the genetic variant (informative of genotype) assessed through capillary electrophoresis.
Genetic material has been treated according to the Italian guidelines for treatment of genetic material (General Authorization No. 8/2013 for the Processing of Genetic Data, from the “Garante per la protezione dei dati personali,” accessible at http://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/2818993).
Statistical Analysis
The data are reported as absolute number and prevalence or median and interquartile (IQR) values for categorical or continuous variables, respectively. GR has been reported as diameter variation over time and expressed as GR per year of observation. Comparisons among groups have been performed with nonparametric statistics (Kruskal–Wallis test). Due to the heterogeneous pattern of aneurysmal growth, even within the same patient, the inference of independent variables has been analyzed for all observations with multiple regression analysis (stepwise method) and, additionally, according to the occurrence or not of GA, as dichotomous variable, with binary logistic regression.
The influence of genotype has been evaluated assuming both an additive and dominant effect of G+ for MMP-1, 5A for MMP-3, and ≥ 22 repeats for MMP-9.
Independent variables for linear and binary logistic regression were gender, age, eventual type of intervention, starting and final aortic diameter (in millimeters), occurrence of GA, duration (in months) of the observation, popliteal and/or thoracic aneurysm, smoking habit (never, former, current smoker of more than 3 cigarettes/day), hypercholesterolemia (total cholesterol > 200 mg/dL or high-density lipoprotein cholesterol < 40 in women or < 35 mg/dL in men), hypertension (systolic and/or diastolic pressure > 140/85 mm/Hg on more measurements), diabetes (fasting blood sugar > 116 mg/dL), weight, height, body mass index, occurrence of coronary heart disease, peripheral obstructive arterial disease, use of antiplatelet, antihypertensive or lipid lowering drugs, MMP-1 (1 = G−/G−, 2 = G−/G+, 3 = G+/G+), MMP-3 (1 = 5A/5A, 2 = 5A/6A, 3 = 6A/6A), and MMP-9 (1 = ≤ 21/≤ 21, 2 = ≤ 21/≥ 22, 3 = ≥ 22/ ≥ 22) polymorphisms and their interactions, considering dominant G + , 5A, and ≥22 CA repeats in MMPs-1, -3, and -9, respectively. A two-tailed p-value less than 0.05 has been considered statistically significant. All statistical analysis has been conducted with SPSS 21.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL).
Results
Out of 137 patients, 36 provided at least two images with measurements of the maximum AAA diameter obtained with the same technique (either US or CT scan) in the appropriate range of time (minimum 3 months apart). These time windows have been named observations. Median duration of the follow-up was 35 months (IQR, 11–69; range, 2–160) and median number of observations for these patients was 3 (IQR, 2–4; range, 2–14). The main clinical characteristics of patients are reported in Table 1. Genotyping was successful in all patients considered, and the allelic frequency in AAA patients did not differ between patients with or without follow-up, and between all AAA patients and a group of blood donors (data not reported).
Table 1. General characteristics of the study group.
| Age at intervention (y) | 76 (72–79) |
| Gender (M/F) | 33/3 |
| Intervention (open/endo/both) | 6/30 |
| BMI (kg/m2) | 26.3 (22.8–28.3) |
| CHD | 15/21 |
| PAOD | 10/26 |
| Popliteal aneurysm | 11/25 |
| Any thoracic aneurysm | 12/24 |
| Antiplatelet agents | 27/9 |
| Antihypertensive drugs | 31/5 |
| Lipid lowering drugs | 25/11 |
| Smoking (N/F/C) | 2/22/12 |
| Hypertension | 32/4 |
| Hypercholesterolemia | 25/11 |
| Diabetes | 5/31 |
| MMP-1 (G − G − /G − G + /G + G + ) | 6/16/4 |
| MMP-3 (5A5A/5A6A/6A6A) | 9/14/3 |
| MMP-9 (≤ 21 ≤ 21/≤ 21 ≥ 22/≥ 22 ≥ 22) | 9/13/4 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; PAOD, peripheral arterial occlusive disease; N/F/C, never a smoker, former smoker, current smoker.
Note: Unless otherwise specified, the figures are occurrence/absence of the condition.
Median GR for each observation was 3 mm/year (IQR, 0–5.8) with 32 observations (34% of total observations) of GA, that is, without increase in diameter. GA episodes occurred mainly (18 out of 32) in patients with G−/G− MMP-1 genotype (χ2 test; p = 0.002; odds ratio [OR], 3.9; 95% confidence interval [CI], 1.6–9.7). Considering the whole group of patients with follow-up (36), the aortic diameter increased from 39.5 (IQR, 34.5–44) to 51 mm (IQR, 45–57) with a median GR per patient of 3.7 mm (IQR, 2.3–6.4). Overall, 47% of patients had at least one episode of GA, 50% of these patients were carriers of genotype G−/G− (χ2 test; p = 0.015; OR, 7.6; 95% CI, 1.3–43). Follow-up data of aneurysms and MMPs polymorphisms are reported in Table 2.
Table 2. Progression of AAA according to the different MMP genotypes.
| MMP-1 | |||||
|---|---|---|---|---|---|
| G − /G− | G − /G+ | G + /G+ | P | P′ | |
| Starting diameter (mm) | 43 (39–47) | 40 (36–42) | 45 (41–50) | a | n.s. |
| Examination time (mo) | 12 (5–15) | 13 (9–28) | 11 (5–14) | b | n.s. |
| GR (mm/y) | 0.0 (0–4.3) | 3.5 (0.4–4.9) | 4.7 (0.4–10) | b | a |
| Growth arrest (#/total observation) | 18/33 | 8/35 | 6/26 | a | a |
| MMP-3 | |||||
| 5A/5A | 5A/6A | 6A/6A | P | P′ | |
| Starting diameter (mm) | 40 (39–45) | 42 (40–48) | 42 (38–43) | n.s. | n.s. |
| Examination time (mo) | 13 (9–17) | 12 (6–16) | 10 (4–13) | n.s. | n.s. |
| GR (mm/y) | 4.3 (0.3–8.2) | 1.7 (0–4.8) | 5.3 (2–15.8) | n.s. | n.s.c |
| Growth arrest (#/total observation) | 6/25 | 24/61 | 2/8 | n.s. | n.s. |
| MMP-9 | |||||
| ≤ 21/≤ 21 | ≤ 21/≥ 22 | ≥ 22/≥ 22 | P | P′ | |
| Starting diameter (mm) | 41.5 (39.5–47) | 41 (39–45) | 44 (38–46) | n.s. | n.s. |
| Examination time (mo) | 12 (9–19) | 12 (8.5–17) | 4 (1.5–7) | a | n.s. |
| GR (mm/y) | 2.6 (0–5.8) | 4 (0–5.2) | 0 (0–9.7) | n.s. | n.s. |
| Growth arrest (#/total observation) | 15/44 | 11/39 | 6/11 | n.s. | n.s. |
Abbreviations: MMP, matrix metalloprotease; n.s., not significant.
Note: Examination time = months elapsed between two observations. P = Kruskal–Wallis test assuming additive influence of each PM, P′ = Mann–Whitney test assuming a dominant effect of G + , 5A, and ≥ 22 Ca repeats for MMPs-1, -3, and -9, respectively.
0.01.
<0.05.
0.042 for homozygote versus heterozygote.
Logistic Regression
Clinical, biometric, and pharmacological variables linked to GR have been considered in linear and binary logistic regression (Table 3).
Table 3. Multiple regression analysis for growth rate (A and C) and binary logistic regression analysis for growth arrest (B).
| Growth rate (all) | |||||
|---|---|---|---|---|---|
| A step | Independent variables | R 2 | B | Beta | p-Value |
| 4 | Constant | 0.380 | −7.095 | 0.025 | |
| Growth arrest | −4.840 | −0.281 | 0.003 | ||
| MMP-1 PM | 4.579 | 0.439 | 0.00003 | ||
| Antihypertensive drugs | 7.705 | 0.418 | 0.00007 | ||
| PAOD | −3.735 | −0.191 | 0.039 | ||
| Growth arrest | |||||
| B step | Independent variables | Accuracy | B | Exp (B) | p-Value |
| 4 | MMP-1 PM | 75 | −1.299 | 0.273 | 0.001 |
| PAOD | 1.732 | 5.651 | 0.009 | ||
| Antihypertensive drug | −1.599 | 0.202 | 0.025 | ||
| Duration of observation | −0.099 | 0.906 | 0.0001 | ||
| Constant | 3.844 | 46.734 | |||
| Growth rate > 0 | |||||
| C step | Independent variables | R 2 | B | Beta | p-Value |
| 3 | Constant | 0.278 | −0.948 | 0.683 | |
| MMP-1 G+/G+ | 8.26 | 0.425 | 0.001 | ||
| Antihypertensive drug | 6.900 | 0.340 | 0.006 | ||
| MMP-3 6A/6A | 7.464 | 0.245 | 0.039 | ||
Abbreviations: MMP, matrix metalloprotease; PAOD, peripheral arterial occlusive disease; PM, polymorphism.
Being GA a main determinant of GR, binary logistic regression of factors associated to such event have been sought, together with those of AAA GR greater than 0 (Table 3, B and C, respectively). Occurrence of GA has been analyzed in stepwise binary logistic regression (forward conditional) with the same independent variables used in linear regression.
Determinants of GR greater than 0 were homozygosis of G insertion for MMP-1 and of 6A for MMP-3 and antihypertensive drugs. Similarity of B values for the three dichotomous independent variables suggested that a simple score system might be effective in assessment of GR. Occurrence or not of each condition generated four groups with 0, 1, 2, or 3 factors present, with 6, 41, 11, and 2 cases, respectively, and progressively increasing GR, going from 2.8 (IQR, 2.5–3.5) mm/year for no factors, to 4.3 (IQR, 3–5.8) for 1, to 9.1 (IQR, 4.6–18.5) for 2, and being 24.8 and 30 mm/year when all factor occurred in the same patient (2 + 3 = 9.5 (IQR, 5–26); p = 0.001; Kruskal–Wallis test among groups, Fig. 1). Such trend was also confirmed when all observations were analyzed with the same score (p = 0.008; Kruskal–Wallis test). Different classes of antihypertensive drugs (α-blockers = 2, β-blockers = 16, calcium antagonists = 5, ACE blockers + sartans = 20) did not influence GR.
Fig. 1.
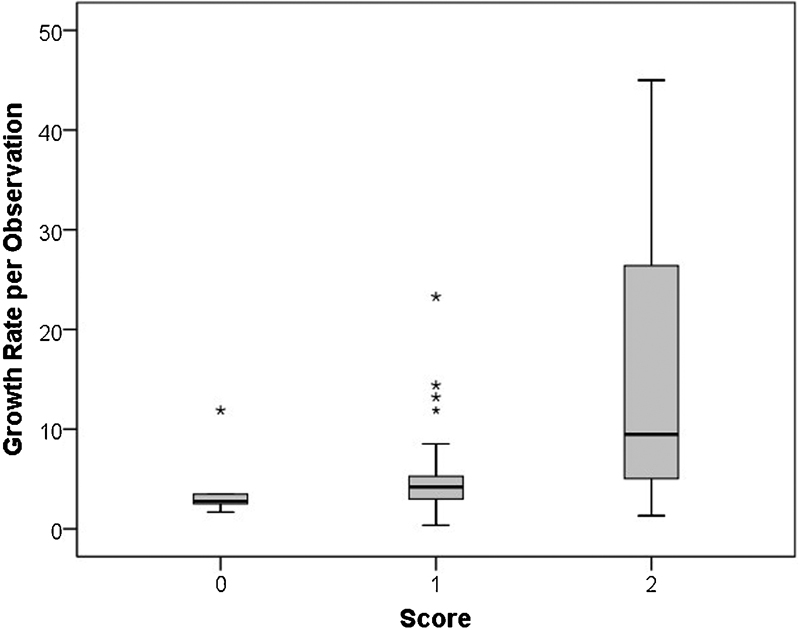
Growth rate (mm/year) according to the proposed score.
Discussion
AAA expansion is the most accurate hallmark of vessel wall thinning and rupture risk, and knowledge of mechanisms involved would allow a more aware follow-up and might hold potential for medical treatment. The present study shows that, in humans, GA is more frequent in patients with genetic variant accounting for reduced MMP-1 expression, and that MMP-1 and MMP-3 genetic variants, together with intake of antihypertensive drugs, account in an additive mode for GR. To the best of our knowledge, this is the first demonstration of a role for these single nucleotide polymorphisms in GR of human aneurysms.
Growth Arrest
GA represents an important confounder in final GR of AAA evaluation, might have important pathophysiological implications, but has been poorly considered, so far. At least one episode of GA has been observed in 65% of patients in a study by Kurvers et al,6 while in our study, it reached 50%. In linear multiple logistic regression analysis, one observation out of three was a GA, and this major negative determinant of the final GR called for a separate subanalysis. Statistical analysis of our patients shows that such arrest is associated to a functional genetic variant of MMP-1, occurrence of peripheral arterial disease, use of antihypertensive drugs, and duration of observation. In the MMP-1 genetic variant, the guanosine insertion creates a binding site for the transcription factor ets which increases by eight or more times the MMP-1 expression.11 On the contrary, the lack of such a binding site (G−) might lessen the activity of this collagenase, AAA expansion and, likely, increase the prevalence of GA, as in our present study. Further support to such a hypothesis comes from experimental data: decoying ets ligands with oligodeoxynucleotides in the aneurysm of an experimental rabbit model, Miyake et al demonstrated aneurysm regression.17 The design of their study did not allow identification of the genes involved but, in light of the present results, MMP-1 might be one of the genes involved in stationary phases of progression of aneurysms.
Other factors, however, contribute to the GA: the occurrence of peripheral arterial disease, likely as a contributor of aortic and aneurysm stiffness, antihypertensive drugs, which show to be protective, and duration of follow-up, since longer time periods between assessments have higher chances of detecting AAA growth.
Abdominal Aortic Aneurysm Progression
Looking at the observations with recognized aneurysm expansion, three conditions are included in the multiple regression equation: homozygosis for G insertion in MMP-1 and 6A in MMP-3 and taking antihypertensive drugs. Similar B values of each determinant allow to infer a simple additive model to estimate GR based on these few variables. The role of such variables of the model is not so obvious. Although the role of MMP-1 genotype has already been discussed, intake of drugs reducing high blood pressure raises the hypothesis that either hypertension is poorly controlled, with occurrence of rebound hypertension episodes, or that these drugs, increasing vascular compliance in the smooth muscle cell-rich upstream aorta segments, also increase mechanical stress in the less compliant aneurysmal segment.18 The association with GR is similar among all the different antihypertensive drugs and there is no association with hypertension itself; this suggests that a very general pharmacodynamics effect of the whole class is involved.
The role of MMP-3 genotype in GR is less clear, and somehow counterintuitive, since the variant accounting for increased GR (6A/6A) is related to lower messenger RNA and protein level in human aorta,12 13 and extracellular matrix degradation. In our study, GR is significantly higher in homozygosis (5A plus 6A), compared with heterozygosis. Such a pattern parallels the aortic stiffness already observed in all homozygous healthy subjects by other authors.12 Aortic stiffness, estimated by pulse pressure velocity, is increased in patients with AAA,19 but its positive association with progression of aortic aneurysm is only reported for another surrogate marker of arterial stiffness, that is, pulse pressure.2 Another, less likely, explanation of the association between GR and MMP-3 polymorphisms is in the genetic linkage disequilibrium of 6A MMP-3 genotype with variants of other MMPs (collagenases) or miRNA.
It could be plausible, in brief, that increased collagenase activity, together with (drug-induced) increased shear stress, and increased aortic stiffness can account for excess of matrix remodeling which, in turn, might fuel GR.
Study Limitations
The main limitation of the present study is the low number of patients observed and its retrospective design. A larger population would have allowed a more precise definition of the role of some clinical variables. A prospective follow-up would have given more information on events and mortality, but we are confident that the variables determining GR have been analyzed also without such a cumbersome approach. Another limitation is in the lack of other clinical variants, particularly pulse wave velocity for the patients and the analysis of the effects of some drugs on arterial stiffness in AAA patients. The retrospective design prevented the collection of such a variable.
Summary
Our study shows a relevant association of AAA progression with genetic variants of metalloproteinases. In particular, MMP-1 and MMP-3 genetic variants might be key elements for GR monitoring and future AAA treatment.
References
- 1.Kontopodis N, Metaxa E, Papaharilaou Y, Georgakarakos E, Tsetis D, Ioannou C V. Value of volume measurements in evaluating abdominal aortic aneurysms growth rate and need for surgical treatment. Eur J Radiol. 2014;83(7):1051–1056. doi: 10.1016/j.ejrad.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Thompson S G, Brown L C, Sweeting M J. et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013;17(41):1–118. doi: 10.3310/hta17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlösser F J, Tangelder M J, Verhagen H J. et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47(6):1127–1133. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Powell J T. Genes predisposing to rapid aneurysm growth. Ann N Y Acad Sci. 2006;1085:236–241. doi: 10.1196/annals.1383.042. [DOI] [PubMed] [Google Scholar]
- 5.Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography—a systematic literature review. Atherosclerosis. 2014;235(1):182–188. doi: 10.1016/j.atherosclerosis.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Kurvers H, Veith F J, Lipsitz E C. et al. Discontinuous, staccato growth of abdominal aortic aneurysms. J Am Coll Surg. 2004;199(5):709–715. doi: 10.1016/j.jamcollsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Trollope A, Moxon J V, Moran C S, Golledge J. Animal models of abdominal aortic aneurysm and their role in furthering management of human disease. Cardiovasc Pathol. 2011;20(2):114–123. doi: 10.1016/j.carpath.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Wills A, Thompson M M, Crowther M, Sayers R D, Bell P RF. Pathogenesis of abdominal aortic aneurysms—cellular and biochemical mechanisms. Eur J Vasc Endovasc Surg. 1996;12(4):391–400. doi: 10.1016/s1078-5884(96)80002-5. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Cassis L A. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(3):429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 10.Newman K M, Jean-Claude J, Li H. et al. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J Vasc Surg. 1994;20(5):814–820. doi: 10.1016/s0741-5214(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 11.Rutter J L, Mitchell T I, Butticè G. et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58(23):5321–5325. [PubMed] [Google Scholar]
- 12.Medley T L, Kingwell B A, Gatzka C D, Pillay P, Cole T J. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92(11):1254–1261. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- 13.Ye S, Watts G F, Mandalia S, Humphries S E, Henney A M. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73(3):209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters D G, Kassam A, St Jean P L, Yonas H, Ferrell R E. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke. 1999;30(12):2612–2616. doi: 10.1161/01.str.30.12.2612. [DOI] [PubMed] [Google Scholar]
- 15.Shimajiri S, Arima N, Tanimoto A. et al. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999;455(1-2):70–74. doi: 10.1016/s0014-5793(99)00863-7. [DOI] [PubMed] [Google Scholar]
- 16.Fiotti N, Moretti M E, Bussani R. et al. Features of vulnerable plaques and clinical outcome of UA/NSTEMI: Relationship with matrix metalloproteinase functional polymorphisms. Atherosclerosis. 2011;215(1):153–159. doi: 10.1016/j.atherosclerosis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Miyake T, Aoki M, Masaki H. et al. Regression of abdominal aortic aneurysms by simultaneous inhibition of nuclear factor kappaB and ets in a rabbit model. Circ Res. 2007;101(11):1175–1184. doi: 10.1161/CIRCRESAHA.107.148668. [DOI] [PubMed] [Google Scholar]
- 18.Jamous M A, Nagahiro S, Kitazato K T, Satoh K, Satomi J. Vascular corrosion casts mirroring early morphological changes that lead to the formation of saccular cerebral aneurysm: an experimental study in rats. J Neurosurg. 2005;102(3):532–535. doi: 10.3171/jns.2005.102.3.0532. [DOI] [PubMed] [Google Scholar]
- 19.Durmus I, Kazaz Z, Altun G, Cansu A. Augmentation index and aortic pulse wave velocity in patients with abdominal aortic aneurysms. Int J Clin Exp Med. 2014;7(2):421–425. [PMC free article] [PubMed] [Google Scholar]


