Abstract
The aim of this study was to examine secondary varicose small pelvic veins (VSPV) and their treatment with micronized purified flavonoid fraction (MPFF). We examined 70 patients with a history of acute iliac thrombosis of > 1 year. Patients with urination difficulties associated with other symptoms (n = 24) received MPFF 1,000 mg once daily for 1 month. Clinical manifestations were assessed by collecting complaints and analyzing results of physician examinations. VSPV was identified in 48 (68.6%) patients, the majority (58%) had grade 2 (7.0–9.0 mm) venous dilation. VSPV severity correlated with time since the thrombotic event. In most women, varicosities were found in the parametrial venous plexus (mean vein diameter 7.91 mm); retrograde flow during the Valsalva maneuver was found in 14 (78%). In men, all varicosities occurred in the paraprostatic plexus (mean vein diameter 7.20 mm); retrograde flow was found in 21 (70%). MPFF significantly reduced VSPV dilation in 18 (75%) patients (p = 0.0863) and returned ultrasonic indices to normal values in the remainder. Patients with bilateral varices decreased from 10 to 2. Only four patients had retrograde flow in the SPV plexus after treatment. MPFF decreased mean paraprostatic vein diameter in men and parametrial vein diameter in women to near-normal values. Clinical improvement was reported in 13 (54%) patients. Patients with pelvic pain decreased from 8 to 1 and patients with urination disorders from 24 to 9. VSPV is common in patients with a history of iliac vein thrombosis. MPFF decreases the diameter of affected veins, improves retrograde flow and pelvic hemodynamics, and significantly reduces the severity of the clinical manifestations.
Keywords: iliac vein thrombosis, micronized purified flavonoid fraction, retrograde flow, varicose small pelvic veins, secondary varicose
The iliac vein and venous networks surrounding the inferior vena cava are a common site for thrombotic events.1 Recently, advances have been made in the treatment of acute iliac thrombosis.2 3 The previous widespread conservative approach to treatment based on the use of anticoagulants has been gradually phased out and replaced by a more intensive treatment approach with local thrombolysis.4 5 6 7 Nevertheless, the long-term risk of impaired blood outflow through the affected veins still persists. The iliac veins collect blood not only from the lower limbs but also from the pelvic veins. The pelvic organs are surrounded by large venous plexuses composed of networks of small pelvic veins (SPVs), which are responsible for the complex hemodynamic conditions in the venous circulation of the pelvic area. Impaired blood outflow can cause varicose disease of the SPV, which in the long-term can result in pelvic organ disorders.
Varicose SPV (VSPV) is a challenging condition to treat. It was first recognized in 1949, when the association between impaired venous blood flow in the uterus and the presence of pelvic pain in women was noted and the term “congestion” (hyperemia or stasis) introduced.8 Although venous congestion of the pelvic organs has been mostly studied in women,8 9 10 11 evidence also points to its existence in men, for example, in urogenital diseases.12
Micronized purified flavonoid fraction (MPFF) is a well-established treatment for various venous disorders, including symptomatic varicose veins of the lower extremities.13 14 15 16 17 18 Some authors have also reported beneficial effects of venoactive drugs in clinically manifest pelvic venous congestion of primary origin.19 The aim of this study was to examine the peculiarities of secondary VSPV caused by iliac vein thrombosis and to investigate the value of MPFF for its treatment.
Methods
Individuals with an acute iliac thrombosis during the period 2002 to 2013 were invited to participate in the study. Inclusion criteria were history of an acute iliac thrombosis at least 1 year previously, and patient's informed consent. Exclusion criteria were neoplasms in the pelvic area; surgery in the pelvic area; pregnancy; VSPV diagnosed before the iliac vein thrombosis; heart, lung, liver, or kidney disease at decompensation stage; and a history of pelvic fracture.
The VSPV were examined by duplex scanning using the SonoScape S6 system (Sonoscape Co Ltd, Shenzhen, China). Intracavity transducers (rectal and vaginal) were used with a frequency of 3.5 to 7.5 MHz in the following modes: B-mode, color coding, energy coding, Doppler spectrogram, and B-flow. Duplex scanning was performed in a supine position, with empty bowel and bladder, at rest, and during the Valsalva maneuver. Pelvic plexus veins with a diameter of less than 5.0 mm were considered normal. The VSPV were classified as grade 1 (5.0 − 6.0 mm), grade 2 (7.0 − 9.0 mm), or grade 3 (≥ 9.0 mm) according to the degree of dilation.
In a first step, all enrolled patients underwent an ultrasound examination to report the characteristics of secondary VSPV. Clinical manifestations were assessed by collecting patients' complaints along with the results of urological, proctological, and gynecological examinations. A general clinical examination was also performed (with analysis of complaints, medical history, physical examination, and digital rectal examination).
In a second step, patients who suffered the most from the disease, that is, those with impaired urination possibly associated with other complaints (pain, dyspareunia in women), received MPFF 1,000 mg once daily in the morning for 1 month. In this group, the results of ultrasound investigation and clinical examination after 1 month of MPFF treatment were compared with baseline values. The International Prostate Symptom Score (IPSS) was also used in men to assess the degree of their urination difficulties. The voiding symptom severity scores were interpreted as follows: 0 to 7, mild difficulties; 8 to 19, moderate difficulties; and 20 to 35, severe urination difficulties.
Statistical analysis was performed using descriptive statistics and nonparametric criteria (2 × 2 table, Mann–Whitney U test).
Results
A total of 70 patients with an acute iliac thrombosis were screened between 2002 and 2013: 46 (65.7%) men and 24 (34.3%) women. Mean age was 52.3 years (54.2 years for men and 48.7 years for women). The mean time since the thrombotic event was 3.8 years. The majority of patients (68%) had left-side iliac vein thrombosis, while 23% had right-side iliac thrombosis, and 9% had bilateral thrombosis (Table 1).
Table 1. Characteristics of patients with iliac thrombosis.
| Characteristics of patients with iliac thrombosis | All screened patients (N = 70) |
|---|---|
| Mean age (y) | 52.3 |
| % Men | 66 |
| Mean time since thrombotic event (y) | 3.8 |
| Left-side iliac thrombosis, N (%) | 48 (68) |
| Right-side iliac thrombosis, N (%) | 16 (23) |
| Bilateral iliac thrombosis, N (%) | 6 (9) |
Characteristics of Secondary VSPV in Our Series
VSPV were identified in 48 (69%) of the 70 screened patients. Of the 46 men with iliac thrombosis, 30 (65%) had VSPV and among the 24 thrombotic women, 18 (75%) had VSPV. In these secondary VSPV patients, the mean time since thrombosis was 3.4 years (95% confidence interval [CI], 1 − 11). Almost half (46%) had left-side thrombosis of the iliac vein, 15% had right-side thrombosis, and 39% had bilateral thrombosis. No contralateral lesions of pelvic veins on the side of the thrombosis were observed (Table 2).
Table 2. Comparison between the population of enrolled patients with VSPV and the group of VSPV patients to be treated with MPFF.
| Characteristics of patients with VSPV | Enrolled subjects (N = 48) | Men (N = 30) | Women (N = 18) | MPFF group (N = 24) | Men (N = 17) | Women (N = 7) | p-Value |
|---|---|---|---|---|---|---|---|
| Mean age (y) | 51.5 | 53.9 | 47.7 | 55.8 | 54.1 | 56.6 | 0.301 |
| Mean time since thrombotic event (y) | 3.4 | 3.4 | 3.4 | 3.9 | 3.9 | 4.0 | 0.232 |
| Location of dilated VSPV, N (%) | NS | ||||||
| Left side | 22 (46) | 9 (30) | 13 (72) | 11 (46) | 6 (35) | 5 (72) | 1.000 |
| Right side | 7 (15) | 4 (13) | 3 (17) | 3 (12) | 2 (12) | 1 (14) | 0.833 |
| Bilateral | 19 (39) | 17 (57) | 2 (11) | 10 (42) | 9 (53) | 1 (14) | 0.912 |
| Of which, contralateral | 0 | 0 | 0 | 0 | 0 | 0 | |
| Degree of dilation, N (%) | NS | ||||||
| Grade 1 (5–6 mm) | 13 (27) | 10 (33) | 3 (17) | 2 (10) | 1 (50) | 1 (50) | 0.124 |
| Grade 2 (7–9 mm) | 28 (58) | 17 (57) | 11 (61) | 20 (80) | 14 (70) | 6 (30) | 0.353 |
| Grade 3 (≥ 9 mm) | 7 (15) | 3 (10) | 4 (22) | 2 (10) | 2 (100) | 0 (0) | 0.501 |
| Mean diameter, mm (95% CI) | 7.64 (5.47–10.93) | 7.20 (5.03–10.49) | 7.91 (5.44–15.83) | 7.59 (5.04–9.70) | 7.52 (5.04–9.70) | 7.79 (5.83–9.19) | |
| Retrograde blood flow, N (% patients) | 35 (73) | 21 (70) | 14 (78) | 16 (67) | 12 (71) | 4 (57) | 0.819 |
Abbreviations: CI, confidence interval; MPFF, micronized purified flavonoid fraction; VSPV, varicose small pelvic veins.
Regarding the degree of VSPV dilation, grade 2 venous dilation prevailed (58%) both in men (57%) and women (61%). Grade 1 venous dilation was observed in 27% of subjects (33% of men and 17% of women), and grade 3 in 15% (10% of men and 22% of women) (Table 2). No statistically significant differences between men and women in grade of venous dilation were reported (p ≥ 0.3).
The dilation of VSPV was proportional to the time since the thrombotic event (Fig. 1). In patients with a history of thrombosis of 1 to 3 years (N = 8), grades 1 and 2 varicosities largely prevailed (93%), while grade 2 varicosities were more common (76%) in patients with a history of thrombosis of 3 to 5 years (N = 21). The majority (77%) of the 13 patients with a history of iliac thrombosis of 5 years or more had grade 2 or 3 dilation.
Fig. 1.
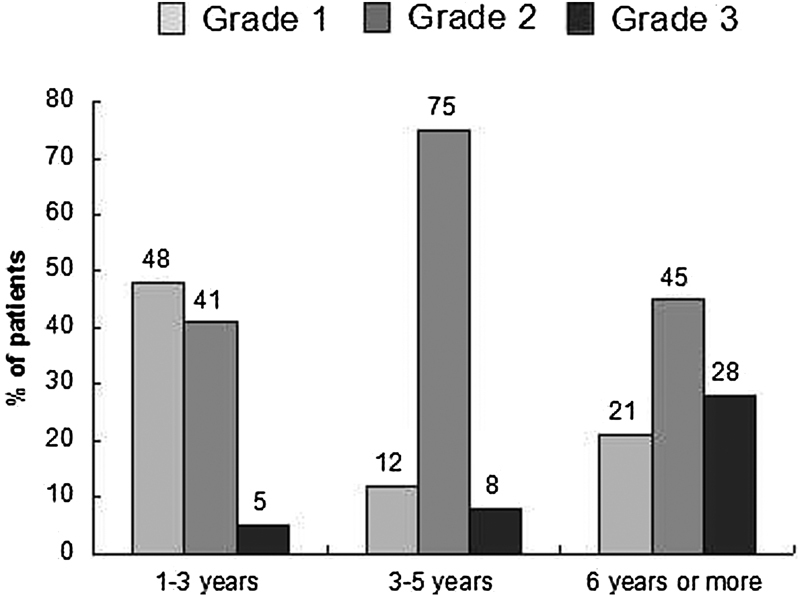
Relation between dilation of the small pelvic veins and time since thrombosis.
In women, varicosities in the parametrial plexus were found in 18 cases and were located on the left side in 72%, on the right side in 17%, and were bilateral in 11% of cases. The mean diameter of the parametrial veins was 7.91 mm (95% CI, 5.44–13.83), and retrograde blood flow was found in 14 (77.8%) of them (Fig. 2; Table 2). Varices in the vesical plexus were found in four (22.2%) cases with a mean vein diameter of 6.86 mm (95% CI, 5.36–8.40) and accompanied in all cases by varices of the parametrial veins. Retrograde flow in the vesical plexus veins during the Valsalva maneuver was found in three (75%) cases. No varicose transformation or hemodynamic disturbances in the ovarian veins were observed.
Fig. 2.
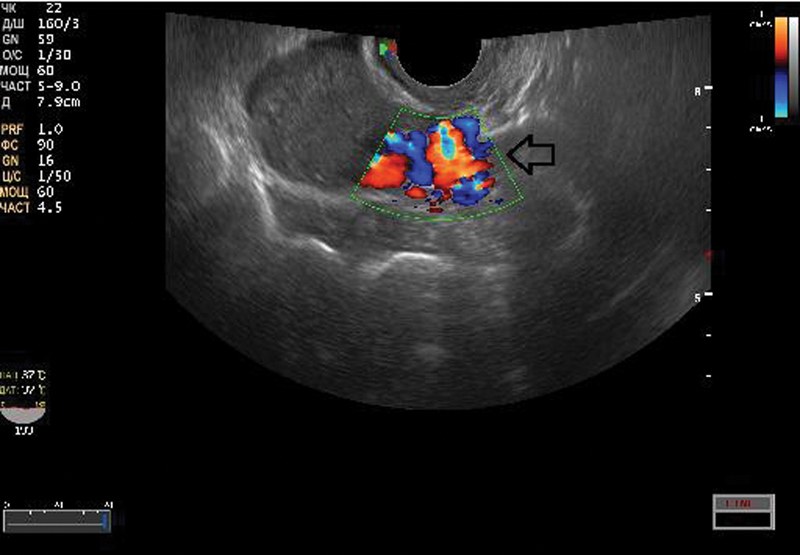
Duplex ultrasound imaging showing left-side varicose disease of the small pelvic veins after left-side iliac vein thrombosis. The arrow points to the reflux wave through the parametrial veins during a Valsalva maneuver.
In men, varicosities were localized exclusively in the paraprostatic plexus. The VSPV were bilateral in 57%, on the left side in 30%, and on the right side in 13% of cases. The mean diameter of the paraprostatic veins was 7.20 mm (95% CI, 5.03–10.49), and retrograde flow was found in 21 (70%) of them (Table 2). No varicose transformation or hemodynamic disturbances in the testicular veins were observed.
Results of MPFF Treatment in Secondary VSPV Patients
A total of 24 VSPV patients, all presenting with impaired urination associated with pain in eight subjects, and dyspareunia in three women, received drug treatment in the form of MPFF 1,000 mg once daily in the morning for 1 month (MPFF group). This group did not significantly differ by age or disease characteristics from the general sample of VSPV patients (Table 2).
Ultrasonic Investigation
Compared with baseline, duplex scanning of the VSPV revealed a significant reduction in the grade of varicose vein dilation in 18 (75%) patients (p = 0.0863), while the mean diameter significantly decreased from 7.59 mm (95% CI, 5.04–9.70) to 5.92 mm (95% CI, 3.40–8.27); p < 0.0001 (Table 3). There were no longer any cases of grade 3 varicosities after treatment, and the number of patients with grade 2 varicosities decreased from 19 (80%) to 11 (46%). However, in the remaining six patients (25%) with a stable grade of VSPV after treatment, ultrasonic indices returned to normal values (Fig. 3).
Table 3. Ultrasonic and clinical results of MPFF treatment in 24 patients with VSPV.
| Characteristics of patients in the MPFF group (N = 24) | Before treatment | After treatment | p-Value | |||
|---|---|---|---|---|---|---|
| Ultrasonic results | ||||||
| Place of dilated VSPV, N (%) | ||||||
| Left side | Unilateral | 11 (46) | 14 (58) | 12 (50) | 16 (67) | 0.0371 |
| Right side | 3 (12) | 4 (17) | ||||
| Bilateral | 10 (42) | 2 (8) | ||||
| No dilation | 0 | 6 (25) | ||||
| Degree of dilation, N (%) | ||||||
| Grade 1 | 2 (10) | 7 (29) | 0.0863 | |||
| Grade 2 | 19 (80) | 11 (46) | ||||
| Grade 3 | 2 (10) | 0 (0) | ||||
| No dilation | 0 | 6 (25) | ||||
| Mean diameter, mm (95% CI) | 7.59 (5.04–9.70) | 5.92 (3.40–8.27) | 0.0001 | |||
| Retrograde blood flow, N (%) | 16 (67) | 4 (17) | 0.0220 | |||
| Clinical results | ||||||
| Impaired urination | 24 (100) | 9 (37) | 0.0310 | |||
| Pelvic pain | 8 (33) | 1 (4) | 0.0408 | |||
| Dyspareunia (women) | 3 (43) | 1 (14) | NS | |||
Abbreviations: CI, confidence interval; MPFF, micronized purified flavonoid fraction; VSPV, varicose small pelvic veins.
Fig. 3.
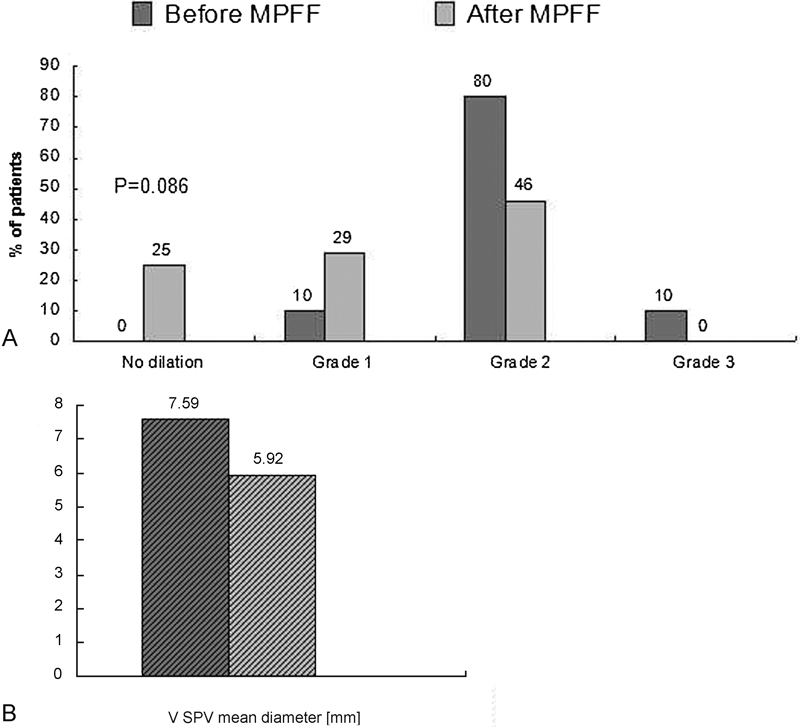
Ultrasonic results for VSPV dilation before and after MPFF treatment. (A) Grades of dilation and (B) mean diameter of VSPV. MPFF, micronized purified flavonoid fraction; VSPV, varicose small pelvic vein.
The number of cases of bilateral varicose veins decreased from 10 (42%) to 2 (8%), while at the same time, the number of cases of ipsilateral varicose veins increased from 14 (58%) to 16 (67%) due to transformation of bilateral lesions into unilateral ones (Table 3).
Bilateral varicosities of the paraprostatic plexus prevailed in men (53%). MPFF treatment reduced the number of cases of VSPV from 17 to 12 (a 30% improved in VSPV) and the number of cases of bilateral varicose disease from 9 to 2. The mean diameter of the paraprostatic veins decreased from 7.52 mm (95% CI, 5.04–9.70) to 5.77 mm (95% CI, 3.40–8.27) after treatment, which is a near-normal value (Fig. 4), while retrograde flow in the paraprostatic plexus decreased from 12 to 3 cases (53% improvement).
Fig. 4.
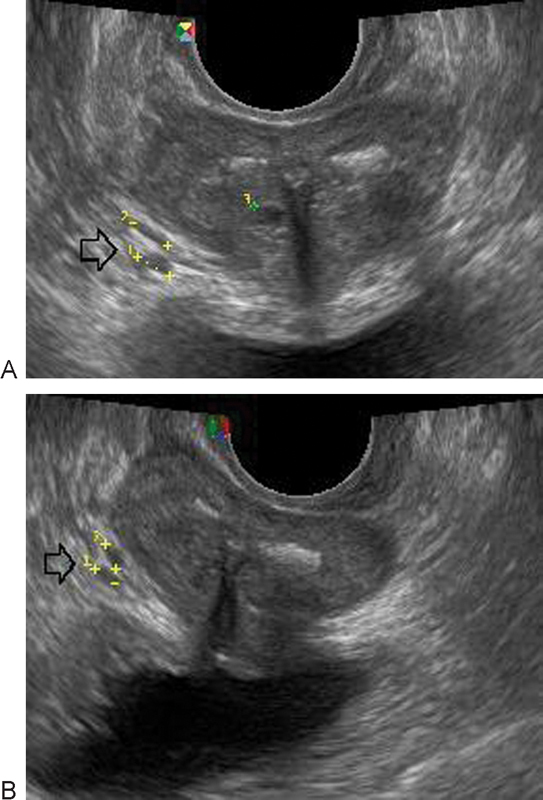
Gray-scale ultrasound imaging showing varicose disease of paraprostatic plexus veins on the right (indicated by arrow) with grade 2 dilation in a man with a history of right-side iliac vein thrombosis. (A) Diameter of vein before treatment 5.10 mm. (B) Diameter of vein after treatment 3.53 mm.
In women, parametrial varicosities were found on the left side in five (72%) patients, and in one woman for both right side and bilateral varicosities. MPFF treatment only reduced the number of VSPV from seven to six cases (NS), but retrograde flow in parametrial plexus veins disappeared in three women (43%). The mean diameter of the parametrial veins decreased from 7.79 mm (95% CI, 5.83–9.19) to 6.28 mm (95% CI, 4.90–7.69), which is close to normal range.
Clinical Results
Treatment with MPFF resulted in a significant improvement in pelvic disorders (Table 3).
Urination disorders: All patients in the treatment group had urination disorders at baseline; for men, the mean IPSS score was 10.6. (95% CI, 1–21). After treatment, the number of patients with impaired urination decreased from 24 to 9 (63% of improved patients; p = 0.03; Fig. 5), and mean IPSS score in men decreased to 4.7.
Pelvic symptoms: The number of patients with pelvic pain significantly decreased (p = 0.04) (Fig. 5).
Hemorrhoid grade: At the baseline proctological examination, hemorrhoids were found in 15 (63%) patients: 11 men and 4 women. Among these patients, 12 had grade 1 or 2 hemorrhoids, and 3 had grade 3 or 4 hemorrhoids. One month of MPFF treatment resulted in a reduction in hemorrhoid grade: only one patient remained with a grade 3 hemorrhoid, while the number with grades 1 and 2 increased to 14 patients.
Dyspareunia: The proportion of women with dyspareunia decreased from 3 at baseline to 1 after MPFF treatment. Given the small sample size, the results were NS.
Fig. 5.
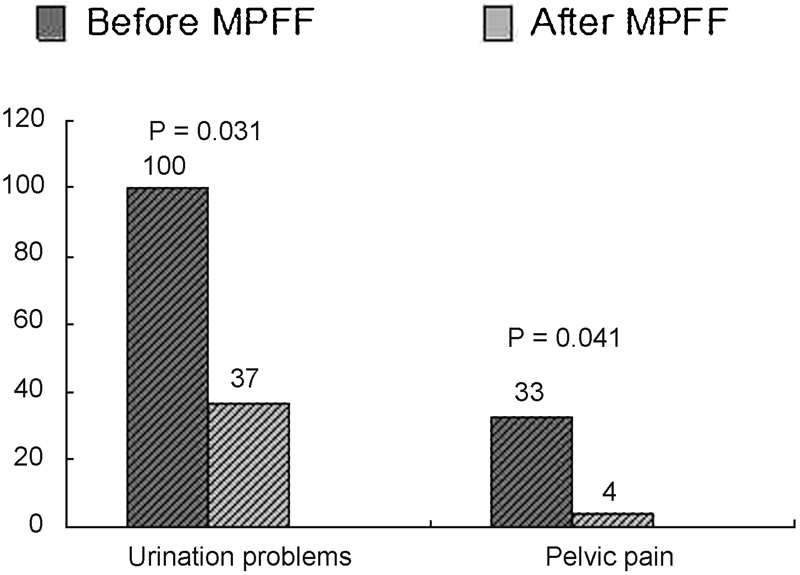
Clinical results for symptomatic patients with varicose small pelvic veins before and after treatment with MPFF. MPFF, micronized purified flavonoid fraction.
Discussion
This study confirms that in the long term, iliac vein thrombosis can cause hemodynamic impairment in the venous system of the pelvic plexuses, with secondary varicose disease.11 20 21 Varicosities initially develop on the side ipsilateral to the prevalent thrombosis, as the narrowing of the iliac vein creates conditions that limit blood outflow from the SPV on the side of thrombosis. However, the number of patients with bilateral VSPV was found to be higher (19 [39%]) than the number of patients with a history of bilateral thrombosis (6 [9%]), suggesting that varicosities of SPV progress with time, involving new veins and plexuses in the varicose process and becoming bilateral, with the development of pelvic venous congestion. Indeed, disease characteristics are associated with the severity of the prevalent thrombosis, the degree and process of thrombus recanalization, and the intensity of therapy in the acute phase of thrombosis as well as the long term. In cases of no or incomplete recanalization, resulting in a persistent limitation of blood outflow from the SPV, extensive varicose disease can develop even in patients with unilateral thrombosis.
In women, varicose disease predominantly affects the parametrial veins. Less often, varicose veins develop in the vesical plexus; however, in our patients, they were part of a complex lesion. This may be explained by the fact that these large venous plexuses of the female pelvis anastomose with each other. It is of interest that VSPV can also develop in men with a history of iliac vein thrombosis. In our study, lesions were exclusively found in the paraprostatic venous plexus. The more extensive varicose disease in women can be explained by the fluctuant hormonal profile due to menstrual cycle, and in some cases, by the use of oral contraceptives and hormone replacement therapy.
The severity of VSPV increased with time since thrombosis. The majority of examined patients with this disorder (35 [73%]) developed retrograde blood flow, a component of pelvic venous congestion syndrome.19 21 22 23
In our study, there were no cases of varicose transformation and hemodynamic disturbances in testicular veins in men and ovarian veins in women with secondary VSPV. This suggests that in the examined patients the varicose transformation of the pelvic veins developed in the absence of a predisposition to varicose disease.
The present study demonstrates the efficacy of MPFF in relieving the clinical manifestations of secondary VSPV and in returning hemodynamic parameters in the venous system of the pelvis to normal. In all patients, 1 month of MPFF treatment reduced the severity of clinical signs and symptoms, such as pelvic pain, urinary disorders, hemorrhoids, and dyspareunia, as well as the grade of varicose veins of the perineum and the anterior abdominal wall.
It is important to note that in 25% of patients, the studied ultrasonic parameters completely returned to normal after MPFF treatment. The efficacy of treatment is clearly illustrated by the decrease in the rate of retrograde blood flow in the venous plexuses. This can be explained by an increase in venous tone and reduction in vein diameter as a result of the venotonic and venoprotective action of MPFF. The beneficial effect of MPFF treatment is demonstrated by a positive trend for a reduction in the rate of VSPV in all patients, and by a significant decrease in the number of patients with bilateral varicosities of the pelvic veins. Moreover, a substantial improvement in the clinical manifestations of pelvic disorders shows that symptoms are mainly determined by the presence of varicosities in the SPV.
A further important finding is the relation between the prevalence of varicose disease and the side of iliac thrombosis. In the majority of patients, bilateral VSPV was transformed into unilateral disease after MPFF treatment. This can be explained by earlier decompensation of venous plexuses on the side of the prevalent thrombosis compared with the contralateral side. As a result, the reverse process occurs with medical therapy with faster recovery of vein hemodynamics and diameter in the venous plexuses on the contralateral side. The substantial beneficial effects of MPFF in this study can be explained by the secondary nature of changes in the venous walls in patients without a predisposition to primary varicose disease.
These data provide a basis for the therapeutic correction of VSPV hemodynamics in patients with a history of iliac vein thrombosis. MPFF, already widely used for the treatment of varicose veins of the lower extremities, is of value in this group of patients by correcting the postthrombotic changes in the iliac veins that limit blood outflow from the VSP.
According to the data of numerous researches, conducted by various medical centers, side effects of MPFF are very few,14 15 17 18 thus this medication was chosen. The results of the work fully correspond with the data—there has been no side effect noticed in the course of the work.
Patients with thrombolysis and stenting were not included in the study group. However, the authors' findings regarding the specific features of the secondary varicose veins of the pelvis, namely, the direct relation between degree and area of lesion, and duration of obstructive process in the iliac vein, as well as the remarkable reversibility of venous abnormalities, primarily on the top of original venoactive drug, Daflon 500, suggest that the earliest elimination of the iliac vein occlusion, achieved by adequate thrombolysis or anticoagulation in acute phase of thrombosis, significantly reduces the risk and extent of the secondary varicose of pelvic veins. Similar results can be expected with the iliac vein stenting due to persistent postthrombotic obstruction of blood flow.
Conclusion
Varicose disease of the SPV develops in about two-thirds of patients with a history of iliac vein thrombosis. It affects mainly the veins of the parametrial plexus in women and the paraprostatic plexus in men, and is associated with retrograde blood flow in the majority of cases. In both men and women, once-daily treatment with MPFF 1,000 mg for 1 month significantly decreased vein diameter and the rate of blood reflux, improving pelvic hemodynamics, and thereby reducing the severity of the clinical manifestations of this secondary varicose disease.
References
- 1.Crisostomo P R, Cho J, Feliciano B, Klein J, Jones D, Dalsing M C. Period frequency of iliofemoral venous occlusive disease by Doppler ultrasound and corresponding treatment in a tertiary care facility. J Vasc Surg. 2010;52(5):1272–1277. doi: 10.1016/j.jvs.2010.05.108. [DOI] [PubMed] [Google Scholar]
- 2.Pollack C V Jr. Advanced management of acute iliofemoral deep venous thrombosis: emergency department and beyond. Ann Emerg Med. 2011;57(6):590–599. doi: 10.1016/j.annemergmed.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Casey E T, Murad M H, Zumaeta-Garcia M. et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(5):1463–1473. doi: 10.1016/j.jvs.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 4.Comerota A J. Quality-of-life improvement using thrombolytic therapy for iliofemoral deep venous thrombosis. Rev Cardiovasc Med. 2002;3 02:S61–S67. [PubMed] [Google Scholar]
- 5.Agnelli G, Becattini C. Treatment of DVT: how long is enough and how do you predict recurrence. J Thromb Thrombolysis. 2008;25(1):37–44. doi: 10.1007/s11239-007-0103-z. [DOI] [PubMed] [Google Scholar]
- 6.Holzheimer R G. Low-molecular-weight heparin (LMWH) in the treatment of thrombosis. Eur J Med Res. 2004;9(4):225–239. [PubMed] [Google Scholar]
- 7.Wong Y M, Quek Y N, Tay J C, Chadachan V, Lee H K. Efficacy and safety of a pharmacist-managed inpatient anticoagulation service for warfarin initiation and titration. J Clin Pharm Ther. 2011;36(5):585–591. doi: 10.1111/j.1365-2710.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor H C Jr. Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; etiology and therapy. Am J Obstet Gynecol. 1949;57(4):654–668. [PubMed] [Google Scholar]
- 9.Hobbs J T. The pelvic congestion syndrome. Br J Hosp Med. 1990;43(3):200–206. [PubMed] [Google Scholar]
- 10.Fernández-Samos R, Zorita A, Ortega J M. et al. Female gonadal venous insufficiency [in Spanish] Angiologia. 1993;45(6):203–209. [PubMed] [Google Scholar]
- 11.Liddle A D, Davies A H. Pelvic congestion syndrome: chronic pelvic pain caused by ovarian and internal iliac varices. Phlebology. 2007;22(3):100–104. doi: 10.1258/026835507780807248. [DOI] [PubMed] [Google Scholar]
- 12.Tsukanov A. Varicose disease of the pelvis as the cause of LUTS in men. J Men's Health. 2011;8(3):208. [Google Scholar]
- 13.Pascarella L. Essentials of Daflon 500 mg: from early valve protection to long-term benefits in the management of chronic venous disease. Curr Pharmaceutic Des. 2007;13(4):431–444. [Google Scholar]
- 14.Nicolaides A N. From symptoms to leg edema: efficacy of Daflon 500 mg. Angiology. 2003;54 01:S33–S44. doi: 10.1177/0003319703054001S05. [DOI] [PubMed] [Google Scholar]
- 15.Ramelet A A. Daflon 500 mg: symptoms and edema clinical update. Angiology. 2005;56 01:S25–S32. doi: 10.1177/00033197050560i105. [DOI] [PubMed] [Google Scholar]
- 16.Goldman M P, Guex J J, Weiss R A. London: Elsevier Saunders; 2011. Sclerotherapy Treatment of Varicose and Telangiectatic Leg Veins. 5th ed; p. 401. [Google Scholar]
- 17.Perrin M, Ramelet A A. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41(1):117–125. doi: 10.1016/j.ejvs.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol. 2005;3(1):1–9. doi: 10.2174/1570161052773870. [DOI] [PubMed] [Google Scholar]
- 19.Burak F, Gonduz T, Simsek M, Taskin O. Chronic pelvic pain associated with pelvic congestion syndrome and the benefit of Daflon 500 mg: a review. Phlebolymphology. 2009;16(3):290–294. [Google Scholar]
- 20.Venbrux A C Chang A H Kim H S et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain J Vasc Interv Radiol 200213(2 Pt 1):171–178. [DOI] [PubMed] [Google Scholar]
- 21.Belardi P, Viacava A, Lucertini G. Iliac vein insufficiency syndrome. Clinical contribution [in Italian] Minerva Cardioangiol. 1998;46(6):211–214. [PubMed] [Google Scholar]
- 22.Bergan J J. Treatment of pelvic venous reflux (pelvic venous congestion) in North America. Vasc Surg. 1997;31(3):255–261. [Google Scholar]
- 23.Koo S, Fan C M. Pelvic congestion syndrome and pelvic varicosities. Tech Vasc Interv Radiol. 2014;17(2):90–95. doi: 10.1053/j.tvir.2014.02.005. [DOI] [PubMed] [Google Scholar]


