Abstract
Minimally invasive surgery (MIS) for upper gastrointestinal (GI) cancer, characterized by minimal access, has been increasingly performed worldwide. It not only results in better cosmetic outcomes, but also reduces intraoperative blood loss and postoperative pain, leading to faster recovery; however, endoscopically enhanced anatomy and improved hemostasis via positive intracorporeal pressure generated by CO2 insufflation have not contributed to reduction in early postoperative complications or improvement in long-term outcomes. Since 1995, we have been actively using MIS for operable patients with resectable upper GI cancer and have developed stable and robust methodology in conducting totally laparoscopic gastrectomy for advanced gastric cancer and prone thoracoscopic esophagectomy for esophageal cancer using novel technology including da Vinci Surgical System (DVSS). We have recently demonstrated that use of DVSS might reduce postoperative local complications including pancreatic fistula after gastrectomy and recurrent laryngeal nerve palsy after esophagectomy. In this article, we present the current status and future perspectives on MIS for gastric and esophageal cancer based on our experience and a review of the literature.
Keywords: Stomach neoplasms, Esophageal neoplasms, Minimally invasive surgical procedures, Postoperative complications, Robotic surgical procedures
Core tip: Minimally invasive surgery (MIS) for upper gastrointestinal cancer reduces intraoperative blood loss and postoperative pain, leading to faster recovery. It also results in better cosmetic outcomes. The impact of MIS on postoperative complications and long-term outcomes has been under debate. We have recently demonstrated that use of da Vinci Surgical System might reduce postoperative local complications including pancreatic fistula after gastrectomy and recurrent laryngeal nerve palsy after esophagectomy.
INTRODUCTION
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer death in the world in 2012[1]. Surgical resection remains the only curative treatment option, and regional lymphadenectomy is recommended as part of radical gastrectomy[2]. According to the Japanese Gastric Cancer Association (JGCA) Gastric Cancer Treatment Guidelines, D2 gastrectomy is recommended for advanced gastric cancer (AGC)[3,4]; however, D2 lymphadenectomy, especially when combined with splenectomy or pancreaticosplenectomy, has been reported to increase morbidity and mortality[5-8].
Esophageal cancer (EC) is the eighth most common malignancy and the sixth leading cause of cancer death in the world in 2012[1]. Similar to GC, surgical resection remains the primary curative treatment option, and regional lymphadenectomy is recommended as part of radical esophagectomy[9-12]. Esophagectomy, which requires thoracolaparotomic manipulation, is one of the most invasive operations in gastrointestinal (GI) surgery, being associated with significant morbidity and mortality[13,14].
Minimally invasive surgery (MIS), which was launched in the late 80’s[15], has been characterized by minimal access using laparoscope or thoracoscope with CO2 insufflation[16]. Although the impact of MIS on postoperative inflammatory response has still been unclear, it has been increasingly used for upper GI malignancies in an attempt to improve postoperative outcomes[13,17,18].
This article provides the updates on laparoscopic gastrectomy (LG) for GC and video-assisted thoracoscopic surgery esophagectomy (VATS-E) for EC, particularly focusing on our twenty-year experience in this field along with a review of previously reported and ongoing large prospective studies.
GASTRIC CANCER
LG for early gastric cancer
Since the first report of LG by Kitano et al[19] in 1994, LG for GC has gained popularity because of its beneficial short-term effects leading to improved quality of life (QoL) in comparison with open gastrectomy (OG), although many controversies still exist due to the lack of solid evidence on its long-term outcomes[20-24]. Therefore, LG had long been recognized as an investigational treatment even for early gastric cancer (EGC) but not as a standard procedure in Japan[25]. However, based on the results of the following multicenter phase II trial conducted by the Japanese Clinical Oncology Group (JCOG) (JCOG0703)[26], the new Japanese Gastric Cancer Treatment Guidelines (ver. 4, issued in 2014) has turned to allow laparoscopic distal gastrectomy (LDG) for clinical stage I disease as a standard treatment option[4].
JCOG0703
JCOG0703[26] was conducted to assess the safety of LDG with D1+ lymph node (LN) dissection for clinical stage I GC. In this well-designed phase II study, to control for the quality of surgery, only surgeons who had performed 30 or more LDGs and 30 or more open distal gastrectomies (ODGs) participated. A central review of the surgical procedure in all the patients was conducted by evaluating photographs taken during the procedure. Between 2007 and 2008, 176 eligible patients from 14 hospitals were enrolled. The incidence of anastomotic leakage or pancreatic fistula was primarily determined, resulting in only 1.7 % (3/173), which was much lower than the pre-specified threshold of 8%. Moreover, morbidity (Common Terminology Criteria for Adverse Events, CTCAE v3.0 Grade 3 or 4)[27] was 5.1%. Thus, the safety of LDG for clinical stage IA/IB disease was securely confirmed.
JCOG0912
On the basis of JCOG0703, a multicenter phase III RCT of LDG vs ODG with D1+ nodal dissection for clinical stage I GC (JCOG0912) has currently been conducted to determine the non-inferiority of LDG to ODG in terms of overall survival[28,29]. For quality control of surgery, surgeons were required to have experience with at least 30 LDGs as well as certification (or its equivalent) from the Japan Society for Endoscopic Surgery (JSES) according to the Endoscopic Surgical Skill Qualification System[30]. Between 2010 and 2013, 921 patients (LDG 462, ODG 459) were enrolled from 33 institutions. Regarding short-term outcomes of this study, LDG significantly improved blood loss, postoperative pain and recovery of bowel movement irrespective of extended operative time. There were no grade 3 or 4 (CTCAE v4.0[31]) intraoperative adverse events in either arm. No difference was observed in the overall proportion of in-hospital, non-hematological grade 3 or 4 adverse events excluding biochemical data (LDG vs ODG, 3.3% vs 3.7%). The proportion of grade 3 or 4 serum AST/ALT increased was higher in LDG than ODG (16.4% vs 5.3%, P < 0.001). Thus, this trial has so far demonstrated that LDG performed by the credentialed surgeons was at least as safe as ODG in terms of adverse event and short-term clinical outcomes. The primary analysis of the long-term outcomes including overall survival and relapse-free survival is planned in 2018[29].
KLASS01
The Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) group 01 trial is another multicenter (13 institutions) RCT to confirm oncological safety of LDG for EGC in comparison with ODG[32,33]. The primary endpoint of this study is 5-year overall survival. Surgeons had to have performed at least 50 cases of both LDG and ODG, and their institution should have performed more than 80 cases of both LDG and ODG, respectively. Between 2006 and 2010, 1416 patients (705 LDGs and 711 ODGs) were enrolled. Regarding short-term outcomes, LDG improved the overall complication rate (LDG vs ODG, 13.0% vs 19.9%, P = 0.001), particularly wound complication (LDG vs ODG, 3.1% vs 7.7%, P < 0.001). The major intra-abdominal complication (LDG vs ODG, 7.6% vs 10.3%, P = 0.095) and mortality rates (LDG vs ODG, 0.6% vs 0.3%, P = 0.687) were similar between the groups. Thus, this trial has so far demonstrated that LDG for patients with clinical stage I GC was sufficiently safe and has a benefit of lower occurrence of wound complication compared with conventional ODG. The long-term outcomes are being awaited.
Laparoscopic total gastrectomy for EGC
These multicenter prospective studies only cover distal gastrectomy. At this moment, both JGCA and JSES have commented that Laparoscopic total gastrectomy (LTG) should be cautiously introduced because of its technical difficulties in complicated alimentary tract reconstruction as well as the LN dissection at the splenic hilum or along the short gastric arteries[4,34]. Since techniques for laparoscopic esophagojejunostomy has recently been established among expert laparoscopic surgeons[35,36], JCOG is planning a phase II study to determine the safety of LTG with D1+ LN dissection for clinical stage I disease[37]. KLASS group has already been conducting a similar phase II trial (KLASS03) to properly evaluate the perioperative morbidity and mortality of LTG for EGC since 2012[17].
LG for AGC
Application of LG for AGC remains to be debated not only because of the lack of evidence on long-term outcomes, but also because of the technical difficulty in performing complete D2 LN dissection and a concern for the innate risk of cancer cell dissemination to the peritoneal cavity[5,16,17,38]. Having said that, acceptable short- and long-term outcomes of the LG for AGC have been reported by a couple of experienced surgeons including us[38-41]. Currently, large-scale multicenter RCTs have been conducted in Japan (The Japanese Laparoscopic Surgery Study Group, JLSSG 0901[42]), Korea (KLASS02[17,43]), and China (The Chinese Laparoscopic Gastrointestinal Surgery Study, CLASS 01[17]) in order to determine the feasibility of LDG for locally AGC (Table 1).
Table 1.
Ongoing randomised controlled trials on laparoscopic distal gastrectomy for advanced gastric cancer
| JLSSG0901 | KLASS02 | CLASS01 | |
| Country | Japan | Korea | China |
| Start year | 2010 | 2011 | 2012 |
| Phase | II/III | III | III |
| Intervention | LDG vs ODG | LDG vs ODG | LDG vs ODG |
| Inclusion criteria | cT2-4a | cT2-4a | cT2-4a |
| cN0-2 (except bulky N2) | cN0/1 | cN0-3 (except bulky LN) | |
| Sample size | II:180, III:500 | 1050 | 1056 |
| Primary endpoint | II: morbidity | 3-year RFS | 3-year RFS |
| III: 3-year RFS |
JLSSG: Japanese Laparoscopic Surgery Study Group; KLASS: Korean Laparoscopic Gastrointestinal Surgery Study; CLASS: Chinese Laparoscopic Gastrointestinal Surgery Study; LDG: Laparoscopic distal gastrectomy; ODG: Open distal gastrectomy; LN: Lymph node; RFS: Relapse-free survival.
LG for AGC at our institute
History: Laparoscopic surgery was launched in the early 90’s in our country[44]. At that time, most laparoscopic surgeons applied laparoscopic surgery, using its minimally invasive nature, to less extended surgery[45]. However, we assumed from the beginning that laparoscopic surgery should be suitable for meticulous LN dissection using laparoscopically enhanced anatomy and reduced venous bleeding via pneumoperitoneum irrespective of the limited range of motion, poor depth perception, and limited tactile sensation[5,46]. Then, we introduced laparoscopic assistance into moderate to advanced GI surgery in combination with a caudocranial and mediolateral approach to overcome those limitations in 1995, and developed techniques for LDG and LTG with D2 dissection for AGC, which were published for the first time in the world[47,48]. Since then, we have performed more than 1500 LGs. At present, the standard type of operation for curable GC at our institute is totally laparoscopic D2 gastrectomy[5].
Suprapancreatic lymph node dissection: Outermost-layer oriented medial approach: D2 dissection entails removal of the LNs in the suprapancreatic area in distal and total gastrectomy[4]. Dissection of this area is technically demanding due to the serious risk of bleeding and/or pancreatic leakage derived from a major vessel or organ injury[49,50]. To improve the safety, efficacy, and reproducibility of suprapancreatic LN dissection, we developed our original methodology called outermost layer-oriented medial approach[46,50]. In this approach, the thin loose connective tissue layer between the autonomic nerve sheaths of the major arteries and the adipose tissue bearing lymphatic tissue is dissected[46,50]. We termed this layer as the outermost layer of the autonomic nerve (Figure 1)[46]. To identify this layer throughout the dissection process, we developed an original surgical theory, “XYZ-axis” theory (Figure 2), consisting of the following three steps: (1) cut the serosal membrane on the suprapancreatic border; (2) dissect suprapancreatic adipose tissue caudocranially towards the junction of the three arteries (zero point) to find the outermost layer; and (3) dissect the target adipose tissue mediolaterally along the outermost layer spreading on the XZ and YZ axes. Using this theory, the outermost layer could easily be found not only at the junction of left gastric, common hepatic, and splenic arteries (Figure 3A), but also at that of gastroduodenal, right gastroepiploic, and anterior superior pancreaticoduodenal arteries (Figure 3B) and that of proper hepatic and right gastric arteries (Figure 3C).
Figure 1.
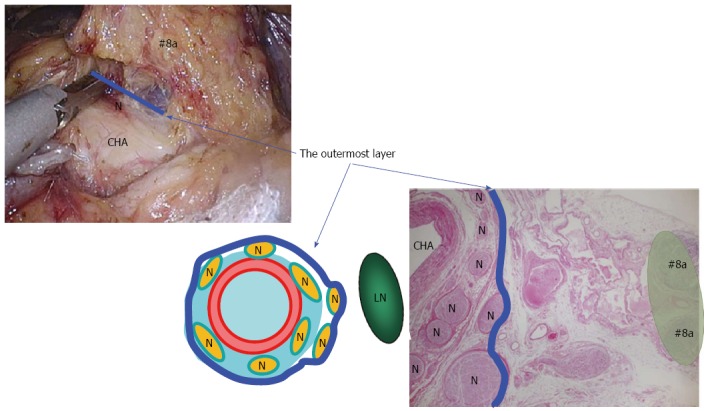
Outermost layer of the autonomic nerve. Shown in the blue line, lies between the vascular sheath of the major arteries and the fat tissue including lymph nodes. Appropriate tension given to this thin loose connective tissue layer generates sufficient space for safe, adequate and reproducible prophylactic lymph node dissection along the major arteries. LN: Lymph node; N: Nerve; CHA: Common hepatic artery.
Figure 2.
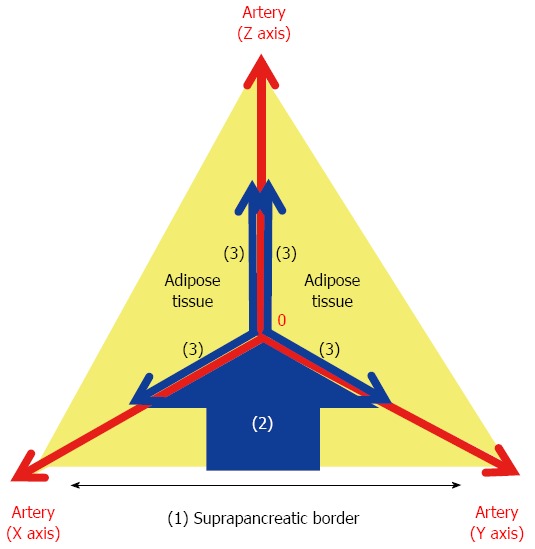
XYZ-axis theory. The following three steps result in effective probing of the outermost layer: (1) dissection of the serosal membrane on the suprapancreatic border; (2) dissection of the fat tissue in the caudo-cranial direction towards the zero point; and (3) dissection of the fat tissue bearing the target LNs in the medio-lateral direction along the outermost layer on the XZ and YZ axes. The outermost layer adjacent to the zero point should be exposed during the 2nd step.
Figure 3.
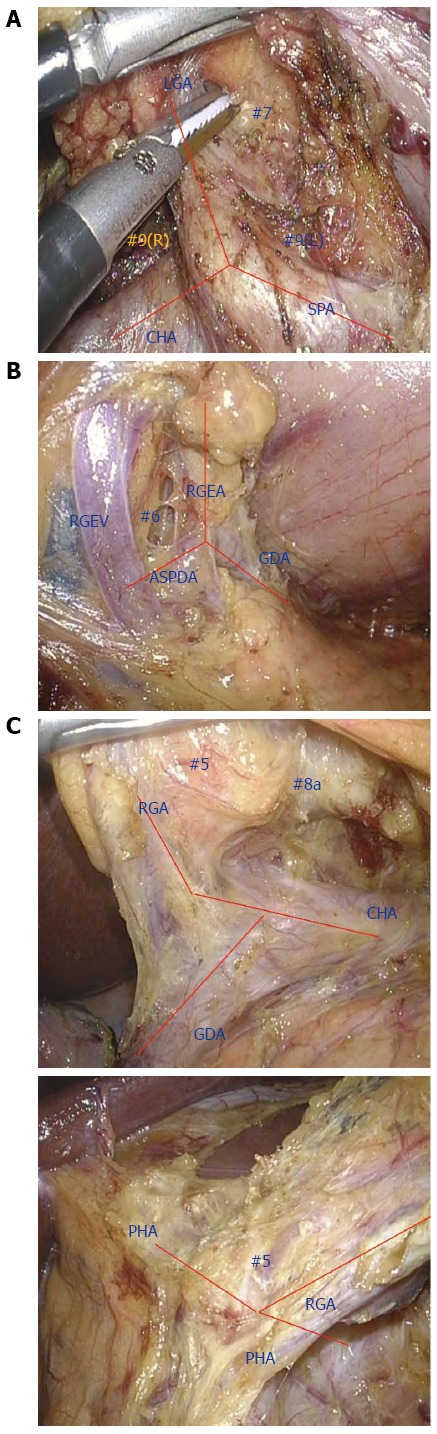
Lymph node dissection along the outermost layer using the XYZ-axis theory. A: No. 7 and 9 dissection, B: No. 6 dissection, C: No. 5 dissection.
LTG for AGC: Splenic hilar lymph node dissection: According to the JGCA guidelines, D2 total gastrectomy is recommended for advanced proximal GC[3,4]; however, as mentioned before, D2 lymphadenectomy combined with splenectomy or pancreaticosplenectomy has been reported to increase morbidity and mortality[5,51,52]. Therefore, the practical importance of station 10 LN dissection and splenectomy in D2 total gastrectomy is controversial[6-8].
We started totally LTG (TLTG) for AGC in 1997[47] and have established a stable and robust methodology, including splenic hilar LN (SHLN) dissection, even though LTG but not LDG has still been one of the independent risk factors for postoperative complications of LG[53,54]. Regarding the extent of SHLN dissection, D2 lymphadenectomy combined with distal pancreaticosplenectomy (D2 + PS) is performed in patients with tumors infiltrating into the pancreatic body or tail. D2 lymphadenectomy combined with splenectomy (D2 + S) is performed in patients with LN metastasis at the station 11 d or 10 or in those with greater curvature invasion. Spleen-preserving D2 lymphadenectomy (D2-S) is performed in patients with tumor depths cT ≥ 3 without LN metastasis at the station 11 d or 10, whereas D2 lymphadenectomy with preservation of station 10 LNs and the spleen (D2-10) is performed in patients without greater curvature invasion and with tumor depths cT2 (Figure 4)[55].
Figure 4.
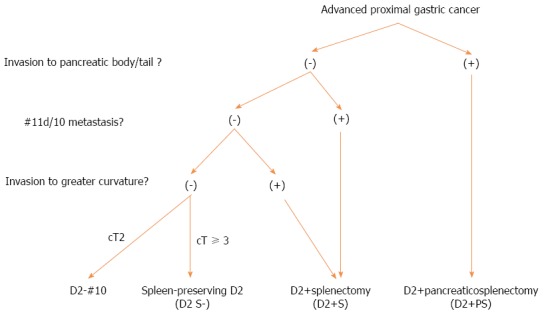
Indication for splenic hilar lymph node dissection at FHU.
Regarding the operating procedures, additional care to control the extent of SHLN dissection in TLTG was given to: (1) the layer on the fusion fascia at the infrapancreatic border of the pancreatic tail; (2) the layer on the subretroperitoneal fascia on the left diaphragmatic crus around the upper pole of the spleen; and (3) the outermost layer of the splenic artery. Using these layers, the aforementioned four different types of SHLN dissection could easily be performed. Procedural details are summarized in our previous literature[55]. In this previous study, multivariate analysis revealed that operative time was the only significant factor associated with postoperative complications. Operative time, morbidity, and pancreatic fistula increased with increasing extent of SHLN dissection. Therefore, the extent of SHLN dissection should be appropriately attenuated if this is allowed by oncological factors. At present, according to the latest JGCA guidelines[4], complete clearance of station 10 nodes by splenectomy should still be considered for potentially curable T2-4 tumors invading the greater curvature of the upper stomach. However, in patients with T2-4/N0-2/M0 GC not invading the greater curvature, the JCOG0110 trial demonstrated that prophylactic splenectomy should be avoided to improve operative safety and survival[2,56].
Intracorporeal anastomosis: To fully utilize the advantages of LG, totally laparoscopic gastrectomy with intracorporeal anastomosis is promising. We have preferred intracorporeal anastomosis with linear staplers because of its handy, quick visible, and reproducible natures. In LDG, we have used delta-shaped anastomosis for Billroth-I reconstruction[57-59], antiperistaltic side-to-side anastomosis for Billroth-II reconstruction, and functional end to end anastomosis for Roux-en-Y reconstruction[5]. In total gastrectomy, we have used functional end to end anastomosis[36] and overlap method[35] for intraabdominal and intrathoracic esophagojejunostomy, respectively. In proximal gastrectomy, modified overlap method with no-knife stapler has been used[60]. The details on intracorporeal anastomosis in LG are well summarized in the review article by Hosogi et al[60].
Outcomes: The short-term and long-term outcomes of LG for AGC at our institute have been satisfactory from both technical and oncological point of view (LG vs OG: mortality, 1.1% vs 0%, P = 0.519; morbidity, 24.2% vs 28.5%, P = 0.402; 5-year disease free survival, 65.8% vs 62.0%, P = 0.737; overall survival, 68.1% vs 63.7%, P = 0.968). Details are demonstrated in our previous reports[38,55].
Robotic gastrectomy
In Japan, da Vinci S HD Surgical System received approval by the Drugs, Cosmetics and Medical Instruments Act in November, 2009. We introduced da Vinci S to our institution in 2009 for the first time in our county, and have been actively using this system for operable patients with resectable upper GI cancer who agreed to uninsured use of the robot[46,53,61].
According to the latest meta-analysis of robotic gastrectomy (RG) vs LG, combining the findings from previous observational studies with small sample size, use of the robot significantly increased operative time and cost, whereas there were no significant differences in other short-term outcomes including blood loss, number of dissected lymph nodes, surgical margins, postoperative complications and duration of hospital stay[62]. The only large non-randomized prospective study (NCT01309256), recently reported from Korea, demonstrated similar outcomes[63]. These reports suggested that use of the robot might even deteriorate the cost-effectiveness[62,63]. In other words, the greatest issue around RG is a lack of clear benefits of the robotic system which corroborate the longer duration of operation and higher cost[63]. However, most of the patients enrolled in these previous studies had pathological stage I diseases, and these studies failed to eliminate the learning effect or the selection bias at least partly generated by more expensive copayment in RG[62,63]. The impact of RG on long-term outcomes has largely been unclear[64,65]. Thus, the advantages of RG for AGC conducted by fully-trained robotic surgeons have never been clarified. In addition, several reports have demonstrated the short learning curve of RG[66-69].
Since 2009, we have performed RG for more than 250 patients not only with EGC but with AGC. Then, according to our retrospective analyses in comparison with LG (EGC vs AGC, 57% vs 43%), RG reduced morbidity down to one fifth including pancreatic fistula, leading to further improvement in short-term postoperative courses, although it slightly increased blood loss and operative time[53,70]. Multivariate analyses clearly demonstrated that conventional LG (non-use of the surgical robot), total gastrectomy (vs distal) and D2 lymphadenectomy (vs D1+) were the significant independent risk factors determining postoperative complications[53]. Moreover, the greater the extent of gastric resection and LN dissection, the more effective the use of the robot[53]. In terms of long-term results, 3-year overall survival did not change between RG and LG[71]. Not only oncological factors including tumor size and clinical stage but also surgical factors including pancreatic fistula were found to be associated with three-year recurrence free survival, indicating the oncological as well as surgical importance of preventing pancreatic fistula[71,72]. These data suggest that the best indication for the use of the robot might be RG for AGC with D2 dissection[53]. Thus, multi-institutional prospective studies conducted by experienced robotic surgeons, in which considerable number of patients with AGC are enrolled, should be required to determine whether use of the robotic system for AGC truly attenuates pancreatic fistula, possibly leading to improvement in long-term outcomes[70].
Since the beginning of October, 2014, we have been conducting a multi-institutional single-arm prospective study (UMIN000015388), which Japanese Ministery of Health, Labor, and Welfare has recently approved for Advanced Medical Technology (“senshiniryo”)[70]. This study was designed to determine the impact of the use of the robot, for minimally invasive radical gastrectomy to treat resectable GC, on short-term outcomes, mainly focusing on postoperative complications, as well as long-term outcomes and cost. The specific hypothesis of this study was that the use of the robot in patients with cStage I or II diseases reduces the morbidity (Clavien-Dindo Classification Grade ≥ III[73]) of 6.4% in conventional LG down to 3.2%. The sufficient sample size was calculated to be 330. All the patients will be registered in 2 years after starting this study and followed up for 3 years, thus the expected study period should be 5 years in total. Interim analyses will be done once the initial 220 cases are registered. As of January 31, 2016, 122 patients from 5 institutions have been registered.
ESOPHAGEAL CANCER
History
Since 1992, when Cuschieri et al[74] first reported on VATS-E, many groups have described various methods[75-79]. In Japan, Akaishi et al[75] first reported on thoracoscopic total esophagectomy with en bloc mediastinal lymphadenectomy in 1996. Kawahara et al[76] demonstrated the details of VATS-E with extended lymphadenectomy in 1999, and Osugi et al[80] clarified the long-term outcomes of VATS-E. We performed prone VATS-E with CO2 insufflation in 2006 for the first time in our country[61].
Indication
The indication for VATS-E is relatively wider than that for LG[10]. VATS-E has currently been applied up to locally advanced EC even after neoadjuvant chemoradiotherapy[9,10]. Only some conditions including T4 tumor, severe intrathoracic adhesion, and one-lung ventilation failure are considered to be excluded from the indication for VATS-E[10,77,81].
Left lateral decubitus vs prone position
Regarding the patient positions used for VATS-E, similar to right transthoracic open esophagectomy (OE), the left lateral decubitus position had been mostly used[75,82]. However, the prone position has increasingly been used recently[74,83-85]. Prone VATS-E is characterized by operating surgeon-friendly sense of use brought by more ergonomic set up as well as a drier operative field given by gravity in combination with the positive intrathoracic pressure generated by CO2 insufflation[61]. To enjoy these advantages of the prone position as well as those of laparoscopic horizontal magnified view with overcoming the laparoscopic limited range of motion, we fully mobilize the “meso-oesophagus”[86] from lower up to upper mediastinum prior to the LN dissection of station 106 recR, 106 recL+tbL, and 112 (Japanese Classification of Esophageal Cancer, the 11th ed[87]) using the 6-trocar system in the hemi-prone position (Figure 5).
Figure 5.
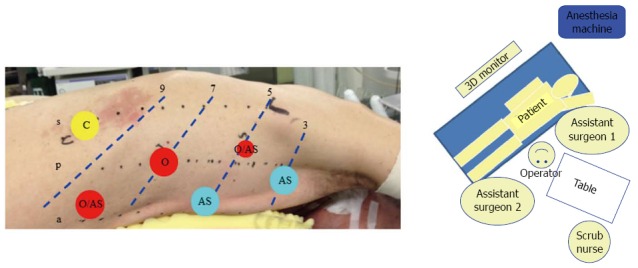
Setup for prone VATS-E at Fujita Health University. A: The patient in the hemi-prone position using the six-trocar system. 12 mm trocars are used except for the trocar in the 5th intercostal space (ICS) behind the posterior axillary line; B: OR setup. s: Scapula angle line; p: Posterior axillary line; a: Anterior axillary line; 3: 3rd ICS; 5: 5th ICS; 7: 7th ICS; 9: 9th ICS; O: Operating surgeon; AS: Assistant surgeon.
Outcomes
To date, a number of single-institution studies have demonstrated acceptable short-term outcomes of VATS-E for thoracic EC regarding operative time, blood loss and postoperative complications, which are comparable with those of conventional OE[13,81]. According to a meta-analysis based on these small case-control studies, VATS-E reduced blood loss, total morbidity and respiratory complications, leading to shorter intensive care unit and hospital stay in comparison with OE[88-90]. In terms of long-term outcomes, a limited number of case-control studies have demonstrated the comparable results with conventional OE[80,89,91,92]. Therefore, to determine the feasibility and beneficial effects of VATS-E, multicenter prospective RCTs are warranted.
ECOG2202: The Eastern Cooperative Oncology Group (ECOG) performed the first prospective phase II multicenter trial (ECOG2202[93]) to assess the feasibility of VATS-E. A total of 110 patients were enrolled at 17 credentialed sites. The primary endpoint was 30-d mortality. 30-d and perioperative mortality was 2.1% and 2.9%, respectively. Grade 3 or 4 (CTCAE v3.0[27]) adverse effects occurred in 49.5% of the eligible 105 patients. Estimated 3-year overall survival was 58.4% (95%CI: 47.7%-67.6%). These data suggested that VATS-E was feasible and safe with acceptable perioperative and oncological outcomes.
Traditional Invasive vs Minimally Invasive Esophagectomy trial: Traditional Invasive vs Minimally Invasive Esophagectomy (TIME) trial is the first multicenter RCT comparing short-term outcomes of prone VATS-E and those of OE, which was conducted by a study group in Europe[77,94]. In this study, 56 and 59 patients were randomly assigned to the OE and the VATS-E group, respectively. As a result, VATS-E reduced, intraoperative blood loss, postoperative pain, postoperative pulmonary infection, and vocal cord palsy, leading to reduced hospital stay and improved postoperative QoL. No siginificant difference was observed in mortality and the number of dissected lymph nodes. These findings suggested the advantages of VATS-E over OE in terms of short-term outcomes.
Robotic esophagectomy
The robotic esophagectomy has been less commonly performed than robotic gastrectomy. Thus, the impact of the use of DVSS on esophagectomy has been assessed mostly in case-series with small sample size[61,95-109]. Various groups have reported on feasibility and safety with good short-term outcomes in a wide-range of approaches to esophagectomy[110]. Van der Sluis et al[111] have reported sufficient oncological long-term outcomes of robotic esophagectomy for advanced esophageal cancer (5-year overall survival, 42%). Hernandez et al[100] demonstrated that the learning curve for a robotic-assisted procedure appears to begin near proficiency after 20 cases for surgeons experienced in conventional minimally invasive approach. Further studies are warranted to determine advantages and disadvantages of robotic esophagectomy.
Since 2009, we have performed robotic radical esophagectomy in the prone position for more than 40 patients. Then, compared to conventional MIS, robotic approach significantly reduced incidence of vocal cord palsy and hoarseness, suggesting that the use of the robot, which promotes more accurate recurrent laryngeal nerve identification and dissection[95], should reduce the chances of recurrent laryngeal nerve injury, resulting in preserved laryngopharyngeal function[61].
DISCUSSION
Although MIS for upper GI cancer has consistently appeared to improve short-term outcomes and at least preserve long-term outcomes, solid evidences that verify feasibility of MIS and even superiority to open surgery have still been lacking. Advantages and disadvantages of LDG for EGC and AGC over ODG will soon be clarified after the ongoing multicenter RCTs are concluded, however, those of LTG and VATS-E will have been unclear for the time being.
One of the principal reasons why surgeons have been attracted to MIS must be the laparoscopically enhanced anatomy provided by the magnified vivid image with high definition in combination with the horizontal view. To fully utilize these advantages of MIS, the disadvantages of MIS including limited range of motion has to be overcome. We believe one of the solutions may be the laparoscopic manipulation in the caudocranial and/or mediolateral manner, and another may be the use of the surgical robot as indicated in our previous reports[46,50,53,55,61].
We wish to further develop MIS for advanced cancer and that requiring advanced skills by actively utilizing novel technologies including the surgical robot, based on the principles and methods grown through conventional MIS and open surgeries.
In conclusion, technical advancements and development of endoscopic instruments will continue to evolve MIS for upper GI cancer. The outcomes should be validated in a scientific fashion.
ACKNOWLEDGMENTS
The authors thank Dr Seiichiro Kanaya for his wonderful contribution to establishing our original method of minimally invasive gastrectomy and esophagectomy.
Footnotes
Conflict-of-interest statement: Suda K and Uyama I are funded by Intuitive Surgical, Inc. in relation to a multi-institutional single-arm prospective study, which Japanese Ministry of Health, Labor, and Welfare has recently approved for Advanced Medical Technology (“Clinical advantage of robotic radical gastrectomy for gastric cancer: multi-institutional prospective cohort study”, INTUITIVE SURGICAL OPERATIONS, INC. 2015 CLINICAL ROBOTIC RESEARCH GRANT, $50000). Nakauchi M, Inaba K and Ishida Y have no commercial association with or financial involvement that might pose a conflict of interest in connection with the submitted article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 4, 2016
First decision: April 1, 2016
Article in press: April 20, 2016
P- Reviewer: Enrico F, Iida T S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. JGCA Gastric Cancer Treatment Guidelines 2014 (ver 4) Kanehara, Tokyo: Japanese Gastric Cancer Association; 2014. pp. 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13:19–25. doi: 10.5230/jgc.2013.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi H, Kawakubo H, Kitagawa Y. Current status of minimally invasive esophagectomy for patients with esophageal cancer. Gen Thorac Cardiovasc Surg. 2013;61:513–521. doi: 10.1007/s11748-013-0258-9. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Sugihara K, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260:259–266. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 12.Mamidanna R, Bottle A, Aylin P, Faiz O, Hanna GB. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg. 2012;255:197–203. doi: 10.1097/SLA.0b013e31823e39fa. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Baba Y, Nagai Y, Baba H. Minimally invasive esophagectomy for esophageal cancer: an updated review. Surg Today. 2013;43:237–244. doi: 10.1007/s00595-012-0300-z. [DOI] [PubMed] [Google Scholar]
- 14.Suda K, Kitagawa Y, Ozawa S, Miyasho T, Okamoto M, Saikawa Y, Ueda M, Yamada S, Tasaka S, Funakoshi Y, et al. Neutrophil elastase inhibitor improves postoperative clinical courses after thoracic esophagectomy. Dis Esophagus. 2007;20:478–486. doi: 10.1111/j.1442-2050.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- 15.Cuschieri A, Dubois F, Mouiel J, Mouret P, Becker H, Buess G, Trede M, Troidl H. The European experience with laparoscopic cholecystectomy. Am J Surg. 1991;161:385–387. doi: 10.1016/0002-9610(91)90603-b. [DOI] [PubMed] [Google Scholar]
- 16.Ng CS, Whelan RL, Lacy AM, Yim AP. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg. 2005;29:975–981. doi: 10.1007/s00268-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 17.Byun C, Han SU. Current status of randomized controlled trials for laparoscopic gastric surgery for gastric cancer in Korea. Asian J Endosc Surg. 2015;8:130–138. doi: 10.1111/ases.12176. [DOI] [PubMed] [Google Scholar]
- 18.Shu ZB, Cao HP, Li YC, Sun LB. Influences of laparoscopic-assisted gastrectomy and open gastrectomy on serum interleukin-6 levels in patients with gastric cancer among Asian populations: a systematic review. BMC Gastroenterol. 2015;15:52. doi: 10.1186/s12876-015-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 20.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 22.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 23.Strong VE. Defining the role of laparoscopic gastrectomy for gastric cancer. J Clin Oncol. 2014;32:613–614. doi: 10.1200/JCO.2013.52.9479. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Yang KH, Guan QL, Cao N, Chen Y, Zhao P, Chen YL, Yao L. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. 2013;27:2466–2480. doi: 10.1007/s00464-012-2758-6. [DOI] [PubMed] [Google Scholar]
- 25.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 26.Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703) Gastric Cancer. 2010;13:238–244. doi: 10.1007/s10120-010-0565-0. [DOI] [PubMed] [Google Scholar]
- 27.Common Terminology Criteria for Adverse Events (CTCAE) v3. 0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [PubMed]
- 28.Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, Ito S, Takagi M, Takagane A, Ninomiya M, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912) Jpn J Clin Oncol. 2013;43:324–327. doi: 10.1093/jjco/hys220. [DOI] [PubMed] [Google Scholar]
- 29.Takagi M, Katai H, Mizusawa J, Nakamura K, Yoshikawa T, Terashima M, Ito S, Teshima S, Koeda K, Sano T, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M, Japan Clinical Oncology Group. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912): Analysis of the safety and short-term clinical outcomes. J Clin Oncol. 2015;33 Suppl:abstr 4017. [Google Scholar]
- 30.Mori T, Kimura T, Kitajima M. Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol. 2010;19:18–23. doi: 10.3109/13645700903492969. [DOI] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events v4. 0 (CTCAE) Available from: http://evsnci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-2006-2014_QuickReference_2015x2017.pdf.
- 32.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01) J Korean Surg Soc. 2013;84:123–130. doi: 10.4174/jkss.2013.84.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park do J, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01) Ann Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 34.Uyama I, Okabe H, Kojima K, Satoh S, Shiraishi N, Suda K, Takiguchi S, Nagai E, Fukunaga T. Gastroenterological Surgery: Stomach. Asian J Endosc Surg. 2015;8:227–238. doi: 10.1111/ases.12220. [DOI] [PubMed] [Google Scholar]
- 35.Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25–e29. doi: 10.1016/j.jamcollsurg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y. Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc. 2009;23:2167–2171. doi: 10.1007/s00464-008-9987-8. [DOI] [PubMed] [Google Scholar]
- 37.Katai H. Current status of a randomized controlled trial examining laparoscopic gastrectomy for gastric cancer in Japan. Asian J Endosc Surg. 2015;8:125–129. doi: 10.1111/ases.12171. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286–294. doi: 10.1007/s00464-012-2442-x. [DOI] [PubMed] [Google Scholar]
- 39.Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548–1553. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 40.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 41.Qiu J, Pankaj P, Jiang H, Zeng Y, Wu H. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:1–7. doi: 10.1097/SLE.0b013e3182747af7. [DOI] [PubMed] [Google Scholar]
- 42.Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901) World J Surg. 2015;39:2734–2741. doi: 10.1007/s00268-015-3160-z. [DOI] [PubMed] [Google Scholar]
- 43.Kim HI, Hur H, Kim YN, Lee HJ, Kim MC, Han SU, Hyung WJ. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [ NCT01283893] BMC Cancer. 2014;14:209. doi: 10.1186/1471-2407-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura T, Kimura K, Suzuki K, Sakai S, Ohtomo Y, Sakuramachi S, Yamashita Y, Ido K, Kitano S, Yazaki Y. Laparoscopic cholecystectomy: the Japanese experience. Surg Laparosc Endosc. 1993;3:194–198. [PubMed] [Google Scholar]
- 45.Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg. 2011;35:1469–1477. doi: 10.1007/s00268-011-1051-5. [DOI] [PubMed] [Google Scholar]
- 46.Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. 2012;36:331–337. doi: 10.1007/s00268-011-1352-8. [DOI] [PubMed] [Google Scholar]
- 47.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 48.Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hasumi A. Laparoscopic D2 lymph node dissection for advanced gastric cancer located in the middle or lower third portion of the stomach. Gastric Cancer. 2000;3:50–55. doi: 10.1007/pl00011690. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–1142. doi: 10.1001/archsurg.2009.223. [DOI] [PubMed] [Google Scholar]
- 50.Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, Ishida Y, Taniguchi K, Isogaki J, Uyama I. Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc. 2011;25:3928–3929. doi: 10.1007/s00464-011-1792-0. [DOI] [PubMed] [Google Scholar]
- 51.Persiani R, Antonacci V, Biondi A, Rausei S, La Greca A, Zoccali M, Ciccoritti L, D’Ugo D. Determinants of surgical morbidity in gastric cancer treatment. J Am Coll Surg. 2008;207:13–19. doi: 10.1016/j.jamcollsurg.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 52.Aoyagi K, Kouhuji K, Miyagi M, Imaizumi T, Kizaki J, Shirouzu K. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol. 2010;2:81–86. doi: 10.4254/wjh.v2.i2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 54.Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer. 2015;18:662–668. doi: 10.1007/s10120-014-0392-9. [DOI] [PubMed] [Google Scholar]
- 55.Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I. Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc. 2015:Epub ahead of print. doi: 10.1007/s00464-015-4726-4. [DOI] [PubMed] [Google Scholar]
- 56.Sano T, Sasako M, Mizusawa J, Katayama H, Katai H, Yoshikawa T, Yabusaki H, Ito S, Kaji M, Imamura H, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): Final survival analysis. J Clin Oncol. 2015;33:103. [Google Scholar]
- 57.Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 58.Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365–371. doi: 10.1007/s10120-011-0054-0. [DOI] [PubMed] [Google Scholar]
- 59.Man-I M, Suda K, Kikuchi K, Tanaka T, Furuta S, Nakauchi M, Ishikawa K, Ishida Y, Uyama I. Totally intracorporeal delta-shaped B-I anastomosis following laparoscopic distal gastrectomy using the Tri-Staple™ reloads on the manual Ultra handle: a prospective cohort study with historical controls. Surg Endosc. 2015;29:3304–3312. doi: 10.1007/s00464-015-4085-1. [DOI] [PubMed] [Google Scholar]
- 60.Hosogi H, Kanaya S. Intracorporeal anastomosis in laparoscopic gastric cancer surgery. J Gastric Cancer. 2012;12:133–139. doi: 10.5230/jgc.2012.12.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suda K, Ishida Y, Kawamura Y, Inaba K, Kanaya S, Teramukai S, Satoh S, Uyama I. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg. 2012;36:1608–1616. doi: 10.1007/s00268-012-1538-8. [DOI] [PubMed] [Google Scholar]
- 62.Hyun MH, Lee CH, Kim HJ, Tong Y, Park SS. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg. 2013;100:1566–1578. doi: 10.1002/bjs.9242. [DOI] [PubMed] [Google Scholar]
- 63.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 64.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today. 2016;46:528–534. doi: 10.1007/s00595-015-1190-7. [DOI] [PubMed] [Google Scholar]
- 66.Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012;26:60–67. doi: 10.1007/s00464-011-1828-5. [DOI] [PubMed] [Google Scholar]
- 67.Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Zhou J, Shi Y, Qian F, Tang B, Hao Y, Zhao Y, Yu P. Cumulative summation analysis of learning curve for robot-assisted gastrectomy in gastric cancer. J Surg Oncol. 2015;111:760–767. doi: 10.1002/jso.23876. [DOI] [PubMed] [Google Scholar]
- 70.Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I. Revising Robotic Surgery for Stomach, Potential benefits Revised II: Prevention of Pancreatic Fistula. Transl Gastrointest Cancer. 2015;4:461–467. [Google Scholar]
- 71.Nakauchi M, Suda K, Susumu S, Kadoya S, Inaba K, Ishida Y, Uyama I. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc. 2016:Epub ahead of print. doi: 10.1007/s00464-016-4904-z. [DOI] [PubMed] [Google Scholar]
- 72.Nagasako Y, Satoh S, Isogaki J, Inaba K, Taniguchi K, Uyama I. Impact of anastomotic complications on outcome after laparoscopic gastrectomy for early gastric cancer. Br J Surg. 2012;99:849–854. doi: 10.1002/bjs.8730. [DOI] [PubMed] [Google Scholar]
- 73.Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–685. doi: 10.1007/s00595-015-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7–11. [PubMed] [Google Scholar]
- 75.Akaishi T, Kaneda I, Higuchi N, Kuriya Y, Kuramoto J, Toyoda T, Wakabayashi A. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg. 1996;112:1533–140; discussion 1533-140;. doi: 10.1016/s0022-5223(96)70012-0. [DOI] [PubMed] [Google Scholar]
- 76.Kawahara K, Maekawa T, Okabayashi K, Hideshima T, Shiraishi T, Yoshinaga Y, Shirakusa T. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc. 1999;13:218–223. doi: 10.1007/s004649900948. [DOI] [PubMed] [Google Scholar]
- 77.Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 78.Luketich JD, Schauer PR, Christie NA, Weigel TL, Raja S, Fernando HC, Keenan RJ, Nguyen NT. Minimally invasive esophagectomy. Ann Thorac Surg. 2000;70:906–911; discussion 911-912. doi: 10.1016/s0003-4975(00)01711-2. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen NT, Follette DM, Lemoine PH, Roberts PF, Goodnight JE. Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2001;72:593–596. doi: 10.1016/s0003-4975(00)02261-x. [DOI] [PubMed] [Google Scholar]
- 80.Osugi H, Takemura M, Higashino M, Takada N, Lee S, Kinoshita H. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003;90:108–113. doi: 10.1002/bjs.4022. [DOI] [PubMed] [Google Scholar]
- 81.Shichinohe T, Hirano S, Kondo S. Video-assisted esophagectomy for esophageal cancer. Surg Today. 2008;38:206–213. doi: 10.1007/s00595-007-3606-5. [DOI] [PubMed] [Google Scholar]
- 82.Osugi H, Takemura M, Higashino M, Takada N, Lee S, Ueno M, Tanaka Y, Fukuhara K, Hashimoto Y, Fujiwara Y, et al. Video-assisted thoracoscopic esophagectomy and radical lymph node dissection for esophageal cancer. A series of 75 cases. Surg Endosc. 2002;16:1588–1593. doi: 10.1007/s00464-002-9019-z. [DOI] [PubMed] [Google Scholar]
- 83.Palanivelu C, Prakash A, Senthilkumar R, Senthilnathan P, Parthasarathi R, Rajan PS, Venkatachlam S. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg. 2006;203:7–16. doi: 10.1016/j.jamcollsurg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Fabian T, McKelvey AA, Kent MS, Federico JA. Prone thoracoscopic esophageal mobilization for minimally invasive esophagectomy. Surg Endosc. 2007;21:1667–1670. doi: 10.1007/s00464-007-9193-0. [DOI] [PubMed] [Google Scholar]
- 85.Noshiro H, Iwasaki H, Kobayashi K, Uchiyama A, Miyasaka Y, Masatsugu T, Koike K, Miyazaki K. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc. 2010;24:2965–2973. doi: 10.1007/s00464-010-1072-4. [DOI] [PubMed] [Google Scholar]
- 86.Cuesta MA, Weijs TJ, Bleys RL, van Hillegersberg R, van Berge Henegouwen MI, Gisbertz SS, Ruurda JP, Straatman J, Osugi H, van der Peet DL. A new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc. 2015;29:2576–2582. doi: 10.1007/s00464-014-3972-1. [DOI] [PubMed] [Google Scholar]
- 87.The Japan Esophageal Society. Japanese Classification of Esophageal Cancer. 11th ed. Kanehara, Tokyo: The Japan Esophageal Society; 2015. [Google Scholar]
- 88.Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621–1629. doi: 10.1007/s00464-009-0822-7. [DOI] [PubMed] [Google Scholar]
- 89.Sgourakis G, Gockel I, Radtke A, Musholt TJ, Timm S, Rink A, Tsiamis A, Karaliotas C, Lang H. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55:3031–3040. doi: 10.1007/s10620-010-1153-1. [DOI] [PubMed] [Google Scholar]
- 90.Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir. 2009;64:121–133. [PubMed] [Google Scholar]
- 91.Smithers BM, Gotley DC, Martin I, Thomas JM. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245:232–240. doi: 10.1097/01.sla.0000225093.58071.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg. 2012;147:768–776. doi: 10.1001/archsurg.2012.1326. [DOI] [PubMed] [Google Scholar]
- 93.Luketich JD, Pennathur A, Franchetti Y, Catalano PJ, Swanson S, Sugarbaker DJ, De Hoyos A, Maddaus MA, Nguyen NT, Benson AB, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg. 2015;261:702–707. doi: 10.1097/SLA.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Biere SS, Maas KW, Bonavina L, Garcia JR, van Berge Henegouwen MI, Rosman C, Sosef MN, de Lange ES, Bonjer HJ, Cuesta MA, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial) BMC Surg. 2011;11:2. doi: 10.1186/1471-2482-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boone J, Schipper ME, Moojen WA, Borel Rinkes IH, Cromheecke GJ, van Hillegersberg R. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg. 2009;96:878–886. doi: 10.1002/bjs.6647. [DOI] [PubMed] [Google Scholar]
- 96.Anderson C, Hellan M, Kernstine K, Ellenhorn J, Lai L, Trisal V, Pigazzi A. Robotic surgery for gastrointestinal malignancies. Int J Med Robot. 2007;3:297–300. doi: 10.1002/rcs.155. [DOI] [PubMed] [Google Scholar]
- 97.Kernstine KH, DeArmond DT, Shamoun DM, Campos JH. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc. 2007;21:2285–2292. doi: 10.1007/s00464-007-9405-7. [DOI] [PubMed] [Google Scholar]
- 98.Kim DJ, Hyung WJ, Lee CY, Lee JG, Haam SJ, Park IK, Chung KY. Thoracoscopic esophagectomy for esophageal cancer: feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg. 2010;139:53–59.e1. doi: 10.1016/j.jtcvs.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 99.Puntambekar SP, Rayate N, Joshi S, Agarwal G. Robotic transthoracic esophagectomy in the prone position: experience with 32 patients with esophageal cancer. J Thorac Cardiovasc Surg. 2011;142:1283–1284. doi: 10.1016/j.jtcvs.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez JM, Dimou F, Weber J, Almhanna K, Hoffe S, Shridhar R, Karl R, Meredith K. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg. 2013;17:1346–1351. doi: 10.1007/s11605-013-2225-2. [DOI] [PubMed] [Google Scholar]
- 101.Weksler B, Sharma P, Moudgill N, Chojnacki KA, Rosato EL. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus. 2012;25:403–409. doi: 10.1111/j.1442-2050.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- 102.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg. 2013;145:90–96. doi: 10.1016/j.jtcvs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 103.Sarkaria IS, Rizk NP, Finley DJ, Bains MS, Adusumilli PS, Huang J, Rusch VW. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg. 2013;43:e107–e115. doi: 10.1093/ejcts/ezt013. [DOI] [PubMed] [Google Scholar]
- 104.Kim DJ, Park SY, Lee S, Kim HI, Hyung WJ. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc. 2014;28:1866–1873. doi: 10.1007/s00464-013-3406-5. [DOI] [PubMed] [Google Scholar]
- 105.Trugeda S, Fernández-Díaz MJ, Rodríguez-Sanjuán JC, Palazuelos CM, Fernández-Escalante C, Gómez-Fleitas M. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot. 2014;10:397–403. doi: 10.1002/rcs.1587. [DOI] [PubMed] [Google Scholar]
- 106.Galvani CA, Gorodner MV, Moser F, Jacobsen G, Chretien C, Espat NJ, Donahue P, Horgan S. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc. 2008;22:188–195. doi: 10.1007/s00464-007-9441-3. [DOI] [PubMed] [Google Scholar]
- 107.Sutherland J, Banerji N, Morphew J, Johnson E, Dunn D. Postoperative incidence of incarcerated hiatal hernia and its prevention after robotic transhiatal esophagectomy. Surg Endosc. 2011;25:1526–1530. doi: 10.1007/s00464-010-1429-8. [DOI] [PubMed] [Google Scholar]
- 108.Dunn DH, Johnson EM, Morphew JA, Dilworth HP, Krueger JL, Banerji N. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus. 2013;26:159–166. doi: 10.1111/j.1442-2050.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 109.Coker AM, Barajas-Gamboa JS, Cheverie J, Jacobsen GR, Sandler BJ, Talamini MA, Bouvet M, Horgan S. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A. 2014;24:89–94. doi: 10.1089/lap.2013.0444. [DOI] [PubMed] [Google Scholar]
- 110.Ruurda JP, van der Sluis PC, van der Horst S, van Hilllegersberg R. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol. 2015;112:257–265. doi: 10.1002/jso.23922. [DOI] [PubMed] [Google Scholar]
- 111.van der Sluis PC, Ruurda JP, Verhage RJ, van der Horst S, Haverkamp L, Siersema PD, Borel Rinkes IH, Ten Kate FJ, van Hillegersberg R. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S1350–S1356. doi: 10.1245/s10434-015-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


