Abstract
AIM: To analyze contrast-enhanced ultrasound (CEUS) features of histologically proven hepatic epithelioid hemangioendothelioma (HEHE) in comparison to other multilocular benign focal liver lesions (FLL).
METHODS: Twenty-five patients with histologically proven HEHE and 45 patients with histologically proven multilocular benign FLL were retrospectively reviewed. Four radiologists assessed the CEUS enhancement pattern in consensus.
RESULTS: HEHE manifested as a single (n = 3) or multinodular (n = 22) FLL. On CEUS, HEHE showed rim-like (18/25, 72%) or heterogeneous hyperenhancement (7/25, 28%) in the arterial phase and hypoenhancement (25/25, 100%) in the portal venous and late phases (PVLP), a sign of malignancy. Eighteen patients showed central unenhanced areas (18/25, 72%); in seven patients (7/25, 28%), more lesions were detected in the PVLP. In contrast, all patients with hemangioma and focal nodular hyperplasia showed hyperenhancement as the most distinctive feature (P < 0.01).
CONCLUSION: CEUS allows for characterization of unequivocal FLL. By analyzing the hypoenhancement in the PVLP, CEUS can determine the malignant nature of HEHE.
Keywords: Guidelines, Recommendations, Liver tumor, Biopsy, Liver transplantation Contrast enhanced ultrasound
Core tip: In this retrospective study, a large cohort of very rare histologically proven hemangioendothelioma (HEHE) was evaluated. Contrast-enhanced ultrasound (CEUS) allowed for improved detection of multilocular HEHE. HEHE showed typical enhancement patterns on CEUS. Therefore, CEUS can help to determine the malignant nature of HEHE.
INTRODUCTION
Hepatic epithelioid hemangioendothelioma (HEHE) is a rare vascular neoplasm of endothelial origin with primary liver involvement and is characterized by the presence of epithelioid endothelial cells[1]. Weiss and Enzinger first reported 41 patients with this unique tumor in 1982[2]. This tumor is histologically characterized by an epithelial appearance and the endothelial nature of the tumor cells[3]. Currently no more than 200 patients with HEHE have been reported since its first description, and most of the studies were small series[4,5].
No definite etiopathogenetic factors, apart from an association with oral contraceptives, trauma, and exposure to vinyl chloride, have to date been ascribed to HEHE[1,6]. The tumor generally affects adults, with a strong female predominance and a peak incidence occurring between 30 and 40 years of age. The clinical manifestations and laboratory data of HEHE are nonspecific, usually presenting with general symptoms, such as right upper quadrant pain or weight loss. Some patients may present with liver failure, Budd-Chiari syndrome, or portal hypertension, while others may be asymptomatic. Its clinical course and prognosis are variable and unpredictable[7]. Due to its nonspecific clinical manifestations and prolonged clinical course, establishing diagnosis even with histopathological findings can often be challenging[8].
HEHE carries intermediate malignant potential, and transplantation may provide a long term cure[9]. Therefore, the recognition of the imaging features of this rare neoplasm may be helpful for the detection and further surgical treatment of this potentially curable disease. In addition, it is important to distinguish HEHE from other primary and secondary benign and malignant hepatic tumors, such as atypical (multilobulated) hemangioma and hemangiomatosis, hepatocellular adenoma and hepatocellular carcinoma, intrahepatic cholangiocarcinomas, lymphoma, and liver metastases[10]. Radiologists should be aware of its imaging findings and raise suspicion in the proper clinical setting[8,11].
Conventional ultrasound is the most commonly used imaging method for real time diagnosis of FLL. However, the most frequent imaging findings of multilocular HEHE are nonspecific[11]. Often, multiple HEHE on conventional ultrasound might be difficult to differentiate from other atypical multilocular FLLs[12,13]. As a result, the final diagnosis of HEHE depends on biopsy and histological findings[14,15].
Contrast enhanced ultrasound (CEUS) allows for the differentiation of most benign and malignant liver tumors in the portal venous and late phases (PVLP). This finding was summarized in the European Federation of Societies for Ultrasound in Medicine and Biology guidelines and recommendations for the use of CEUS in liver[16,17]. Benign FLLs are typically iso- or hyperenhancing in the PVLP; whereas malignant primary and secondary liver tumors almost always show hypoenhancement in the PVLP, since they do not contain the respective specific hepatic vessels. This hypoenhancement in the PVLP is decisive for determining if a lesion should be biopsied[18,19]. In addition, CEUS findings of HEHE have not been well addressed. Therefore, the aim of our study is to analyze the CEUS features of histologically proven HEHE and to compare these features to those of other multilocular benign FLLs, including hemangiomas and focal nodular hyperplasia (FNH), since they are the most important for differential diagnosis. We assessed the clinical value of CEUS to define the malignant nature of the disease with hypoenhancement in the PVLP. To our best knowledge, this is the first report on the CEUS features of HEHE.
MATERIALS AND METHODS
Patients
Hemangioendothelioma: Between September 2004 and October 2015, 25 patients (eight male, 17 female, mean age 46 ± 14 years; range 24-78 years) were retrospectively analyzed. In this retrospective study, lesions were histologically proven by hepatic surgery (n = 6) or by 18-gauge core needle biopsy (n = 19).
Three patients had a single FLL, whereas 22 patients had multiple FLLs (Table 1). In patients with multiple FLLs, the selected lesions were those in which biopsies had been performed.
Table 1.
Baseline characteristics of patients included in our study
| Characteristic | HEHE (n = 25) | Hemangioma and FNH (n = 45) |
| Age (yr) | ||
| mean ± SD | 46 ± 14 | 46 ± 14 |
| Range | 24-78 | 23-74 |
| Male/female | 8/17 | 9/36 |
| Number of FLL (single/multiple) | 3/22 | 0/45 |
| Histological results | ||
| hepatic surgery | 6 | 0 |
| core needle biopsy | 19 | 45 |
HEHE: Hepatic epithelioid haemangioendothelioma; FNH: Focal nodular hyperplasia; FLL: Focal liver lesions.
Multilocular hemangioma and FNH
Forty-five patients (nine male, 36 female, mean age 46 ± 14 years; range 23-74 years) with multilocular hemangioma and FNH were also retrospectively analyzed. All lesions were histologically proven by 18-gauge core needle biopsy.
Examination technique
Conventional ultrasound and CEUS were performed by five ultrasound systems: LOGIQ E9 (GE Healthcare, Milwaukee, WI, United States; C1-5 convex array probes, 1-5MHz), Acuson Sequoia (Siemens Healthcare, Erlangen, Germany, 3.5 MHz), Philips iU22 unit (Philips Healthcare, Bothell, WA, United States; C5-1 convex array probes, 1-5MHz), Technos MPX Scanner, and MyLab70 (Esaote, Genova, Italy; ca431 convex array probe 1-8 MHz).
CEUS was performed using contrast harmonic real time imaging at a low MI 0.05-0.30. Each examination lasted about 5 min after the bolus injection. The contrast agent used was SonoVue® (Bracco Imaging Spa, Milan, Italy). For each CEUS examination, a dose of 1.5-2.4 mL of SonoVue® was injected as a quick bolus via a 20 gauge intravenous catheter placed in the cubital vein, followed by 5-10 mL of 0.9% normal saline flush. Repeated injection of SonoVue® was performed when necessary.
To characterize the lesion, SonoVue® enhancement during the arterial phase (10-30 s), portal venous (20-120 s), and late vascular phases (120-300 s) were evaluated[17]. All examinations were digitally recorded.
Image analysis
All HEHE images were read by four independent radiologists (15, 17, 23, and 27 years of experience with abdominal ultrasound imaging) blinded to clinical and pathologic data in consensus. Criteria evaluated included number of lesions, maximum diameter, echogenicity (hyperechoic, hypoechoic, or isoechoic; homogeneous or heterogeneous; which were visually compared with the echogenicity of the surrounding liver parenchyma), shape (regular or lobulated), margin (ill- or well defined appearance), and color Doppler imaging features. Using CEUS, the pattern of contrast enhancement of the lesion in comparison to the surrounding liver parenchyma (hypoenhancing, hyperenhancing, isoenhancing), homogeneity of enhancement (homogeneous, heterogeneous), and additional features of enhancement during the arterial, portal venous, and late phases were noted as well, e.g., rim-like or peripheral nodular enhancement, central or eccentric arterial enhancement).
CEUS features of 45 patients of histologically proven multilocular liver hemangioma and FNH were also retrospectively evaluated to compare the CEUS features for differential diagnosis. Digital cineloops were registered both during baseline and post contrast US scanning. All cineloops were digitally stored in a PC based workstation connected to the ultrasound systems.
Pathologic examination
The final pathologic diagnosis was based on hematoxylin-eosin stained sections and immunohistochemical staining results. The immunohistochemical staining included endothelial markers, such as CD 34, CD 31, and factor VIII-related antigen (FVIII Ag)[20].
Statistical analysis
Data are expressed as mean ± SD. All statistical analyses were performed with SPSS 17.0 software package (SPSS, Chicago, IL, United States). The χ2 test was used to compare HEHE with liver hemangiomas and FNH in terms of enhancement pattern. For the features that played a statistically significant role in the differentiation diagnosis, we calculated sensitivity and specificity. A difference was considered statistically significant with P < 0.05.
RESULTS
Clinical and general pathologic features
All patients were incidentally found to have hepatic lesions by conventional ultrasound screening. Conventional laboratory tests [including transaminases, bilirubin, and gamma-glutamyl transpeptidase (gGT)] were within normal limits or only slightly elevated in all patients. Alpha-fetoprotein, carcinoembryonic antigen, and cancer antigen 19-9 as well as hepatitis B surface antigen and hepatitis C virus were negative respective of normal in all patients.
Final pathologic diagnosis of HEHE showed the typical fibrosclerotic center and cellular periphery on hematoxylin-eosin staining. Immunohistochemically, tumors were positive for at least one endothelial marker, including CD 34 (n = 20), CD 31 (n = 20), or FVIII Ag (n = 11).
Features with conventional ultrasound in HEHE
HEHE manifested as single (3/25, 12%) or multiple FLLs (22/25, 88%) with ill-defined margins on grayscale ultrasound. The lesions were mainly hypoechoic (23/25, 92%) to adjacent liver parenchyma, but a heterogeneous echogenicity with hypo- or hyperechoic FLL was observed (2/25, 8%).
Color Doppler imaging detected branched intralesional vessels in 84% (21/25) of HEHE. The Doppler spectrum was measured in 13 patients. The mean value of resistive index (RI) was 0.64 ± 0.07 (Figure 1 and Table 2).
Figure 1.
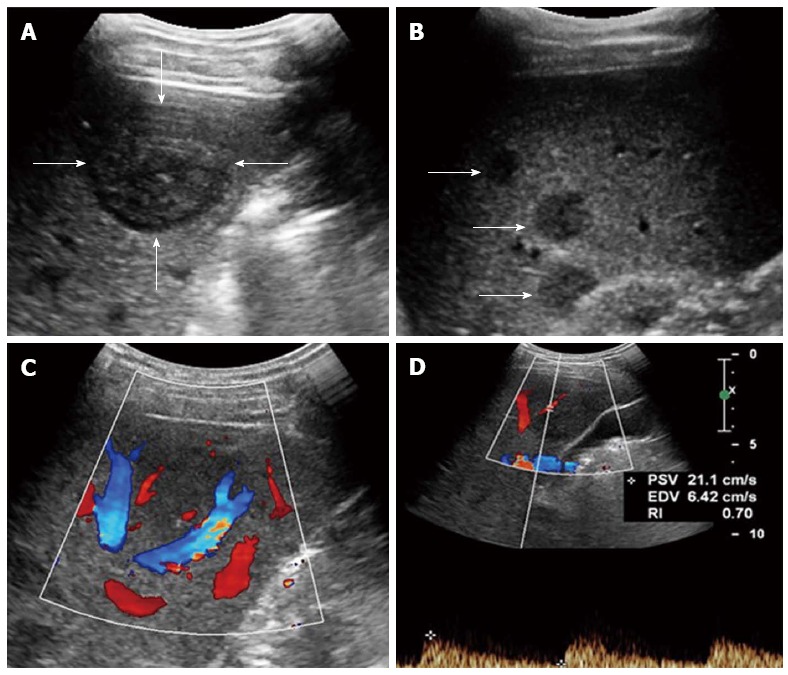
Multiple hepatic epithelioid hemangioendotheliomas in a 31 year female. A: Grayscale ultrasound showed a distinct hypoechoic focal liver lesion (FLL) (arrow); B: Multiple hypoechoic lesions (arrows) were also detected in this patient; C: Color Doppler imaging (CDFI) showed peripheral and intra-lesion color flow signals; D: The resistive index (RI) of color flow was 0.70.
Table 2.
Conventional ultrasound features of hepatic epithelioid haemangioendothelioma and hemangioma/Focal nodular hyperplasia n (%)
| Characteristic | HEHE (n = 25) | Hemangioma/FNH(n = 45) |
| Number of nodules (single/multiple) | 3/22 | 0/45 |
| Size of nodules (mm) | ||
| mean ± SD | 41.5 ± 25.6 | 50.4 ± 25.7 |
| range | 12-120 | 20-138 |
| Echogenicity of nodules | ||
| Hyperechoic | 2 (8) | 19 (42.2) |
| Hypoechoic | 23 (92) | 9 (20.0) |
| Isoechoic | 0 | 17 (37.8) |
| Homogenous/heterogeneous | 9/16 | 15/30 |
HEHE: Hepatic epithelioid haemangioendothelioma; FNH: Focal nodular hyperplasia.
CEUS features
On CEUS, HEHE presented peripheral rim-like (18/25, 72%) (Figure 2) or heterogeneous hyperenhancement (7/25, 28%) at the arterial phase (Figure 3) and hypoenhancement (100%, 25/25) at PVLP (Figure 4). Central unenhanced areas were observed in 72% (18/25) of HEHE in the late phases. After CEUS, more lesions could be detected in seven patients of HEHE than with conventional ultrasound. Liver hemangioma typically demonstrated peripheral nodular contrast enhancement in all patients, whereas FNH showed central or eccentric arterial blood supply in the arterial phase. In addition, in all patients, both entities showed hyperenhancement in the PVLP, a sign of the benign nature of the lesion. Compared to multilocular liver hemangioma and FNH, characteristic CEUS features of HEHE were peripheral rim-like hyperenhancement in the arterial phase and quick washout in the PVLP with a central unenhanced area in the late phase (P < 0.01) (Table 3).
Figure 2.
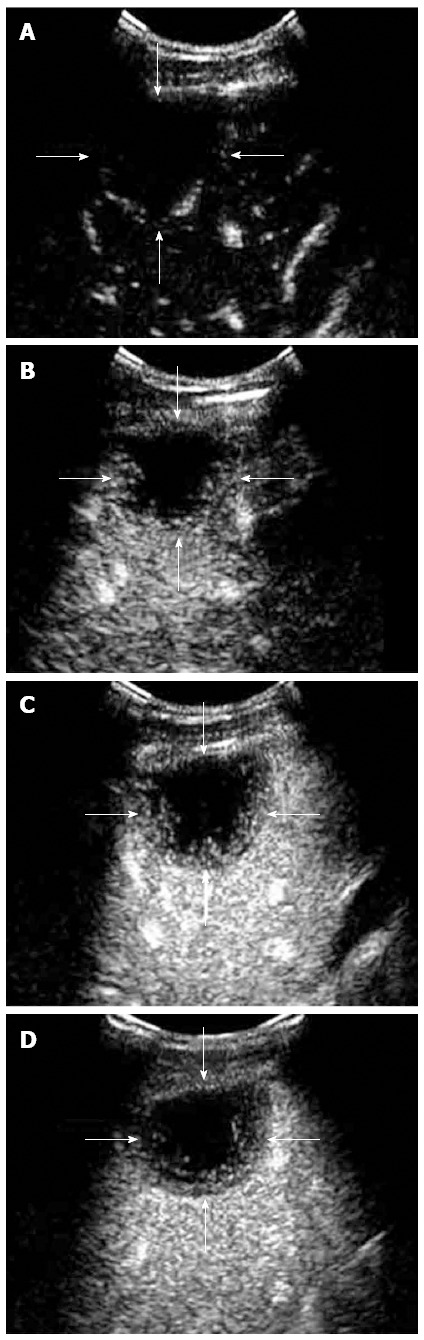
Contrast-enhanced ultrasound feature of hepaticepithelioid hemangioendothelioma in a 31 year female. A: Rim-like enhancement. In arterial phase (16 s after injection of SonoVue), peripheral rim-like enhancement was demonstrated; B: In peak enhancement (24 s after injection of SonoVue), the degree of the rim-like enhancement was equivalent to the liver parenchyma; C: In portal venous phase (45 s after injection of SonoVue), the lesion washed out quickly and showed hypoenhancement; D: In late phase (65 s after injection of SonoVue), the lesion remained hypoenhanced with central unenhanced area.
Figure 3.
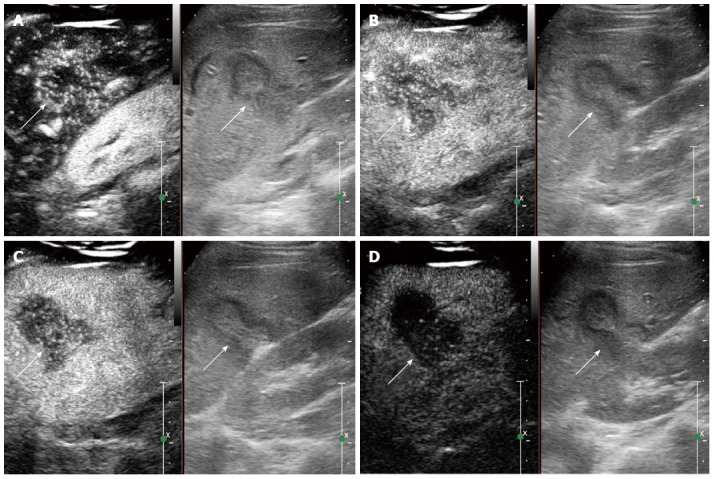
Contrast-enhanced ultrasound feature of hepatic epithelioid hemangioendothelioma in a 25 year female. A: Heterogeneous enhancement pattern. In the arterial phase (16 s after injection of SonoVue), the lesion showed heterogeneous enhancement; B: The enhancement gradually decreased (22 s after injection of SonoVue); C: In the portal venous phase (40 s after injection of SonoVue), the lesion washed out fast than the liver parenchyma and showed hypoenhancement. D: In the late phase (165 s after injection of SonoVue), the lesion remained hypoenhanced.
Figure 4.
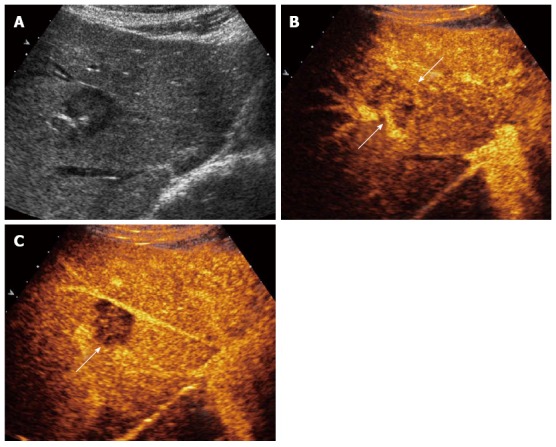
Contrast-enhanced ultrasound feature of hepatic epithelioid hemangioendothelioma in right lobe of liver. A: Grayscale ultrasound showed a hypoechoic focal liver lesions (FLL); B: In the arterial phase the lesion showed heterogeneous enhancement (22 s after injection of SonoVue); C: In the portal venous phase (53 s after injection of SonoVue), the lesion washed out fast and showed hypoenhancement.
Table 3.
Contrast enhanced ultrasound imaging features of hepatic epithelioid haemangioendothelioma and multilocular hemangioma/ focal nodular hyperplasia n (%)
| Characteristic | HEHE(n = 25 patients) | Hemangioma/FNH(n = 45 patients) |
| Arterial phase | ||
| Rim like hyperenhancement | 18 (72) | 0 |
| Heterogeneously hyperenhancement | 7 (28) | 6 (13.3) |
| Peripheral nodular enhancement | 0 | All hemangioma |
| Central arterial blood supply | All FNH | |
| Portal-venous phase | ||
| Hyperenhancement | 0 | 100 (100.0%) |
| Hypoenhancement | 25 (100) | 0 |
| Isoenhancement | 0 | 0 |
| Late phase | ||
| Hyperenhancement | 0 | 45 (100.0%) |
| Hypoenhancement | 25 (100) | 0 |
| Isoenhancement | 0 | 0 |
| Sensitivity | ||
| Rim like hyperenhancement | 18/25 (72) | 0 |
| Hypoenhancement at portal venous phase | 25/25 (100) | 0 |
| Central unenhanced area at late phase | ||
| Yes | 18 (72) | 13 (28.9) |
| No | 7 (28) | 32 (71.1) |
CEUS: Contrast enhanced ultrasound; HEHE: Hepatic epithelioid haemangioendothelioma; FNH: Focal nodular hyperplasia.
The sensitivity for peripheral rim-like hyperenhancement at the arterial phase was 72%; for quick washout in the PVLP, it was 100%; for central unenhanced area at late phase, it was 72%; and for the combination of both, it was 85% (Table 3).
DISCUSSION
To the best of our knowledge, CEUS features of HEHE have not been well characterized. To date, only a few imaging studies have investigated HEHE, and most of them were limited patients series[4,15,21], and CEUS features of HEHE have been described only in a few patients[15,21,22]. In many patients, CEUS is the first and decisive imaging technique for detecting and characterizing liver tumors[23-25]. The use of ultrasound contrast agents improved detection and made it possible to assess the benign or malignant nature of liver tumors in most patients[13,26-28]. Previously, three forms of HEHE have been described: single nodular, multifocal nodular, and the diffuse type[1]. Consistent with our current study, most HEHE present as multiple FLL. After CEUS, more lesions could be detected in 7/25 (28%) patients[29]. As HEHE has ill-defined margins on grayscale ultrasound, CEUS may be helpful to detect more lesions with sharper and clearer margins.
In our current retrospective study, we discovered that CEUS reliably showed typical signs of HEHE in most patients with hyperenhancement in the arterial phase and hypoenhancement in the PVLP, which might be useful in determining whether a biopsy is necessary for suspected malignant lesions. In correlation with pathologic classification, histologically, HEHE possesses two distinctive characteristics, which are directly related to the echogenicity and enhancement pattern of HEHE on ultrasound images[1,20,30,31]. First, HEHE are composed of dendritic and epithelioid cells with intracytoplasmic lumina containing red blood cells. However, the peripheral proliferation remains active and forms numerous arterial-venous shunts, which could account for the fast rim-like enhancement in the arterial phase and quick washout in the PVLP during CEUS[7]. Second, tumor cells and stroma of HEHE exist in variable proportions, and the central stromal portion of the lesion can vary from myxoid to densely fibrotic. With the growth of the tumor, the central stroma degenerate gradually and become sclerotic as the blood supply decreases[20]. In our results, hypoenhancement with central unenhanced area at PVLP of CEUS was mostly common in HEHE. Moreover, additional lesions were detected at CEUS, leading to improvements in liver staging.
Alomari et al[32] first described the lollipop sign as a new cross-sectional sign of HEHE on computed tomography (CT) and magnetic resonance imaging (MRI): a well-defined peripherally enhancing (or non-enhancing) lesion with an avascular core on enhanced images (the candy in the lollipop) and a histologically occluded vein (the stick). Concerning the CT imaging, focal calcifications were reported in 20% of patients; capsular retraction was in 10%-25% of patients[20]. The lesions demonstrated peripheral rim-like hyperenhancement in the arterial phase with even stronger enhancement in the portal venous phase by contrast enhanced MRI. Central areas of reduced signal may correspond to areas of hemorrhage, coagulation necrosis, and calcification[7]. We showed that peripheral rim-like hyperenhancement in the arterial phase and hypoenhancement in the PVLP with central unenhanced areas could be detected in 72% HEHE patients. Therefore, the contrast enhanced image modalities demonstrate a similar enhancement pattern of this disease. CEUS can be considered at least equal to, and in some ways (real time observation, no radiation, less expensive) superior to, CT and MRI as a diagnostic tool[33].
Most of the HEHE lesions were multinodular (88%) and hypoechoic (92%) in our current study. As set out in the current literature and in textbooks, the origin of hypoechoic lesions is considerably more varied and confusing than other lesions[13,23]. All hypoechogenic lesions should be investigated using a contrast enhanced imaging technique[16,18]. Evaluation with CEUS in the PVLP is determinant in this context, and contrast medium hypoenhancement in the late phase is a decisive indication for liver biopsy[23].
HEHE has a variable clinical and biological course compared to benign endothelial tumors (hemangiomas) and malignant angiosarcomas with a slowly progressive phenotype. The tumor can even be difficult to diagnose based on biopsy specimens[34]. CEUS differentiation of different liver tumors is essential because of different therapeutic approaches[35]. HEHE should be differentiated from atypical multilocular liver hemangioma and FNH, because both of them could demonstrate as multilocular hypoechoic liver lesions. Although benign FLLs are commonly iso- or hyperenhancing in the PVLP, malignant primary and secondary liver tumors almost always show hypoenhancement in the PVLP[18,19]. Based on results of our retrospective analysis, we believe that peripheral rim-like hyperenhancement at the arterial phase and quick washout at the PVLP with central unenhanced area are hallmark features that suggest a diagnosis of possible HEHE. In contrast, both multilocular hemangiomas and FNH showed hyperenhancement and remained iso or hyperenhanced in PVLP.
Furthermore, in the clinical setting, factors helpful for the differential diagnosis of HEHE are a medical history without extrahepatic malignant tumor, patients with no symptoms, and laboratory tests[35].
In conclusion, CEUS imaging findings reliably compile typical signs of HEHE, allowing for effective differentiation with other multilocular hypoechoic hepatic lesions, including liver hemangioma and FNH. CEUS can help to improve the diagnostic confidence of HEHE, a rare hepatic tumor, and the liver staging of the disease to guide additional diagnostic work-up.
COMMENTS
Background
To our best knowledge, contrast-enhanced ultrasound (CEUS) features of hepatic epithelioid hemangioendothelioma (HEHE), a rare hepatic tumor, have not been well characterized. To date, only a few imaging studies have investigated HEHE, and most of them were limited patients series.
Research frontiers
This is the first report on the CEUS features of HEHE.
Innovations and breakthroughs
CEUS imaging findings reliably compile typical signs of HEHE and differentiate effectively it from other multilocular hypoechoic hepatic lesions, including liver hemangioma and focal nodular hyperplasia.
Applications
CEUS can help to improve the diagnostic confidence and liver staging of HEHE to guide additional diagnostic work-up.
Terminology
CEUS allows for the differentiation of most benign and malignant liver tumors in the portal venous and late phases.
Peer-review
The aim of this retrospective study was to analyze the CEUS features of histologically proven HEHE in comparison to other multilocular benign focal liver lesions, which might be important differential diagnosis, and to assess the clinical value of CEUS to define the malignant nature of HEHE with hypoenhancement in the portal-venous and late phase.
Footnotes
Institutional review board statement: This study was a Retrospective Study, Institutional review board statement was applicable to our current study.
Informed consent statement: This study was a Retrospective Study, patients Informed consent statement was applicable to our current study.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: Each author gives permission to the graphic designers to alter the visual aspect of figures, tables, or graphs.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 10, 2016
First decision: February 18, 2016
Article in press: April 7, 2016
P- Reviewer: Balaban YH, Kaya M, Zhang XC S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang DN
References
- 1.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562–582. doi: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Furui S, Itai Y, Ohtomo K, Yamauchi T, Takenaka E, Iio M, Ibukuro K, Shichijo Y, Inoue Y. Hepatic epithelioid hemangioendothelioma: report of five cases. Radiology. 1989;171:63–68. doi: 10.1148/radiology.171.1.2648478. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Yu RS, Qiu LL, Jiang DY, Tan YB, Fu YB. Contrast-enhanced multiple-phase imaging features in hepatic epithelioid hemangioendothelioma. World J Gastroenterol. 2011;17:3544–3553. doi: 10.3748/wjg.v17.i30.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadei Gardini A, Pieri F, Fusaroli P, Oboldi D, Passardi A, Monti M, Rosetti P, Calpona S, Valgiusti M, Ragazzini A, et al. Hemangioblastoma of the gastrointestinal tract: a first case. Int J Surg Pathol. 2013;21:192–196. doi: 10.1177/1066896912475082. [DOI] [PubMed] [Google Scholar]
- 6.Gelin M, Van de Stadt J, Rickaert F, De Prez C, Levarlet M, Adler M, Lambilliotte JP. Epithelioid hemangioendothelioma of the liver following contact with vinyl chloride. Recurrence after orthotopic liver transplantation. J Hepatol. 1989;8:99–106. doi: 10.1016/0168-8278(89)90168-2. [DOI] [PubMed] [Google Scholar]
- 7.Amin S, Chung H, Jha R. Hepatic epithelioid hemangioendothelioma: MR imaging findings. Abdom Imaging. 2011;36:407–414. doi: 10.1007/s00261-010-9662-0. [DOI] [PubMed] [Google Scholar]
- 8.Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal N, Parajuli S, Zhao P, Satoskar R, Laurin J, Azumi N, Matsumoto C, Shetty K. Liver transplantation in the management of hepatic epithelioid hemangioendothelioma: a single-center experience and review of the literature. Transplant Proc. 2011;43:2647–2650. doi: 10.1016/j.transproceed.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Orlando G, Adam R, Mirza D, Soderdahl G, Porte RJ, Paul A, Burroughs AK, Seiler CA, Colledan M, Graziadei I, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation. 2013;95:872–877. doi: 10.1097/TP.0b013e318281b902. [DOI] [PubMed] [Google Scholar]
- 11.Perera B, Østbye T. Prevalence and correlates of sexual abuse reported by late adolescent school children in Sri Lanka. Int J Adolesc Med Health. 2009;21:203–211. doi: 10.1515/ijamh.2009.21.2.203. [DOI] [PubMed] [Google Scholar]
- 12.Fröhlich E, Jenssen C, Schuler A, Dietrich CF. [Contrast-enhanced ultrasound for characterisation of focal liver lesions, practical advice] Z Gastroenterol. 2015;53:1099–1107. doi: 10.1055/s-0035-1553491. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich CF, Cui XW, Boozari B, Hocke M, Ignee A. Contrast-enhanced ultrasound (CEUS) in the diagnostic algorithm of hepatocellular and cholangiocellular carcinoma, comments on the AASLD guidelines. Ultraschall Med. 2012;33 Suppl 1:S57–S66. doi: 10.1055/s-0032-1312903. [DOI] [PubMed] [Google Scholar]
- 14.Azzam RI, Alshak NS, Pham HP. AIRP best cases in radiologic-pathologic correlation: Hepatic epithelioid hemangioendothelioma. Radiographics. 2012;32:789–794. doi: 10.1148/rg.323115010. [DOI] [PubMed] [Google Scholar]
- 15.Cui XW, Ignee A, Woenckhaus M, Dietrich CF. Hemangioendothelioma, an Imaging Challenge. Endheu. 2014;27:34–36. [Google Scholar]
- 16.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 17.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, Friedrich-Rust M, Bernatik T. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS) Ultraschall Med. 2009;30:376–382. doi: 10.1055/s-0028-1109672. [DOI] [PubMed] [Google Scholar]
- 19.Bernatik T, Seitz K, Blank W, Schuler A, Dietrich CF, Strobel D. Unclear focal liver lesions in contrast-enhanced ultrasonography--lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med. 2010;31:577–581. doi: 10.1055/s-0029-1245649. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Ji Y. CT and MRI diagnosis of hepatic epithelioid hemangioendothelioma. Hepatobiliary Pancreat Dis Int. 2010;9:154–158. [PubMed] [Google Scholar]
- 21.Mermuys K, Vanhoenacker PK, Roskams T, D’Haenens P, Van Hoe L. Epithelioid hemangioendothelioma of the liver: radiologic-pathologic correlation. Abdom Imaging. 2004;29:221–223. doi: 10.1007/s00261-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer N, Soudah B, Gebel M, Manns MP, Boozari B. Gray scale and contrast-enhanced ultrasound imaging of malignant liver tumors of vascular origin. United European Gastroenterol J. 2015;3:63–71. doi: 10.1177/2050640614560604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich CF, Sharma M, Gibson RN, Schreiber-Dietrich D, Jenssen C. Fortuitously discovered liver lesions. World J Gastroenterol. 2013;19:3173–3188. doi: 10.3748/wjg.v19.i21.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich CF. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012;33 Suppl 1:S22–S30. doi: 10.1055/s-0032-1312892. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich CF, Cui XW, Schreiber-Dietrich DG, Ignee A. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med. 2012;33 Suppl 1:S11–S21. doi: 10.1055/s-0032-1312890. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich CF, Ignee A, Trojan J, Fellbaum C, Schuessler G. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401–405. doi: 10.1136/gut.2003.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich CF, Cui XW, Barreiros AP, Hocke M, Ignee A. EFSUMB guidelines 2011: comment on emergent indications and visions. Ultraschall Med. 2012;33 Suppl 1:S39–S47. doi: 10.1055/s-0032-1312895. [DOI] [PubMed] [Google Scholar]
- 28.Seitz K, Strobel D, Bernatik T, Blank W, Friedrich-Rust M, Herbay Av, Dietrich CF, Strunk H, Kratzer W, Schuler A. Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions - prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreiländertreffen 2008, Davos. Ultraschall Med. 2009;30:383–389. doi: 10.1055/s-0028-1109673. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, Vossas U, Hauenstein K, Koch W, Blank W, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol. 2006;12:1699–1705. doi: 10.3748/wjg.v12.i11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EH, Rha SE, Lee YJ, Yoo IeR, Jung ES, Byun JY. CT and MR imaging findings of hepatic epithelioid hemangioendotheliomas: emphasis on single nodular type. Abdom Imaging. 2015;40:500–509. doi: 10.1007/s00261-014-0229-3. [DOI] [PubMed] [Google Scholar]
- 31.Miller WJ, Dodd GD, Federle MP, Baron RL. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53–57. doi: 10.2214/ajr.159.1.1302463. [DOI] [PubMed] [Google Scholar]
- 32.Alomari AI. The lollipop sign: a new cross-sectional sign of hepatic epithelioid hemangioendothelioma. Eur J Radiol. 2006;59:460–464. doi: 10.1016/j.ejrad.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Chiorean L, Cantisani V, Jenssen C, Sidhu PS, Baum U, Dietrich CF. Focal masses in a non-cirrhotic liver: The additional benefit of CEUS over baseline imaging. Eur J Radiol. 2015;84:1636–1643. doi: 10.1016/j.ejrad.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Liu YI, Brown SS, Elihu A, Bonham CA, Concepcion W, Longacre TA, Kamaya A. Hepatic epithelioid hemangioendothelioma. Dig Dis Sci. 2011;56:303–306. doi: 10.1007/s10620-010-1470-4. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139–1145. doi: 10.1002/hep.21615. [DOI] [PubMed] [Google Scholar]


