Abstract
AIM: To compare hybrid therapy (HT) with traditional sequential therapy (ST) and concomitant therapy (CT) for Helicobacter pylori (H. pylori) eradication.
METHODS: We performed an electronic search of PubMed, Embase, and the CENTRAL database. Randomized controlled trials (RCTs) of HT were included in the meta-analysis. The primary outcome was the eradication rate of H. pylori. The secondary outcomes included the compliance rate and adverse event rate. Effect estimates were pooled using the random-effects model.
RESULTS: Twelve studies were included. Pooled results showed no significant differences in eradication rate between HT and ST in per-protocol (PP) analysis (RR = 1.03, 95%CI: 0.94-1.12, P = 0.59) or in intention-to-treat (ITT) analysis (RR = 1.00, 95%CI: 0.89-1.12, P = 0.94). HT and ST showed similarly high compliance rate (96% vs 98%, P = 0.55) and acceptable adverse event rate (30.3% vs 28.2%, P = 0.63). No significant results were seen in the eradication rate between HT and CT in PP analysis (RR = 1.01, 95%CI: 0.96-1.05, P = 0.76) or in ITT analysis (RR = 0.99, 95%CI: 0.95-1.03, P = 0.47). HT displayed a slightly higher compliance rate than CT (95.8% vs 93.2%, P < 0.05). The adverse event rates of HT and CT were similar (39.5% vs 44.2%, P = 0.24).
CONCLUSION: Compared with ST or CT, HT yields a similar eradication rate, high compliance rate, and acceptable safety profiles.
Keywords: Hybrid therapy, Sequential therapy, Concomitant therapy, Helicobacter pylori, Meta-analysis
Core tip: This meta-analysis of randomized controlled trials compared the novel hybrid therapy with sequential and concomitant therapy in the treatment of Helicobacter pylori. The eradication rate, compliance rate and the adverse event rate were investigated as the main outcomes and were compared. Overall, similar results were shown regarding these outcomes by hybrid and sequential therapy, and by hybrid and concomitant therapy. Hybrid therapy could be an effective and safe alternative to sequential or concomitant therapy.
INTRODUCTION
Approximately 50% of the global population are infected with Helicobacter pylori (H. pylori). The presence of H. pylori in the stomach is directly associated with a series of gastric diseases, including chronic gastritis, peptic ulcer, and gastric cancer[1]. Triple therapy, consisting of one proton pump inhibitor (PPI), amoxicillin, and clarithromycin, has been established as the standard first-line treatment for H. pylori eradication since the 1997 Maastricht Conference[2]. However, the eradication rates have decreased to unacceptable levels (less than 80%) in many countries[3]. Growing resistance of H. pylori strains to clarithromycin and metronidazole is the major cause of treatment failure[4,5].
Worldwide efforts led to the development of new regimens to improve the eradication rate. Sequential therapy is one of the latest innovations, which was introduced by Zullo et al[6] in 2003. It entails the use of a PPI and amoxicillin for the first 5-7 d, followed by 5-7 d of PPI-clarithromycin-metronidazole (or tinidazole)[2,3]. With less clarithromycin resistance[3], the sequential regimen was more effective than standard triple therapy for H. pylori eradication[7,8]. However, some researchers argued that the benefit of sequential therapy only resulted from additional antibiotic therapy. Thus, it has been postulated that the four components of sequential therapy could be administered concurrently as concomitant therapy comprising PPI-clarithromycin-amoxicillin-metronidazole over several days[9]. The latest guideline recommends sequential and concomitant therapies as alternative first-line treatment in areas with a high rate of clarithromycin resistance[2].
Hybrid therapy entails administration of amoxicillin and a PPI for 5-7 d, followed by a PPI, amoxicillin, metronidazole, and clarithromycin for 5-7 d[10]. The recent randomized clinical trials (RCTs) of hybrid therapy showed conflicting results. Two studies showed that hybrid therapy outperformed sequential therapy in H. pylori eradication[11]. However, similar eradication rates were presented by other studies[12-14]. Furthermore, the duration of sequential or concomitant therapy was inconsistent between the studies. Therefore, we conducted this meta-analysis to evaluate the efficacy of hybrid therapy. We compared the efficacy, compliance, and safety of this new therapy with sequential or concomitant therapy.
MATERIALS AND METHODS
Search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement[15]. Two reviewers independently performed systematic literature search of PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception through October 2015. The search strategy is shown in Table 1. We used the following keywords or MESH Terms: “helicobacter pylori” or “H. pylori”, “hybrid” or “sequential-concomitant”. The language was limited to English. We also manually searched the references of eligible studies in case of any omission.
Table 1.
Characteristics of included studies involving hybrid therapy
| Ref. | Region | Design | No. of patients | Age, mean or range, yr | Men, % | Hybrid group | Control group | Confirmation of infection | Confirmation of eradication | Follow-up | Jadad score |
| Hsu et al[10] (2011) | Taiwan | Single-arm | 117 | 54 | 50 | E 40 mg + A 1g, bid, 7d; E 40 mg + A 1g + C 500 mg + M 500 mg, bid, 7d | NA | RUT, UBT, and histology | UBT | 8w | NA |
| Sardarian et al[25] (2012) | Iran | RCT | 420 | 43 | 48 | P 40 mg + A 1g, bid, 7d; P 40 mg + A 1 g + C 500mg + T 500 mg, bid, 7d | Sequential therapy (P 40 mg + A 1g, bid, 5d; P 40 mg + C 500mg + T 500 mg, bid, 5d) | RUT and/or histology | UBT | 8w | 3 |
| Molina-Infante et al[27] (2013) | Spain, Italy | RCT | 343 | 18-87 | 49 | O 40 mg + A 1g bid, 7d; O 20 mg +A 1 g + C 500 mg + N 500 mg, bid, 7d | Concomitant therapy (O 20 mg + A 1 g + C 500 mg + N 500 mg, bid, 14d) | UBT or any two of RUT, histology, or culture | UBT | 8w | 3 |
| Zullo et al[6] (2013) | Italy | RCT | 270 | 49 | 41 | O 40 mg + A 1 g bid, 7d; O 20 mg + A 1 g + C 500 mg + T 500 mg, bid, 7d | Concomitant therapy (O 20 mg + A 1 g + C 500 mg + T 500 mg, bid, 5d); sequential therapy (O 20 mg + A 1 g, bid, 5d; O 20 mg + C 500mg + T 500 mg, bid, 5d) | RUT and histology | UBT | 6w | 3 |
| Oh et al[13] (2014) | Korea | RCT | 184 | 57 | 37 | R 20mg + A 1g bid, 7d; R 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 7d | Sequential therapy (R 20 mg + A 1g, bid, 7d; R 20 mg + M 500 mg, bid, Mo 500 mg, qd, 7d) | RUT or histology | UBT | 6w | 3 |
| De Francesco et al[12] (2014) | Italy | RCT | 440 | 47 | 42 | O 20 mg + A 1g bid, 7d; O 20 mg + A 1 g + C 500 mg + T 500 mg, bid, 7d | Concomitant therapy (O 20 mg + A 1 g + C 500 mg + M 500 mg + T 500 mg, bid, 5d or 14d); sequential therapy (R 20 mg + A 1 g, bid, 7d; R 20 mg + M 500 mg, bid, Mo 500 mg, qd, 7d) | RUT+histology | UBT | 6-8w | 2 |
| Wu et al[23] (2014) | Taiwan | RCT | 220 | 53 | 49 | E 20 mg + A 1g bid, 3d; E 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 7d | Hybrid therapy (E 20 mg + A 1g bid, 5d/7d; E 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 7d) | RUT, UBT, histology, or culture | UBT or triple negative (RUT + histology + culture) | 8w | 3 |
| Cuadrado-Lavin et al[28] (2015) | Spain | RCT | 300 | 44 | 38 | O 20 mg + A 1 g bid, 5d; O 20 mg + A 1 g + C 500 mg + M 500 mg, bid,5d | Concomitant therapy (O 20 mg + A 1g + C 500 mg + M 500 mg, bid, 10d) | RUT, UBT, or histology | UBT | 4w | 3 |
| Heo et al[29] (2015) | Korea | RCT | 422 | 57 | 59 | E 20 mg + A 1g bid, 5d; E 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 5d | Concomitant therapy (E 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 10d) | Any two of UBT, histology, or RUT | UBT | 4w | 3 |
| Hwang et al[26] (2015) | Korea | RCT | 284 | 59 | 46 | R 20 mg + A 1g bid, 7d; R 20 mg + A 1g + C 500 mg + M 500 mg, bid, 7d | Sequential therapy (R 20 mg + A 1g, bid, 7d; R 20 mg + M 500 mg, bid, Mo 500 mg, qd, 7d) | UBT, histology, or RUT | UBT | 4w | 3 |
| Chen et al[11] (2015) | Taiwan | RCT | 175 | 53 | 37 | R 20 mg + A 1g bid, 7d; R 20 mg + A 1 g + C 500 mg + M 500 mg, bid, 7d | Sequential therapy (R 20 mg + A 1g, bid, 5d; R 20 mg + C 500 mg + M 500 mg, bid, 5d) | RUT + histology, culture | RUT + histology or UBT | 8w | 2 |
| Metanat et al[24] (2015) | Iran | RCT | 270 | 46 | 44 | P 40 mg + A 1g, bid, 5d; P 40 mg + A 1 g + C 500 mg + T 500 mg, bid, 5d | Sequential therapy (P 40 mg + A 1 g, bid, 7d; P 40 mg + A 1 g + C 500 mg + T 500 mg, bid, 7d) | RUT, histology | UBT | 8w | 2 |
A: Amoxicillin; C: Clarithromycin; E: Esomeprazole; M: Metronidazole; Mo: Moxifloxacin; N: Nitroimidazole; O: Omeprazole; P: Pantoprazole; R: Rabeprazole; RUT: Rapid urease test; T: Tinidazole; UBT: 13C-urea breath test.
Inclusion criteria
Studies meeting the following inclusion criteria were included in the meta-analysis: (1) comparison of hybrid therapy (proton-pump inhibitors and amoxicillin for 5 to 7 d, followed by proton-pump inhibitors, amoxicillin, clarithromycin, and metronidazole for another 5 to 7 d) with other treatment regimens (sequential therapy, concomitant therapy, or triple therapy) in patients with H. pylori infection, or comparing different durations of hybrid therapy; (2) randomized controlled trials (RCTs); (3) H. pylori infection was diagnosed with rapid urease test, 13C-urea breath test, histology, or culture; and (4) comparison of the eradication rate, compliance, and/or adverse events. The H. pylori eradication was assessed by UBT at least 4 wk after treatment.
Data extraction and quality assessment
Two authors independently abstracted the data using a standardized form. The following data were collected from each study: author and year, study design, country, sample size, gender, comparison arms, diagnosis of H. pylori, eradication of H. pylori, and follow-up. The quality of the included study was evaluated by the Jadad scale, which assessed the study quality by randomization (2 points), blinding (2 points), and attrition information (1 point)[16].
Statistical analysis
The effect size was calculated as the relative risk (RR) and the 95% confidential interval (CI) for each dichotomous outcome. The meta-analysis was conducted using the STATA software (StataCorp LP, College Station, TX, United States). The eradication rate, compliance rate and side effects rate were pooled by the Comprehensive Meta-Analysis statistical package (CMA Version 2.2, Biostat, Englewood, NJ, United States). The random-effects model using the DerSimonian and Laird method was employed for pooling the data because of suspected heterogeneity[17]. The heterogeneity was evaluated by the Cochran’s Q statistic (statistical significance defined as P < 0.05), and the I2 statistic (significant heterogeneity defined as I2 > 50%)[18]. Intention-to-treat (ITT) analysis was preferred to a per-protocol (PP) approach. The non-compliant patients or withdrawals were included in the ITT analysis to minimize bias[19]. Sensitivity analysis was performed by excluding the studies one by one. Subgroup analyses were conducted by stratifying the duration of therapy. The publication bias was assessed by the Egger’s test and the funnel plot. P < 0.05 was considered statistically significant.
RESULTS
Study selection
Our initial search identified 229 publications in total, including 108 articles from PubMed, 100 from Embase, and 21 from the CENTRAL database. Ninety-four duplicate publications were excluded. We discarded 81 irrelevant studies, 31 reviews or comments, and 10 conference abstracts. Fifteen records were eligible for full-text evaluation, of which one was a single-arm hybrid therapy study[20], and three were systematic reviews[4,21,22]. In the final meta-analysis, two studies compared different durations of hybrid therapy[23,24]. Six studies compared hybrid therapy with sequential therapy[11-14,25,26], and 5 studies compared hybrid therapy with concomitant therapy[12,14,27-29]. The selection process is shown in Figure 1. The characteristics of included studies are shown in Table 1. In the quality assessment, the blinding item was least fulfilled as no study used placebo or declared blinding to treatment regimen for patients or researchers. Except for three RCTs[11,12,24], most RCTs described the method of randomization. All studies clearly presented the follow-up data and conducted ITT analysis.
Figure 1.
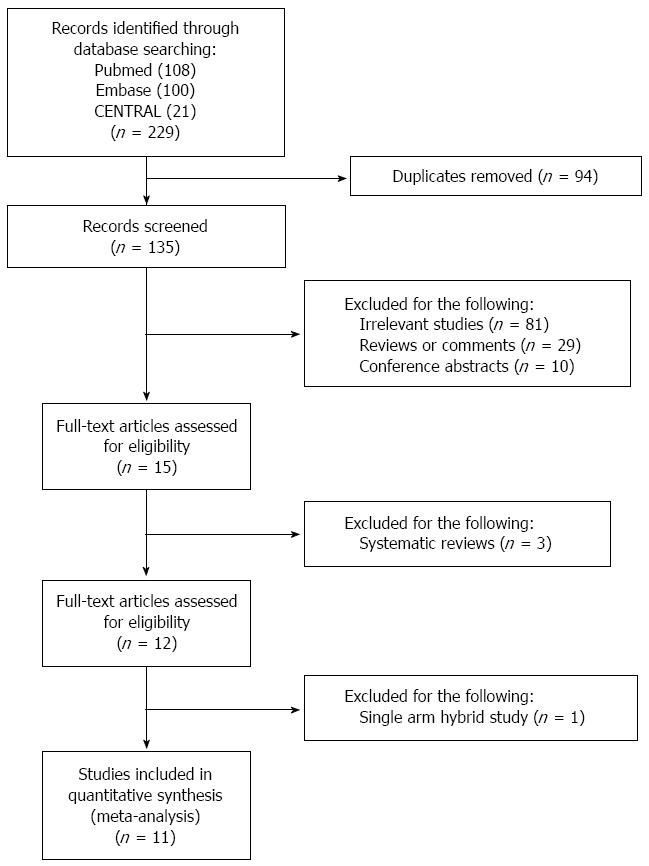
Study selection process of the meta-analysis.
Overall eradication rate of hybrid therapy
The eradication rate was reported in 12 studies. In PP analysis, the overall eradication rate was 91.2% (88.5%-93.4%), with significant heterogeneity (I2 = 63.9%, P < 0.05). In subgroup analyses, the pooled rate was 91.1% (87.4%-93.8%) for 10 studies using the 14-d regimen, and 90.3% (84.6%-94.1%) for 4 studies using the 10-d regimen (Figure 2A). In ITT analysis, the pooled eradication rate was 85.2% (82.1%-87.8%). For 10-d regimen (4 records) and 14-d regimen (10 records), the pooled rate was 82.2% (75.7%-87.2%) and 86.5% (82.6%-89.7%), respectively.
Figure 2.
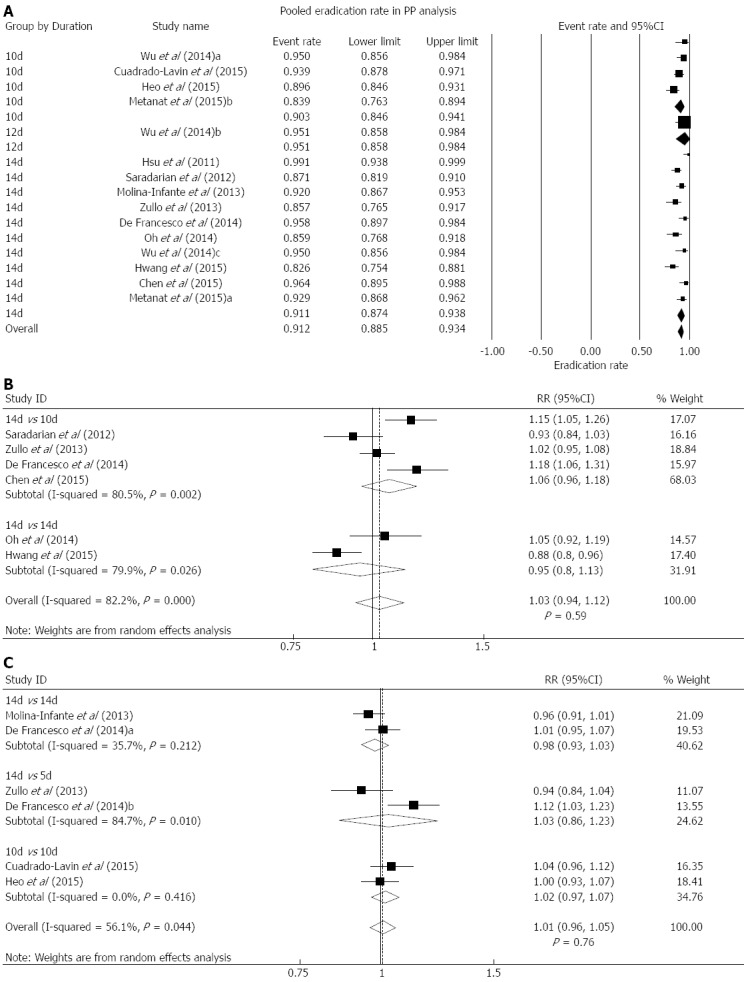
Per-protocol analysis. Forest plot showing the overall eradication rate of Helicobacter pylori (H. pylori) using hybrid therapy based on data from PP analysis. Subgroup analyses were conducted based on different durations of hybrid regimen. B: Forest plot comparing hybrid with sequential therapy in H. pylori eradication using data from PP analysis. Subgroup analyses were conducted based on different durations of sequential regimen. C: Forest plot comparing hybrid with concomitant therapy in H. pylori eradication using the data from PP analysis. Subgroup analyses were conducted based on different durations of concomitant regimen.
Different durations of hybrid therapy
Only two RCTs compared the hybrid therapies lasting 10 and 14 d, respectively[23,24]. In PP analysis, the 14-d regimen did not show significantly higher eradication rate compared with 10-d regimen (RR = 1.04, 95%CI: 0.92-1.18, P > 0.05). Significant heterogeneity was presented (I2 = 73.4%, P = 0.05). In ITT analysis, no significant superiority was found for the 14-d regimen compared with the 10-d regimen (RR = 1.08, 95%CI: 0.99-1.19, P > 0.05), without heterogeneity (I2 = 0%, P > 0.05).
Hybrid therapy vs sequential therapy
Eradication rate: Six studies were available[11-14,25,26]. Two Korean RCTs[13,26], and 2 Italian RCTs[12,14], were conducted by the same groups, during different study periods. In PP analysis, the eradication rate was 88.6% (95%CI: 83.6%-92.3%) for hybrid therapy and 87.8% (95%CI: 79.9%-92.9%) for sequential therapy. No statistically significant difference was found between the hybrid and sequential therapies, with significant heterogeneity (RR = 1.03, 95%CI: 0.94-1.12, P = 0.59; I2 = 82.2%, P < 0.05) (Figure 2B). In ITT analysis, the eradication rate was 84.3% (95%CI: 79.3%-88.2%) for hybrid therapy and 85.1% (95%CI: 78.4%-89.9%) for sequential therapy. No significant differences were seen with hybrid therapy compared with sequential therapy (RR = 1.00, 95%CI: 0.89-1.12, P = 0.94). Significant heterogeneity was found (I2 = 85.2%, P < 0.05) (Table 2).
Table 2.
Summary of meta-analyses: hybrid therapy vs sequential and concomitant therapy
| Outcomes | Studies, n | Hybrid group | Control group | RR (95%CI) | I2 | P value for heterogeneity |
| Hybrid vs sequential | ||||||
| Eradication rate (PP) | 6 | 88.6% | 87.8% | 1.03 (0.94-1.12) | 82.2% | < 0.05 |
| Eradication rate (ITT) | 6 | 84.3% | 85.1% | 1.00 (0.89-1.12) | 85.2% | < 0.05 |
| Compliance rate | 5 | 96.0% | 98.0% | 0.99 (0.96-1.02) | 50.4% | > 0.05 |
| Side effect rate | 6 | 30.3% | 28.2% | 1.05 (0.86-1.02) | 37.8% | > 0.05 |
| Hybrid vs concomitant | ||||||
| Eradication rate (PP) | 5 | 91.3% | 92.4% | 1.01 (0.96-1.05) | 56.1% | < 0.05 |
| Eradication rate (ITT) | 5 | 84.8% | 86.7% | 0.99 (0.95-1.03) | 0 | > 0.05 |
| Compliance rate | 4 | 95.8% | 93.2% | 1.03 (1.00-1.05) 1 | 0 | > 0.05 |
| Side effect rate | 4 | 39.5% | 44.2% | 0.93 (0.82-1.05) | 0 | > 0.05 |
Statistically significant results. ITT: Intention-to-treat; PP: Per-protocol.
Sensitivity analyses were carried out by excluding the studies one by one. Notably, no significant change was shown for PP or ITT results. Regarding sequential therapy, 4 studies used the 10-d regimen[11,12,14,25], and 2 studies used the 14-d regimen[13,26]. Based on the different durations, subgroup analysis of PP data did not find statistically significant changes for the 10-d regimen (RR = 1.06, 95%CI: 0.96-1.18) or for the 14-d regimen (RR = 0.95, 95%CI: 0.80-1.13) (Figure 2B). Similarly, subgroup analysis of ITT data revealed no significant alteration for the 10-d regimen (RR = 1.03, 95%CI: 0.88-1.20) or for the 14-d regimen (RR = 0.93, 95%CI: 0.79-1.09).
Compliance: Five studies evaluated the compliance[11,13,14,25,26]. Both therapies displayed a high compliance rate [96% (95%CI: 93%-98%)] for hybrid therapy, and 98% (95%CI: 95%-99%) for sequential therapy. No significant difference was observed (RR = 0.99, 95%CI: 0.96-1.02, P = 0.55; I2 = 50.4%, P > 0.05) (Table 2).
Side effects: The overall adverse effect rate was 30.3% (95%CI: 20.9%-41.6%) for the hybrid therapy, and 28.2% (95%CI: 15.7%-45.4%) for the sequential therapy. The hybrid therapy did not show significantly lower incidence of adverse effect (RR = 1.05, 95%CI: 0.86-1.02, P = 0.63). No significant heterogeneity was observed (I2 = 37.8%, P > 0.05) (Table 2).
Hybrid therapy vs concomitant therapy
Eradication rate: Five studies were available[12,14,27-29]. In PP analysis, the eradication rate of hybrid and concomitant regimen was 91.3% (95%CI: 87.7%-93.9%) and 92.4% (95%CI: 89.2%-94.7%), respectively. In ITT analysis, the eradication rate of hybrid and concomitant regimen was 84.8% (95%CI: 78.9%-89.2%) and 86.7% (95%CI: 80.7%-91.0%), respectively. In PP analysis, no statistically significant difference was observed between hybrid therapy and concomitant therapy (RR = 1.01, 95%CI: 0.96-1.05, P = 0.76; I2 = 56.1%, P < 0.05) (Figure 2C). In ITT analysis, no significant difference was found between the two regimens, and no heterogeneity was observed (RR = 0.99, 95%CI: 0.95-1.03, P = 0.47; I2 = 0%, P > 0.05) (Table 2).
In sensitivity analysis by excluding studies one by one, no significant change was seen in PP or ITT analysis. For concomitant therapy, two studies presented results of the 14-d regimen[12,27], 2 of the 10-d regimen[28,29], and 2 of the 5-d regimen[12,14]. Subgroup analyses based on different durations of concomitant therapy revealed no significant difference.
Compliance: Four studies were relevant[14,27-29]. The compliance rate was 95.8% (95%CI: 93.2%-97.4%) for hybrid therapy, and 93.2% (95%CI: 89.7%-95.6%) for concomitant therapy. Patients receiving hybrid therapy showed significantly higher rate of compliance when compared with concomitant therapy (RR = 1.03, 95%CI: 1.00-1.05, P < 0.05). No heterogeneity was revealed (I2 = 0%, P > 0.05) (Table 2).
Side effects: Four studies were included[12,14,27,28]. The overall side effect rate was 39.5% (95%CI: 21.7%-60.7%) for hybrid therapy, and was 44.2% (95%CI: 26.7%-63.2%) for concomitant therapy. No significant difference was seen between hybrid therapy and concomitant therapy (RR = 0.93, 95%CI: 0.82-1.05, P = 0.24). No heterogeneity was observed (I2 = 0%, P > 0.05) (Table 2).
Publication bias
Publication bias was representatively evaluated for PP data. For hybrid vs sequential therapy, the funnel plot was symmetrical, with a non-significant result in Egger’s test (P = 0.74) (Figure 3A). In hybrid vs concomitant therapy, the funnel plot was symmetrical (Figure 3B). No statistical significance was revealed by Egger’s test (P = 0.48).
Figure 3.
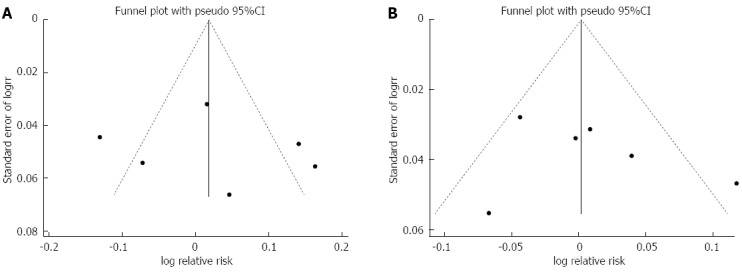
Publication bias. A: Funnel plot of studies comparing hybrid with sequential therapy; B: Funnel plot of studies comparing hybrid with concomitant therapy.
DISCUSSION
Eradication rate plays a pivotal role in evaluating the success of H. pylori treatment. The efficacy of H. pylori eradication was graded as follows: (1) excellent (> 95%); (2) good (90-95%); (3) fair (85-89%); (4) bad (81%-84%); and (5) unacceptable (< 80%)[30]. In ITT and PP analyses, therapeutic significance was achieved when the eradication rates exceeded 80% and 90%, respectively[26]. In this meta-analysis, hybrid therapy yielded a good eradication rate (91%) in PP analysis, and fair (85%) in ITT analysis, both exhibiting significant therapeutic values. The pooled data showed similar treatment success (an eradication rate closer to 90%) with hybrid, sequential, and concomitant therapies against H. pylori. Hybrid therapy had good compliance to medications, which was similar to sequential therapy and slightly better than concomitant therapy. The differences in adverse event rates were small between hybrid, sequential, and concomitant therapies. All the three therapies showed acceptable safety profile. The 10-d hybrid regimen did not show significant inferiority with respect to the eradication rate. Meta-analyses have shown that the eradication outcome was duration dependent[9]. However, the differences in eradication rate across all subgroups stratified by duration were minimal.
Currently, in the absence of any new drugs against H. pylori, different combination regimens, including sequential, concomitant, and hybrid therapies, have been investigated extensively. Hybrid therapy evolved from sequential therapy and concomitant therapy. Compared with sequential therapy, hybrid therapy extended the duration of amoxicillin. Prolonging the duration of traditional triple therapy from 7 to 10-14 d improved the eradication success rate by approximately 5%[2]. The prescription of PPI and amoxicillin was similar for concomitant and hybrid therapies. However, clarithromycin and metronidazole were used over a shorter duration of hybrid therapy. The adverse effects of metronidazole included nausea and regurgitation. Furthermore, both metronidazole and clarithromycin may cause bitter tastes[29]. With decreased pill burden, hybrid therapy was superior in cost-effectiveness over concomitant therapy.
The participants included in the RCTs were residents of Taiwan, Iran, Italy, Spain, and Korea, which represent regions with a high prevalence of antibiotic-resistant H. pylori strains[5,11]. Worldwide increase of H. pylori resistance to antibiotics, especially clarithromycin and metronidazole, is the most important determinant of eradication failure in traditional triple therapy[31]. For sequential therapy, the eradication rate of clarithromycin-resistant and metronidazole-resistant strains was 72.8% and 86.4%, respectively. However, the rate decreased to just 37% for dual-resistant strains[32]. Concomitant regimen outperformed sequential regimen in areas with a high incidence of clarithromycin and/or metronidazole resistance[33,34]. However, eradication was expected to fail (< 90%) when the prevalence of dual clarithromycin-metronidazole resistant strains was > 15%[34]. Compared with concomitant therapy, hybrid therapy initially prescribed amoxicillin, which may prevent the occurrence of secondary clarithromycin resistance[35,36]. Compared with sequential therapy, hybrid regimen extended the duration of amoxicillin exposure. Hybrid therapy combined the advantages of sequential and concomitant therapy. Unfortunately, very few studies conducted antimicrobial susceptibility testing before hybrid treatment. Chen et al[11] showed that sequential therapy resulted in a 71.4% (5/7) eradication rate in patients harboring strains with dual resistance. Hybrid therapy yielded a 100% (4/4) eradication rate. Molina-Infante et al[34] revealed that for clarithromycin-resistant and dual-resistant strains, the concomitant regimen resulted in a 100% (8/8 and 3/3, respectively) eradication rate. By constrast, hybrid therapy only achieved a rate of 75% (6/8) and 33% (1/3), respectively. Nevertheless, the very small number of patients with resistant strains precluded definite conclusions.
Our meta-analysis represented the most comprehensive review of hybrid therapy and an update of two similar meta-analyses[21,22]. Notably, five studies have only recently been published, which were not included in previous meta-analyses[11,24,26,28,29]. The number of studies for meta-analysis doubled that of the previous studies, generating more robust conclusions, albeit with similar non-significant results between different regimens. Additionally, it was the first time that hybrid therapy was compared with different durations of sequential or concomitant therapy. The overall eradication rate with durations of hybrid therapy was demonstrated.
This study had several limitations. The number of included trials was still small, and the sample size was not large enough for the majority of studies. For example, although we did not detect the impact of different durations of sequential or concomitant therapy, the results should be extrapolated with caution as only few studies were included. Most RCTs did not report blinding to treatment regimen. Lack of blinding may influence compliance and the reporting of side effects. The quality of included studies was low. The majority of studies did not conduct susceptibility tests to determine antibiotic resistance[12,25,28]. In fact, we have tried to assess the eradication efficacy in resistant strains. However, we had insufficient related data and very small sample sizes of resistant patients. A number of confounding factors may play a role in determining the H. pylori eradication rates. Except for the disparity between different regions regarding the prevalence of resistant strains, the rates were influenced by genetic differences in the PPIs metabolism, degree of gastritis, administration of probiotics, and the nature of the underlying disease[37]. Additionally, different types of PPIs and nitroimidazole medications, and varying duration of follow-up may potentially generate small amounts of bias[27,28]. Participation in an RCT enhanced the patient compliance, and the compliance gap between hybrid therapy and other treatment regimens might be wider in clinical practice[29].
In conclusion, hybrid therapy yielded good eradication efficacy for H. pylori in regions with a high prevalence of antibiotic-resistant strains. Hybrid regimens achieved equivalent eradication rates compared with sequential or concomitant therapy. The compliance and adverse events were not different between hybrid, sequential or concomitant therapies. The 14-d and 10-d hybrid therapy showed similar eradication rates. Further studies are urgently required to clarify important differences in eradication of H. pylori in the setting of varying patterns of antibiotic resistance.
COMMENTS
Background
Previous trials reported inconsistent results regarding the efficacy, compliance rate and adverse events following the use of hybrid therapy when compared with traditional sequential therapy and concomitant therapy for the eradication of H. pylori.
Research frontiers
The emerging resistance of H. pylori strains is the major cause of treatment failure. Hybrid therapy represented a renewal of sequential therapy and concomitant therapy, and the efficacy and safety of hybrid therapy need to be investigated.
Innovations and breakthroughs
Our meta-analysis represented the most comprehensive review of hybrid therapy and an update of two similar meta-analyses. The authors for the first time, compared hybrid therapy following different durations of sequential or concomitant therapy. They also compared the different durations of hybrid therapy, and demonstrated the overall eradication rate of hybrid therapy.
Applications
Hybrid therapy showed a good eradication rate, high compliance rate, and acceptable safety profiles compared with traditional sequential therapy and concomitant therapy. These findings may represent a future strategy for the treatment of patients with H. pylori infection.
Peer-review
The study is a meta-analysis comparing hybrid therapy with traditional sequential therapy and concomitant therapy against H. pylori infection. The present manuscript included 5 additional studies published in 2015 and therefore strengthens the outcomes of previous meta-analyses.
Footnotes
Supported by National Science and Technology Pillar Program of 12th Five-Year Plan in China, No. 2012BAI06B02; Clinical Key Projects of Peking University Third Hospital, No. Y76493-03; Key Laboratory for Helicobacter pylori Infection and Upper Gastrointestinal Diseases in Beijing, No. BZ0371.
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 10, 2015
First decision: December 30, 2015
Article in press: January 30, 2016
P- Reviewer: Altun B, Kato K S- Editor: Qi Y L- Editor: Ma JY E- Editor: Zhang DN
References
- 1.Yazbek PB, Trindade AB, Chin CM, Dos Santos JL. Challenges to the Treatment and New Perspectives for the Eradication of Helicobacter pylori. Dig Dis Sci. 2015;60:2901–2912. doi: 10.1007/s10620-015-3712-y. [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 3.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 4.Li BZ, Threapleton DE, Wang JY, Xu JM, Yuan JQ, Zhang C, Li P, Ye QL, Guo B, Mao C, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ. 2015;351:h4052. doi: 10.1136/bmj.h4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 6.Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, De Francesco V, Menegatti M, Tampieri A, Perna F, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17:719–726. doi: 10.1046/j.1365-2036.2003.01461.x. [DOI] [PubMed] [Google Scholar]
- 7.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079; quiz 1080. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Ji JS, Choi H, Kim JH. Sequential therapy or triple therapy for Helicobacter pylori infection in Asians: systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:118–125. doi: 10.1016/j.clinre.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011;34:604–617. doi: 10.1111/j.1365-2036.2011.04770.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011;16:146–152. doi: 10.1111/j.1523-5378.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen KY, Lin TJ, Lin CL, Lee HC, Wang CK, Wu DC. Hybrid vs sequential therapy for eradication of Helicobacter pylori in Taiwan: A prospective randomized trial. World J Gastroenterol. 2015;21:10435–10442. doi: 10.3748/wjg.v21.i36.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 2014;63:748–752. doi: 10.1099/jmm.0.072322-0. [DOI] [PubMed] [Google Scholar]
- 13.Oh DH, Lee DH, Kang KK, Park YS, Shin CM, Kim N, Yoon H, Hwang JH, Jeoung SH, Kim JW, et al. Efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014;29:1171–1176. doi: 10.1111/jgh.12518. [DOI] [PubMed] [Google Scholar]
- 14.Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D’Ambrosio P, Castorani L, Bonfrate L, Vannella L, Hassan C, Portincasa P. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013;37:647–650. doi: 10.1016/j.clinre.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139–145. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, Deng T, Luo H. Meta-analysis of sequential, concomitant and hybrid therapy for Helicobacter pylori eradication. Intern Med. 2015;54:703–710. doi: 10.2169/internalmedicine.54.3442. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Wang YH, Lv ZF, Xiong HF, Wang H, Yang Y, Xie Y. Review: efficacy and safety of hybrid therapy for Helicobacter pylori infection: a systematic review and meta-analysis. Helicobacter. 2015;20:79–88. doi: 10.1111/hel.12180. [DOI] [PubMed] [Google Scholar]
- 23.Wu JY, Hsu PI, Wu DC, Graham DY, Wang WM. Feasibility of shortening 14-day hybrid therapy while maintaining an excellent Helicobacter pylori eradication rate. Helicobacter. 2014;19:207–213. doi: 10.1111/hel.12113. [DOI] [PubMed] [Google Scholar]
- 24.Metanat HA, Valizadeh SM, Fakheri H, Maleki I, Taghvaei T, Hosseini V, Bari Z. Comparison Between 10- and 14-Day Hybrid Regimens for Helicobacter pylori Eradication: A Randomized Clinical Trial. Helicobacter. 2015;20:299–304. doi: 10.1111/hel.12202. [DOI] [PubMed] [Google Scholar]
- 25.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129–134. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 26.Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. Efficacy of moxifloxacin-based sequential and hybrid therapy for first-line Helicobacter pylori eradication. World J Gastroenterol. 2015;21:10234–10241. doi: 10.3748/wjg.v21.i35.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121–128.e1. doi: 10.1053/j.gastro.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Cuadrado-Lavín A, Salcines-Caviedes JR, Diaz-Perez A, Carrascosa MF, Ochagavía M, Fernandez-Forcelledo JL, Cobo M, Fernández-Gil P, Ayestarán B, Sánchez B, et al. First-line eradication rates comparing two shortened non-bismuth quadruple regimens against Helicobacter pylori: an open-label, randomized, multicentre clinical trial. J Antimicrob Chemother. 2015;70:2376–2381. doi: 10.1093/jac/dkv089. [DOI] [PubMed] [Google Scholar]
- 29.Heo J, Jeon SW, Jung JT, Kwon JG, Lee DW, Kim HS, Yang CH, Park JB, Park KS, Cho KB, et al. Concomitant and hybrid therapy for Helicobacter pylori infection: A randomized clinical trial. J Gastroenterol Hepatol. 2015;30:1361–1366. doi: 10.1111/jgh.12983. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 31.Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: meeting the challenge of antimicrobial resistance. World J Gastroenterol. 2014;20:9898–9911. doi: 10.3748/wjg.v20.i29.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. doi: 10.1136/bmj.f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Park SM, Kim BW. Sequential or concomitant therapy for eradication of Helicobacter pylori infection: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2015;30:1338–1345. doi: 10.1111/jgh.12984. [DOI] [PubMed] [Google Scholar]
- 34.Molina-Infante J, Gisbert JP. Optimizing clarithromycin-containing therapy for Helicobacter pylori in the era of antibiotic resistance. World J Gastroenterol. 2014;20:10338–10347. doi: 10.3748/wjg.v20.i30.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002;19:67–70. doi: 10.1016/s0924-8579(01)00456-3. [DOI] [PubMed] [Google Scholar]
- 37.Vakil N. Are there geographical and regional differences in Helicobacter pylori eradication? Can J Gastroenterol. 2003;17 Suppl B:30B–32B. doi: 10.1155/2003/903494. [DOI] [PubMed] [Google Scholar]


