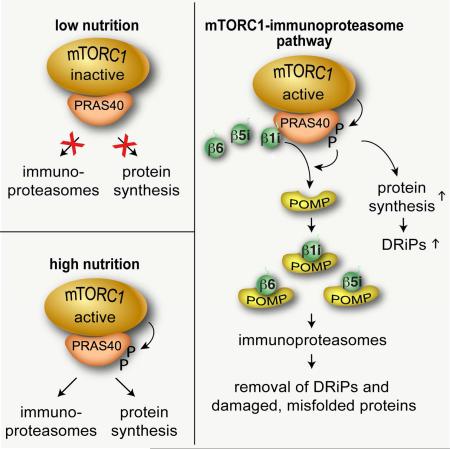
SUMMARY
Reduction of translational fidelity often occurs in cells with high rates of protein synthesis, generating defective ribosomal products. If not removed, such aberrant proteins can be a major source of cellular stress causing human diseases. Here, we demonstrate that mTORC1 promotes the formation of immunoproteasomes for efficient turnover of defective proteins and cell survival. mTORC1 sequesters precursors of immunoproteasome β subunits via PRAS40. When activated, mTORC1 phosphorylates PRAS40 to enhance protein synthesis and simultaneously facilitate the assembly of the β subunits for forming immunoproteasomes. Consequently, the PRAS40 phosphorylations play crucial roles in clearing aberrant proteins that accumulate due to mTORC1 activation. Mutations of RAS, PTEN, and TSC1, which cause mTORC1 hyperactivation, enhance immunoproteasome formation in cells and tissues. Those mutations increase cellular dependence on immunoproteasomes for stress response and survival. These results define a mechanism by which mTORC1 couples elevated protein synthesis with immunoproteasome biogenesis to protect cells against protein stress.
Graphical Abstract
INTRODUCTION
Protein stress, such as misfolded protein aggregates and oxidized proteins, occurs in many human diseases, including cancer, diabetes, neurodegeneration, and age-related pathologies. One major source of protein stress originates when the ribosomal protein synthesis is upregulated with a consequential reduction of translational fidelity, producing defective ribosomal products. Prevalent in many cancers, mutations of PTEN (phosphatase and tensin homolog), RAS, TSC1 (tuberous sclerosis complex 1) or TSC2 commonly enhance the activity of mTORC1 (mechanistic target of rapamycin complex 1), the central regulator of the ribosomal protein synthesis (Hara et al., 2002; Kim et al., 2002; Loewith et al., 2002). Hyperactivation of mTORC1 can reduce translational fidelity as a result of increased rates of ribosomal elongation (Conn and Qian, 2013). The resulting aberrant proteins, if not properly removed, can cause cell death or contribute to human diseases.
Eukaryotic cells have two major degradation systems to remove aberrant proteins: the autophagy-lysosome system (ALS) and the ubiquitin-proteasome system (UPS). The ALS is responsible for bulk degradation of cytosolic proteins and protein aggregates, whereas the UPS is responsible for degradation of individual proteins that are poly-ubiquitinated, misfolded, or oxidized. The UPS plays crucial roles not only for clearance of aberrant proteins but also for control of many cellular processes, such as cell cycle progression, transcription, and apoptosis. The UPS is frequently upregulated in cancer cells, and its impaired regulation can lead to accumulation of protein aggregates and cell death. Within the UPS machinery, the 26S proteasome is responsible for the proteolytic cleavages of poly-ubiquitinated (poly-Ub) proteins. The 26S proteasome consists of the 20S catalytic core and the 19S regulatory particle. Many proteasome subunit genes are transcriptionally regulated by Nrf1 (nuclear factor, erythroid 2 like 1, gene NFE2L1) (Radhakrishnan et al., 2010; Sha and Goldberg, 2014; Steffen et al., 2010), and mTORC1 was shown to regulate expression of some proteasomal genes via Nrf1 (Zhang et al., 2014).
Conserved throughout eukaryotes, the 20S core contains four heptameric rings of α and β subunits that form two outer and two inner rings, respectively (Figure 1A). In addition to the seven β subunits expressed constitutively in most tissues and cell types that form constitutive proteasomes (c-proteasomes), vertebrates also express three β subunits – β1i (LMP2), β2i (MECL1) and β5i (LMP7) – that replace the c-proteasomal β1, β2 and β5 to form immunoproteasomes (i-proteasomes, the term used here for any type of proteasomes that contains at least one of the three i-proteasome-specific β (iβ) subunits). Some subtypes of i-proteasomes contain only one or two of the iβ subunits (Vigneron and Van den Eynde, 2012). I-proteasomes are most highly expressed in immune cells but also occur in many other cell types. About 30-50% of all the proteasomes in normal human liver, kidney and gut are i-proteasomes (Guillaume et al., 2010). I-proteasomes produce antigenic peptides presented by the major histocompatibility complex class I molecules, and play roles in protein stress clearance, differentiation and wound healing (Cui et al., 2014; Ferrington et al., 2013; Seifert et al., 2010).
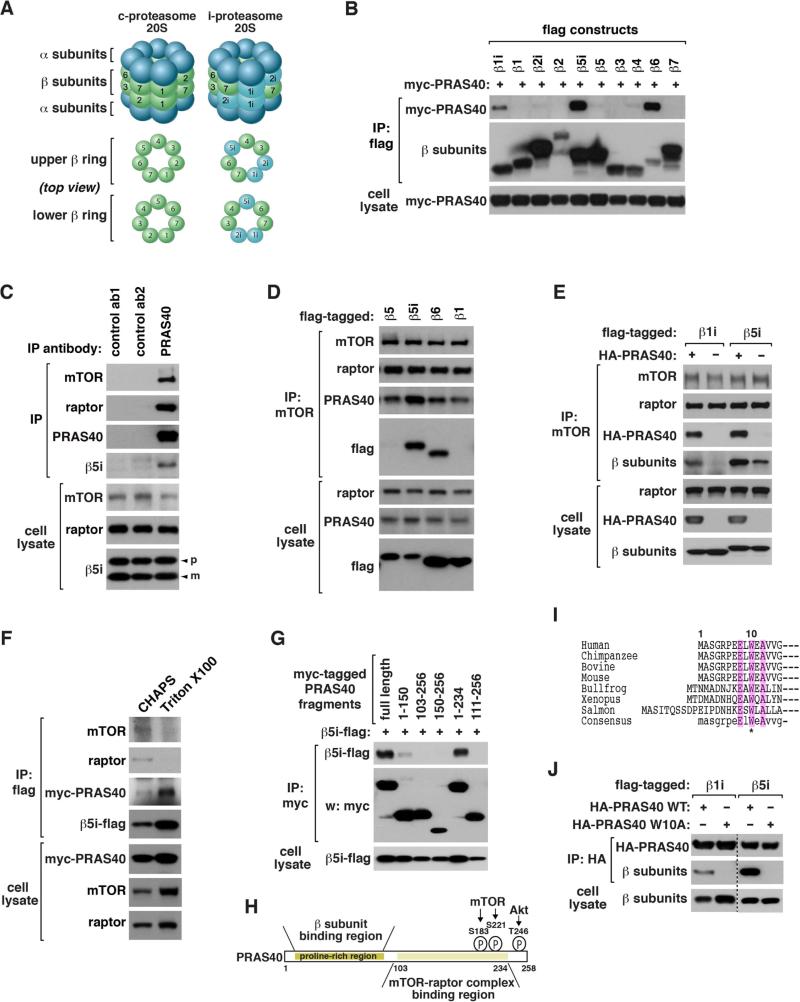
Figure 1. mTORC1 binds to i-proteasomal β1i, β5i and β6.
(A) Comparison of c-proteasome and i-proteasome 20S.
(B) β1i, β5i and β6 bind to PRAS40. C-terminal flag-tagged β subunits were expressed with myc-PRAS40 in HEK293T cells. Myc-PRAS40 recovered with flag immunoprecipitates was analyzed by western blotting (WB).
(C) Endogenous PRAS40 interacts with β5i. An untagged form of β5i was expressed in HEK293T cells. IP was conducted using antibodies for PRAS40, insulin receptor β (ab1) or GAPDH (ab2). “p” and “m” indicate the precursor and mature forms, respectively.
(D) Endogenous mTOR interacts with β5i and β6. C-terminal flag-tagged β subunits were expressed in HEK293T cells.
(E) PRAS40 enhances binding of mTOR to β1i or β5i. Flag-tagged β1i or β5i was expressed with or without HA-PRAS40 in HEK293T cells.
(F) Triton X100 does not disrupt the PRAS40-β5i interaction. Flag-β5i and myc-PRAS40 were transiently expressed in HEK293T cells. IP was conducted in the presence of CHAPS or Triton X100.
(G) β5i binds to an N-terminal region of PRAS40. The indicated constructs were expressed in HEK293T cells.
(H) PRAS40 uses two separate regions for binding to iβ subunits and mTOR/raptor. mTOR and Akt target sites are located near the C-terminus.
(I) N-terminal sequences of PRAS40 from different species.
(J) W10A disrupts binding of PRAS40 to β1i and β5i. Co-IP was conducted using proteins expressed in HEK293T cells.
See also Figure S1.
In this study, we demonstrate that mTORC1 promotes the assembly of i-proteasomes, adapting cells to protein stress. This finding defines a pathway through which mTORC1 regulates the inducible proteolytic degradation machinery to prevent accumulation of protein stress.
RESULTS
mTORC1 binds to β1i, β5i and β6 via PRAS40
PRAS40 (proline-rich Akt substrate 40 kDa; also called Akt1s1, Akt1 substrate 1) is a component of mTORC1 whose functions remain unclear. Through a yeast two-hybrid screen, we identified β6 as a binding protein of PRAS40 (Figure S1A). We confirmed that PRAS40 interacts with β6 in HEK293T cells by co-immunoprecipitation (co-IP) assay (Figures S1B and S1C). Considering the similar structures of the proteasomal β subunits (Figure S1D), we tested if other β subunits interact with PRAS40. We found that PRAS40 also binds to β1i and β5i as well as β5t of the thymoproteasomes (Figures 1B, S1E, and S1F). We confirmed that endogenous PRAS40 interacts with β6 and β5i (Figures 1C, S1G, and S1H).
We asked if the interaction involves other components of mTORC1. β5i and β6 were co-immunoprecipitated with mTOR (Figure 1D), and endogenous raptor was co-immunoprecipitated with β5i and β6 (Figure S1I). PRAS40 overexpression enhanced binding of mTOR and raptor to β1i, β5i and β6 (Figures 1E and S1J-L), whereas PRAS40 knockdown (KD) reduced binding of mTOR and raptor to β5i (Figures S1M and S1N). The PRAS40-β5i interaction still occurred in the presence of Triton X100 (Figure 1F), the detergent that disrupts the PRAS40-mTOR/raptor interaction (Vander Haar et al., 2007), indicating that PRAS40 can bind to β5i without mTOR and raptor. Delimitation analysis revealed a region of PRAS40 within residues 1-102 important for interaction with β5i and β6 (Figures 1G, 1H, and S1O). Mutation of a conserved residue Trp10 to alanine (W10A) abolished the interaction of PRAS40 with β1i, β5i and β6 without affecting the interaction with raptor (Figures 1I, 1J, and S1P-R). Since mTOR and raptor bind to a C-terminal region of PRAS40 (Vander Haar et al., 2007), PRAS40 likely uses two separate regions for binding to the β subunits and mTOR/raptor. However, the weaker binding affinity of the N-terminal half compared to the full length indicates that C-terminal residues influence the interaction with the β subunits.
PRAS40 binds to β1i, β5i and β6 precursors and suppresses i-proteasome formation
All the proteasome β subunits, except β3 and β4, are synthesized as precursors containing N-terminal pro-regions. The pro-regions are cleaved off at the completion of 20S formation by three catalytic subunits (β1, β2 and β5 for c-proteasomes; β1i, β2i and β5i for i-proteasomes). According to the molecular sizes, it was the precursor form of β5i that binds to mTORC1 (Figures 1C and 2A). Using a C-terminal flag-tagged β5i construct, we confirmed that PRAS40 binds to β5i precursor but not its mature form (Figure 2B). PRAS40 interacted with β6 or β5i precursor only when coexpressed but not expressed separately in HEK293T cells (Figures 2C). The separately-expressed PRAS40 was intact in the structural integrity, as it could bind to raptor. We confirmed that other components of mTORC1 are not required for the PRAS40-β5i interaction using proteins expressed in E. coli (Figures 2D and S2A).
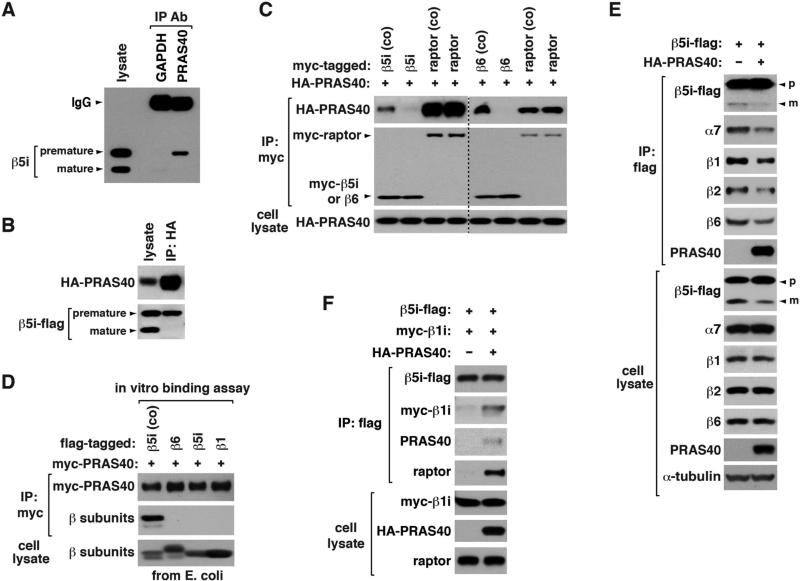
Figure 2. PRAS40 binds to iβ precursors during their folding and suppresses de novo assembly of the 20S core.
(A) Endogenous PRAS40 binds to β5i precursor. An untagged form of β5i was expressed in HEK293T cells, and its binding to PRAS40 was analyzed by co-IP. GAPDH IP was analyzed as control.
(B) β5i-flag was expressed with HA-PRAS40 in HEK293T cells.
(C) PRAS40 binds to β5i and β6 precursors during their folding. For coexpression (co), myc-tagged β subunit or raptor was coexpressed with HA-PRAS40 in HEK293T cells. Otherwise, myc-tagged construct was expressed in HEK293T cells then combined with cell lysate containing HA-PRAS40.
(D) PRAS40 can interact with β5i precursor without other components of mTORC1. For coexpression (co), β5i-flag was expressed with myc-PRAS40 in E. coli. Otherwise, flag-tagged β subunits were expressed in E. coli then combined with E. coli extracts containing myc-PRAS40.
(E) PRAS40 suppresses de novo biogenesis of proteasomes. β5i-flag was expressed with or without HA-PRAS40 in HEK293T cells. Proteasomal components associated with β5i-flag were analyzed by WB.
(F) PRAS40 enhances binding of β5i to β1i. Proteins were transiently expressed in HEK293T cells.
See also Figure S2.
Knowing that the β precursors bind to PRAS40, we predicted that the interaction might influence the ability of the precursors to form proteasomes. Coexpression of PRAS40 with β5i-flag or β6-flag in HEK293T cells suppressed de novo assembly of proteasomes containing the recombinant β subunits (Figures 2E and S2B). PRAS40 induced interactions between β1i, β5i, and β6 precursors (Figures 2F, S2C, and S2D). Given that PRAS40 coexpression suppressed the formation of proteasomes, the interactions between the iβ precursors induced by PRAS40 might form an off-pathway complex that does not go into the i-proteasome assembly machinery.
mTORC1 promotes i-proteasome formation
We asked if mTORC1 activity has any effect on the interaction between PRAS40 and iβ precursors. Torin1 suppressed PRAS40 S183 phosphorylation and increased the PRAS40-β5i interaction within 1 h (Figure 3A). Overexpression of Rheb, a positive regulator of mTORC1, had the opposite effect (Figure 3B). In addition, Rheb overexpression increased the mature form of β5i. To further clarify if mTORC1 promotes iβ maturation, we tested the effect of knockout (KO) of TSC1, a negative regulator of mTORC1, in mouse embryonic fibroblasts (MEFs). Since MEFs do not express iβ subunits in basal conditions, we treated the cells with interferon γ (IFNγ) to induce iβ expression. TSC1 KO increased the mature forms of β5i and β1i and the mature-to-precursor ratio for β1i in MEFs (Figure 3C). β2i, whose maturation depends on β1i (Groettrup et al., 1997), showed a similar change as β1i. Such increases were not observed with c-proteasome β (cβ) subunits. Since the mature forms can be generated only when the β subunits form the 20S core (Chen and Hochstrasser, 1996; Murata et al., 2009), the cleaved forms may reflect the 20S core assembly.
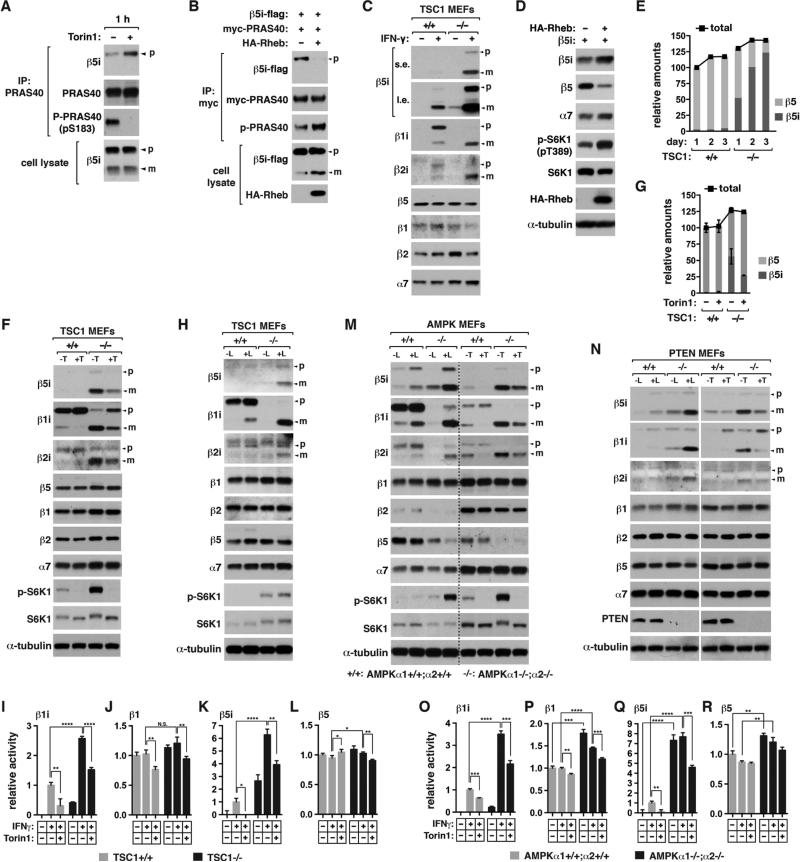
Figure 3. mTORC1 promotes the formation of i-proteasomes.
(A) Torin1 enhances the PRAS40-β5i interaction. HEK293T cells expressing an untagged form of β5i was treated with Torin1 for 1 h.
(B) Rheb overexpression suppresses the PRAS40-β5i interaction and increases the mature form of β5i in HEK293T cells.
(C) TSC1 KO increases the mature forms of iβ subunits. TSC1 MEFs were treated with IFNγ or vehicle (−) for 16 h. Cell lysate was analyzed by WB.
(D) Rheb overexpression increases the mature form of β5i along with reduction of β5. Rheb was transiently expressed with β5i in HEK293T cells for 48 h.
(E) TSC1 MEFs were treated with IFNγ for the indicated days. The relative amounts of β5i and β5 were quantified using purified proteasomes as standard (see Figure S3C).
(F) Torin1 suppresses the maturation of iβ subunits. MEFs were treated with IFNγ in the presence or absence of Torin1 (T) for 16 h.
(G) Quantification of the precursor and mature forms of β5i from (F). Values are means ± SD.
(H) Leucine is necessary for iβ subunit maturaton. TSC1 MEFs were treated with IFNγ in the presence or absence of leucine (L) for 16 h.
(I-L) Proteolytic activities of i-proteasomes and c-proteasomes in TSC1 MEFs. Cells were treated with IFNγ in the presence or absence of Torin1 for 24 h. Fluorogenic peptides were used to detect the specific activities of β1i, β1, β5i, and β5.
(M, N) Deficiency of AMPK (M) or PTEN (N) enhances the mature forms of iβ subunits, which is reversed by leucine deprivation or Torin1. MEFs were treated with IFNγ in the presence or absence of leucine or Torin1 for 16 h.
(O-R) Proteolytic activities of i-proteasomes and c-proteasomes in AMPK MEFs. Final concentrations of IFNγ, Torin1 and leucine weret 50 ng/ml, 250 nM, and 52 μg/ml, respectively.
For I-L and O-R, values are means ± SD (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; N.S., not significant; n=3).
See also Figure S3.
To exclude a possibility that the effects of TSC1 KO involve any IFNγ-induced factor, we used T24 bladder cells that express iβ subunits in basal conditions. TSC1 KD in T24 cells greatly increased the mature forms of β5i and β1i (Figure S3A). TSC1 KD reduced the β5 level but not the β1 level, indicating that a large amount of β5i interacted with β1 in the KD cells. This reflects the ability of β5i to assemble with either β1i or β1 (Guillaume et al., 2010). The effects of TSC1 KD were mimicked by transient or stable overexpression of Rheb in HEK293T cells (Figures 3D and S3B). Using purified proteasomes as standard, we quantified that about 85% of total proteasomes contained β5i in TSC1−/− MEFs at day 3 of IFNγ treatment compared to only 2% in TSC1+/+ MEFs (Figures 3E and S3C). TSC1 KO also increased the mature forms of β1i and β2i, although the increase was less extensive than that of β5i (Figures S3D and S3E).
mTORC1 inhibition suppresses i-proteasome formation
Torin1 or leucine deprivation, which inhibits mTORC1, drastically reduced the mature iβ subunits in TSC1−/− MEFs without affecting cβ subunits (Figures 3F-H and S3F-I). We analyzed β5i processing by monitoring changes in the amounts of its precursor and mature forms after protein synthesis was blocked. β5i precursor transiently expressed in HEK293 cells was processed to the mature form with a rate of ~0.0833 h−1, which was completely blocked by Torin1 (Figures S3J and S3K). Rapamycin did not show such a suppressive effect but interestingly it increased β1i maturation (Figures S3L and S3M). An Akt inhibitor also did not suppress iβ maturation (Figure S3M). Rapamycin and the Akt inhibitor only partially reduced PRAS40 S183 phosphorylation, indicating that S183 is a rapamycin-less-sensitive site like several other mTORC1 targets (Kang et al., 2013). The different effects on the phosphorylation might explain why Torin1, but not rapamycin, suppressed iβ maturation. The difference between Torin1 and rapamycin could also be due to the effect of rapamycin in perturbing the interaction between PRAS40 and mTOR/raptor, as revealed previously (Vander Haar et al., 2007). TSC1 KO significantly increased the proteolytic activities of β1i and β5i, which were reversed by Torin1, whereas the KO effects on the activities of β1 and β5 were much less significant (Figures 3I-L). The reduction of the cβ subunit proteolytic activities by Torin1 treatment for 24 h could be due to reduction of cβ subunit expression or the increase of the PRAS40-β6 interaction, which can suppress the formation of c-proteasomes.
We also found that deficiency of AMPK, a negative regulator of mTORC1, in MEFs drastically increased the iβ mature forms (Figures 3M and S3N). The increase was seen even without IFNγ when both AMPKα isoforms were deficient. Leucine deprivation or Torin1 drastically reduced the iβ mature forms in AMPK KO MEFs (Figures 3M and S3O). AMPK KO largely reduced β5 level (Figures 3M, S3N, and S3P), indicating that β5i replaced β5 in proteasomes. Deficiency of PTEN, a negative regulator of Akt/mTOR signaling, also largely increased the iβ mature forms in IFNγ-treated MEFs, which was reversed by leucine deprivation or Torin1 (Figures 3N and S3Q). Rapamycin increased β1i maturation in PTEN KO MEFs as similarly as in TSC1 KO MEFs (Figure S3R). The regulation of i-proteasome contents was corroborated by changes in the i-proteasome proteolytic activities in AMPK and PTEN KO MEFs (Figures 3O-R and S3S).
We observed that mTORC1 regulates the levels of some iβ subunit mRNAs in MEFs (Figure S3T-V). However, the change of the gene expression did not correlate with iβ maturation, supporting a transcription-independent mechanism for i-proteasome formation. Such a mechanism was further supported by the results obtained using recombinant iβ subunits (Figures 2E, 3B, 3D, and S3B). This is consistent with the recent report that Nrf1, which was shown to be regulated by mTORC1 (Zhang et al., 2014), does not regulate expression of iβ subunits (Sha and Goldberg, 2014).
mTORC1 promotes i-proteasome formation in cancer cells and immortalized cells
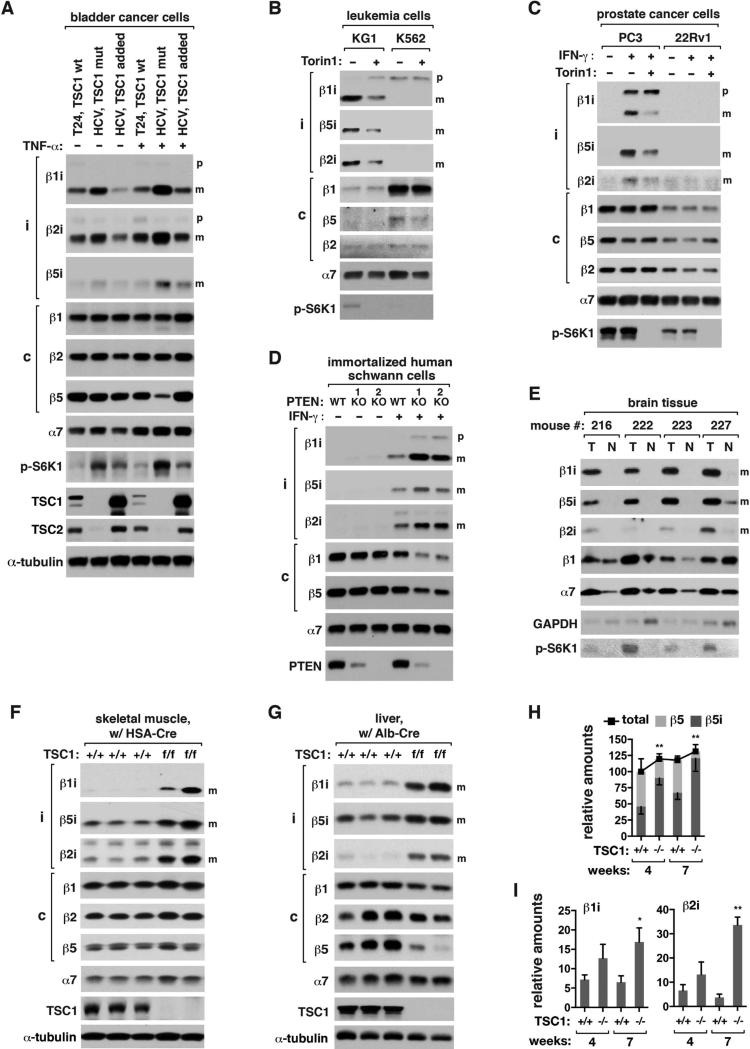
We asked if mTORC1 promotes i-proteasome formation in cancer cells. HCV bladder cancer cells, which do not express TSC1, contained higher levels of iβ subunits compared to T24 cells that express wild type TSC1 (Figure 4A). Adding exogenous TSC1 in HCV cells reduced the iβ mature forms. The expression status of TSC1 did not affect the amounts of cβ subunits. The effect became greater when the cells were treated with tumor necrosis factor α (TNFα), a cytokine that can increase expression of iβ subunits.
Figure 4. mTORC1 promotes i-proteasome formation in cancers, immortalized cells and in vivo.
(A) The amounts of iβ mature forms depend on TSC1 in bladder cancer cells. T24 (TSC1 wt), HCV (TSC1 mut), and HCV (TSC1 added) cells were treated with TNFα (20 ng/ml) or vehicle (−) for 24 h.
(B) The amounts of iβ mature forms depend on NRAS mutation and mTOR activity. KG1 and K562 leukemia cells were treated with IFNγ and Torin1 for 16 h.
(C) PC3 and 22Rv1 prostate cancer cells were treated with IFNγ and Torin 1 for 16 h.
(D) PTEN KO in HSC1λ cells increases the mature forms of iβ subunits and reduces cβ levels. Two different clones of HSC1λ PTEN KO cells were obtained by TALEN technique.
(E) NRAS G12V mutation-induced brain tumors have a high level of i-proteasomes. Brain tumors (T) harboing the mutation and neighboring normal tissues (N) were analyzed.
(F, G) Tissue-specific depletion of TSC1 enhances the mature forms of iβ subunits in mouse tissues. Skeletal muscle- and liver-specific depletions of the TSC1 gene were achieved by breeding TSC1 floxed mice with HSA-Cre and Albumin-Cre mice, respectively. WT (TSC1+/+, Cre) and KO (TSC1f/f, Cre) male mice from the same cohorts at 7 weeks of age were analyzed.
(H, I) Quantification of proteasome subunits in the liver tissue. Values are means ± SD. The amounts of β5i, β1i, and β2i were statistically analyzed (*, p<0.05; **, p<0.01 vs TSC1+/+; n=3).
Final concentrations of IFNγ and Torin1 were 50 ng/ml and 250 nM, respectively.
See also Figure S4.
KG1 acute myelogenous leukemia cells harbor NRAS G12V mutation that upregulates the PI3K-mTORC1 pathway. KG1 cells had higher levels of the iβ mature forms than K562 immortalized leukemia cells that do not harbor the mutation (Figure 4B). About 95 and 45% of total proteasomes contained β5i and β1i, respectively, in KG1 cells compared to only 3 and 0% in K562 cells (Figures S4A and S4B). Torin1 drastically reduced the iβ mature forms in KG1 cells. We could not observe any strong correlation between iβ subunit mRNA levels and the mTORC1 activity in the leukemia cells (Figure S4C).
PC3 prostate cancer cells deficient of PTEN expression showed induction and maturation of iβ subunits in response to IFNγ (Figure 4C). Torin1 reduced the iβ mature forms without affecting cβ subunits. We could not observe iβ subunits in 22Rv1 prostate cancer cells that express PTEN. There was no clear correlation between iβ mRNA levels and the mTORC1 activity in the prostate cancer cells (Figure S4D). Supporting the PTEN-dependent changes of i-proteasomes, immortalized human schwann cells (HSC1λ) with a biallelic deletion of PTEN showed a large induction and maturation of iβ subunits in response to IFNγ (Figure 4D). The increases were accompanied with decreases of cβ subunit amounts.
mTORC1 promotes i-proteasome formation in vivo
To investigate if mTORC1 regulates i-proteasome formation in vivo, we induced tumor in the mouse brain by overexpressing NRAS G12V mutant using the Sleeping Beauty transposon system (Wiesner et al., 2009). The mutation-driven tumor showed high amounts of mature iβ subunits along with a large increase of mTORC1 activity (pS6K1) relative to neighboring normal tissues (Figure 4E). In contrast, β1 did not show such a drastic change. The level of α7 was increased in tumor tissues, indicating that the total amount of proteasomes was increased.
We also investigated if mTORC1 regulates i-proteasome formation in non-tumor tissues. When the TSC1 gene was deleted in the mouse skeletal muscle, the iβ mature forms were drastically increased in the muscle tissue (Figure 4F). Liver-specific depletion of the TSC1 gene also greatly increased the iβ mature forms along with reductions of cβ subunits in the liver tissue (Figures 4G-I and S4E-G). TSC1 KO in the liver increased β5i-containing proteasomes up to 95% of total proteasomes in 7 weeks old mice (Figures 4H and S4E-G). TSC1 KO did not change the phosphorylation of p38 at Y180/Y182 (Figure S4H), indicating that the KO effect might not be due to inflammation.
I-proteasomes are important for viability of mTORC1 hyperactive cells
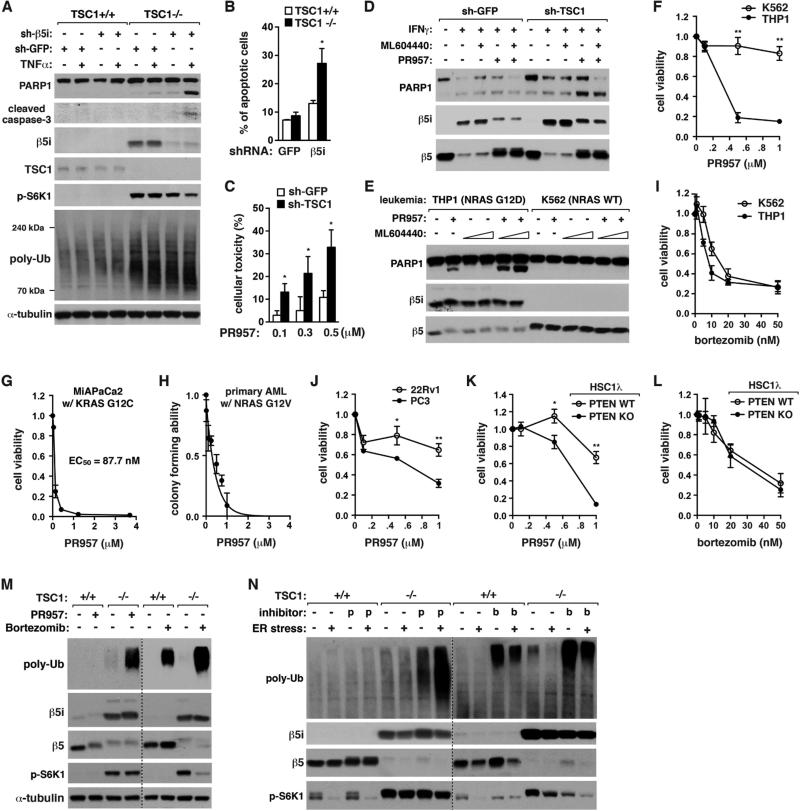
Knowing that mTORC1 promotes i-proteasome formation, we wondered about the functional consequence of the regulation. β5i KD by shRNA induced the cleavages of PARP1 and caspase-3 in TSC1−/− MEFs but not TSC1+/+ MEFs in response to TNFα (Figure 5A). TNFα-induced apoptosis was also induced to a significantly greater extent by β5i KD in TSC1−/− MEFs than TSC1+/+ MEFs (Figures 5B, S5A, and S5B). Similarly, cell death and PARP1 cleavage were induced by PR957, a β5i inhibitor (Muchamuel et al., 2009), to a greater extent in TSC1-silenced T24 cells (Figures 5C and 5D), and in THP1 and KG1 cells compared to K562 cells (Figures 5E, 5F, S5C-G). ML604440, a β1i inhibitor (Basler et al., 2012), did not induce PARP1 cleavage but potentiated the effect of PR957 (Figures 5E and S5D). The RAS mutation-dependent effect of PR957 was further confirmed with MIAPaCa2 pancreatic cancer cells that harbor KRAS G12C mutation (Figure 5G), and mouse primary AML cells expressing NRAS G12V mutant (Sachs et al., 2014) (Figure 5H). In contrast, bortezomib, which inhibits all types of proteasomes, induced apoptosis regardless of TSC1 or RAS mutation (Figures 5I and S5H-J). PR957 also showed a greater toxic effect on PTEN-mutated PC3 cells compared to PTEN-intact 22Rv1 cells (Figure 5J). The PTEN dependence was confirmed using PTEN-deficient HSC1λ cells (Figures 5K, 5L, and S5K).
Figure 5. I-proteasomes are important for viability of mTORC1 hyperactive cells.
(A) β5i KD induces apoptosis and enhances accumulation of poly-Ub proteins in TSC1−/− MEFs but not TSC1+/+ MEFs. Cells were treated with TNFα (20 ng/ml) for 6 h.
(B) β5i KD enhances apoptosis to a significantly greater extent when TSC1 is deficient. MEFs treated with TNFα (20 ng/ml) for 24 h were stained with Annexin V-FITC and propidium iodide and analyzed by FACS.
(C) Toxic effect of PR957 (48 h) is significantly increased when TSC1 is silenced in T24 cells. MTT assay was conducted for cell viability.
(D) I-proteasome inhibition induces PARP1 cleavage to a greater extent when TSC1 is silenced in T24 cells. Cells were treated with IFNγ (50 ng/mL), ML604440 (500 nM) and/or PR957 (100 nM) for 72 h.
(E) I-proteasome inhibition induces apoptosis for THP1 cells but not K562 cells. The leukemia cells were treated with PR957 (100 nM) and/or ML604440 (100 nM or 500 nM) for 24 h.
(F) PR957 (4 days) induces cell death for THP1 cells but not for K562 cells. Cell viability was measured by MTT assay.
(G) MIAPaCa2 pancreatic cancer cells are highly sensitive to PR957 (2 days) for cell viability.
(H) PR957 suppresses self-renewal capacity of primary leukemia cells from AML mice.
(I) Bortezomib (2 days) induces cell death to similar extents between K562 and THP1 cells.
(J) PC3 cells are more sensitive to PR957 (3 days) than 22Rv1 cells for viability.
(K) PTEN KO sensitizes HSC1λ cells to PR957 (24 h) for viability.
(L) Bortezomib (48 h) induces HSC1λ cell death regardless of PTEN status.
(M, N) PR957, but not bortezomib, has a selective effect on TSC1−/− MEFs for accumulation of poly-Ub proteins. Cells were treated with IFNγ (50 ng/mL) for 72 h, then PR957 (100 nM) or bortzomib (10 nM) for additional 24 h in the presence or absence of tunicamycin (10 μM), an ER stress inducer.
For every graph in Figure 5, values are means ± SD (*, p<0.05; **, p<0.01; n=3).
See also Figure S5.
I-proteasomes alleviate protein stress in mTORC1 hyperactive cells
Since mTORC1 hyperactivation made cells to depend more on i-proteasomes for viability, we predicted that i-proteasomes might play a role in reduce protein stress, such as defective ribosomal products (DRiPs), that can be toxic to cells. As previously shown (Conn and Qian, 2013), TSC1 KO induced accumulation of poly-Ub proteins in MEFs (Figure 5A). TNFα further increased poly-Ub protein accumulation. β5i KD induced the accumulation to a similar extent as TNFα treatment in TSC1−/− MEFs (lanes 6 and 7). TNFα greatly increased the KD effect in TSC1−/− MEFs but not in TSC1+/+ MEFs. This result suggests that i-proteasomes might be important for degradation of poly-Ub DRiPs in mTORC1-hyperactive cells. β5i KD also increased protein oxidation in TSC1−/− MEFs but not in TSC1+/+ MEFs (Figure S5L). Similar to β5i KD, i-proteasome inhibitors greatly enhanced accumulation of poly-Ub proteins in TSC1−/−, THP1 and AMPK−/− cells but not in TSC1+/+, K562 and AMPK+/+ cells (Figures 5M, S5D, and S5M). Bortezomib induced accumulation of poly-Ub proteins to a greater extent than PR957 but it did not show such a selective effect.
The endoplasmic reticulum (ER) stress, which was induced by tunicamycin, drastically enhanced the level of poly-Ub proteins in TSC1−/− MEFs relative to TSC1+/+ MEFs (Figure 5N). PR957 potentiated the effect of tunicamycin in TSC1−/− MEFs but not in TSC1+/+ MEFs, indicating that i-proteasomes might be important to attenuate the ER stress in mTORC1-hyperactive cells. Bortezomib did not show such a TSC1-dependent effect. Rapamycin suppressed β5i KD-induced accumulation of poly-Ub proteins and PARP1 cleavage in TSC1−/− MEFs (Figure S5N). This rapamycin effect might be due to inhibition of protein synthesis.
mTORC1 regulation of i-proteasome formation is mediated by PRAS40
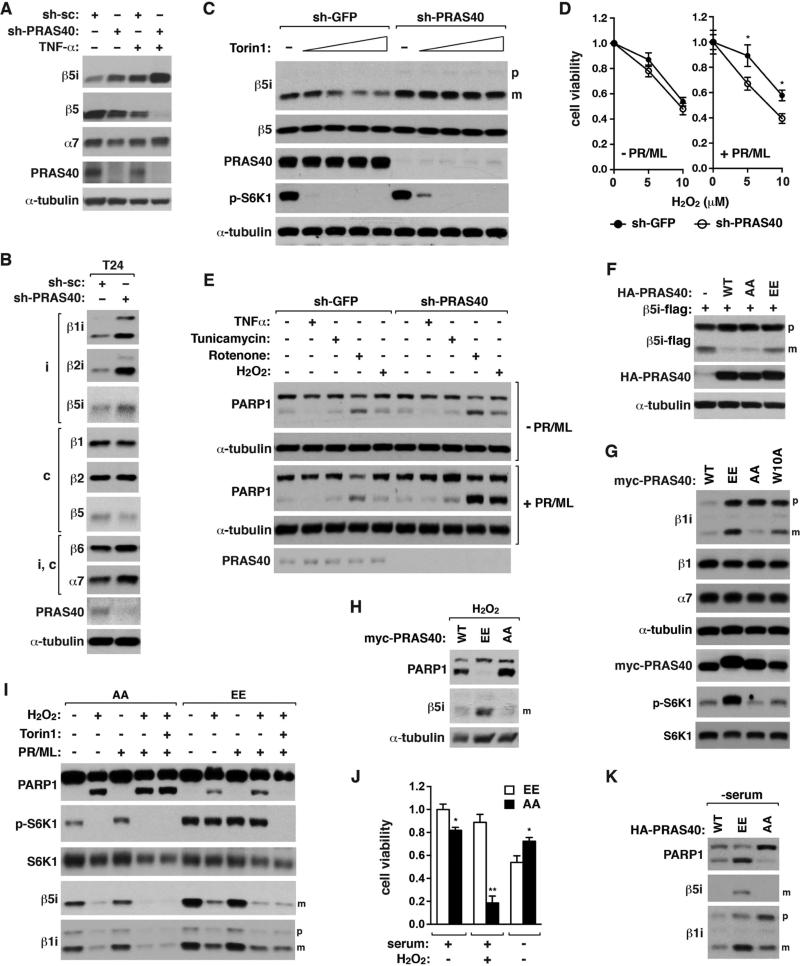
Since PRAS40 binds to iβ subunits, we predicted that PRAS40 might be important for mTORC1 regulation of i-proteasome formation. PRAS40 KD increased the iβ mature forms in multiple cell types (Figures 6A, 6B, and S6A). Furthermore, PRAS40 KD made the maturation of β1i and β5i less responsive to Torin1 (Figures 6C, S6A-D). PRAS40 KD cells depended more on i-proteasomes for survival under oxidative stress (Figure 6D). While PRAS40 KD only moderately increased PARP1 cleavage in KG1 cells exposed to oxidative stress, the KD effect became greater when i-proteasomes were inhibited (Figures 6E and S6E). These results suggest that mTORC1 regulation of i-proteasome formation depends on PRAS40 and the regulation is important for cell viability under oxidative stress.
Figure 6. mTORC1 regulation of i-proteasome formation depends on PRAS40 and is important for cell survival against stress.
(A) PRAS40 KD enhances the mature form of β5i along with reduction of β5 in 3T3-L1 cells. 3T3-L1 cells were stably transduced by shRNA and treated with TNF-α (20 ng/mL) for 24 h.
(B) PRAS40 KD enhances the mature forms of iβ subunits in T24 cells.
(C) PRAS40 KD reduces the suppressive effect of Torin1 on β5i maturation. HCT116 cells stably transduced by shRNA were treated with IFN-γ (50 ng/mL) and Torin1 at 0, 20, 50, 100, and 250 nM for 16 h.
(D) PRAS40 KD makes cells to depend on i-proteasomes for viability under oxidative stress. KG1 cells transduced by shRNA were treated with H2O2 in the presence or absence of PR957 (PR, 100 nM) and ML604440 (ML, 500 nM) for 48 h. MTT assay was conducted for viability. Values are means ± SD (*, p<0.05; n=3).
(E) I-proteasome inhibition enhances oxidative stress-induced PARP1 cleavage to a greater extent when PRAS40 is silenced. KG1 cells transduced by shRNA were treated with TNFα (20 ng/mL), tunicamycin (1 μM), rotenone (1 μM) and/or H2O2 (5 μM) for 16 h in the presence (+PR/ML) or absence (−PR/ML) of i-proteasome inhibitors.
(F) PRAS40 phosphorylations at S183 and S221 are important for β5i maturation. Flag-tagged β5i was transiently expressed alone (−) or together with PRAS40 contructs in HEK293T cells.
(G) PRAS40 phosphorylations are important for β1i maturation and S6K1 phosphorylation. Myc-PRAS40 construct was stably reconstituted in PRAS40-silenced HCT116 cells.
(H) PRAS40 phosphorylations are important for cellular resistance to oxidative stress. HCT116 cells reconstituted with PRAS40 construct were treated with IFN-γ (1 ng/ml) and H2O2 (100 μM) for 24 h.
(I) PRAS40 phosphorylations are important for oxidative stress response and S6K1 phosphorylation. AA or EE HCT116 cells were exposed to IFN-γ (50 ng/mL) for 24 h then treated with H2O2 (100 μM), Torin1 (250 nM), PR957 (100 nM), and/or ML604440 (500 nM) for 24 h.
(J) AA or EE HCT116 cells were treated with IFN-γ (50 ng/mL) with or without H2O2 (100 μM) for 48 h, or with IFN-γ (50 ng/mL) for 24 h then starved of serum for 24 h. Values are means ± SD (*, p<0.05; **, p<0.01 vs EE; n=3).
(K) EE mutation induces PARP1 cleavage under serum-starved condition. HCT116 cells reconstituted with WT or mutant PRAS40 were treated with IFN-γ (0.1 ng/mL) for 4 days then starved of serum for 24 h.
See also Figure S6.
To further clarify the underlying mechanism, we considered involvement of PRAS40 phosphorylations at S183 and S221 by mTOR (Fonseca et al., 2007; Oshiro et al., 2007; Wang et al., 2008). When a PRAS40 mutant (EE) with glutamate substitutions of S183 and S221 was expressed in HEK293T cells, the amount of the mature β5i was much higher compared to that in wild type PRAS40 (WT)-expressing cells (Figure 6F). In contrast, cells expressing a PRAS40 mutant (AA) harboring alanine substitutions barely showed the mature β5i. Consistently, reconstitution of EE in PRAS40-silenced HCT116 cells greatly increased β1i maturation (Figure 6G). W10A also enhanced β1i maturation compared to WT but to a lesser extent than EE. The EE cells also showed a higher level of S6K1 phosphorylation and cell proliferation (Figures 6G and S6F). This result suggests that the PRAS40 phosphorylations might have a dual function, stimulating both protein synthesis and i-proteasome formation.
mTORC1 phosphorylations of PRAS40 protect cells against stress
We asked if the phosphorylational regulation of PRAS40 is important for cellular stress response. Oxidative stress induced PARP1 cleavage in WT- or AA-reconstituted HCT116 cells but barely in EE cells (Figures 6H and 6I). Consistently, EE cells were resistant to oxidative stress-induced cell death (Figure 6J). H2O2 suppressed IFNγ-induced upregulation of i-proteasomes in EE cells, explaining why the i-proteasome inhibitors had only a marginal effect on PARP1 cleavage in EE cells (Figure 6I, lanes 7 and 9). Torin1 blocked the PARP1 cleavage in EE cells even in the presence of both oxidative stress and i-proteasome inhibitors (lane 10), indicating that reducing the mTORC1 activity made cells less dependent on i-proteasomes. In contrast, Torin1 did not suppress PARP1 cleavage in AA cells (lane 5). We speculate that AA cells might have accumulated protein stress highly regardless of mTORC1 activity or have a critical cell survival factor inhibited. H2O2 suppressed S6K1 phosphorylation in AA cells but not in EE cells (lanes 7 and 9), indicating that the oxidative stress-induced suppression of S6K1 phosphorylation might require dephosphorylation of S183 and/or S221.
If PRAS40 phosphorylations are important for cell viability under nutrient-enriched conditions, we reasoned that their dephosphorylation state might be important for cell survival under unfavorable growth conditions. As reasoned, AA-reconstituted HCT116 cells were more viable than EE cells when cells were deprived of serum (Figure 6J). Consistently, serum starvation under inflammatory conditions induced PARP1 cleavage in EE cells but not in AA cells (Figures 6K and S6G). EE cells showed higher levels of S6K1 phosphorylation and poly-Ub proteins than AA cells even in serum starvation (Figure S6G). Torin1 reduced the amount of poly-Ub proteins and PARP1 cleavage in EE cells, suggesting that mTOR is responsible for the protein stress in EE cells. Combined, these results demonstrate that the phosphorylational regulation of PRAS40 is crucial for viability of cells in response to growth conditions and stress.
mTORC1 phosphorylations of PRAS40 facilitate i-proteasome formation
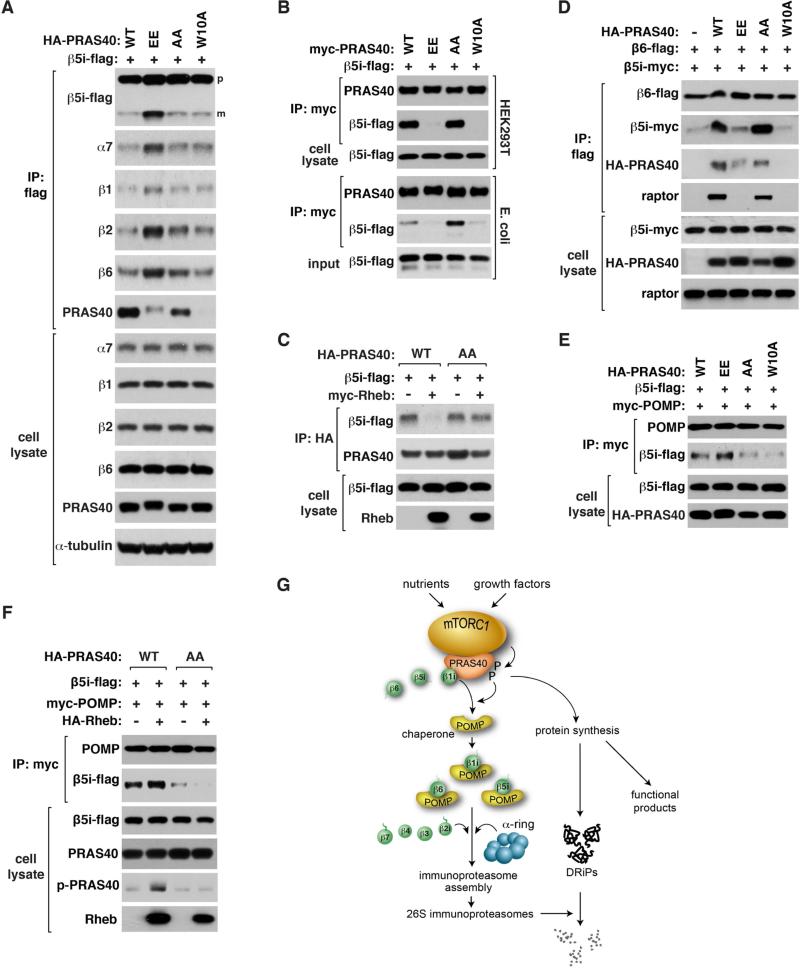
To clarify how PRAS40 phosphorylations promote i-proteasome formation, we expressed β5i-flag in HEK293T cells together with PRAS40 constructs and analyzed proteasome formation. EE, compared to WT and AA, drastically increased the formation of proteasomes containing β5i-flag (Figure 7A). W10A did not show such an increase, indicating that dissociation of iβ precursors from PRAS40 might not be enough to induce the formation of i-proteasomes. Interestingly, EE made β5i and β1i precursors to be dissociated from PRAS40 (Figures 7B, S7A, and S7B). The dissociation was also seen between the proteins expressed in E. coli (Figure 7B). Rheb overexpression disrupted binding of β5i to WT but not AA (Figure 7C). This result indicates that PRAS40 phosphorylations might be required for mTORC1-mediated regulation of the PRAS40-β5i interaction.
Figure 7. mTORC1 facilitates de novo biogenesis of i-proteasomes by phosphorylating PRAS40.
(A) EE PRAS40 enhances de novo synthesis of proteasomes.
(B) EE does not form a stable interaction with β5i. β5i-flag was coexpressed with myc-PRAS40 constructs in HEK293T cells or E. coli. Cell extracts were used for IP.
(C) AA PRAS40 prevents Rheb from destabilizing the PRAS40-β5i interaction.
(D) EE and W10A suppress the β6-β5i interaction.
(E) Binding of POMP to β5i is enhanced by EE and reduced by AA.
(F) Rheb enhances binding of POMP to β5i, which is suppressed by AA.
(G) Model for the role of mTORC1 in promoting i-proteasome formation to reduce protein stress.
For A-F, the indicated tagged proteins were transiently expressed in HEK293T cells.
See also Figure S7.
Since the phosphorylational status of S183 can affect the PRAS40-raptor interaction (Oshiro et al., 2007), we wondered whether raptor binding is involved in the regulation. PRAS40 F129A mutation, which disrupts the PRAS40-raptor interaction (Oshiro et al., 2007; Wang et al., 2007), did not alter the PRAS40-β5i interaction and only marginally increased β5i maturation (Figure S7C). This indicates that raptor binding might not be critical for the phosphorylational effect. The PRAS40-induced interaction between β5i and β6 precursors, which we speculated as forming an off-pathway complex (Figure S2D), was increased by AA but reduced by EE (Figure 7D). Based on this finding, we speculate that the PRAS40 phosphorylations might promote i-proteasome formation by altering the interaction between the β precursors.
POMP (proteasome maturation protein/proteassemblin) is a chaperone that binds to β precursors and facilitates the 20S core assembly (Burri et al., 2000; Griffin et al., 2000; Witt et al., 2000). We found that PRAS40 suppresses the interaction between POMP and iβ precursors (Figure S7D). The suppressive effect became greater with AA compared to WT (Figure 7E). In contrast, EE enhanced the POMP-β5i interaction. W10A did not enhance the POMP-β5i interaction, indicating that dissociation of β5i from PRAS40 is not enough to induce the binding of β5i to POMP. Supporting the positive role of PRAS40 phosphorylations in promoting the POMP-β5 interaction, Rheb overexpression enhanced the POMP-β5i interaction along with PRAS40 S183 phosphorylation (Figure 7F). This did not occur with AA cells, suggesting that the PRAS40 phosphorylations might promote the i-proteasome formation through enhancing the POMP binding.
DISCUSSION
In this study, we have discovered that mTORC1 promotes the assembly of i-proteasomes and thereby increases the cellular capacity to attenuate protein stress (Figures 7G, S7E, and S7F). According to the current model (Murata et al., 2009), the i-proteasome assembly starts with a stable pre-proteasome complex composed of a seven-member α-ring (Figure S7F). After sequential incorporation of seven β subunits is complete on the α-ring, the two half proteasomes dimerize at the β ring interface. By recruiting β1i, β5i and β6 before they enter into the i-proteasome assembly machinery, mTORC1 functions as a checkpoint kinase to regulate the ordered, stoichiometric assembly of the subunits in response to cell growth conditions or stress. mTORC1 catalyzes the transfer of bound β precursors to the i-proteasome assembly machinery. When mTORC1 is inhibited, the halted catalysis blocks the influx of new β precursors to mTORC1. Unbound to mTORC1, β precursors accumulate or are degraded depending on their stability. β1i precursor, which is the first β subunit added for the assembly, is likely more stable than other subunits, making it readily available for i-proteasome assembly when mTORC1 is activated. mTORC1 could also affect c-proteasome assembly via interacting with β6. However, c-proteasomes have a much slower rate of assembly (82 vs. 21 min half-time) and a longer half-life (133 vs. 27 h in HeLa cells) compared to i-proteasomes (Heink et al., 2005; Yewdell, 2005). Thus, i-proteasomes are likely more suited than c-proteasomes for regulation by mTORC1 in response to environmental fluctuations.
Our study supports the role of i-proteasomes in efficient clearance of protein aggregates and oxidized proteins (Pickering et al., 2010; Seifert et al., 2010). Such a role might be prevalent in many cell types of our body, including non-immune cells, given the dynamic nature of i-proteasomes that can be transiently induced by oxidative stress (Ding et al., 2003; Hussong et al., 2010). In this regard, it is possible that IFNγ or TNFα could induce i-proteasomes as a response to oxidative stress rather than an immune response. Indeed, i-proteasomes are found in many cell types with no apparent link to immunity, such as photoreceptor cells, Purkinje cells, and oligodendrocytes (Ferrington and Gregerson, 2012).
Our findings imply that nutritional factors activating mTORC1 might induce a shift of the proteasome population toward i-proteasomes. I-proteasomes with different β compositions may produce different functional products, as supported by the distinct roles of β1i and β5i in myoblast differentiation (Cui et al., 2014). The shift of the proteasome population may also affect the immune system by modulating recognition of virus-infected cells or cancer cells by cytotoxic T cells or altering antigens linked to autoimmune diseases. Since PRAS40 binds to β5t, mTORC1 might also regulate the positive or negative selections of T cells in the thymus. Given the potential link between mTORC1 and immunity, it is worth noting that rapamycin did not suppress i-proteasome assembly but rather enhanced β1i maturation. Thus, i-proteasomes might be an important factor in the efficacy of rapamycin as an immunopressive agent or a drug to treat diseases.
How the C-terminal phosphorylations of PRAS40 affect binding of iβ precursors to PRAS40 N-terminus is unclear. The N-terminal region shows sequence homology only within vertebrates, and since i-proteasomes are found only in vertebrates, this implies that the proteolytic machinery might have converged with the mTORC1 pathway during vertebrate evolution. This would have allowed vertebrates to acquire the ability to coordinate mTORC1 signaling with the proteolytic machinery that regulates immunity and stress response. Supporting the notion, i-proteasomes are involved in human diseases related to metabolism and inflammation, such as rheumatoid arthritis and colitis (Basler et al., 2010) and diabetes (Zaiss et al., 2011). According to our results, PRAS40 phosphorylations by mTORC1 are the key event for the coordination, as we found that the phosphorylations are important for both S6K1 phosphorylation and i-proteasome formation.
The coupled regulation between protein synthesis and i-proteasome formation reveals therapeutic potential of i-proteasome inhibitors in treating many human diseases. For example, inhibitors of i-proteasomes may be effective in selectively killing cancer cells that harbor mutations of PTEN, PI3K, AKT or RAS. However, application of i-proteasome inhibitors to human diseases would require a better understanding of the functions of i-proteasomes beyond their role in clearing protein stress. Elucidating further the functions of the mTORC1-regulated proteolytic machinery may shed light on therapeutic strategies for human diseases related to mTORC1 dysregulation.
EXPERIMENTAL PROCEDURES
In vitro binding assay
Untagged or myc-tagged PRAS40 and flag-tagged β precursors were cloned in pGEX6P2 (GE Healthcare, Little Chalfont, UK) and expressed in ArcticExpress bacteria (Agilent Technologies, Santa Clara, CA). Untagged PRAS40 was purified using glutathione-sepharose 4B beads and PreScission protease (GE Healthcare). Myc-PRAS40 and flag-β precursors in bacterial cell extract were used for IP. For coexpression analysis, PRAS40 and β precursors were cloned in pRSFDuet-1 vector (EMD Millipore Chemicals, Billerica, MA). More details are described in the Supplemental Experimental Procedures.
Proteasomal activity assay
The i-proteasomal activities specific to β1i and β5i were assessed using Ac-PAL-AMC and Ac-ANW-AMC as substrates, respectively, in the presence or absence of i-proteasome inhibitors. The c-proteasomal activities specific to β1 and β5 were assessed using Ac-Nle-Pro-Nle-Asp-AMC and Ac-WLA-AMC as substrates, respectively. The reaction was monitored with excitation at 380 nm and emission at 460 nm. A detailed procedure is described in the Supplemental Experimental Procedures.
Brain tumor induction
FVB Neonatal mice less than 2 days of age were used. Tumors were induced by administration of PEI/plasmid DNA mix by intracranial injection into the right lateral cerebral ventricle of neonatal mice as described previously (Wiesner et al., 2009). A detailed procedure is described in the Supplemental Experimental Procedures.
Genetic ablation of TSC1 in skeletal muscle and liver
Tissue-specific KO of TSC1 was made by Cre-Lox recombination approach. TSC1 flox mice (005680), HSA-Cre line (006139), and Albumin-Cre line (003574) were obtained from Jackson Laboratory. Singly transgenic mice were crossed to obtain double transgenic mice carrying each transgene of floxed allele and Cre gene. Double transgenic mice were interbred to obtain experimental (fl/fl, Cre) and control (+/+, Cre) cohorts. All mice were maintained in a specific pathogen-free facility.
Other Experimental Procedures
Other experimental procedures, including materials, yeast two-hybrid screen, plasmid construction, cell culture, transfection, co-IP, WB, lentiviral preparation, viral infection, stable cell generation, qPCR, flow cytometric analysis, cell viability assay, colony formation assay, and statistical analysis, are described in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
mTORC1 binds to immunoproteasome β subunit precursors via PRAS40.
mTORC1 promotes immunoproteasome formation via PRAS40 phosphorylation.
Immunoproteasomes are important for viability of mTORC1-hyperactive cells.
The mTORC1-immunoproteasome pathway is important for cell survival against stress.
ACKNOWLEDGEMENTS
We thank C. Tucker for yeast two-hybrid screen; V. Stambolic for PTEN MEFs; D. Kwiatkowski for TSC1 MEFs and bladder cancer cells; B. Viollet for AMPK MEFs; D. Ferrington, H. Towle, A. Hertzel and Kim lab members for critical reading and helpful comments; R. Foncea for lentivirus prep. This study was supported by the Korea Research Foundation (KRF-2008-357-C00121) (to Y.S.Y.); the NIH (DK050456, GM097057) and the DoD (W81XWH-13-1-0060) (to D-.H.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Y.S.Y., Z.S., L.C., D.L., and D.-H.K. designed experiments, Y.S.Y., K.H.K., B.T., K.E.N.-O., B.S.M., T.A., R.D., J.W., and D.-H.K. conducted experiments, and Y.S.Y. and D.-H.K. wrote the paper.
REFERENCES
- Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol. 2010;185:634–641. doi: 10.4049/jimmunol.0903182. [DOI] [PubMed] [Google Scholar]
- Basler M, Lauer C, Moebius J, Weber R, Przybylski M, Kisselev AF, Tsu C, Groettrup M. Why the structure but not the activity of the immunoproteasome subunit low molecular mass polypeptide 2 rescues antigen presentation. J Immunol. 2012;189:1868–1877. doi: 10.4049/jimmunol.1103592. [DOI] [PubMed] [Google Scholar]
- Burri L, Hockendorff J, Boehm U, Klamp T, Dohmen RJ, Levy F. Identification and characterization of a mammalian protein interacting with 20S proteasome precursors. Proc Natl Acad Sci U S A. 2000;97:10348–10353. doi: 10.1073/pnas.190268597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Conn CS, Qian SB. Nutrient signaling in protein homeostasis: an increase in quantity at the expense of quality. Sci Signal. 2013;6:ra24. doi: 10.1126/scisignal.2003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Hwang SM, Gomes AV. Identification of the immunoproteasome as a novel regulator of skeletal muscle differentiation. Mol Cell Biol. 2014;34:96–109. doi: 10.1128/MCB.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003;546:228–232. doi: 10.1016/s0014-5793(03)00582-9. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Gregerson DS. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Roehrich H, Chang AA, Huang CW, Maldonado M, Bratten W, Rageh AA, Heuss ND, Gregerson DS, Nelson EF, et al. Corneal wound healing is compromised by immunoproteasome deficiency. PLoS One. 2013;8:e54347. doi: 10.1371/journal.pone.0054347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- Griffin TA, Slack JP, McCluskey TS, Monaco JJ, Colbert RA. Identification of proteassemblin, a mammalian homologue of the yeast protein, Ump1p, that is required for normal proteasome assembly. Mol Cell Biol Res Commun. 2000;3:212–217. doi: 10.1006/mcbr.2000.0213. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B, Chapiro J, Stroobant V, Colau D, Van Holle B, Parvizi G, Bousquet-Dubouch MP, Theate I, Parmentier N, Van den Eynde BJ. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc Natl Acad Sci U S A. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Heink S, Ludwig D, Kloetzel P-M, Krüger E. IFN-γ-induced immune adaptation of the proteasome system is an accelerated and transient response. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong SA, Kapphahn RJ, Phillips SL, Maldonado M, Ferrington DA. Immunoproteasome deficiency alters retinal proteasome's response to stress. Journal of neurochemistry. 2010;113:1481–1490. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature medicine. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- Murata S, Yashiroda H, Tanaka K. Molecular mechanisms of proteasome assembly. Nature reviews. Molecular cell biology. 2009;10:104–115. doi: 10.1038/nrm2630. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. The Biochemical journal. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs Z, LaRue RS, Nguyen HT, Sachs K, Noble KE, Mohd Hassan NA, Diaz-Flores E, Rathe SK, Sarver AL, Bendall SC, et al. NRASG12V oncogene facilitates self-renewal in a murine model of acute myelogenous leukemia. Blood. 2014;124:3274–3283. doi: 10.1182/blood-2013-08-521708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, Prozorovski T, Lange N, Steffen J, Rieger M, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Current biology : CB. 2014;24:1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen J, Seeger M, Koch A, Kruger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Vigneron N, Van den Eynde BJ. Proteasome subtypes and the processing of tumor antigens: increasing antigenic diversity. Current opinion in immunology. 2012;24:84–91. doi: 10.1016/j.coi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Harris TE, Lawrence JC., Jr. Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence JC., Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, Long C, Demorest ZL, Zamora EA, Low WC, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer research. 2009;69:431–439. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt E, Zantopf D, Schmidt M, Kraft R, Kloetzel PM, Kruger E. Characterisation of the newly identified human Ump1 homologue POMP and analysis of LMP7(beta 5i) incorporation into 20 S proteasomes. J Mol Biol. 2000;301:1–9. doi: 10.1006/jmbi.2000.3959. [DOI] [PubMed] [Google Scholar]
- Yewdell JW. Immunoproteasomes: Regulating the regulator. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9089–9090. doi: 10.1073/pnas.0504018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, Bekker CP, Grone A, Lie BA, Sijts AJ. Proteasome immunosubunits protect against the development of CD8 T cell-mediated autoimmune diseases. J Immunol. 2011;187:2302–2309. doi: 10.4049/jimmunol.1101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.