Abstract
In this study, we used event-related potentials (ERPs) to examine how dimensions of emotion – valence and arousal – influence different stages of word processing under different task demands. In two experiments, two groups of participants viewed the same single emotional and neutral words while carrying out different tasks. In both experiments, valence (pleasant, unpleasant, and neutral) was fully crossed with arousal (high and low). We found that task made a substantial contribution to how valence and arousal modulated the Late Positive Complex (LPC), which is thought to reflect sustained evaluative processing (particularly of emotional stimuli). When participants performed a semantic categorization task in which emotion was not directly relevant to task performance, the LPC showed a larger amplitude for high-arousal words than low-arousal words, but no effect of valence. In contrast, when participants performed an overt valence categorization task, the LPC showed a large effect of valence (with unpleasant words eliciting the largest positivity), but no effect of arousal. These data show not only that valence and arousal act independently to influence word processing, but that their relative contributions to prolonged evaluative neural processes are strongly influenced by situational demands (and individual differences, as revealed in a subsequent analysis of subjective judgments).
Keywords: event-related potentials (ERP), LPC, LPP, late positivity, emotion, valence, arousal, language, word processing, task
Emotional stimuli elicit a rapid and coordinated set of responses. They can capture attention, guide evaluative judgments, and mobilize autonomic reflexes quickly and powerfully. These emotion responses are generally described as having an appetitive/aversive direction, called “valence”, and a level of activation, called “arousal”. In this study, we used event-related potentials (ERPs), a direct measure of online neural activity, to ask how valence and arousal influence different stages of emotional word processing under different tasks demands.
According to the Circumplex Model of emotion, “valence” and “arousal” reflect two orthogonal underlying dimensions of emotion, which together explain most of the variation in how emotional stimuli are evaluated (Abelson & Sermat, 1962; Osgood, Suci, & Tannenbaum, 1967; Russell, 1980). The valence dimension ranges from pleasant to neutral to unpleasant, and reflects the general motivational significance of a stimulus1. The arousal dimension ranges from high (or active) to low (or passive), and expresses the degree to which a particular stimulus prepares a person for action, e.g. by eliciting an autonomic sympathetic response (Bradley & Lang, 2007) or capturing and focusing attention (Mather & Sutherland, 2011).
While there is evidence that the dimensions of valence and arousal can explain unique variance across explicit evaluative ratings of mood, facial expressions, and words (Russell, 1980), in practice these ratings are consistently correlated with one another: stimuli that are overtly rated as extremely valenced (pleasant or unpleasant) tend also to be rated as highly arousing, while stimuli rated as less valenced (more neutral) tend to be rated as less arousing, leading to a “U-shaped” relationship between the two (Lang, Bradley, & Cuthbert, 2005). This close correlation between overt evaluations of valence and arousal raises a straightforward question: does the brain actually distinguish between these two dimensions of emotion during word processing? Or alternatively, does arousal fall naturally out of the dimension of valence such that as “pleasantness” or “unpleasantness” increases, so does arousal?
To address this question, several researchers have investigated whether arousal and valence exert independent effects on behavior. There is now fairly strong evidence that, even when valence is held constant (Aquino & Arnell, 2007; Arnell, Killman, & Fijavz, 2007), high-arousal stimuli capture and hold attention more than low-arousal stimuli (Anderson, 2005; Mather & Sutherland, 2011; Vogt, De Houwer, Koster, Van Damme, & Crombez, 2008), in addition to eliciting systemic arousal (Bradley & Lang, 2007). The effects of valence, independent of arousal, however, have been more mixed. Some studies report larger or stronger attentional and autonomic effects on unpleasant than pleasant stimuli that are matched on arousal (Peeters & Czapinski, 1990). This is known as a negativity bias (Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001; Ito, Larsen, Smith, & Cacioppo, 1998; Taylor, 1991): the “tendency for the negative motivational system to respond more intensely than the positive motivational system to comparable increases in input” (Ito & Cacioppo, 2005). Others, however, have reported no difference between behavioral responses to pleasant and unpleasant stimuli, or even stronger responses to pleasant stimuli (see Kousta, Vinson, & Vigliocco, 2009 for discussion of inconsistencies).
ERP Studies
Behavioral responses like those discussed above typically reflect the culmination of multiple stages of neural processing. An alternative approach is to directly measure the neural activity evoked by a given stimulus as it is processed in real time. Event-related potentials (ERPs) enable just this, and there have now been a number ERP studies examining the neurocognitive processes recruited in response to emotional stimuli, including words. Some of these studies report very early effects of emotion, within the first 150ms of word-onset, with effects reported on the N1 component (Briesemeister, Kuchinke, & Jacobs, 2014; Hinojosa, Méndez-Bértolo, & Pozo, 2010; Hofmann, Kuchinke, Tamm, Võ, & Jacobs, 2009; Kissler & Herbert, 2013; Wang, Bastiaansen, Yang, & Hagoort, 2013), the P2 component (González-Villar, Triñanes, Zurrón, & Carrillo-de-la-Peña, 2014; Herbert, Kissler, Junghöfer, Peyk, & Rockstroh, 2006; Kanske & Kotz, 2007; Ortigue et al., 2004; Wang et al., 2013), or on other early perceptual components (Bayer, Sommer, & Schacht, 2012; Bernat, Bunce, & Shevrin, 2001; Keuper et al., 2014; Scott, O'Donnell, Leuthold, & Sereno, 2009; Zhang et al., 2014). This has been taken to reflect a very early influence of emotion on initial perceptual processing. These findings, however, have been quite variable, with the majority of such studies failing to find such early effects.2
The most consistent effect of emotion in ERP studies is on a positive-going ERP component that begins at around 400-500ms after word onset and extends for several hundred milliseconds (Citron, 2012; Hajcak, MacNamara, & Olvet, 2010; Kissler, Assadollahi, & Herbert, 2006) – henceforth referred to as the emotional LPC (for Late Positive Complex). While there is debate about the precise functional significance of the emotional LPC (Gable, Adams, & Proudfit, 2015), for the purposes of this paper, we assume that it is likely related to the sustained evaluation of the motivational significance of a salient stimuli (Hajcak et al., 2010; Hajcak, Weinberg, MacNamara, & Foti, 2012; Weinberg & Hajcak, 2011). As such, the emotional LPC has been theorized to be part of the P300 family of components (Crites, Cacioppo, Gardner, & Berntson, 1995; Delplanque, Silvert, Hot, Rigoulot, & Sequeira, 2006), which can reflect the attentional demands of evaluating task-relevant oddball or other similarly salient stimuli (Donchin & Coles, 1988; Nieuwenhuis, Aston-Jones, & Cohen, 2005; Polich, 2012). Importantly, just as for the P300, the sustained evaluative processing reflected by the emotional LPC is not static, and depends on both task and context (Dunning & Hajcak, 2009; Fields & Kuperberg, 2012; Fischler & Bradley, 2006; Hajcak, Dunning, & Foti, 2009; Holt, Lynn, & Kuperberg, 2009; Schindler, Wegrzyn, Steppacher, & Kissler, 2014; Schupp et al., 2007).
Several studies have reported a larger LPC on high arousal than low arousal words (and pictures: Leite et al., 2012), even when valence is kept constant (Bayer et al., 2012; Delplanque et al., 2006; Recio, Conrad, Hansen, & Jacobs, 2014), though others have found no differences (Bayer, Sommer, & Schacht, 2010) or more complex interactions between valence and arousal (Citron, Weekes, & Ferstl, 2013). Importantly, two recent studies (Bayer et al., 2012; Recio et al., 2014) suggest that the LPC arousal effect can also be elicited using neutrally-valenced stimuli, where high-arousal words like “joust”, “scrimmage”, and “samurai” elicit a larger LPC amplitude than low-arousal words like “table” and “sculpture”. High-arousal neutral words have not traditionally been included in many behavioral or ERP studies of emotion processing. However, they are an important component of the Circumplex model (Russell, 1980), where variation in arousal is distinct from variation in valence. Though these dimensions of emotion were derived primarily from factor analyses of how moods and emotional stimuli were overtly evaluated, it's plausible that the circumplex model may also describe how emotional stimuli are actually processed, and the relative orthogonality of valence and arousal processing would be an important indicator to this effect.
There is also evidence for effects of valence on the LPC, independent of arousal. Most have reported a larger LPC on valenced words (both pleasant and unpleasant) than neutral words that are matched for low levels of arousal (Citron, 2012). Others have reported a negativity bias, with a larger (Ito et al., 1998) and/or longer (Hajcak & Olvet, 2008) LPC to unpleasant than pleasant stimuli matched for high levels of arousal (for examples using word stimuli, see Delaney-Busch & Kuperberg, 2013; Fields & Kuperberg, 2012; Holt et al., 2009). Other studies, however, describe larger LPC amplitudes on pleasant than unpleasant arousal-matched stimuli (Bayer et al., 2012; Briggs & Martin, 2009; Herbert, Junghöfer, & Kissler, 2008; Kissler & Herbert, 2013; Kissler, Herbert, Winkler, & Junghöfer, 2009; Recio et al., 2014), and still others have reported an interactive pattern between valence and arousal (Citron et al., 2013; Feng et al., 2014) or no effects at all.
In sum, existing data suggest that while both arousal and valence can independently contribute to behavioral and neural responses, these effects are quite variable. There are several possible reasons for this variability. For instance, some studies have not controlled for potentially confounding factors (discussed by Kissler et al., 2006) such as orthographic neighborhood or concreteness (e.g. Scott et al., 2009; Zhang et al., 2014), although others have used very well controlled stimuli (e.g. Bayer et al., 2012; Recio et al., 2014). Most relevant to the questions of the present study, previous investigations have used different tasks that impose quite different situational demands.
Further. task goals have long been known to influence behavioral reactions to emotional stimuli (see Lai, Hagoort, & Casasanto, 2012 for a particularly elegant example). And, as noted above, consistent with its relationship with the P300, task is also known to modulate the amplitude of the LPC evoked by emotional stimuli (Dunning & Hajcak, 2009; Hajcak et al., 2009; Hajcak, Moser, & Simons, 2006; Hajcak et al., 2012). For example, attending to non-emotional features of words, such as during a word/nonword lexical decision task, reduces the overall effect of emotion on the LPC, while attending to emotional features, such as during explicit valence judgments, increases the effect (Fischler & Bradley, 2006).
The Present Study
While previous studies provide evidence that the emotional relevance of a task can increase sustained evaluative processing to emotional stimuli, it remains unclear whether or how such sustained evaluative processing, as reflected by the LPC, is differentially influenced by the task relevance of the dimensions of emotion: valence and arousal. For instance, how does the arousal effect change when valence is made task-relevant (versus when neither are task- relevant)?
To address these questions, we carried out two ERP experiments using identical sets of stimuli but different tasks. In Experiment 1, participants performed a semantic monitoring task in which neither the valence or arousal properties of the words were overtly task relevant. In Experiment 2, participants explicitly judged the valence of each word. In both experiments, we used a 3 × 2 factorial design that fully crossed three levels of valence (pleasant, unpleasant, and neutral) with two levels of arousal (high and low). This design meant that we not only included low-arousal but also high-arousal neutral words. Such high-arousal neutral words consistently appear in large ratings studies (Bradley & Lang, 1999; Redondo, Fraga, Padrón, & Comesaña, 2007; Võ, Jacobs, & Conrad, 2006; Warriner, Kuperman, & Brysbaert, 2013). Although there remains debate about their precise functional implications (see General Discussion), these high arousal ratings cannot be easily explained by simple valence ambiguity (Bayer, Sommer, & Schacht, 2011), and can be empirically identified in the same manner as any other condition.3 Finally, we also controlled for a number of possibly confounding features across our six conditions, including frequency, concreteness, word length, orthographic neighborhood size, bigram frequency, and word class.
Our primary focus for these experiments was on the specific contributions of valence and arousal to word processing on the LPC, which reflects sustained evaluative processing. We considered two broad possibilities for the influence of task relevance. The first was that similar effects of valence and arousal would be seen in both tasks. This would indicate that sustained evaluative processing of each emotion dimension is evoked by the inherent emotional properties of words, regardless of task relevance (e.g. as a necessary consequence of emotion perception). The second overall possibility was that the relative effects of valence and arousal on the LPC would differ depending on task demands. For example, in Experiment 1, in which emotion was irrelevant to task performance, any sustained evaluative processing might be driven more by the arousal properties of the words than their valence (as discussed below). In Experiment 2, however, where valence is overtly task-relevant, sustained evaluative processing might be driven more by valence (González-Villar et al., 2014) than by their arousal. These findings would indicate that the sustained evaluative processing evoked by emotional stimuli is dynamic, reflecting in part the relevance of valence and arousal dimensions to the current situational demands.
Experiment 1
Experiment 1 aimed to determine when and how the valence and arousal properties of words would influence neural processing when they were both irrelevant to task performance. There is now a large body of literature suggesting that emotional stimuli can capture attention and distract from task requirements in many different experimental contexts (Carretié, 2014; Lang, Bradley, & Cuthbert, 1997; Okon-Singer, Lichtenstein-Vidne, & Cohen, 2013). This is the case even when participants are explicitly asked to ignore the emotional features of such stimuli (Arnell et al., 2007). Much of this ‘distracting’ effect has been attributed to the arousal properties of emotional stimuli (Mather & Sutherland, 2011). In support of this idea, previous ERP studies using a lexical decision task report an effect of arousal (high > low) on the LPC evoked by both valenced (pleasant and unpleasant) words (Carretié et al., 2008; Hinojosa et al., 2010; Hofmann et al., 2009; Kanske & Kotz, 2007) and neutral words (Bayer et al., 2012; Recio et al., 2014). Clearly, arousal does not need to be task-relevant in order to elicit evaluative processing.
What is less clear, however, is whether the valence properties of words can also elicit sustained evaluative processing when they are not relevant to task performance, and whether such processing is also dependent on arousal (e.g. whether both high- and low-arousal words would show the same valence effect when valence is not task relevant). Some previous ERP studies using lexical decision tasks report no effect of valence on the LPC evoked by words that are matched on arousal (Carretié et al., 2008; Hinojosa et al., 2010; Hofmann et al., 2009; Kanske & Kotz, 2007). Others, however, report a larger LPC on pleasant than unpleasant arousal-matched words (Bayer et al., 2012; Recio et al., 2014) — a “positivity bias” — with no clear interaction between arousal and valence.
Most of these previous studies have used a lexical decision task. Categorizing letter-strings as words or non-words, however, does not require full semantic processing (Milberg & Blumstein, 1981): it is possible to decide whether a string of letters is a word or a non-word through recognition of familiar orthographic or phonological features. Moreover, there is evidence that valence information can actually help participants with lexical categorization (Kissler & Herbert, 2013).
To encourage deep semantic processing, in our first experiment, we used a semantic monitoring task in which participants had to decide whether or not each word referred to an animal, and to press a button whenever they saw such a word (animal words were not analyzed but were added as fillers, e.g. Kreher, Goff, & Kuperberg, 2009). This task therefore required participants to access the semantic features of all words, and categorize all words according to group membership. However, neither valence nor arousal were directly relevant to task performance (such a task was also utilized by Fischler & Bradley, 2006).
Based on the previous literature described above, we predicted that high-arousal words would capture attention and elicit sustained evaluative processing, manifesting as a larger LPC to high arousal than low arousal words, regardless of valence. The key question we asked was whether valence would also capture attention and trigger sustained evaluative processing under these task conditions. We considered three possibilities. The first was that valence would act independently of arousal to influence the LPC, (e.g. a positivity bias or negativity bias effect, as with a lexical decision task, see Bayer et al., 2012; Recio et al., 2014). The second was that valence would enhance any effect of arousal, leading to a larger arousal LPC effect on valenced words than on neutral words, indicating that only valenced words might elicit strong arousal effects under such conditions. The third was that there would be no effect of valence at all on the LPC, indicating that when emotion is simply a distraction of the task at hand, arousal effects prevail and valence effects are minimized during the LPC time window.
Methods
Construction of stimuli
Valence (pleasant, unpleasant, neutral) was fully crossed with Arousal (high, low) to generate six conditions in total, see Table 1 for examples. To generate words for each category of Valence and Arousal, a series of rating studies were carried out with participants who did not take part in the ERP experiment. We also collected ratings of Concreteness in order to match our six experimental conditions (see below). Some ratings had been collected for previous published experiments in a similar manner (Delaney-Busch & Kuperberg, 2013; Fields & Kuperberg, 2012; Holt et al., 2009), but the majority were collected specifically for this study. In all rating studies, participants (20-50 per rating study) were recruited through online postings. Informed consent was obtained for all participants, who were compensated for their time. Participants completed a guided practice prior to each survey. They were asked to rate each word on the specified dimension, on a scale of 1-7. Responses were excluded if there was early language exposure other than English, self-reported psychiatric illness, neurological illness, neurological damage including stroke and concussion, or current treatment with psychoactive medication. In addition, “catch” questions were used to identify and omit bots (computer programs designed to automatically complete paid surveys). Finally, outlying participants were omitted from the ratings, as defined by being an average of two standard deviations or more away from the mean rating for each word.
Table 1. Stimulus properties and examples.
Valence, Arousal, and Concreteness were all pre-rated using 7-point likert scales (from “most negative” to “most positive”, “least arousing” to “most arousing”, and “abstract” to “concrete”, respectively). Frequency was defined as the log of the HAL frequency per million (Balota et al., 2007). Length was defined as the number of letters. Number of orthographic neighbors (“Orth”) and the number of wordforms that share the same constrained bigrams (“N2_C”) were drawn from the MCWord database (Medler & Binder, 2005), along with the mean log frequency of the orthographic neighbors (“Orth_F”) and the bigrams (“N2_F”). Values are listed as “mean (standard deviation)”.
| Unpleasant Low Arousal | Neutral Low Arousal | Pleasant Low Arousal | Unpleasant High Arousal | Neutral High Arousal | Pleasant High Arousal | |
|---|---|---|---|---|---|---|
| Valence | 2.26 (0.42) | 4.20 (0.46) | 5.40 (0.36) | 1.97 (0.41) | 4.12 (0.588) | 5.66 (0.394) |
| Arousal | 3.47 (0.43) | 3.34 (0.38) | 3.39 (0.45) | 4.61 (0.56) | 4.43 (0.491) | 4.75 (0.490) |
| Frequency | 7.81 (1.72) | 7.98 (1.82) | 8.23 (2.03) | 7.91 (1.58) | 7.88 (1.806) | 7.86 (1.710) |
| Concreteness | 3.82 (0.91) | 4.01 (1.07) | 3.92 (1.15) | 3.79 (0.95) | 3.87 (1.073) | 3.69 (1.000) |
| Length | 7.06 (2.18) | 7.13 (1.47) | 6.96 (1.98) | 6.92 (1.61) | 7.12 (1.546) | 7.31 (1.514) |
| Orth | 2.06 (2.78) | 1.88 (3.45) | 2.15 (3.15) | 1.73 (3.21) | 1.68 (2.844) | 1.45 (2.458) |
| Orth_F | 8.44 (19.1) | 6.13 (17.3) | 22.90 (50.8) | 13.80 (43.4) | 8.26 (21.1) | 15.85 (56.9) |
| N2_C | 110.31 (89.7) | 132.94 (93.1) | 111.20 (93.6) | 130.75 (108.4) | 124.16 (99.7) | 132.07 (97.5) |
| N2_F | 811.73 (617.2) | 936.24 (678.3) | 1035.03 (806.5) | 1010.47 (765.7) | 884.03 (600.9) | 1001.64 (919.0) |
| Examples | Stingy | Pacify | Serenity | Atrocity | Splashed | Flourish |
| Anxiety | Feminine | Loyal | Brutal | Mythical | Delicious | |
| Ignorance | Random | Peace | Hate | Radical | Success | |
| Gangster | Sculpture | Tulips | Tyrant | Spicy | Caressed | |
| Vomit | Apples | Sapphire | Bombs | Samurai | Fireworks | |
| Garbage | Coffee | Food | Murder | Alien | Champion |
The final stimulus set included 468 experimental words (159 adjectives, 168 nouns, and 141 verbs; no hyphenated words), 78 per condition (3 levels of Valence × 2 levels of Arousal), and 52 animal word fillers (see Table 1 for exemplars). Valence ratings were matched across both levels of arousal, and arousal ratings were matched across all three levels of valence. In addition, concreteness ratings, HAL log frequency values (Balota et al., 2007), orthographic neighborhood size and bigram frequency (Medler & Binder, 2005) were matched across all six conditions. Word class was also matched across conditions, as confirmed by a log-linear analysis of word counts for Valence, Arousal, and Class categories (all ps > 0.3). Finally, word length (number of letters) was calculated for each word, and was matched across levels of arousal, see Table 1.
52 animal words were then distributed among the experimental words, comprising 10% of the total stimulus set of 520. These probe words were matched on word length and HAL frequency to the 468 experimental words (none of which was the name of an animal). While the animal words were all nouns (compared to the experimental materials which were only about one third nouns), the matched word class across experimental conditions prevented word class from confounding Valence and Arousal effects even if participants could use word class as an implicit heuristic to assist in task performance.
ERP study participants
All participants were recruited through online postings at Tuftslife.com. Data from 26 young adults (13 men) were collected. All participants were right-handed native English speakers (having learned no other language before the age of 5) between the ages of 18 and 25. No participants were taking neuropsychiatric medications and none reported a history of psychiatric or neurological disorders or head trauma. All participants had normal or corrected to normal vision. They were compensated for their time and provided informed consent in accordance with the procedures of the Institutional Review Board of Tufts University.
Task and experimental procedure
Each trial started with a “blink sign”, written as “(- -)”, and began when the participant pressed the “advance” button with their right index finger. After pressing this button, a fixation cross appeared at the center of the screen for 1 second, and then disappeared, leaving a blank screen for 500ms. Then, a word was presented on the screen for 800ms. Participants were directed to press the “target” button with their right thumb as quickly as possible if the word was identified as an animal word. For all other words, participants were told not to press a button. Each word was followed by 300ms of blank screen, followed by a pause at another blink sign.
The full stimulus set was divided into 25 self-paced blocks. Between each block, the experiment was paused while “READY” was shown on the screen. Participants were told that they could move their head or hands during this pause only, and that they could continue to the next word by pressing the “advance” button.
EEG recording
Twenty-nine tin electrodes were held in place on the scalp by an elastic cap (Electro-Cap International, Inc., Eaton, OH). Electrodes were also placed below the left eye and at the outer canthus of the right eye to monitor vertical and horizontal eye movements, and on the left and right mastoids. Target impedance was below 5 kΩ for all scalp and mastoid electrode sites and below 10 kΩ for the two eye channels. The EEG signal was collected with a left-mastoid reference, and was amplified by an Isolated Bioelectric Amplifier System Model HandW-32/BA (SA Instrumentation Co., San Diego, CA) with a bandpass of 0.01 to 40 Hz and was continuously sampled at 200 Hz by an analogue-to-digital converter. The stimuli and behavioral responses were simultaneously monitored by a digitizing computer. Trials were rejected if they captured a blink, head movement, disconnected electrode, missing data, or other artifact between 200ms pre-onset and 800ms post-onset. The overall artifact rejection rate was 4.76% (SD 3.91%). Data were then subject to a 15Hz low-pass butterworth filter before analysis, which filtered out frequency domains that were roughly an order of magnitude faster than the a priori amplitude modulations of interest (i.e. the LPC, which is typically averaged over several hundred milliseconds). No additional offline high-pass filter was used due to possibility that the anticipated LPC modulation could induce illusory early effects (Acunzo, Mackenzie, & van Rossum, 2012; Rousselet, 2012).
Statistical analysis
After artifact rejection, the EEG was time-locked to word onset and the amplitudes were averaged into the six word conditions (see Table 1 for examples). All waveforms were analyzed using a -100ms to 0ms baseline.
Following several previous studies using both emotional and non-emotional stimuli (Delaney-Busch & Kuperberg, 2013; Fields & Kuperberg, 2015; Kreher et al., 2009; Osterhout & Holcomb, 1992), we adopted the following approach for systematic statistical analysis: the scalp was subdivided spatially into a number of comparatively-shaped regions, each consisting of three electrodes that were averaged together. Because of the strong a priori expectation that the LPC (and other) components would peak near the midline, we first carried out a “mid-regions omnibus ANOVA” that included five of these regions arranged down the anterior-posterior axis, along the center of the scalp, as shown in dark gray in figure 1. This mid-regions omnibus ANOVA included Region (prefrontal, frontal, central, parietal, and occipital), Valence (pleasant, neutral, unpleasant), and Arousal (high, low) as within-subjects factors. In addition, to extend coverage of the scalp without compromising the simplicity or utility of the a priori mid-regions test, we carried out a “peripheral omnibus ANOVA”. This included a second set of 4 regions along the left and right scalp periphery (as shown in light gray on figure 1). The within-subject factors in this second omnibus ANOVA were Anteriority (anterior, posterior), Hemisphere (left, right), Valence (pleasant, neutral, unpleasant), and Arousal (high, low).
Fig. 1. Scalp regions.
For the purposes of statistical analyses, the scalp was divided into three-electrode regions. Regions in dark gray were part of the mid-regions omnibus ANOVA and regions in light gray were part of the peripheral regions omnibus ANOVA.
For both omnibus tests, significant interactions between Valence and Arousal were followed up by simple effects ANOVAs at each level of each experimental variable. Interactions that involved spatial factors (Region, Anteriority, or Hemisphere) were followed up within each level. Significant simple effects of Valence were also followed by pairwise effect testing using the Fisher-Hayter method. Alpha was set to 0.05 for all hypothesis testing, and all effects were corrected using the Greenhouse-Geisser method, where applicable (Greenhouse & Geisser, 1959).
Results for the LPC time window are reported below. Earlier effects are reported in supplementary materials.
Results
Behavioral responses
Participants' mean accuracy in recognizing the 52 animal words was 94.3% (SD 4.8%), and their mean reaction time was 679ms (SD 66ms). The mean rate of false alarms (button presses to words that were not animals) was 0.3%. All included participants had accuracy rates higher than 75%, mean reaction times lower than 800ms, and three or fewer false alarms.
ERP results
There were no main effects or two-way interactions for Valence and Arousal between 0-100ms or between 100-200ms in either mid-regions omnibus ANOVAs (all ps > 0.3) or peripheral regions omnibus ANOVAs (all ps > 0.1). A P2 component and anterior negativity were apparent in the waveforms, and effects in these time windows are reported in supplementary materials.
LPC: 500-800ms
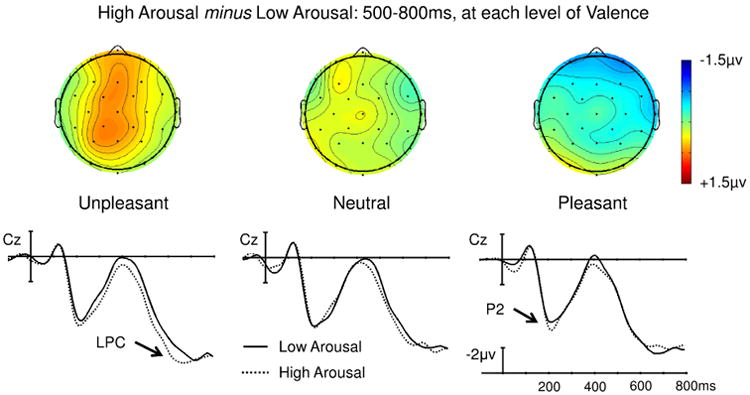
A main effect of Arousal was observed in the mid-regions omnibus ANOVA (F(1,23) = 8.24, p = 0.009) and approached significance in the peripheral omnibus ANOVA (F(1,23) = 4.23, p = 0.051). This effect was driven by a larger LPC to high than low arousal words. An interaction between Arousal and Region also reached significance in the mid-regions ANOVA (F(4,92) = 5.52, p = 0.010). To follow-up this interaction, we assessed the effect of Arousal at each of the mid-regions. The main effect of Arousal was maximal over occipital sites (F(1,23) = 14.6, p < 0.001) and remained significant over parietal, central, and frontal sites (ps < 0.025), but each step towards the anterior decreased the size of the effect (see Figure 2) until it was no longer significant in the prefrontal mid-region (p = 0.495). Main effects and interactions involving Valence did not reach significance in either omnibus ANOVA (see Figure 3).
Fig. 2. Experiment 1, Semantic monitoring task: Effect of Arousal at each level of Valence.
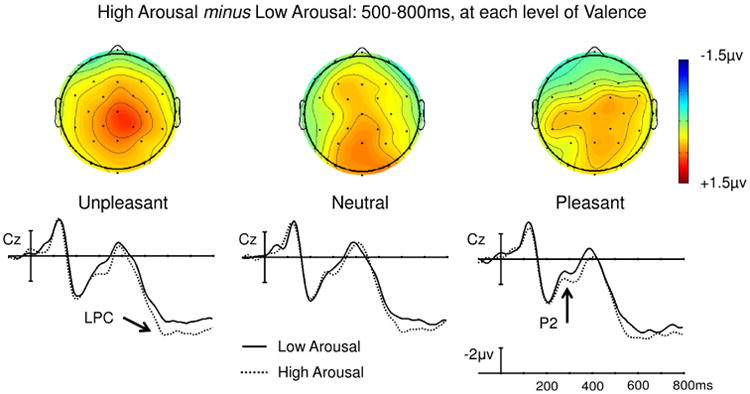
Unpleasant, Neutral, and Pleasant words each yielded an Arousal effect that did not statistically differ in amplitude (as with the waveforms shown for electrode Cz) and appeared to show comparable distributions (as shown with the voltage maps).
Fig. 3. Experiment 1, Semantic monitoring task: Effect of Valence at each level of Arousal.
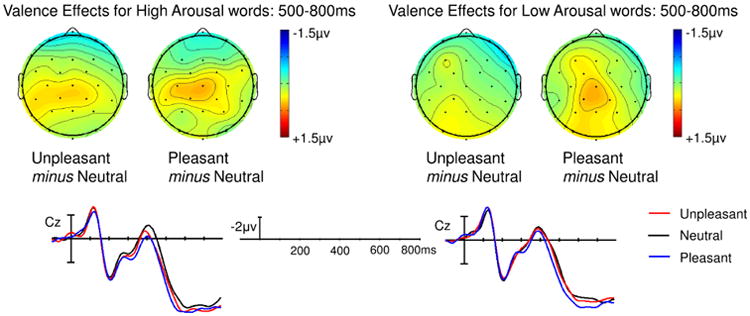
No Valence effects reached significance on the LPC (500-800ms).
Experiment 1 Discussion
Experiment 1 was designed to determine the effects of valence and arousal during the processing of single emotional words when neither dimension of emotion was relevant to the task at hand. We found a main effect of arousal on the LPC. This effect was independent of valence: the arousal effects on pleasant, unpleasant, and neutral words were statistically indistinguishable in their magnitude and direction, and qualitatively appeared to have comparable spatial distributions that were consistent with an LPC effect (see Figure 2).
The clear effects of arousal on the LPC replicates previous work using lexical decision and passive reading tasks (Bayer et al., 2012; Recio et al., 2014). Our findings add to these previous studies by providing evidence that, when emotion was not overtly relevant to task performance, the arousal property of emotional words captured attention and elicited sustained evaluative processing, even when participants were engaged in deep semantic processing. We also found an enhanced P2 to high-arousal (versus low-arousal) words, regardless of valence (reported in supplementary materials), which might reflect an early orientation of attention to these high-arousal words (Crowley & Colrain, 2004; Kanske, Plitschka, & Kotz, 2011). A similar pattern of concurrent P2 and LPC arousal effects across posterior electrodes was observed by Bayer and colleagues (2012) with a lexical decision task.
The absence of any effect of valence or any interaction between valence and arousal on the LPC (or the P2) suggests that, with these task demands, valence alone was not sufficient to elicit sustained evaluative processing. This result differs from what has been reported in some previous studies (Bayer et al., 2012; Recio et al., 2014), where a larger LPC to pleasant words than to unpleasant or neutral arousal-matched words was found. As noted in the Introduction, however, valence may have been implicitly relevant to carrying out the lexical decision task in these experiments (Kissler & Herbert, 2013; Kissler & Koessler, 2011; Kousta et al., 2009; Schacht & Sommer, 2009).
In sum, these findings suggest that, during deep semantic processing, when emotion is not relevant to task performance, arousal, but not valence, captures attention and leads to sustained evaluative processing. The wider implication of this finding is that, considering the similarities and differences to the previous effects seen using lexical decision tasks, the relative contributions of valence and arousal to neural processing may depend on task demands. If this is correct, then valence may trigger sustained evaluative processing if it is made relevant to the task at hand, whereas arousal might have a relatively smaller effect by comparison. Testing this hypothesis was the goal of Experiment 2.
Experiment 2
In Experiment 2, the same stimulus materials were shown to a new group of participants. This time, participants were asked to overtly evaluate the valence of each word, designating them as “pleasant”, “unpleasant”, or “neutral” in a forced-choice categorization task (with no time pressure). Based on previous research demonstrating that ERP emotion effects on the LPC are largest when attention is overtly oriented towards valence (Fischler & Bradley, 2006), we expected a larger LPC to high-arousal valenced words (in general) than to low-arousal neutral words. Our question pertained to how each particular dimension of emotion contributes to this effect. We considered three possibilities.
The first was that, as for Experiment 1, the LPC would be driven primarily by arousal. This would imply that sustained evaluative processing is elicited by the attention-grabbing high-arousal words in a relatively fixed manner, irrespective of task requirements. The second was that the LPC would be driven by a combination of valence and arousal. For example, the effect of arousal on the LPC might be larger for unpleasant and pleasant words than for neutral words. This would suggest that, when valence is task relevant, valence and arousal contribute in tandem to sustained evaluative processing.
The third possibility was that, with this explicit valence evaluation task, the LPC would be driven predominantly by valence instead of arousal. This could appear as a negativity bias (Ito et al., 1998), positivity bias (Bayer et al., 2012), or an overall tendency of valenced words (versus neutral words). This inversion of the main effects found in Experiment 1, using the same stimuli, would provide strong evidence that the relative contributions of valence and arousal to the LPC are, in part, determined by the demands of a given situation, and are not fixed consequences of the words themselves.
Methods
The same 468 experimental words were used in a 3 Valence (pleasant, unpleasant, neutral) × 2 Arousal (high, low) experimental design, but the 52 animal words from Experiment 1 were omitted. Data from 26 young adults (13 men) were initially collected, but 4 participants were rejected because of excessive artifact, leaving 22 participants (11 men, mean age 20.3). Procedures were the same as in Experiment 1, except that participants were directed to classify each word as “positive”, “neutral”, or “negative”. Participants made their responses following a “?” cue, which appeared after the 800ms word presentations (following an interstimulus interval of 300ms), with no time pressure. All responses were made using the right thumb, with the “negative” button slightly to the left of center, “neutral” in the middle, and the “positive” button slightly to the right (to correspond with the intuitions of right-handed participants and reduce errors).
Data processing and statistical analysis of ERP data were the same as in Experiment 1. ERPs were averaged according to the original categorizations (pleasant, neutral, and unpleasant) from the ratings studies in order to retain the careful counterbalancing. However, we also report a post-hoc analysis of ERPs averaged by the idiosyncratic behavioral classifications of each individual participant. Average artifact rejection rate was 5.06% per participant (SD 4.10%).
Results
ERP results
There were no main effects or two-way interactions for Valence and Arousal between 0-100ms or between 100-200ms in either mid-regions omnibus ANOVAs (all ps > 0.3) or peripheral regions omnibus ANOVAs (all ps > 0.1). A P2 component and anterior negativity were apparent in the waveforms, and the effects within these time windows are reported in supplementary materials.
LPC: 500-800ms
A main effect of Valence was significant in both the mid-regions (F(2,42) = 4.62, p = 0.015) and the peripheral regions (F(2,42) = 5.73, p = 0.006) omnibus ANOVAs. The magnitude of this Valence effect varied across the scalp, reflected by interactions between Valence and Region in the mid-regions ANOVA (F(8,168) = 3.42, p = 0.016), and between Valence and Anteriority in the peripheral regions ANOVA (F(2,42) = 6.64, p = 0.003). Follow-up ANOVAs revealed significant effects of Valence only in central, parietal (mid and lateral), and occipital regions (ps < 0.01), due to a larger LPC on unpleasant words than on either pleasant (all ps < 0.05) or neutral (all ps < 0.01) words (see Figure 5). There were no significant differences between pleasant and neutral words in any of these regions. Main effects and interactions involving Arousal were not significant in either omnibus ANOVA (see Figure 4).
Fig. 5. Experiment 2, Valence judgment task: Effect of Valence at each level of Arousal.
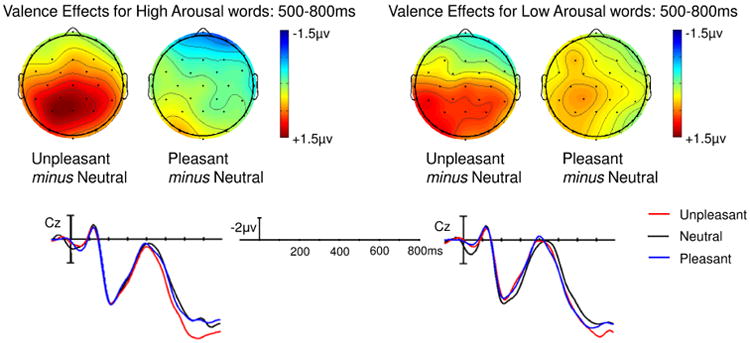
Over central-posterior electrodes, unpleasant words elicited a larger LPC than neutral and pleasant words. This valence effect did not significantly differ between high and low arousal words.
Fig. 4. Experiment 2, Valence judgment task: Effect of Arousal at each level of Valence.
No Arousal effects reached significance on the LPC (500-800ms).
LPC to each participant's valence judgments
We also assessed the Valence effects to ERPs elicited by words individually classified as “positive”, “negative”, or “neutral”, regardless of pre-rated category (see Figure 6). This analysis reflects a test of “perceived valence category” on LPC amplitudes, with the caveat that each participant had a different number of trials per condition, and the careful counterbalancing of potentially confounding factors between conditions was no longer maintained. In addition to main effects of Valence (mid-region: F(2, 42) = 7.85, p = 0.002; peripheral: F(2,42) = 7.94, p = 0.002), we observed interactions between Valence and Region in the mid-regions ANOVA (F(8, 168) = 5.36, p < 0.001) that was echoed by an interaction between Valence and Anteriority in the peripheral regions ANOVA (F(2, 42) = 9.78, p < 0.001). Follow-ups showed that Valence effects were maximal over posterior regions (with parietal, occipital, and posterior peripheral regions showing the most significant effects, with ps < 0.001). Here, however, the pattern was somewhat different from that described above. Once again, unpleasant words evoked a larger LPC than neutral words (all ps < 0.001), but as shown in Figure 6, pleasant words also evoked a larger LPC amplitude than neutral words (all ps < 0.05), and unpleasant words evoked a larger LPC than pleasant words only over the posterior periphery (p = 0.007).
Fig. 6. Experiment 2. Effects of Self-Reported Valence.
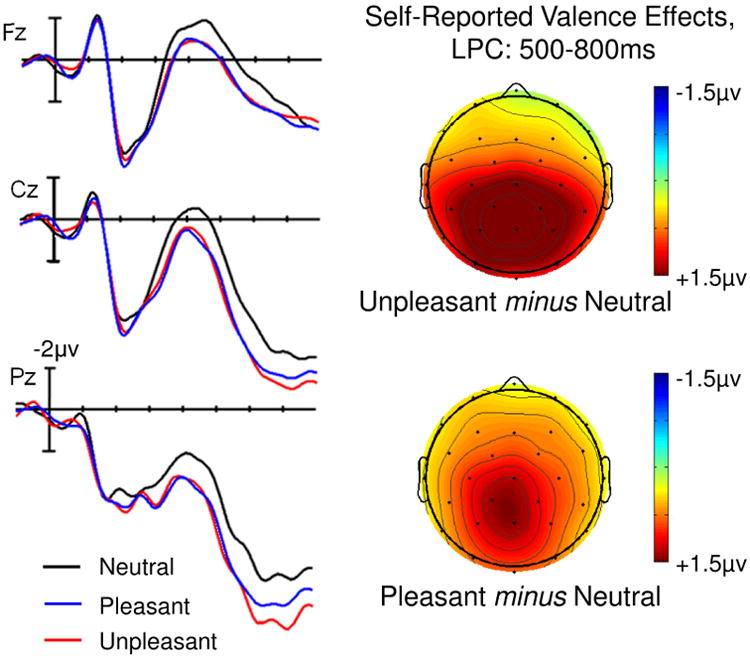
Words that participants categorized as pleasant or unpleasant elicited larger LPCs than words they categorized as neutral.
Relationship between participants' valence judgments and ERP data to pre-rated valence categories
The correspondences between each participant's own valence categorization during the ERP experiment and our prior classification of the three emotional conditions (using the norming data described under Experiment 1) are shown in Table 2a. As seen here, participants tended to misclassify neutral words more often than emotional words. The breakdown of classifications indicates that misclassified neutral words were most likely to be misclassified as “positive” (median 54.0 out of 156 neutral words) rather than as “negative” (median 17.5 out of 156 neutral words).
Table 2. Experiment 2 Valence categorizations.
156 words from each of the pre-rated valence categories were shown to participants (leftmost column in 2a), but some were classified by participants as a different valence from the pre-rated norms. Because the extent of deviation from the pre-rated norms had a skewed distribution, the median number of categorizations is shown in Table 2a instead of the mean. A false positive was defined as a valence judgment that did not coincide with the pre-rated norms. A d′ score (shown here in 2b) was calculated for each person at each level of arousal for both the pleasant versus neutral and the unpleasant versus neutral boundary (omitting data from the third valence category in each case). Values are listed as “mean (standard deviation)”.
| 2a) Median number of Valence categorizations across participants | |||
|---|---|---|---|
| Pre-rated valence category | “Positive” | “Neutral” | “Negative” |
| Pleasant | 127 | 23.5 | 2 |
| Neutral | 54 | 79.5 | 17.5 |
| Unpleasant | 2 | 11 | 140.5 |
| 2b) D′ score for discriminating Valenced words from Neutral words | |||
| Pleasant from Neutral | Unpleasant from Neutral | ||
| High Arousal | 1.68 (0.38) | 2.44 (0.48) | |
| Low Arousal | 1.21 (0.23) | 2.63 (0.46) | |
To quantify the extent to which participants tended to discriminate unpleasant and pleasant words from neutral words, we carried out a d′ analysis (see Table 2b). This confirmed that a participant's ability to discriminate unpleasant and neutral words was significantly better than their ability to discriminate pleasant and neutral words (t = 6.12, p < 0.001 for high arousal words; t = 15.04, p < 0.001 for low-arousal words).
Finally, to directly test the hypothesis that participants' ability to discriminate pleasant or unpleasant words from neutral words was related the size of the LPC effect for that person, we calculated a Pearson's correlation coefficient between each participant's d′ scores (averaged across high and low arousal words) and the amplitude of each participant's pairwise valence effect (unpleasant vs. neutral, and pleasant vs. neutral) in each of the three regions where the LPC was largest (central, parietal, and occipital mid-regions). We found that the more discriminable the unpleasant words were from the neutral words (i.e. the larger the d′ score), the larger the LPC effect to unpleasant (versus neutral) words over the occipital mid-region (rˆ2 = 0.198, p = 0.038). Similarly, the more discriminable the pleasant words were from the neutral words (i.e. the larger the d′ score), the larger the LPC effect to pleasant (versus neutral) words over central (rˆ2 = 0.224, p = 0.026) and parietal (rˆ2 = 0.223, p = 0.027) mid-regions. In other words, the size of the LPC valence effect was directly related to the perceived distinction between pre-rated valence categories (as measured by overt valence judgments).
Between-subjects task effects
To overtly test the influence of experimental task on the processing of valence and arousal, we conducted a group-level ANOVA, with Task as a between-subjects factor and Valence and Arousal as within-subjects factors. To limit unacceptable inflation of the familywise error rate, we restricted our analysis to a single test at the parietal mid-region, where Valence and Arousal effects on the LPC tend to be maximal (Citron, 2012), as was found for the present experiments as well.
The combined data revealed an interaction between Task and Valence (F(2, 88) = 3.88, p = 0.026), indicating that the Valence effect differed significantly between experiments. However, the Task by Arousal interaction did not reach significance (F(1, 44) = 2.66, p = 0.110). The three-way interaction between Task, Valence, and Arousal was also non-significant (F(2,88) = 0.55, p = 0.577). As expected, there were significant within-subjects main effects of both Valence (F(2, 88) = 7.15, p = 0.002) and Arousal (F(1, 44) = 7.75, p = 0.008), with no interaction between the two (F(2, 88) = 1.98, p = 0.146).
Experiment 2 Discussion
Experiment 2 aimed to determine whether valence could influence sustained evaluative processing of the same emotional words which previously only elicited an arousal effect under different task demands. We found this to be the case. There was a clear main effect of valence on the LPC, which was statistically indistinguishable between high-arousal and low-arousal words (Figure 5). This main effect of valence was driven by a larger LPC on unpleasant words, relative to both neutral and pleasant words, over the regions that typically capture the LPC (Hajcak et al., 2010; Hajcak et al., 2012).
The larger LPC amplitude to unpleasant than to neutral words is consistent with most previous studies (Citron, 2012; Kissler et al., 2006). Here, we extend this result to words matched at both high and low levels of arousal. The larger LPC amplitude to unpleasant than to pleasant words is also consistent with several previous ERP studies, both with words (Delaney-Busch & Kuperberg, 2013; Fields & Kuperberg, 2012; Holt et al., 2009) and pictures (Ito et al., 1998) across a variety of valence evaluation and comprehension tasks. This effect has been interpreted as a neural correlate of an inherent attentional bias toward negative stimuli — a ‘negativity bias’ (Carretié, 2014; Carretié, Albert, López-Martin, & Tapia, 2009; Ito et al., 1998). While a negativity bias on the LPC in general is not novel, the present ERP study is, to our knowledge, the first to show a negativity bias for low-arousal words. Specifically, the LPC was larger to low-arousal unpleasant words like “moldy” than low-arousal pleasant words like “bouquet”.
As one might expect, the way any particular individual classified the valence of given word during the ERP experiment did not necessarily mirror our prior ratings-based categorizations of that word. Given that our prior categorizations were based on average ratings, this is not surprising: there is bound to be some variability in how different individuals perceive the valence of different words. For example, while “fireworks” — a word that fell in the pleasant category based on our prior ratings — might be perceived as pleasant by most people, some people might be indifferent to fireworks and perceive the word as neutral, while others may perceive fireworks as startling and altogether unpleasant. Similarly, there was significant natural variation in where individuals implicitly defined the boundary between valence categories while making their judgments. For instance, some participants tended to classify a relatively large proportion of the words as pleasant or unpleasant (indicating that most stimuli were perceived as highly valenced), while other participants tended to classify a relatively small proportion of words as pleasant or unpleasant (indicating that significantly fewer stimuli were perceived as highly valenced). Finally, some discrepancies between our prior ratings-based categorizations and participants' classifications during the ERP experiment may have arisen as a result of differences in task requirements (rating on a likert scale of 1-7 versus classifying into one of three nominal categories during the ERP experiment) and broader experimental context (differences in the nature of the surrounding words).
Examination of the pattern of participants' average behavioral classifications during the ERP experiment revealed some insights into the nature of the negativity bias. It showed that, whereas participants tended to classify the unpleasant words in a way that was consistent with our prior ratings-based categorizations, this was less true of the pleasant words, which were often categorized as neutral (see Table 2a). These data therefore raise the possibility that the larger LPC to unpleasant words than neutral words — the negativity bias, which was also based on average data — was driven by the relatively clear and universal distinction between unpleasant and neutral words (versus between pleasant and neutral words). This suggests that words clearly perceived as pleasant by particular participants may also generally elicit larger LPC amplitudes than words perceived as neutral, regardless of pre-rated norms. Examination of the same ERP data categorized by participant's subjective valence classifications of each word during the experiment itself (rather than our prior ratings-based categorizations) supported this interpretation: the negativity bias disappeared. Instead, the amplitude of the LPC was larger to both unpleasant and pleasant words than to neutral words, with no difference between pleasant and unpleasant words at most sites (Figure 6).
This result carries implications for the interpretation of the negativity bias effect on the LPC. Classically, the larger LPC to unpleasant than pleasant arousal-matched words has been interpreted as reflecting the “tendency for the negative motivational system to respond more intensely than the positive motivational system to comparable amounts of activation” (Ito et al., 1998, p. 888) – a difference in scale among otherwise equivalent valence effects (Rozin & Royzman, 2001; Taylor, 1991). Our data suggest an alternative contribution: that the negativity bias could reflect the extent to which unpleasant words were more distinct or discriminable (from neutral words) than pleasant words given the particulars of the context and demands of the situation.
More generally, these findings also have important implications for the understanding the functional significance of the emotional LPC: they suggest that the LPC might be primarily sensitive to individual participants' perceived, subjective valence. In this study, given the requirement to categorize the words into one of three valence groups, this proxied to each individual's propensity to discriminate valenced words (both pleasant and unpleasant) from neutral words. In support of this interpretation, we found that d′ scores, reflecting each individual's ability to discriminate unpleasant words from neutral words, as well as pleasant words from neutral words, correlated with the magnitude of the LPC effect to both unpleasant and pleasant (versus neutral) words, respectively.
In sum, the valence task led to a robust valence effect, where the LPC may have reflected subjective perceived valence. Taken together with the data from Experiment 1, these data indicate that task can heavily influence the processing of emotional words. We turn to the functional significance of these differences between the two experiments next in the General Discussion.
General Discussion
This study had two related aims. The first was to determine how valence and arousal contributed to the neural processes engaged during the online evaluation of emotional words, using a design that fully crossed two levels of arousal (high, low) and three levels of valence (pleasant, unpleasant, neutral) in a large, carefully controlled sample of words (see also Recio et al., 2014; Bayer et al., 2012). The second was to determine how these effects of emotional properties are influenced by their task relevance. Our findings were clear. We showed that valence and arousal both can elicit an effect on the LPC, but that the particular pattern of their respective contributions depended significantly on task. In Experiment 1, when participants performed a semantic categorization task in which emotion was not relevant to task performance, the LPC showed a large effect of arousal (Figure 2), but no effect of valence (Figure 3). In contrast, in Experiment 2, where participants performed an overt valence categorization task, the LPC showed a large effect of valence (Figure 5), but no effect of arousal (Figure 4).
These data are fully consistent with the large body of previous research reporting overall “emotion” effects to words on the LPC under a broad array of experimental conditions (Citron, 2012; Kissler et al., 2006): in both Experiment 1 and Experiment 2, high-arousal valenced words elicited larger LPC amplitudes than low-arousal neutral words. The results of Experiment 1 are also consistent with previous research reporting effects of arousal within a single level of valence (Bayer et al., 2012; Delplanque et al., 2006; Recio et al., 2014), though we extend these previous findings to a novel task, and to neutral words that also showed an arousal effect comparable to the pleasant and unpleasant words. The results of Experiment 2 are consistent with research reporting LPC effects of valence within a single level of arousal (Bayer et al., 2012; Hajcak & Olvet, 2008; Ito et al., 1998; Recio et al., 2014), though we extend these previous findings to a novel task, and establish that a negativity bias can be elicited using both high and low arousal words. Finally, like previous research (Bayer et al., 2012; Recio et al., 2014), we found no interaction between valence and arousal on the LPC in either experiment, and extend the previous work on the independence between these two factors during word processing to two new tasks.
What is most novel about our findings is that we show, for the first time, that changes in task can lead to robust changes in the relative contributions of valence and arousal to the LPC. Previous ERP studies have reported that “emotion” effects on the LPC elicited by emotional (vs. neutral) words (Fischler & Bradley, 2006) and pictures (Dunning & Hajcak, 2009; Hajcak et al., 2009; Hajcak et al., 2010; Olofsson et al., 2008) can grow or shrink depending on task demands. Other work, such as that by Bayer and colleagues (2012), has reported effects of both valence and arousal on the LPC during a lexical decision task (see also Recio et al.) and a passive reading task, suggesting that valence and arousal may be processed independently during reading. In this study, however, we show that a change in task relevance can completely invert the observed pattern of effects: when emotion was task-irrelevant, only an arousal effect manifested on the LPC, but when valence was overtly relevant, only a valence effect manifested on the LPC. A between-subjects analysis additionally indicated that the valence effect may have been the primary contributor to this difference in outcomes, as the valence effect was significantly larger in the valence task than in the semantic task, while the apparent complementary change in the arousal effect was not distinguishable from chance (meaning that despite the difference in the overall pattern of results, we cannot conclude that arousal effects in semantic categorization tasks are generally larger than arousal effects in valence judgment tasks).
We attribute these differences in ERP modulation across the two experiments to the differences in the relative relevance of valence and arousal to task performance. In Experiment 1, participants needed to make a decision about each word on the basis of its semantic features (whether or not they were animal words), and so the emotional features of each word (both their valence and arousal) were essentially a distraction from effectively carrying out this task. Under these task conditions, high-arousal words seemed to engage attention (as indicated by the P2, see Supplementary Materials) and elicit sustained evaluative processing (as indicated by the LPC), consistent with the known impact of arousal in lure and distraction paradigms (Mather & Sutherland, 2011). In contrast, in Experiment 2, participants made decisions about each word on the basis of its valence, and valenced words elicited more sustained evaluative processing (as reflected by the LPC) than neutral words. The arousal effects on the LPC (and the P2), however, disappeared entirely, possibly because every emotional word was already attended and overtly evaluated as part of the task demands.
It is, however, possible that other differences between the two tasks contributed to the different pattern of effects, such as differences in their requirements for overt motor responses. Specifically, in Experiment 1, although participants made semantic decisions on each word, they were not required to make overt motor response on the experimental items themselves (these were essentially no-go trials). In Experiment 2, however, participants were required to make motor responses on all items. However, this alone is unlikely to have driven the difference in ERP findings. Although the P300 family of components (including the LPC) is thought to be related to decision-making and the identification of salient targets (reviewed by Pritchard, 1981; Twomey, Murphy, Kelly, & O'Connell, 2015), it does not specifically reflect motor response demands or response selection (Mccarthy & Donchin, 1981). Further, in Experiment 2, we introduced a forced delay before button presses on the experimental items in order to reduce any motor contamination on the ERP responses. It is, however, possible that this forced delay introduced a second task-related difference: in Experiment 1, participants were under more time pressure to make their decisions than in Experiment 2. It is possible that this requirement for a speeded response contributed to the selective effect of arousal in Experiment 1 versus 2 by emphasizing haste (while Experiment 2 emphasized more deliberate evaluations).
Neural Implications
These findings have two important neural implications. First, they indicate that the dimensions of valence and arousal can act independently to influence the online neurocognitive evaluation of emotional words. The fact that valence and arousal each modulated a similar LPC component, which qualitatively showed a similar time course and topography across the two experiments, suggests that they may have independently influenced the recruitment of a common neurocognitive mechanism, such as a general re-evaluation or reanalysis of the eliciting stimulus in relation to its context (context updating), as hypothesized for the related P300 component (Donchin, 1981; Donchin & Coles, 1988). Of course, it is possible that distinct neuroanatomical networks contributed to the effect of arousal in Experiment 1 and the effect of valence in Experiment 2: because the spatial resolution of ERPs is poor and multiple sources are likely to contribute to the emotional LPC (Foti, Hajcak, & Dien, 2009) this study alone cannot address this question.
Second, these findings show that the neurocognitive processes elicited by emotional words are not fixed. Rather, we seem to engage in quite different modes of processing to the identical set of words depending on task demands (see Lai et al., 2012 for behavioral evidence for such a malleable and dynamic emotion-evaluation system). Specifically, when emotion was not overtly relevant to task demands, as in Experiment 1, highly arousing stimuli triggered a sustained emotion evaluation from the bottom-up (as indexed by the LPC). However, in situations where emotion was relevant to task demands, sustained evaluative processing was driven primarily by valence, regardless of arousal. This illustrates a subtle but critical theoretical point: dimensions of emotional significance are not strictly a property of the eliciting stimulus, but rather reflect the relationship between the stimulus, the perceiver, and the context (Okon-Singer et al., 2013).
Theoretical Implications
Our findings also have implications for theoretical models discussing how various dimensions of emotional stimuli influence the processing of emotional words. We suggest that they are generally consistent with the Circumplex model of the structure of affect, which proposes that bipolar dimensions of valence and arousal are separate and orthogonal, and each uniquely contributes to aspects of our evaluations of emotional stimuli (Bradley & Lang, 2007; Osgood et al., 1967; Russell, 1980). Although the Circumplex model was initially derived from explicit behavioral semantic evaluations, our data show that these dimensions seem to correspond to online neural processing as well, albeit in a dynamic fashion. In particular, our finding in Experiment 1 of a clear effect of arousal even on neutral items (see also Bayer et al., 2012; and Recio et al., 2014), shows that a stimulus does not need to be clearly pleasant or unpleasant in order for it to lead to prolonged neural processing. It is sufficient for it simply to be arousing.
On the other hand, we think it is likely that “neutral” stimuli are never actually completely devoid of appetitive or aversive significance (Lebrecht, Bar, Barrett, & Tarr, 2012). Instead, we see “neutral valence” as a useful category for stimuli with very low motivational significance, or “micro-valences” (Lebrecht et al., 2012). As such, high-arousal neutral words like “alien” and “rouse” could elicit sustained evaluative processing in a similar manner to high-arousal pleasant and unpleasant words. The activation (arousal) is comparable, while the motivational significance (valence) is smaller (though never completely nonexistent).
In contrast, our data are less consistent with models (such as the Evaluative Space Model or ESM) that have expressed an affect structure in which valence is integrated with arousal during emotion processing (Norris, Gollan, Berntson, & Cacioppo, 2010). These models propose that arousal increases as a natural consequence of increases in pleasantness or unpleasantness. Although this model of affect structure parsimoniously describes much of human and animal behavior (action preparedness often seems to require a direction of motivation - see reviews by Cacioppo & Bernston, 1994; Norris et al., 2010), it would have predicted correlated effects of valence and arousal during word processing in the present study, for which we found no evidence. On the other hand, although we provide evidence against the integration of valence and arousal expressed in the most recently articulated versions of the ESM, the present study does not address whether the structure of valence alone is bipolar (ranging from pleasant to unpleasant) or unipolar (ranging from neutral to pleasant and neutral to unpleasant), which is the much more central hypothesis of the ESM. It remains possible that unipolar “positivity” and “negativity” dimensions remain orthogonal to a distinct arousal dimension during the processing of emotional words.
Finally, these data are also somewhat inconsistent with other interactive accounts of valence and arousal, which have argued that low levels of arousal or pleasant stimuli trigger approach motivations, while high levels of arousal or unpleasant stimuli trigger avoidance motivations (Robinson, Storbeck, Meier, & Kirkeby, 2004). If manifested during word processing, this account may predict that high-arousal pleasant stimuli and low-arousal unpleasant stimuli would elicit larger LPCs than low-arousal pleasant or high-arousal unpleasant stimuli because the motivational signals are in conflict and require extended consideration to resolve the ambiguity. Although some studies have found evidence of this interaction (Citron et al., 2013; Feng et al., 2014), the large studies by Recio et al. (2014) and Bayer et al. (2012) provided contrary evidence using lexical decision and passive reading tasks. The present study now extends this pattern (of no interactions) to semantic categorization and valence evaluation tasks.
Open Questions
Our results and interpretation raise important questions for future research. Particularly, given the apparent malleability of evaluative processing, it will be important to look beyond the role of task instructions to the effects of other aspects of context on the LPC. There is already strong behavioral evidence that local context can dramatically influence emotional word processing (Lai et al., 2012), and recent ERP experiments have shown that the processing of emotional words is influenced by preceding single words (Delaney-Busch, 2013; Herring, Taylor, White, & Crites Jr, 2011), sentence contexts (Ding, Wang, & Yang, 2014; Holt et al., 2009), and discourse contexts (Delaney-Busch & Kuperberg, 2013; Moreno & Vázquez, 2011), with similar findings for pictures (Foti & Hajcak, 2008). It is also possible that the wider structure experimental environment can influence how the LPC is modulated. For example, a recent study manipulated the presence or absence of taboo words within the wider experimental context (Fogel, Midgley, Delaney-Busch, & Holcomb, 2013; manuscript in prep), finding attenuated LPC emotion effects when the surrounding stimuli were more extreme. Local and broad context, manner of presentation, and individual differences between participants remain important and understudied avenues for future research (Kuppens, Tuerlinckx, Russell, & Barrett, 2013).
Conclusions
Our results were clear. When emotion was irrelevant to task performance (Experiment 1), the high-arousal words elicited a larger LPC than low-arousal words, indicating a sustained evaluation of emotional and motivational significance, regardless of valence. When valence was overtly relevant to the given task (Experiment 2), unpleasant words elicited a greater LPC than neutral words, regardless of level of arousal (and words perceived by individuals as pleasant or unpleasant elicited a larger late positivity than words perceived as neutral). As a whole, these data suggest that valence and arousal act independently to influence word processing, and that these dimensions of emotion are not simply useful mathematical derivations of meaning, but that they actually reflect how emotional words are processed by the brain. Above all, these experiments illustrate the importance of task: the evaluative systems underlying the LPC not only wax and wane along with the relevance of emotion in general, but also mediate the relative contributions of valence and arousal to word processing in tune with situational demands.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of Mental Health (R01MH071635 to G.R.K.) and the Sidney Baer Trust. We thank several people who contributed to constructing the experimental materials, to data collection, and technical support, including Arim Choi, Allison Fogel, Vivian Haime, Ju Hyung Kim, and Ann Yacoubian. We also thank Marianna Eddy, Eric Fields, Phil Holcomb, Ellen Lau, Katherine Midgley, Heather Urry, and three gracious anonymous reviewers for their insightful comments and technical guidance.
Footnotes
In the present paper, we will use “pleasant” and “unpleasant” to express levels of valence, in order to distinguish them from positive and negative ERP voltages. This terminology is also recommended in Watson and Tellegen's proposed naming conventions for a bipolar valence dimension (Watson & Tellegen, 1985).
Other ERP studies have reported reliable effects of emotion on an Early Posterior Negativity (or EPN), a tempero-parietal component between 200 and 300 stimuli (Citron, 2012). This has been interpreted as reflecting a prioritized deployment of selective attention towards emotional meaning at an early stage of lexico-semantic processing. However, this is generally seen when data are analyzed using whole-brain average reference electrodes, rather than when using mastoid references (Olofsson, Nordin, Sequeira, & Polich, 2008). The electrode array and averaged mastoid references used in the present experiments were chosen to capture the LPC well, but precluded appropriate analysis of the EPN.
We do not intend to argue that arousal is a meaningful concept in the absence of motivational significance (see General Discussion).
References
- Abelson RP, Sermat V. Multidimensional scaling of facial expressions. Journal of experimental psychology. 1962;63(6):546–554. doi: 10.1037/h0042280. [DOI] [PubMed] [Google Scholar]
- Acunzo DJ, Mackenzie G, van Rossum MC. Systematic biases in early ERP and ERF components as a result of high-pass filtering. J Neurosci Methods. 2012;209(1):212–218. doi: 10.1016/j.jneumeth.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of experimental psychology: General. 2005;134(2):258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Aquino JM, Arnell KM. Attention and the processing of emotional words: Dissociating effects of arousal. Psychonomic bulletin & review. 2007;14(3):430–435. doi: 10.3758/BF03194084. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7(3):465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, et al. Treiman R. The English Lexicon Project. Behavior Research Methods. 2007;39(3):445–459. doi: 10.3758/BF03193014. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5(4):323–370. doi: 10.1037//1089-2680.5.4.323. [DOI] [Google Scholar]
- Bayer M, Sommer W, Schacht A. Reading emotional words within sentences: The impact of arousal and valence on event-related potentials. International Journal of Psychophysiology. 2010 doi: 10.1016/j.ijpsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Bayer M, Sommer W, Schacht A. Emotional words impact the mind but not the body: evidence from pupillary responses. Psychophysiology. 2011;48(11):1554–1562. doi: 10.1111/j.1469-8986.2011.01219.x. doi:10.1111/j.1469-8986.2011.01219.x; 10.1111/j.1469-8986.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- Bayer M, Sommer W, Schacht A. P1 and beyond: Functional separation of multiple emotion effects in word recognition. Psychophysiology. 2012;49(7):959–969. doi: 10.1111/j.1469-8986.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42(1):11–34. doi: 10.1016/S0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings (Vol C-1) Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Bradley MM, Lang PJ. Emotion and Motivation. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. 3rd. 2007. p. 581. [Google Scholar]
- Briesemeister BB, Kuchinke L, Jacobs AM. Emotion word recognition: discrete information effects first, continuous later? Brain research. 2014;1564:62–71. doi: 10.1016/j.brainres.2014.03.045. [DOI] [PubMed] [Google Scholar]
- Briggs KE, Martin FH. Affective picture processing and motivational relevance: arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology. 2009;72(3):299–306. doi: 10.1016/j.ijpsycho.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Bernston GG. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115(3):401–423. doi: 10.1037/0033-2909.115.3.401. [DOI] [Google Scholar]
- Carretié L. Exogenous (automatic) attention to emotional stimuli: a review. Cognitive, affective & behavioral neuroscience. 2014;14(4):1228–1258. doi: 10.3758/s13415-014-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Albert J, López-Martin S, Tapia M. Negative brain: An integrative review on the neural processes activated by unpleasant stimuli. International Journal of Psychophysiology. 2009;71(1):57–63. doi: 10.1016/j.ijpsycho.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Albert J, López-Martin S, De La Gándara BS, Igoa JM, Sotillo M. Modulation of ongoing cognitive processes by emotionally intense words. Psychophysiology. 2008;45(2):188–196. doi: 10.1111/j.1469-8986.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- Citron FM. Neural correlates of written emotion word processing: a review of recent electrophysiological and hemodynamic neuroimaging studies. Brain and language. 2012;122(3):211–226. doi: 10.1016/j.bandl.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Citron FM, Weekes BS, Ferstl EC. Effects of valence and arousal on written word recognition: Time course and ERP correlates. Neuroscience letters. 2013;533(0):90–95. doi: 10.1016/j.neulet.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Crites SL, Jr, Cacioppo JT, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorization: II. A late positive brain potential that varies as a function of attitude registration rather than attitude report. Journal of personality and social psychology. 1995;68(6):997–1013. doi: 10.1037/0022-3514.68.6.997. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clinical neurophysiology. 2004;115(4):732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Delaney-Busch N. The processing of emotional features in single and primed words. (Master's of Science), Tufts University; 2013. [Google Scholar]
- Delaney-Busch N, Kuperberg GR. Friendly drug-dealers and terrifying puppies: affective primacy can attenuate the N400 effect in emotional discourse contexts. Cognitive, affective & behavioral neuroscience. 2013;13(3):473–490. doi: 10.3758/s13415-013-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60(3):315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang L, Yang Y. The dynamic influence of emotional words on sentence processing. Cognitive, affective & behavioral neuroscience. 2014 doi: 10.3758/s13415-014-0315-6. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11(03):357–374. doi: 10.1017/S0140525X00058027. [DOI] [Google Scholar]
- Dunning JP, Hajcak G. See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46(1):28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Feng C, Li W, Tian T, Luo Y, Gu R, Zhou C, Luo YJ. Arousal modulates valence effects on both early and late stages of affective picture processing in a passive viewing task. Social neuroscience. 2014;9(4):364–377. doi: 10.1080/17470919.2014.896827. [DOI] [PubMed] [Google Scholar]
- Fields EC, Kuperberg GR. It's All About You: an ERP study of emotion and self-relevance in discourse. NeuroImage. 2012;62(1):562–574. doi: 10.1016/j.neuroimage.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields EC, Kuperberg GR. Loving yourself more than your neighbor: ERPs reveal online effects of a self-positivity bias. Social cognitive and affective neuroscience. 2015;10(9):1202–1209. doi: 10.1093/scan/nsv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler I, Bradley MM. Event-related potential studies of language and emotion: words, phrases, and task effects. Progress in brain research. 2006;156:185–203. doi: 10.1016/S0079-6123(06)56009-1. [DOI] [PubMed] [Google Scholar]
- Fogel A, Midgley K, Delaney-Busch N, Holcomb PJ. Processing emotion and tabooness in a native vs a second language: an ERP study. Paper presented at the 20th Annual Meeting of the Cognitive Neuroscience Society 2013 [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of cognitive neuroscience. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46(3):521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Gable PA, Adams DL, Proudfit GH. Transient tasks and enduring emotions: the impacts of affective content, task relevance, and picture duration on the sustained late positive potential. Cognitive, affective & behavioral neuroscience. 2015;15(1):45–54. doi: 10.3758/s13415-014-0313-8. [DOI] [PubMed] [Google Scholar]
- González-Villar AJ, Triñanes Y, Zurrón M, Carrillo-de-la-Peña MT. Brain processing of task-relevant and task-irrelevant emotional words: An ERP study. Cognitive, affective & behavioral neuroscience. 2014;14(3):939–950. doi: 10.3758/s13415-013-0247-6. [DOI] [PubMed] [Google Scholar]
- Greenhouse S, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Clinical neurophysiology. 2009;120(3):505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8(2):250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP components. New York: Oxford University Press; 2012. [Google Scholar]
- Herbert C, Junghöfer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45(3):487–498. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Herbert C, Kissler J, Junghöfer M, Peyk P, Rockstroh B. Processing of emotional adjectives: Evidence from startle EMG and ERPs. Psychophysiology. 2006;43(2):197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Herring DR, Taylor JH, White KR, Crites SL., Jr Electrophysiological responses to evaluative priming: the LPP is sensitive to incongruity. Emotion. 2011;11(4):794–806. doi: 10.1037/a0022804. [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Méndez-Bértolo C, Pozo MA. Looking at emotional words is not the same as reading emotional words: Behavioral and neural correlates. Psychophysiology. 2010;47(4):748–757. doi: 10.1111/j.1469-8986.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- Hofmann MJ, Kuchinke L, Tamm S, Võ ML, Jacobs AM. Affective processing within 1/10th of a second: High arousal is necessary for early facilitative processing of negative but not positive words. Cognitive, affective & behavioral neuroscience. 2009;9(4):389–397. doi: 10.3758/9.4.389. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Lynn SK, Kuperberg GR. Neurophysiological correlates of comprehending emotional meaning in context. Journal of cognitive neuroscience. 2009;21(11):2245–2262. doi: 10.1162/jocn.2008.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Cacioppo JT. Variations on a human universal: Individual differences in positivity offset and negativity bias. Cognition and Emotion. 2005;19(1):1–26. doi: 10.1080/02699930441000120. [DOI] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of personality and social psychology. 1998;75(4):887–900. doi: 10.1037/0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain research. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kanske P, Plitschka J, Kotz SA. Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia. 2011;49(11):3121–3129. doi: 10.1016/j.neuropsychologia.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Keuper K, Zwanzger P, Nordt M, Eden A, Laeger I, Zwitserlood P, et al. Dobel C. How ‘love’ and ‘hate’ differ from ‘sleep’: using combined electro/magnetoencephalographic data to reveal the sources of early cortical responses to emotional words. Human brain mapping. 2014;35(3):875–888. doi: 10.1002/hbm.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J, Assadollahi R, Herbert C. Emotional and semantic networks in visual word processing: insights from ERP studies. Progress in brain research. 2006;156:147–183. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C. Emotion, Etmnooi, or Emitoon?--Faster lexical access to emotional than to neutral words during reading. Biological psychology. 2013;92(3):464–479. doi: 10.1016/j.biopsycho.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Winkler I, Junghöfer M. Emotion and attention in visual word processing — An ERP study. Biological psychology. 2009;80(1):75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kissler J, Koessler S. Emotionally positive stimuli facilitate lexical decisions-an ERP study. Biological psychology. 2011;86(3):254–264. doi: 10.1016/j.biopsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Kousta ST, Vinson DP, Vigliocco G. Emotion words, regardless of polarity, have a processing advantage over neutral words. Cognition. 2009;112(3):473–481. doi: 10.1016/j.cognition.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kreher DA, Goff D, Kuperberg GR. Why all the confusion? Experimental task explains discrepant semantic priming effects in schizophrenia under “automatic” conditions: evidence from Event-Related Potentials. Schizophrenia research. 2009;111(1-3):174–181. doi: 10.1016/j.schres.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P, Tuerlinckx F, Russell JA, Barrett LF. The relation between valence and arousal in subjective experience. Psychological Bulletin. 2013;139(4):917–940. doi: 10.1037/a0030811. [DOI] [PubMed] [Google Scholar]
- Lai VT, Hagoort P, Casasanto D. Affective Primacy vs. Cognitive Primacy: Dissolving the Debate. Frontiers in psychology. 2012;3:243. doi: 10.3389/fpsyg.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. Attention and orienting: Sensory and motivational processes. 1997:97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Lebrecht S, Bar M, Barrett LF, Tarr MJ. Micro-valences: perceiving affective valence in everyday objects. Frontiers in psychology. 2012;3:107. doi: 10.3389/fpsyg.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Galdo-Alvarez S, Alves J, Sampaio A, Gonçalves ÓF. Affective picture modulation: Valence, arousal, attention allocation and motivational significance. International Journal of Psychophysiology. 2012;83(3):375–381. doi: 10.1016/j.ijpsycho.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on psychological science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy G, Donchin E. A Metric for Thought - a Comparison of P300 Latency and Reaction-Time. Science. 1981;211(4477):77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An On-Line Orthographic Database of the English Language. 2005 Web Page. [Google Scholar]
- Milberg W, Blumstein SE. Lexical decision and aphasia: evidence for semantic processing. Brain and language. 1981;14(2):371–385. doi: 10.1016/0093-934X(81)90086-9. [DOI] [PubMed] [Google Scholar]
- Moreno EM, Vázquez C. Will the glass be half full or half empty? Brain potentials and emotional expectations. Biological psychology. 2011;88(1):131–140. doi: 10.1016/j.biopsycho.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the p3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131(4):510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biological psychology. 2010;84(3):422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Lichtenstein-Vidne L, Cohen N. Dynamic modulation of emotional processing. Biological psychology. 2013;92(3):480–491. doi: 10.1016/j.biopsycho.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigue S, Michel CM, Murray MM, Mohr C, Carbonnel S, Landis T. Electrical neuroimaging reveals early generator modulation to emotional words. NeuroImage. 2004;21(4):1242–1251. doi: 10.1016/j.neuroimage.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Osgood CE, Suci GJ, Tannenbaum P. The Measurement of Meaning. University of Illinois Press; 1967. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. Journal of Memory and Language. 1992;31(6):785–806. [Google Scholar]
- Peeters G, Czapinski J. Positive-negative Asymmetry in Evaluations: The distinction between affective and informational negativity effects. European Review of Social Psychology. 1990;1(1):33–60. doi: 10.1080/14792779108401856; M3: doi: 10.1080/14792779108401856. [Google Scholar]
- Polich J. Neuropsychology of P300. In: Luck SJ, Kappenman ES, editors. Oxford handbook of event-related potential components. 2012. pp. 159–188. [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89(3):506. [PubMed] [Google Scholar]
- Recio G, Conrad M, Hansen LB, Jacobs AM. On pleasure and thrill: The interplay between arousal and valence during visual word recognition. Brain and language. 2014;134:34–43. doi: 10.1016/j.bandl.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Redondo J, Fraga I, Padrón I, Comesaña M. The Spanish adaptation of ANEW (affective norms for english words) Behavior Research Methods, Instruments & Computers. 2007;39(3):600–605. doi: 10.3758/bf03193031. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Storbeck J, Meier BP, Kirkeby BS. Watch out! That could be dangerous: Valence-arousal interactions in evaluative processing. Personality & social psychology bulletin. 2004;30(11):1472–1484. doi: 10.1177/0146167204266647. [DOI] [PubMed] [Google Scholar]
- Rousselet GA. Does Filtering Preclude Us from Studying ERP Time-Courses? Frontiers in psychology. 2012;3:131. doi: 10.3389/fpsyg.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity Bias, Negativity Dominance, and Contagion. Personality and Social Psychology Review. 2001;5(4):296–320. doi: 10.1207/s15327957pspr0504_2. [DOI] [Google Scholar]
- Russell JA. A Cicumplex Model of Affect. Journal of personality and social psychology. 1980;39(6):1161–1178. doi: 10.1037/h0077714. [DOI] [Google Scholar]
- Schacht A, Sommer W. Time course and task dependence of emotion effects in word processing. Cognitive, affective & behavioral neuroscience. 2009;9(1):28–43. doi: 10.3758/CABN.9.1.28. [DOI] [PubMed] [Google Scholar]
- Schindler S, Wegrzyn M, Steppacher I, Kissler J. It's all in your head - how anticipating evaluation affects the processing of emotional trait adjectives. Frontiers in psychology. 2014;5:1292. doi: 10.3389/fpsyg.2014.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Selective visual attention to emotion. The Journal of neuroscience. 2007;27(5):1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GG, O'Donnell PJ, Leuthold H, Sereno SC. Early emotion word processing: evidence from event-related potentials. Biological psychology. 2009;80(1):95–104. doi: 10.1016/j.biopsycho.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: the mobilization-minimization hypothesis. Psychological Bulletin. 1991;110(1):67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Twomey DM, Murphy PR, Kelly SP, O'Connell RG. The classic P300 encodes a build-to-threshold decision variable. European journal of neuroscience. 2015;42(1):1636–1643. doi: 10.1111/ejn.12936. [DOI] [PubMed] [Google Scholar]
- Võ M, Jacobs A, Conrad M. Cross-validating the Berlin Affective Word List. Behavior Research Methods. 2006;(4):606–609. doi: 10.3758/BF03193892. [DOI] [PubMed] [Google Scholar]
- Vogt J, De Houwer J, Koster EH, Van Damme S, Crombez G. Allocation of spatial attention to emotional stimuli depends upon arousal and not valence. Emotion. 2008;8(6):880–885. doi: 10.1037/a0013981. [DOI] [PubMed] [Google Scholar]
- Wang L, Bastiaansen M, Yang Y, Hagoort P. ERP evidence on the interaction between information structure and emotional salience of words. Cognitive, affective & behavioral neuroscience. 2013;13(2):297–310. doi: 10.3758/s13415-012-0146-2. [DOI] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behavior Research Methods. 2013;45(4):1191–1207. doi: 10.3758/s13428-012-0314-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98(2):219–235. doi: 10.1037/0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of cognitive neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Zhang D, He W, Wang T, Luo W, Zhu X, Gu R, et al. Luo YJ. Three stages of emotional word processing: an ERP study with rapid serial visual presentation. Social cognitive and affective neuroscience. 2014;9(12):1897–1903. doi: 10.1093/scan/nst188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


