Abstract
Background
The mechanisms of right ventricular (RV) failure in pulmonary arterial hypertension (PAH) are poorly understood. Abnormalities in fatty acid (FA) metabolism have been described in experimental models of PAH, but systemic and myocardial FA metabolism have not been studied in human PAH.
Methods and Results
We used human blood and RV tissue and non-invasive imaging to characterize multiple steps in the FA metabolic pathway in PAH subjects and controls. Circulating FFAs and long-chain acylcarnitines were elevated in PAH patients versus controls. Human RV long chain FAs were increased and long chain acylcarnitines were markedly reduced in PAH versus controls. Using proton magnetic resonance spectroscopy, in vivo myocardial triglyceride content was elevated in human PAH versus controls (1.4 ± 1.3 %TG vs. 0.22 ± 0.11 %TG, p = 0.02). Ceramide, a mediator of lipotoxicity, was increased in PAH RVs versus controls. Using an animal model of heritable PAH we demonstrated reduced fatty acid oxidation via failure of palmitoylcarnitine to stimulate oxygen consumption in the PAH RV.
Conclusions
Abnormalities in fatty acid metabolism can be detected in the blood and myocardium in human PAH and are associated with in vivo cardiac steatosis and lipotoxicity. Murine data suggests that lipotoxicity may arise from reduction in fatty acid oxidation.
Keywords: Lipid and lipoprotein metabolism, pulmonary circulation and disease, pulmonary hypertension, right ventricle, lipid toxicity, magnetic resonance spectroscopy, fatty acid
INTRODUCTION
Right ventricular (RV) failure is the predominant cause of death in pulmonary arterial hypertension (PAH), but no RV-specific therapies exist because the underlying mechanisms are poorly understood. Abnormalities of glucose homeostasis and insulin resistance are well described in PAH1–4 but less is known about lipid metabolism despite the interrelated nature of glucose and lipid homeostasis. Abnormalities in fatty acid metabolism have been described in experimental models of PAH5,6 but systemic and myocardial fatty acid metabolism have not been studied in human PAH.
Given the heart’s preference for fatty acids (FA) as an energy source7, understanding FA metabolism may be particularly relevant to understanding RV adaptation to elevated afterload in PAH. We recently showed that RV failure is associated with myocardial steatosis and accumulation of the lipotoxic and pro-apoptotic mediator ceramide in human heritable PAH (HPAH) due to mutation in bone morphogenetic protein receptor type II (BMPR2)8. Others and we have also shown indirect evidence of abnormal fatty acid oxidation (FAO) in experimental models of PAH9–11. The generalizability of these abnormalities in fatty acid metabolism to idiopathic PAH (IPAH) and whether they are a systemic feature in human PAH are unknown.
We hypothesized that reduced fatty acid metabolism is ubiquitous in PAH and associated with lipotoxic cardiac steatosis in the RV. We tested this hypothesis by studying blood, RV tissue, and in vivo myocardial triglyceride imaging in patients with PAH and controls.
METHODS
Study Approval
The Institutional Animal Care and Use Committee of Vanderbilt University School of Medicine approved all animal procedures. Human studies were approved by the Institutional Review Board of Vanderbilt University School of Medicine (IRB numbers 9401, 111157, and 90782). All patients gave written informed consent.
Study Populations
PAH patients for this study were enrolled from the Vanderbilt Center for Pulmonary Vascular Disease. PAH patients were diagnosed by experienced clinicians according to consensus guidelines12, defined as an invasively measured mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg as well as a pulmonary wedge pressure (PWP) or left ventricular end diastolic pressure ≤ 15 mmHg. PAH patient inclusion was restricted to IPAH or HPAH. Control subjects were enrolled in the prospective Vanderbilt Pulmonary Hypertension Research Cohort at Vanderbilt University Medical Center after recruitment via research network email solicitation.
Sample Collection and Analysis
Fasting peripheral blood samples were obtained at the time of clinic visits or at the Vanderbilt General Clinical Research Center. Plasma samples were collected into ethylenediaminetetracetic acid (EDTA) plasma tubes. EDTA tubes were centrifuged within 45 minutes at 4000 rpm and the plasma fraction immediately aliquoted as 200μL aliquots and stored at −80ºC. Plasma acylcarnitine samples were analyzed as described previously 13. The Hormone Assay Core of the Mouse Metabolic Phenotypic Center (MMPC) at Vanderbilt University quantified plasma free fatty acids using standard enzymatic reactions.
RV Gene Expression Array
RNA isolation and Microarray techniques have been described previously 8. All array results have been submitted to the National Center for Biotechnology Information gene expression and hybridization array data repository (GEO, http://www.ncbi.nlm.nih.gov/geo/) as series GSE67492. Arrays were analyzed as previously described14.
Human Tissue Metabolite Studies
Human PAH hearts were obtained at autopsy after informed consent from patients or their families. The right ventricular free wall was immediately dissected and flash frozen in liquid nitrogen. Right ventricular samples from unmatched donor hearts and explanted hearts from patients with non-ischemic dilated cardiomyopathy at the time of transplantation were collected in the operating room and processed and stored in the same manner. Unmatched donor hearts exhibited no gross cardiac defects and no significant abnormal findings on echocardiography. Donor hearts were excluded for transplantation due to one or more of the following reasons: intravenous drug use, abnormal T waves during stroke, possible pericarditis, known coronary artery disease, and history of smoking. All myocardial samples were kept in a −80°C freezer until used for this study. Human RV long-chain acylcarnitines, ceramides, and carnitine palmitoyltransferase I activity were quantified using standard liquid chromatography/mass spectrometric methods as described in the supplemental materials and Supplemental Tables 4 and 5.
Histology
Immunolocalization for ceramide was performed on paraffin-embedded sections of human RV tissue as described in the supplemental materials. Differences in samples size between experiments reflect the tissue available for testing.
Human Tissue Western Blot
Western blots were performed on tissue homogenates according to standard protocols 15. Antibodies for carnitine palmitoyltransferase 1B1 and carnitine palmitoyltransferase 2 were purchased from Novus (product number NBP1-59576).
Transgenic Murine Model
The Rosa26-rtTA2 x TetO7-Bmpr2R899X FVB/N mouse model (called BMPR2R899X for brevity) has been previously described and shown to have RV lipotoxicity 8,16. Expression of the human mutant BMPR2 gene occurs only after administration of doxycycline. Transgene-negative mice were used as littermate controls and were also administered doxycycline. At the time of sacrifice, tissue was harvested and preserved in formalin, snap frozen, or immediately prepared for high resolution respirometry.
Myocardial High Resolution Respirometry
Cardiac Magnetic Resonance and Proton Magnetic Resonance Imaging Protocol
Cardiac imaging and proton spectroscopy were performed between 5/2013-5/2014 on non-fasting subjects. This technique can distinguish triglyceride that is found in the aqueous cardiomyocyte from triglyceride in adipose tissue17. Studies were performed using a 3.0T Philips Achieva (Philips Medical Systems, The Netherlands) equipped with a package for localized cardiac spectroscopy. Imaging parameters and spectroscopy analysis are detailed in the supplemental materials
Statistics
All values are reported as mean ± standard deviation or median (interquartile range) and categorical variables as absolute value and percent unless otherwise stated. Between-group differences were calculated using Mann-Whitney U test or Chi-Square test, as appropriate. The one-way ANOVA and Student’s t-test were used as appropriate to compare myocardial acylcarnitines and gene expression data after log-transformation to achieve a normal distribution. To account for multiple testing using the 17 different acylcarnitines in the human plasma samples, we used a Bonferroni corrected significance threshold of p < 0.003 (0.05/17). Correlation coefficients were calculated using Spearman’s method. Statistical analyses were performed using SPSS 22 software (SPSS Inc, Chicago, IL).
RESULTS
Evidence of Peripherally Detectable Alterations in Fatty Acid Metabolism in PAH
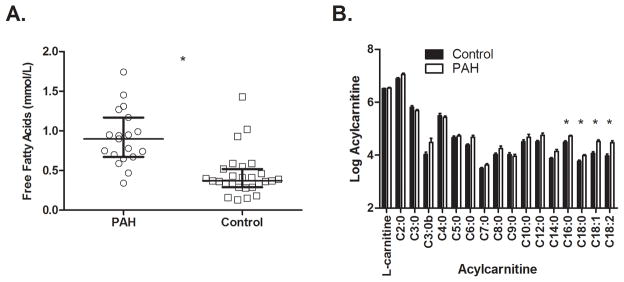
Given the accumulating evidence that PAH is a systemic metabolic disease 18,19, we tested the hypothesis that peripheral blood would demonstrate evidence of altered lipid metabolism. We measured non-esterified FFAs and acylcarnitines, which shuttle fatty acids across membranes and have been associated with insulin resistance and adverse cardiovascular outcomes in other populations13,20. Fatty acid esters of free carnitine, acylcarnitines are classified as short (2 to 4 carbons), medium (5 to 12 carbons) and long chain (defined as ≥ 14 carbons). Control subjects were selected from our prospective registry to achieve a similar distribution of age, gender, and BMI as PAH subjects. Demographic and clinical data for the FFA and acylcarnitine group comparisons are found in Supplemental Table 1. The PAH patients have severe disease based on functional capacity impairment, medical treatment (50% on prostanoid therapy), and advanced RV dysfunction and remodeling. FFAs were nearly two-fold higher in 19 PAH patients compared with 22 control subjects (0.91±0.35 vs. 0.48±0.30, p < 0.001; Figure 1A). Acylcarnitines (C2–C18) were measured in 11 PAH patients and 19 healthy subjects. Because acylcarnitine values can be influenced by renal function, we selected only PAH patients with normal creatinine (0.8±0.2mg/dL). Control subjects were assumed to have normal renal function. Only the long chain acylcarnitines palmitoylcarnitine (C16), stearoylcarnitine (C18), oleoylcarnitine (C18:1), and linoleoylcarnitine (C18:2) reached the pre-specified significance threshold, measuring 1.5–2 fold higher in PAH patients compared with healthy subjects (Figure 1B and Supplemental Table 2; p < 0.003 for all). We found no significant differences in short or medium chain acylcarnitines between PAH and controls. Total acylcarnitines were increased 1.3 fold in PAH compared with controls (p = 0.008). Differences in long-chain acylcarnitines remained significant after adjustment for carnitine levels (Supplemental Table 2). We quantified long-chain fatty acid profiles in the controls and PAH patients with acylcarnitine measurements. The unsaturated fatty acids (C14:0, C:16:0, and C18:0) were lower in patients with PAH versus control subjects, whereas saturated fatty acids (C16:1, C18:1, C18:2) were increased in patients with PAH (Supplemental Table 3).
Figure 1.
Elevated Free Fatty Acids and Long-chain Acylcarnitines in PAH Patients Versus Controls. A. Ratio of fasting free fatty acids (FFA) in PAH versus age, gender, and BMI-matched control subjects. FFAs were nearly two-fold higher in 19 PAH patients compared with 22 controls (*p < 0.001). Between-group comparisons performed using the Mann-Whitney U test and data presented as mean ± standard error. B. Log-transformed long chain acylcarnitines in PAH (n = 11) versus age, gender, and BMI-matched controls (n =19). The long-chain acylcarnitines palmitoylcarnitine (C16), stearoylcarnitine (C18), oleoylcarnintine (C18:1), and linoleylcarnitine (C18:2) were 1.5-2 fold higher in PAH patients compared to control subjects (** p < 0.003 for all). The significance threshold for this experiment was p < 0.003 (0.05/17) after Bonferroni adjustment for multiple comparisons, including all 17 measured acylcarnitines. No other acylcarnitines met the significance threshold. Raw data for all acylcarnitines are provided in Supplemental Table 2. Between-group comparisons performed using the Mann-Whitney U test and data presented as mean ± standard error.
Isolated elevation in long-chain acylcarnitines is a feature of insulin resistance and has been attributed to incomplete FAO20,21, which we corroborate in data presented subsequently. Our PAH population was insulin resistant as measured by the Homeostatic Index of Insulin Resistance (HOMA-IR), but PAH patients did not differ from our controls (3.5 ± 2.7 vs. 3.5 ± 4.6, p = 0.99), who were also insulin resistant. We found no association between FFAs or acylcarnitines and HOMA-IR with our relatively small sample size. We found no association between acylcarnitine levels and RV function. However, when RV function is dichotomized as normal/mild versus moderate/severe, worse RV function was association with a greater than 2 fold increase in HOMA-IR (5.1 ± 3.3 versus 2.3 ± 1.0, p = 0.017) and numerically higher FFAs (0.84 ± 0.25 versus 0.65 ± 0.24, p = 0.14). Worse New York Heart Association class was associated with elevation in the long chain acylcarnitines palmitoylcarnitine (C16), stearoylcarnitine (C18), oleoylcarnitine (C18:1), and linoleoylcarnitine (C18:2) (rs = 0.54, 0.45, 0.57 and 0.45, respectively; p < 0.02 for all; Supplemental Figure 1). These data show that abnormalities in FA metabolism in PAH are demonstrable in plasma and may have an influence on RV function and functional capacity, leading us next to test the effect of these abnormalities on the myocardium directly.
Increased Right Ventricular Long Chain Fatty Acids and Decreased Acylcarnitines in Human PAH
To determine whether elevated peripheral FFAs in PAH were associated with increased lipid accumulation in the myocardium, we measured long-chain fatty acids (LCFA) in human RV tissue in PAH, unmatched donor hearts, and explanted hearts from patients with dilated cardiomyopathy (DCM) and RV dysfunction on echocardiography. DCM patients with RV dysfunction were included as a disease control group to separate the metabolic effects of RV dysfunction generally from PAH-specific effects. LCFAs were generally increased in PAH compared with control, particularly palmitate (C16) and palmiolate (C16:1), which were increased approximately 70% (Figure 2A). In contrast, linoleate (C18:2) was reduced by 31% in PAH. Linoleate must be consumed in the diet; therefore reduced levels in the PAH RV may reflect differences in consumption between groups. Values from DCM hearts were largely intermediate between PAH and controls.
Figure 2.
Long chain fatty acid and Acylcarnitine Profiles in the Human PAH Right Ventricle. A. Long Chain Fatty Acids are Increased in Human PAH Right Ventricle. We measured long-chain fatty acids in human right ventricular tissue from PAH patients (n = 3), unmatched donor hearts (n = 5), and dilated cardiomyopathy patients (n = 2). The long chain fatty acids palmitate (C16), stearate (C18), and oleate (C18:1) were increased by 26–37% in PAH compared with control whereas linoleate (C18:2) was 50% higher in control. Values in the DCM RVs tended to be intermediate between PAH and control but linoleate was similarly elevated. ** P < 0.05 and * P = 0.05 for comparisons between PAH and control. No statistical comparisons were made with DCM group due to availability of only 2 DCM samples. Between-group comparisons performed using the Mann-Whitney U test and data presented as mean ± standard error. B. Long Chain Acylcarnitines are Reduced in the Human PAH Right Ventricle. The long chain acylcarnitines palmitoylcarnitine (C16), stearoylcarnitine (C18), oleoylcarnitine (C18:1), and linoleylcarnitine were markedly reduced (5 to 355-fold lower) in the PAH right ventricle (n = 3) compared with unmatched donor (n = 7) and DCM (n = 6) RVs. Acylcarnitines were measured in ng/mL and log-transformed due to non-normal distribution. **P < 0.05 for all comparisons between PAH and control and ## P < 0.05 vs. DCM. There were no significant differences between DCM and control for any metabolites (p > 0.1 for all). Data presented as mean ± standard error.
The first step in the metabolism of LCFAs to undergo beta-oxidation is the conversion to acylcarnitines, which allows LCFAs to cross the mitochondrial membranes. We therefore quantified human RV long-chain acylcarnitines, which were reduced nearly 100-fold in PAH compared with both control groups (Figure 2B).
These data implicate a fundamental problem in the utilization of LCFAs to produce energy in the PAH RV, despite adequate delivery. Importantly, these observations closely parallel our previous findings in the BMPR2R899X murine model of PAH indicating this model strongly recapitulates the RV metabolic phenotype in human disease22.
Mitochondrial Fatty Acid Transport in PAH
One potential mechanism to explain LCFA excess in cardiomyocytes with suppression of long-chain acylcarnitines is failure to transport FAs into the mitochondria. Using human RV gene expression arrays, we found no difference in the major mitochondrial carnitine transporters carnitine palmitoyltransferase 1 (CPT1), carnitine palmitoyltransferase 2 (CPT2), solute carrier family 22 (organic cation/carnitine transporter) member 5, or carnitine-acylcarnitine translocase in the PAH RV versus dilated cardiomyopathy or unmatched donor RVs (Supplemental Figure 2). In addition, we found similar between-group expression of long-chain acyl-coA dehydrogenase, the enzyme responsible for β-oxidation of LCFAs into acetyl-CoA (Supplemental Figure 2).
Using a mouse model of universal expression of a human BMPR2 mutation associated with PAH (BMPR2R899X)16, we next confirmed normal carnitine transporter protein content in the PAH RV compared with control (Supplemental Figure 3A,B). Finally, we used a mass spectrometry technique to show that CPT1 activity in the human RV was similar among human PAH, DCM, and control RVs (Supplemental Figure 4). CPT1 is the rate-limiting step in acylcarnitine formation and the major point of inhibition of FAO by the glucose oxidation pathway. These data suggest that the mitochondrial transport enzymes may not play a primary role in accumulation of FAs and reduced long-chain acylcarnitines.
Failure of PAH RV Myocardium to Use A Long-chain Acylcarnitine for Oxidative Metabolism
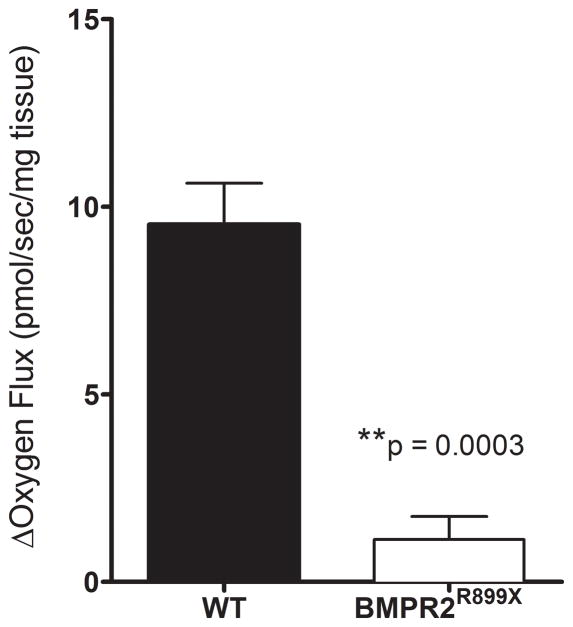
Others and we have previously shown indirect evidence of impaired FAO in the PAH RV but reduced oxygen consumption using a fatty acid substrate has not been shown8–10,23. We next tested directly the ability of the PAH RV mitochondria to utilize a long-chain acylcarnitine as a mitochondrial fuel source. We performed high-resolution respirometry on isolated RVs from BMPR2R899X mice and littermate controls (n = 4 per group). Fatty acid supplementation in this system should increase oxygen consumption via mitochondrial fatty acid oxidation. We found, however, that the proportional mitochondrial oxygen consumption rate attributable to the oxidation of palmitoylcarnitine in the BMPR2R899X RV was reduced nearly 9-fold compared to wild type RV (Figure 3), with the increase over and above Complex I-linked respiration being nearly undetectable in the BMPR2 mutant RV.
Figure 3.
Failure of BMPR2R899X Right Ventricle to Augment Oxygen Consumption with Long-chain Acylcarnitine Supplementation. We performed high-resolution respirometry on freshly isolated RV from BMPR2R899X mice and wild type littermates under baseline conditions and in response to palmitoylcarnitine supplementation (n = 4 per group). When supplemented with palmitoylcarnitine, the wild type right ventricle (RV) augments oxygen consumption rate nearly 9-fold, whereas the PAH RV shows only a modest increase in oxygen consumption (p = 0.0003). Data presented as mean ± standard error. Between-group comparisons performed using the Mann-Whitney U test.
Evidence for Lipotoxicity in Human PAH RV
To determine whether FA accumulation is associated with lipotoxic lipids in the RV in PAH, we stained human tissue for ceramide, a well-described mediator of cardiac apoptosis and lipotoxicity8,24–26. We performed immunohistochemistry analysis in 5 PAH and 8 control RVs demonstrating an increase in ceramide (113% on average) in PAH compared with control RVs (p = 0.006; Figure 4). We validated this finding by mass spectrometry of a long-chain (C16:0) and a very long-chain (C24:1) ceramide (Figure 4). This finding suggests that lipotoxicity is a functional consequence of RV lipid accumulation in human PAH.
Figure 4.

Human Right Ventricular Ceramide in PAH and Controls. A) We performed immunohistochemistry for ceramide and RVs from humans with PAH (n = 5) and control unmatched donor hearts (n = 8). Ceramide was over two-fold higher in the PAH RVs compared with controls (p = 0.006). B) Mass spectrometric quantification confirmed increase of a long chain (C16:0) and very long chain ceramide (C24:1) in the human PAH RV (n = 5) versus controls (n = 6). Ceramides have previously been observed to cause myocardial lipotoxicty in the left ventricle, but have not been reported in the right ventricle. Data are shown as mean ± standard error. Between-group comparisons performed using the Mann-Whitney U test.
Detection of Elevated In Vivo Myocardial Triglyceride Content in PAH Patients
We have previously shown abundant myocardial lipid accumulation in explanted RV samples from patients with HPAH, but whether lipid accumulation occurs in the living human with PAH and whether it is present in living patients with non-end stage disease is unknown. Proton MRS is a non-invasive tool used to quantify in vivo intracellular triglyceride content that has been validated in healthy subjects and patients with obesity and/or diabetes27,28. We performed cardiac proton MRS on 6 PAH subjects (46±19 years, 67% female, NYHA class 1.8±0.8) and 8 healthy controls (34±8 years, 50% female) without diabetes or known cardiopulmonary disease. We demonstrated a high degree of intra-subject reproducibility measured on 2 controls and 1 PAH subject at < 0.05 percent triglyceride %TG. Three PAH patients were on continuous intravenous infusion of epoprostenol and 3 were on oral-only regimens. All controls had %TG levels consistent with published values (range 0.1–0.4 %TG) for healthy subjects17. PAH patients had on average 7 fold higher %TGs compared to controls (Figure 5; 1.4 ± 1.3 %TG vs. 0.22 ± 0.11 %TG, p = 0.02). To put myocardial lipid content in context with functional status, we found a moderate inverse relationship between %TG and six-minute walk distance, however this relationship was not statistically significant (rs = −0.7, p=0.13). Of note, one PAH patient with NYHA Class I functional status and normal RV size and function had a value of 1.5%, 50% higher than average values reported from an obese, diabetic population27. This observation suggests that myocardial lipid accumulation may occur early in the disease process.
Figure 5.

Human Proton Magnetic Resonance Spectroscopy in PAH and Controls. Non-fasting in vivo myocardial triglyceride content was quantified using proton magnetic resonance spectroscopy in PAH patients (n = 6) compared with control subjects (n = 8). Triglyceride content is expressed a percent of triglyceride (%TG) compared with water in a 6cm3 voxel placed in the interventricular septum. A) Representative Cardiac MRI and proton magnetic resonance spectroscopy (MRS) of a control subject demonstrates normal RV function and small lipid peak (LP). PAH patient has RV dilation and hypertrophy with a prominent lipid peak (LP). B) On average, %TG was 7 fold higher in PAH compared with control (1.4±1.3 %TG versus 0.22±0.11, P = 0.02). Intra-patient reproducibility performed on 2 controls and 1 PAH subject was within 0.05%TG. Analysis performed using the Mann-Whitney U test and data presented as mean ± SEM. LP = lipid peak; WP = water peak.
DISCUSSION
Right ventricular failure is the leading cause of death in PAH and often progresses independent of the hemodynamic response to pulmonary vasodilators.29 The lack of available RV-specific therapies largely reflects an incomplete understanding of why RV failure develops in this disease. To date, investigation into the contribution of metabolic dysregulation to RV failure in PAH has been limited to animal models19. Here, we use plasma samples, RV tissue, and non-invasive imaging to show evidence of abnormal systemic and myocardial metabolism of fatty acids in human PAH. We demonstrate that circulating FFAs and long chain acylcarnitines are significantly elevated in PAH patients and associated with FA accumulation in the myocardium. In RV tissue from humans with PAH, we show a two-fold increase in ceramide, a lipotoxic and pro-apoptotic triglyceride metabolite1,26,30. High resolution respirometry in a murine model of PAH suggests that the mechanism underlying lipid accumulation is reduced FAO. Finally, applying proton MRS for the first time in this population, we demonstrate increased in vivo myocardial triglyceride accumulation in PAH.
The emphasis on human data sources in this work provides important, new evidence that animal models of PAH faithfully recapitulate human PAH RV pathophysiology. These observations lend support to the use of animal models of PAH for pre-clinical metabolic interventions. Moreover, we have identified potential therapeutic pathways of interest in humans that can be targeted by existing, well-tolerated metabolic therapies. Finally, we have established a non-invasive imaging tool to study myocardial lipid metabolism in vivo in PAH.
Clinical relevance of the present study
Our data point to two novel mechanisms of myocardial lipid accumulation in PAH: increased delivery and reduced mitochondrial capacity to use FAs to produce energy (Figure 6)31. In LV failure, insulin resistance – highly prevalent in PAH 1,3 – and increased adrenergic tone lead to increased circulating FFAs. FFAs are then delivered to the myocardium in excess of mitochondrial oxidative capacity. As a result, lipotoxic triglyceride metabolites and reactive oxygen species accumulate causing apoptosis26. Our study suggests that abnormal peripheral FA metabolism is coupled with failure of FA utilization at the mitochondrial level. Elevation of saturated long-chain FAs in the plasma in PAH suggests a possible compensatory mechanism for reduced FAO because saturated FAs are more easily oxidized (whereas unsaturated FAs are more readily used for synthesis); however, this explanation is speculative and would require further investigation. The uniform elevation of fatty acids and reduction of acylcarnitines in the RV suggests that the metabolic abnormalities are most pronounced at the tissue level.
Figure 6.
Possible Mechanisms of Right Ventricular Lipotoxicity in Pulmonary Arterial Hypertension. Insulin resistance and adrenergic tone contribute to increased circulating free fatty acids (FA), leading to increased delivery to the myocardium. Inside the cell, FAs can undergo beta-oxidation or be stored as triglyceride. Excess FA delivery in the presence of impaired beta-oxidation leads to FA accumulation. Toxic triglyceride intermediates are generated, leading to cellular and ultimately organ dysfunction. Markedly reduced myocardial acylcarnitines with normal transporter function suggest either shunting of FAs away from mitochondrial transport or acylcarnitine export. Acylcarnitine export has been described from the kidney and liver31, which may explain elevated plasma levels but decreased myocardial levels. Finally, failure of the BMPR2R899X right ventricle to augment oxygen consumption when directly supplemented with a long-chain acylcarnitine implicates a primary abnormality of mitochondrial respiration.
Long-chain acylcarnitines were markedly reduced in the PAH RV despite normal activity, gene expression, and protein content of mitochondrial transport proteins. By supplementing the RV directly with a long-chain acylcarnitine, we showed that failure of FA utilization is not simply a result of excess delivery or suppression by glucose oxidation but a primary abnormality of the mitochondria. Direct supplementation of palmitoylcarnitine to permeabilized RV tissue negates the need for CPT1b activity, which is the major point of inhibition by the glucose oxidation pathway 32. Therefore, when the influence of glucose oxidation is removed from the experiment, FAO remains markedly reduced. Thus paired defects in peripheral and myocardial metabolism appear to contribute to RV failure in PAH and both may be viable therapeutic targets.
This is the first study to demonstrate in vivo myocardial lipid accumulation in PAH. Proton MRS has most widely been used to study the effects of systemic metabolic diseases such as diabetes and obesity on the heart17,33. LV steatosis has recently been observed in the setting of increased afterload (severe aortic stenosis)34, although mean triglyceride content in that study was about half that observed in our PAH cohort. Steatosis decreased after surgical correction of aortic stenosis raising the possibility that steatosis in our study is driven by increased afterload. However, we have previously shown absence of steatosis in a pulmonary artery banding model of isolated RV afterload 8 indicating that pressure elevation alone is not causatory. The inverse relationship between %TG and six minute walk distance in our cohort may reflect an adverse effect of lipid accumulation on both cardiac and peripheral muscle, which we have shown in the BMPR2R899X model8. Of note, we found elevated RV lipid in one PAH patient with no functional limitation and normal RV structure and function. This suggests that steatosis is present in subclinical disease and may be a cause and not just a consequence of RV failure. Studies of larger cohorts are underway to determine the relationship between myocardial steatosis and RV afterload, RV function, and clinical outcomes. It is unclear whether RV lipid deposition might be a modifiable risk factor for right heart failure in PAH. The observation of increased ceramide in the human PAH RV suggests that lipid accumulation may have deleterious functional consequences raising its attractiveness as a therapeutic target.
Elevation of long chain acylcarnitines has also been described in obese, insulin resistant populations. This has been ascribed to increased lipid flux and incomplete FAO, both of which we demonstrated in the PAH RV. We previously showed no association between BMI and insulin resistance in PAH1, indicating that adiposity does not explain the mechanism of insulin resistance in this population. Recent evidence suggests that long chain acylcarnitines may directly impair insulin signaling, but this has not been tested in humans with PAH or experimental models. In contrast to elevated long chain acylcarnitines in plasma, we found reduced RV long chain acylcarnitines. Reverse function of the acylcarnitine transporter has been described as a mitochondrial detoxification function with active transport into the extracellular space 35. It is possible active export is occurring in the myocardium, although our study did not directly test this hypothesis. More likely, elevated plasma acylcarnitines reflects altered lipid metabolism in other highly metabolically active tissue such as liver and skeletal muscle.
Previous studies have shown reduced myocardial and pulmonary vascular FAO in various experimental models of PAH. For example, we have previously shown reduced acylcarnitines and reduced expression of carnitine transporter genes in human pulmonary microvascular endothelial cells expressing a mutation in BMPR211. The RV in the monocrotaline model of PAH demonstrates increased glycolysis and reduced glucose and fatty acid oxidation10. In the SU5416/hypoxia model, genes encoding acyl-CoA dehydrogenases are downregulated 9. It is unclear whether reduced FAO in these models is adaptive or maladaptive. When FAO is further inhibited either genetically (knock-out of malonyl-coenzyme A decarboxylase) or pharmacologically (trimetazidine, dichloroacetate) the PH phenotype and RV function improve 5,36,37. A clinical trial is underway to test the effects of dichloroacetate on RV function in PAH (NCT01083524). Given the inability of the PAH RV to use palmitoylcarnitine as a substrate for FAO in our study, it is unclear whether further suppression of FAO will improve myocardial energetics in humans.
Limitations
We were unable to determine the temporal relationship between plasma abnormalities and RV abnormalities because our PAH cohorts predominantly had advanced disease. Despite this limitation, our cohorts are clinically relevant because many PAH patients present with advanced disease and RV dysfunction38. Our study involves relatively small sample sizes, especially the experiments involving explanted human tissue. These tissues are critical for translation of findings in experimental models, but are difficult to obtain. RV samples from our control populations were obtained at the time of transplant whereas PAH samples were obtained at autopsy. Although lipid species are unlikely to undergo significant degradation in the time to autopsy, we cannot exclude the possibility that differences in time to storage of RV samples influenced our results. We were underpowered to draw any conclusions about the relative abundance of myocardial lipid accumulation in HPAH compared with IPAH, though this is a focus of future studies. Nonetheless, we demonstrated the feasibility of this technique to assess myocardial metabolism in PAH, even in patients on continuous intravenous medications. We measured myocardial lipid accumulation in the interventricular septum because voxel size requirements of the MRS technique do not currently allow direct measurement of the RV free wall. The relative contribution of the RV and LV to the septum is a matter of dispute, but there is evidence that the septum shares a common embryologic origin with the right ventricle39. Regardless, whatever metabolic abnormalities may exist in the RV, they clearly extend to the septum in our study. Finally, we used RV tissue from the BMPR2R899X mouse to measure carnitine transporter protein and oxygen consumption. It is possible that RV metabolism in this model differs from human disease; however, we have shown that fatty acid and acylcarnitine profiles are strikingly similar in the BMPR2R899X and human PAH RVs.
Conclusions
Using human blood, RV tissue, and non-invasive imaging, we demonstrate that human PAH is associated with abnormalities in systemic and myocardial FA metabolism. This study highlights specific metabolic pathways of potential therapeutic interest and establishes a tool to study their activity in vivo. More detailed study of FA metabolic pathways and their functional significance in human PAH is needed to draw conclusions about their potential as therapeutic targets.
Supplementary Material
Clinical Perspectives.
Right ventricular (RV) failure is the predominant cause of death in patients with pulmonary arterial hypertension (PAH), but the underlying mechanisms are poorly understood. Metabolic dysfunction has recently been implicated as a mechanism of RV failure but studies of myocardial metabolism have been limited to experimental models. In this study, we used blood, tissue samples, and a non-invasive imaging technique to demonstrate that human PAH is associated with circulating and myocardial markers of abnormal fatty acid metabolism. The importance of this work for the practicing clinician is that some of the abnormalities we identified may be effectively targeted with existing and well-tolerated metabolic therapies. Further studies are needed to identify the mechanisms of reduced fatty acid oxidation in the PAH RV, but our findings may support pursuing early phase clinical trials using metabolic therapies to target insulin resistance and fatty acid oxidation. We also identified in vivo myocardial lipid accumulation in humans with PAH using proton magnetic resonance spectroscopy. This technique offers a non-invasive means of studying myocardial lipid metabolism and may be a valuable adjunct to clinical outcomes in future studies of metabolic therapies in PAH. Lastly, metabolic abnormalities in the human PAH RV recapitulate prior findings in experimental models providing support for using animal models to study RV failure in PAH.
Acknowledgments
The authors would like to thank Yan Ru Su, MD, Tarek Absi, MD, and Kelsey Tomasek, BS for assistance in collecting human right ventricular samples.
Funding Sources: American Heart Association Fellow to Faculty Grant #13FTF16070002, Pulmonary Hypertension Association Proof-of-Concept Award, and Actelion Entelligence Young Investigator Award (Brittain), National Institutes of Health [K08 HL093363 (Hemnes), K23 HL098743 (Austin), K08 HL121174 (Fessel), R01 122417-01 (Hemnes), 1P0 HL108800-01A1 (Brittain, Hemnes, Pugh, Newman, Austin), and NCRR/NIH [1 UL1 RR024975 (Vanderbilt)]. This work was supported in part by the Core Laboratory for Cardiovascular Translational and Clinical Research and the Vanderbilt Heart Bio-repository.
Footnotes
Disclosures: ELB reports research funding from Actelion Pharmaceuticals through the Entelligence program. ARH reports a grant from Pfizer and serves as a consultant to Pfizer, United Therapeutics. JPF reports research funding from Actelion Pharmaceuticals through the Entelligence program. MEP is a Gilead advisory board member. All other authors report no conflicts of interest
References
- 1.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–911. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for Insulin resistance in experimental pulmonary hypertension. Eur Resp J. 2013;41:861–871. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Resp J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokhari S, Raina A, Berman Rosenweig E, Schulze PC, Bokhari J, Einstein AJ, Barst RJ, Johnson LL. PET Imaging May Provide a Novel Biomarker and Understanding of Right Ventricular Dysfunction in Patients With Idiopathic Pulmonary Arterial Hypertension. Circ Cardiovasc Imaging. 2011;4:641–647. doi: 10.1161/CIRCIMAGING.110.963207. [DOI] [PubMed] [Google Scholar]
- 5.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JRB, Michelakis ED. Fatty Acid Oxidation and Malonyl-CoA Decarboxylase in the Vascular Remodeling of Pulmonary Hypertension. Science Transl Med. 2010;2:44ra58–44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 6.Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med (Berl) 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T, West J. Evidence for Right Ventricular Lipotoxicity in Heritable Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2014;189:325–34. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, Remedios dos CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, Chalfant CE, Bigbee J, Abbate A, Lesnefsky EJ, Bogaard HJ, Voelkel NF. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6:136–144. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2010;88:1011–1020. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, Rhee EP. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc. 2013;2:e000542. doi: 10.1161/JAHA.113.000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin ED, Menon S, Hemnes AR, Robinson LR, Talati M, Fox KL, Cogan JD, Hamid R, Hedges LK, Robbins I, Lane K, Newman JH, Loyd JE, West J. Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression. Pulm Circ. 2011;1:389–398. doi: 10.4103/2045-8932.87308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West JD, Austin ED, Gaskill C, Marriott S, Baskir R, Bilousova G, Jean J-C, Hemnes AR, Menon S, Bloodworth NC, Fessel JP, Kropski JA, Irwin D, Ware LB, Wheeler L, Hong CC, Meyrick B, Loyd JE, Bowman AB, Ess KC, Klemm DJ, Young PP, Merryman WD, Kotton D, Majka SM. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol, Cell Physiol. 2014;307:C415–30. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 17.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac Steatosis in Diabetes Mellitus: A 1H-Magnetic Resonance Spectroscopy Study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 18.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 20.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Brittain EL, Talati M, Zhu H, West J, Fessel JP, Penner N, Funke M, Lewis GD, Gerszten RE, Hamid R, Pugh ME, Austin ED, Newman JH, Hemnes AR. Dysregulated Fatty Acid Metabolism in Pulmonary Arterial Hypertension is Associated with Right Ventricular Steatosis and Lipotoxicity. Circulation. :A18041. Abstract. [Google Scholar]
- 23.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013;91:1315–1327. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 24.Park T-S, Hu Y, Noh H-L, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang X-C, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10–18. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 28.Reingold JS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005;289:E935–E939. doi: 10.1152/ajpendo.00095.2005. [DOI] [PubMed] [Google Scholar]
- 29.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 30.Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FGS, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lysiak W, Toth PP, Suelter CH, Bieber LL. Quantitation of the efflux of acylcarnitines from rat heart, brain, and liver mitochondria. J Biol Chem. 1986;261:13698–13703. [PubMed] [Google Scholar]
- 32.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 34.Mahmod M, Bull S, Suttie JJ, Pal N, Holloway C, Dass S, Myerson SG, Schneider JE, De Silva R, Petrou M, Sayeed R, Westaby S, Clelland C, Francis JM, Ashrafian H, Karamitsos TD, Neubauer S. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging. 2013;6:808–816. doi: 10.1161/CIRCIMAGING.113.000559. [DOI] [PubMed] [Google Scholar]
- 35.Violante S, Ijlst L, Brinke Te H, Tavares de Almeida I, Wanders RJA, Ventura FV, Houten SM. Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J. 2013;27:2039–2044. doi: 10.1096/fj.12-216689. [DOI] [PubMed] [Google Scholar]
- 36.Piao L, Fang Y-H, Cadete VJJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piao L, Sidhu VK, Fang Y-H, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, Archer SL. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013;91:333–346. doi: 10.1007/s00109-012-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deaño RC, Glassner-Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg-Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;173:887–893. doi: 10.1001/jamainternmed.2013.319. [DOI] [PubMed] [Google Scholar]
- 39.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.