Abstract
Patients with schizophrenia often suffer from sleep disturbances such as excessive sleeping and insomnia. Common medications for schizophrenia can have a sedative effect on patients. Not all antipsychotic medications have the same sedative effect, which is related to dosage and affinity for histamine H1 receptors. Studies have shown that, compared with conventional antipsychotics, atypical antipsychotics such as risperidone, olanzapine, quetiapine, and ziprasidone generally cause less sedation yet are as effective in controlling psychosis and agitation. Sedation can be troublesome to patients who are trying to become reintegrated into society and interfere with their treatment regimen. Both persistent sedation and chronic insomnia can be managed by the physician.
Sleep disturbances, including insomnia, excessive sleeping, and changes in sleep patterns, are frequent in patients with schizophrenia and many other psychiatric disorders. The actual process of sleep is also different in patients with schizophrenia compared with that in individuals with no psychiatric disorders. In addition, medications commonly given for psychosis can worsen excessive sleeping by sedating patients. Selecting a less sedating antipsychotic for patients with excessive sleeping and managing sleep disturbances carefully can improve outcome for antipsychotic-treated patients.
SLEEP DISTURBANCES IN ANTIPSYCHOTIC-TREATED PATIENTS
Sleep disturbances in schizophrenia have been well studied. Tandon et al.1 examined sleep disturbances in 40 patients with schizophrenia and found they had a longer sleep latency, a higher number of arousals during sleep, and increased periods of wakefulness after sleep onset compared with those in a nonpsychiatric control group, resulting in a lower sleep efficiency rating for the patients with schizophrenia. The control group had a 95% sleep efficiency, while drug-naïve schizophrenic patients had a 78% sleep efficiency rating and previously treated patients had a 72% sleep efficiency rating.
Benson and Zarcone2 studied sleep in 18 schizophrenic patients and 13 nonpsychiatric control subjects. They found that the individuals with schizophrenia had an increase in the duration of stage 1 sleep compared with the control group, as well as a decrease in the duration of stages 3 and 4 (slow wave) sleep. The patients with schizophrenia had a total sleep efficiency rating of 83%, while the control group had a sleep efficiency rating of 95%. Because of these changes in sleep patterns, patients with schizophrenia often have inadequate sleep.
In addition to these sleep difficulties caused by schizophrenia, the antipsychotic medication a patient is taking may be causing sedation. Sedation is a common occurrence with conventional antipsychotics, but its effects can sometimes be mistaken for the negative symptoms of schizophrenia such as avolition, amotivation, asociality, withdrawal, and anhedonia. Before treating a patient for these symptoms, it is important to determine whether they are caused by antipsychotic medication or by the disorder itself. Sedation can also be mistaken for cognitive impairment. It may be difficult to determine whether an individual's cognitive impairment is caused by schizophrenia or the antipsychotic medication.
Sedation can impair a person's ability to function normally over the long term. It may interfere with the efforts of patients who are trying to go back to work or school or otherwise have normal socialization. Sedation can also prevent patients from benefiting from other treatments such as psychosocial training and psychiatric rehabilitation.
Sedation is an especially common problem in elderly patients who take antipsychotic medications. Elderly patients become more heavily sedated and are sedated for longer periods of time than younger patients taking the same doses of the same medications. Sedation can impair arousal levels during the day and lead to an increased number of falls.
ANTIPSYCHOTICS AND SLEEP DISTURBANCES
The physician's choice of antipsychotic medication can affect the patient's sleep disturbances. A study of sleep measures in schizophrenic patients3 who were receiving either the atypical antipsychotic risperidone alone (N = 5) or the conventional antipsychotic haloperidol alone (N = 5) revealed a significant difference in slow wave sleep between the 2 groups. Patients taking risperidone spent 27% of their time asleep in slow-wave sleep, while patients taking haloperidol spent 20% in slow-wave sleep. This was the only significant difference between the 2 groups (p < .05).
Risperidone may lengthen the amount of slow wave sleep in patients because it has a higher affinity for serotonin 5-HT2 receptors than does haloperidol.4 5-HT2 receptors have been reported to be involved in controlling sleep quality.5 Olanzapine, another atypical antipsychotic, also has a high affinity for 5-HT2 receptors.4 Thus, while the sedative effect of some antipsychotic medications may have a negative impact on patients, atypical antipsychotics such as risperidone and olanzapine may have the potential to improve the quality of sleep in individuals with schizophrenia.
ANTIPSYCHOTICS AND SEDATION
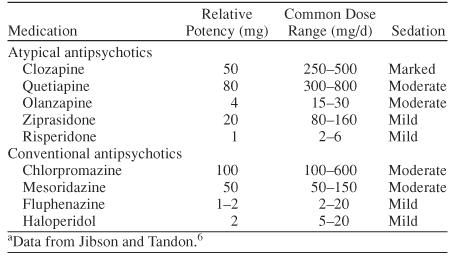
Sedation is a common effect of conventional antipsychotics, especially when they are taken at high doses. Some of the atypical antipsychotic medications can also cause sedation, although it is generally less frequent and less severe than with conventional antipsychotics. Not all conventional antipsychotics have the same sedative effect, nor do all atypical antipsychotics. In general, the high-milligram, low-potency antipsychotics, such as chlorpromazine and mesoridazine, produce more sedation than the low-milligram, high-potency antipsychotics such as haloperidol and fluphenazine (Table 1).6 This principle tends to hold true for the atypical antipsychotics as well. For example, the high-potency, low-dose atypical antipsychotic risperidone is less sedating than the lower-potency, high-dose atypical antipsychotics quetiapine and clozapine. However, dose does not always determine sedation. Olanzapine, which has a common dose range of 15 to 30 mg/day, is more sedating than ziprasidone, which has a common dose range of 80 to 160 mg/day.
Table 1.
Comparison of Potency, Dose, and Sedation in Antipsychotic Medications
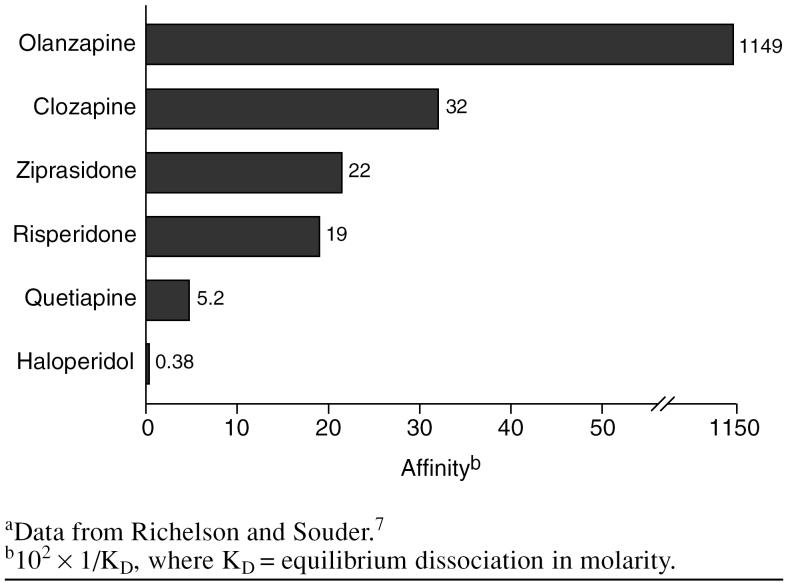
Studies have indicated that sedation may also be related to the affinity of the medication for the histamine H1 receptors. The antipsychotics vary in their ability to block these receptors.4,7 A study by Richelson and Souder7 of the binding profiles of antipsychotic medications found that olanzapine has the highest affinity for the histamine H1 receptors, followed by clozapine (Figure 1). This may explain why olanzapine has a relatively large sedative effect even though it is a high-potency medication. Of the antipsychotics studied, haloperidol had the lowest affinity for the histamine H1 receptors. Quetiapine and risperidone had the lowest affinity of the atypical antipsychotics.
Figure 1.
Affinity of Antipsychotic Medications for the Histamine H1 Human Brain Receptora
Although both dosage and affinity for histamine H1 receptors play a part in the sedative effect of a medication, what ultimately determines sedative effect is the amount of the drug reaching the histamine H1 receptors in the central nervous system. For example, quetiapine, which has little affinity for the histamine H1 receptors, is a less potent antipsychotic medication and requires many more milligrams to be effective than do higher-potency medications such as risperidone and ziprasidone. Because of this, quetiapine has a greater sedative effect on patients in clinical use than do risperidone and ziprasidone.
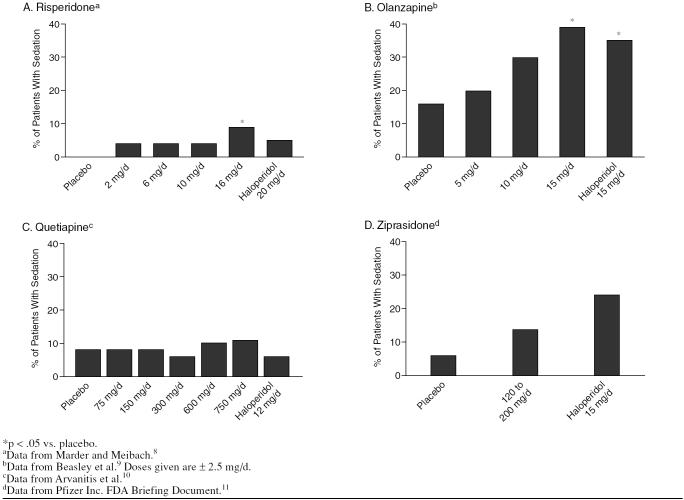
When analyzing the relative effects of several medications, it is useful to choose studies that used a second medication and a placebo as controls. For example, the sedative properties of risperidone and other atypical antipsychotics can be seen by comparing their effects with those of haloperidol in clinical trials (Figure 2).
Figure 2.
Sedation Rates of Atypical Antipsychotics Compared With Placebo and Haloperidol
During a study of risperidone for the treatment of schizophrenia,8 4.5% of patients taking 20 mg/day of haloperidol (N = 66) reported somnolence. Of the patients taking risperidone, 3.2% taking 2 mg/day (N = 63) reported somnolence, 3.1% taking 6 mg/day (N = 64) reported somnolence, and 3.1% taking 10 mg/day (N = 65) reported somnolence. Of the patients taking 16 mg/day of risperidone (N = 64), 9.4% reported somnolence. Patients taking placebo reported no somnolence.
In a study of olanzapine,9 20% of patients (N = 65) taking a range of low doses of olanzapine (2.5, 5, or 7.5 mg/day) reported somnolence, while 29.7% of patients (N = 64), taking a range of medium doses of olanzapine (7.5, 10, or 12.5 mg/day) reported somnolence, and 39.1% of patients (N = 69) taking a range of high doses (12.5, 15, or 17.5 mg/day) reported somnolence. In comparison, 34.8% of patients (N = 69) taking a range of 10, 15, or 20 mg/day of haloperidol reported somnolence. Among patients taking placebo (N = 68), 16.2% reported somnolence.
In a study of quetiapine,10 8% of patients taking either 75 mg/day (N = 53) or 150 mg/day (N = 48) reported somnolence, 6% of patients taking 300 mg/day (N = 52) reported somnolence, 10% of patients taking 600 mg/day (N = 51) reported somnolence, and 11% of patients taking 750 mg/day (N = 54) reported somnolence. Six percent of patients taking 12 mg/day of haloperidol reported somnolence, as did 8% of those taking placebo.
In a study on the treatment-emergent adverse events of ziprasidone,11 14.4% of patients taking 120 to 200 mg/day (N = 702) reported somnolence, while 23.5% of patients taking 15 mg/day of haloperidol (N = 85) reported somnolence, and 6.6% of patients taking placebo (N = 273) reported somnolence.
In each of these studies, the atypical antipsychotic (risperidone, olanzapine, quetiapine, and ziprasidone) caused less sedation than did the conventional antipsychotic used for comparison, haloperidol, except in the highest doses. Physicians can avoid sedation and still have a range of medications to choose from in treating their patients.
Controlling Positive Symptoms Without Sedation or Agitation
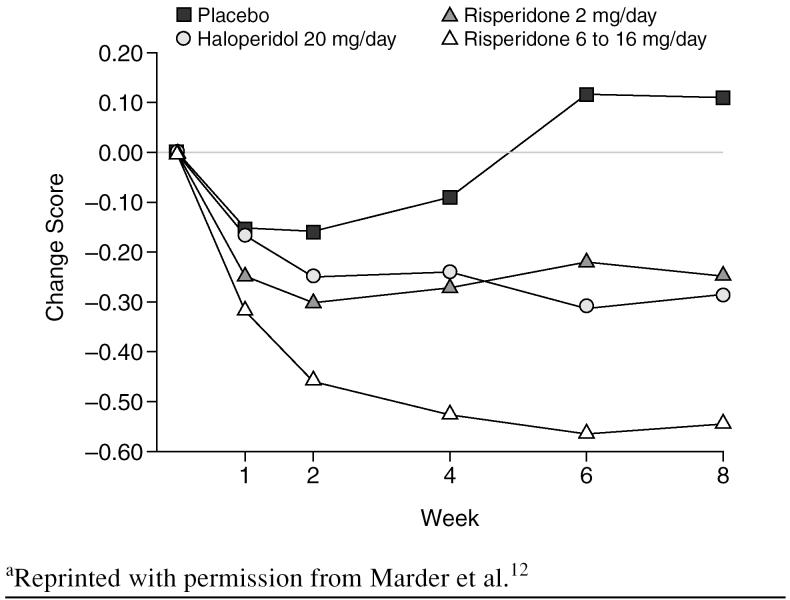
Sedation used to be considered necessary for the efficacy of antipsychotic medications in controlling the positive symptoms of schizophrenia, such as psychosis, but, with the atypical agents, psychosis and acute agitation can be controlled without sedation. A review by Marder et al.12 of 2 North American trials of risperidone found that it was as effective as haloperidol at reducing the positive symptoms of schizophrenia. Two-mg/day and 6- to 16-mg/day doses of risperidone were compared with a 20-mg/day dose of haloperidol. The 2-mg/day dose of risperidone had approximately the same effect on positive symptoms of schizophrenia as the 20-mg/day dose of haloperidol (Figure 3). The severity of positive symptoms improved similarly in the 2-mg/day risperidone-treated group and the haloperidol-treated group, according to the changes in scores on the positive symptoms factor of the Positive and Negative Syndrome Scale (PANSS). Neither haloperidol nor risperidone has a high affinity for H1 receptors, but risperidone is effective at lower doses, which may make a difference in its sedative effect on patients.
Figure 3.
Mean Changes in Scores on the Positive Symptoms Factor of the Positive and Negative Syndrome Scalea
When the effects of risperidone and haloperidol on uncontrolled hostility and excitement were compared,12 20 mg/day of haloperidol and 2 mg/day of risperidone produced a similar reduction in symptoms on the PANSS subscale. Risperidone causes little sedation, which indicates that it can effectively reduce hostility and excitement through a mechanism other than sedation.
As shown in Figure 2, results from the studies on quetiapine,10 olanzapine,9 and ziprasidone11 are similar: psychosis can be effectively controlled with atypical antipsychotics, while generally causing similar or less sedation than conventional antipsychotics.
The atypical antipsychotics have also been found to be as effective against agitation as conventional antipsychotics, with the possibility of less sedation. Currier and Simpson13 conducted an open-label study in patients with acute psychosis presenting to the emergency department who received either 2 mg of oral liquid concentrate risperidone and 2 mg of oral lorazepam or 5 mg of intramuscular (IM) haloperidol and 2 mg of IM lorazepam. Both groups showed similar improvements in agitation with no significant difference in somnolence.
Battaglia et al.14 studied the calming versus sedative effect of IM olanzapine in agitated patients. In a double-blind study, 131 patients were given 10.0 mg of IM olanzapine, 126 patients were given 7.5 mg of IM haloperidol, and 54 patients were given IM placebo. Response to the medications was measured using the excited subscale of the PANSS and the Agitation Calmness Evaluation Scale (ACES), which measures agitation, calmness, and sedation on a scale of 1 to 9, with 1 being the most agitated and 9 being the most sedated. Even when participants whose ACES scores were higher than 6 (moderate calmness) were removed from the results, patients treated with IM olanzapine and IM haloperidol showed significant reductions in scores on the PANSS subscale, indicating that sedation is not necessary to reduce symptoms of agitation.
TREATMENT OF SLEEP DISTURBANCES
Sedation and chronic insomnia are common sleep disturbances found in patients treated with antipsychotics. Both can be controlled with proper treatment.
Sedation
If sedation is bothersome to patients taking antipsychotic medications, physicians can take steps to minimize it. According to the 1999 Expert Consensus Guidelines on the treatment of schizophrenia,15 physicians should consider eliminating other sedating agents from the patient's list of medications. This includes antidepressants, such as the tricyclics and mirtazapine, and mood-stabilizing medications such as valproic acid. Instructing the patient to take his or her medication at bedtime can also reduce daytime sedation. If the entire dose cannot be given at bedtime, then the majority of the dose should be taken at night. If necessary, the physician should consider reducing the dose of the antipsychotic medication, but this should be done slowly and cautiously. The physician could also consider switching the patient to a less sedating antipsychotic. Also, the physician might consider checking the patient for hypothyroidism, which can cause individuals to feel sedated. If these efforts do not work, caffeine or bupropion might help the patient feel more alert. Many patients taking antipsychotic medications drink several cups of coffee every morning to feel less sedated.
The 1999 guidelines recommended prescribing stimulants for patients who were persistently sedated, but this has become highly controversial. Generally, I do not recommend prescribing stimulants for psychotic patients because sedation can usually be controlled using other means and the physician may be held liable for the patient's actions while medicated with stimulants.
A medication that has recently emerged as an option to treat drug-induced sedation is modafinil. The treatment mechanism of modafinil is unknown, and although it is a schedule IV controlled substance, it is not a stimulant. It has been used successfully in clinical settings to combat sedation, but there is concern that it may worsen psychotic symptoms. Modafinil was reported to have exacerbated psychosis in 1 patient who was taking a dose of 200 mg 4 times daily,16 but no adverse effects were reported in 3 patients who were taking 200 mg/day along with antipsychotic medications.17
Chronic Insomnia
Physicians also need to treat patients who suffer from the opposite problem of sedation, chronic insomnia. According to the 1999 Expert Consensus Guidelines, the physician could switch the patient to a more sedating antipsychotic such as olanzapine, quetiapine, or clozapine. However, sedation alone should not be a reason to switch to clozapine. The physician should also consider adding a sedative to the patient's medications. Benzodiazepines are helpful, but caution should be taken when prescribing to patients with comorbid substance abuse disorders. Other available sedatives include trazodone, zolpidem, chloral hydrate, and antihistamines such as diphenhydramine and hydroxyzine.
CONCLUSION
Sleep disturbances and sedation are common in patients with schizophrenia. There are differences in the actual sleep process between patients with schizophrenia and individuals with no psychiatric disorders. Many antipsychotic medications cause sedation, but not all medications have the same sedative effect. Sedation is related to the amount of medication reaching the central nervous system, which is determined by dosage and the drug's affinity for histamine H1 receptors. Atypical antipsychotics often cause less sedation than do conventional antipsychotics while providing similar or greater reduction in symptoms. Sedation can cause problems for patients and is often unnecessary, as both psychosis and agitation can be managed without sedation. If persistent sedation is a problem, there are steps that physicians can take to minimize the sedative effect of antipsychotic medication. Chronic insomnia can also be treated by the physician.
Drug names: chlorpromazine (Thorazine, Sonazine, and others), clozapine (Clozaril and others), diphenhydramine (Benadryl, Ambenyl, and others), fluphenazine (Prolixin, Permitil, and others), haloperidol (Haldol and others), hydroxyzine (Vistaril, Atarax, and others), mesoridazine (Serentil), mirtazapine (Remeron and others), modafinil (Provigil), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal), trazodone (Desyrel and others), valproic acid (Depakene, Myproic Acid, and others), ziprasidone (Geodon), zolpidem (Ambien).
Footnotes
This article was derived from the planning roundtable “Using Atypical Antipsychotics in Primary Care,” which was held June 2–4, 2002, in Washington, D.C., and supported by an unrestricted educational grant from Janssen Pharmaceutica, L.P.
Disclosure of off-label usage: The author of this article has determined that, to the best of his knowledge, diphenhydramine and hydroxyzine are not approved by the U.S. Food and Drug Administration for the treatment of sedation and modafinil is not approved for counteracting antipsychotic-induced sedation.
REFERENCES
- Tandon R, Shipley JE, and Taylor S. et al. Electroencephalographic sleep abnormalities in schizophrenia: relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992 49:185–194. [DOI] [PubMed] [Google Scholar]
- Benson KL, Zarcone VP. Rapid eye movement sleep eye movements in schizophrenia and depression. Arch Gen Psychiatry. 1993;50:474–482. doi: 10.1001/archpsyc.1993.01820180076008. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Morinobu S, and Yamawaki S. et al. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Res. 2002 109:137–142. [DOI] [PubMed] [Google Scholar]
- Collaborative Working Group on Clinical Trial Evaluations. Measuring outcome in schizophrenia: differences among the atypical antipsychotics. J Clin Psychiatry. 1998 59suppl 12. 3–9. [PubMed] [Google Scholar]
- Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;378:164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- Jibson MD, Tandon R. An overview of antipsychotic medications. CNS News Special Edition. 2001;3:49–54. [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors: focus on newer generation antipsychotics. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–835. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Tollefson G, and Tran P. et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996 14:111–123. [DOI] [PubMed] [Google Scholar]
- Arvanitis LA, Miller BG. and the Seroquel Trial 13 Study Group. Multiple fixed doses of Seroquel (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry. 1997 42:233–246. [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. Briefing Document for Zeldox Capsules (ziprasidone HCl). Submitted to FDA Psychopharmacological Drugs Advisory Committee. 19July2000 Available at: http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3619b1a.pdf. Accessed Aug. 1, 2003. [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Currier GW, Simpson GM. Risperidone liquid concentrate and oral lorazepam versus intramuscular haloperidol and intramuscular lorazepam for treatment of psychotic agitation. J Clin Psychiatry. 2001;62:153–157. doi: 10.4088/jcp.v62n0303. [DOI] [PubMed] [Google Scholar]
- Battaglia J, Lindborg S, and Alaka K. et al. Calming versus sedative effects of intramuscular olanzapine in agitated patients. Am J Emerg Med. 2003 21:192–198. [DOI] [PubMed] [Google Scholar]
- Expert Consensus Guideline Series. Treatment of Schizophrenia 1999. J Clin Psychiatry. 1999 60suppl 11. 1–80. [PubMed] [Google Scholar]
- Narendran R, Young CM, and Valenti AM. et al. Is psychosis exacerbated by modafinil? [letter]. Arch Gen Psychiatry. 2002 59:292–293. [DOI] [PubMed] [Google Scholar]
- Makela EH, Miller K, Cutlip WD II. Three case reports of modafinil use in treating sedation induced by antipsychotic medications [letter] J Clin Psychiatry. 2003;64:485–486. doi: 10.4088/jcp.v64n0420h. [DOI] [PubMed] [Google Scholar]