Abstract
Purpose
Many patients cannot effectively increase water intake and urine volume to prevent urinary stones. Tolvaptan, a V2 receptor antagonist, blocks water reabsorption in the collecting duct and should reduce urinary supersaturation (SS) of stone forming solutes, but this has never been proven.
Materials and Methods
We conducted a double blind, randomized, placebo-controlled, crossover study in 21 adult calcium urinary stone formers stratified as majority calcium oxalate(CaOx, n=10) or calcium phosphate(CaP, n=11). Patients received tolvaptan 45 mg/day or placebo for 1 week, followed by a washout week and crossover to tolvaptan or placebo for week 3. A 24h urines was collected at the end of weeks 1 and 3.
Results
Tolvaptan vs. placebo decreased urinary osmolality (204±96 vs 529±213 mOsm/kg, P<0.001) and increased urinary volume (4.8±2.9 vs 1.8±0.9 L, P<0.001). The majority of urinary solute excretion rates including sodium and calcium did not significantly change, although oxalate secretion slightly increased (23±8 to 15±8 mg/24h, P = 0.009). Urinary CaOx SS (−0.01±1.14 vs 0.95±0.87 DG, P<0.001), CaP SS (−1.66±1.17 vs −0.13±1.02 DG, P<0.001) and Uric Acid SS (−2.05±4.05 vs −5.24±3.12 DG, P=0.04) all dramatically decreased. Effects did not differ between CaOx and CaP groups (P>0.05 for all interactions).
Conclusions
Tolvaptan increases urine volume and decreases urinary SS in calcium stone formers. Further study is needed to determine if long term use of V2 receptor antagonists results in fewer stone events.
Keywords: fluid intake, kidney stones, urinary concentration, vasopressin, water
INTRODUCTION
Urinary stone disease (USD) is a common metabolic disorder with a prevalence of 7.2 – 7.7% in the adult population, and a 10 year recurrence rate of 30% or more.1, 2 The costs for treatment of USD exceed $10.3 billion.3 Evidence suggests that USD incidence is increasing in industrialized countries with an estimated global prevalence now between 10%–15%.4 The precise biologic pathways to calcium stone formation are many.5 However, a common underlying feature is often increased urinary supersaturation (SS) for calcium oxalate (CaOx), calcium phosphate (CaP), and/or uric acid (UA).6
Increasing urinary volume is a useful measure to decrease kidney stone risk since this will dilute the components that drive urinary SS. Indeed, a recent meta-analysis7 confirmed that increasing urinary volume by drinking more fluid reduced the risk of both incident as well as recurrent events. Nevertheless, not all patients can effectively increase fluid intake despite consistent counseling.
Tolvaptan, an orally administered arginine vasopressin (AVP) V2 receptor antagonist, causes dose-related polyuria.8 Thus urinary free water losses increase. This in turn leads to a slight increase in serum osmolality and stimulates thirst and increased water intake to replace that lost in the urine.8 Therefore, tolvaptan could potentially induce urinary stone patients to drink more fluid. However, the effect of tolvaptan on urinary excretion of calcium, oxalate and other determinant of urinary SS is untested. This study was performed to evaluate the net result of short-term tolvaptan on overall urinary SS and the excretion of key components of calcium-based stones.
MATERIALS AND METHODS
This randomized, double-blind placebo-controlled, crossover study was conducted at the Mayo Stone Clinic in Rochester, MN between September, 2013 and January 2015. The Mayo Institutional Review Board approved the study (Clinical Trials.gov NCT02096965).
Study Population
We enrolled 21 adult (age ≥18 years) idiopathic calcium kidney stone formers. Patients were stratified to achieve near equal numbers of majority CaOx and CaP. Patients with chronic kidney disease (estimated glomerular filtration rate (GFR) < 60 ml/min/1.73m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation); history of hypo- or hypernatremia, hypotension or orthostatic dizziness, or congestive heart failure; and pregnant women were excluded. Stones were confirmed by computerized tomography (CT) and analysis by infrared spectroscopy in the Mayo Metals Laboratory.
Tolvaptan Regimen and Dosage
After informed written consent subjects received tolvaptan (Samsca®) or placebo in a randomized order. A daily dose of 45 mg (30 mg at 8 AM and 15 mg at 4 PM) was chosen since this dose had adequate tolerability (96% patients willing to adhere to this regimen for the rest of their lives) and efficacy (60% of patients maintaining persistently hypotonic urines throughout the 24 hours) in a phase 2 autosomal dominant polycystic kidney disease (ADPKD) trial (156-04-250).9, and this dose could be safely initiated in outpatients.10 This varied dose is designed to produce a maximal AVP inhibition on waking with a gradual fall-off of effect during overnight to allow better sleep.
Patients were maintained at each phase for one week since to allow for a new steady state (Figure 1).11 All patients completed a baseline 24-hour urine collection, received drug or placebo for one week, and then completed another urine collection. After a washout week a third week of drug or placebo (whichever they did not receive initially) and a final urine collection followed (Figure 1). The order of drug and placebo were randomized. Serum electrolytes were monitored at each stage of the study. All patients were instructed to drink to thirst and maintain their usual diet. No medications were altered, including those already taken for urinary stone disease.
Figure 1. Study design.
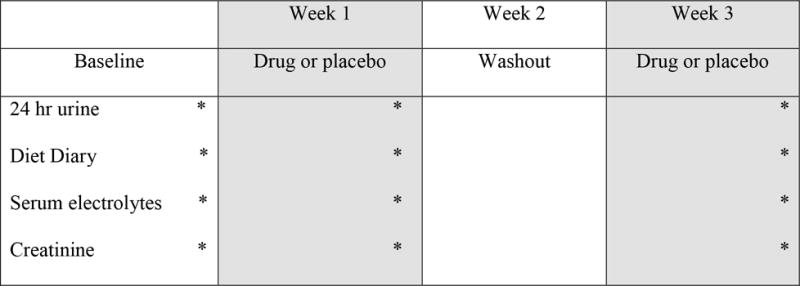
Subjects collected two baseline 24 urine samples, were randomized to placebo or tolvaptan for one week, then completed 2 more 24 hour urine collections. After a washout week subjects crossed over tolvaptan or placebo, then completed two final 24 hour urine collections.
Outcomes
The primary outcome was urinary CaOx and CaP SS on drug versus placebo. Secondary outcomes included urinary UA SS; volume and osmolality; urinary excretions of calcium, oxalate, citrate, uric acid, and phosphate; urinary pH; and serum sodium.
Urinary concentrations (24 h) of oxalate, calcium, and other determinants of SS were measured in the Mayo Clinic Renal Testing Laboratory. Serum electrolytes were measured in the Mayo Central Clinical Chemistry Laboratory. SS was calculated using the EQUIL2 program.12
Statistical analysis
Continuous variables were reported as means with standard deviations (SD) or medians with interquartile ranges (IQR), as appropriate. All categorical variables are reported as counts with percentages. In the event of missing information, data was not imputed. A Wilcoxon matched-pairs signed-ranks test was used to compare the endpoint changes while on drug versus placebo. Linear regression was used to test for the potential effect of the baseline level and a treatment order effect. The primary endpoint analysis was performed for the entire group to maximize power. Interactions between treatment group and stone type (CaOx vs CaP) on urinary SS profiles were assessed by adding interaction terms to the linear regression models. A two-sided P value <0.10 was considered statistically significant for the carryover effect test, since this test has low power. Otherwise, a two-sided P value of <0.05 was considered statistically significant. All analyses were performed using JMP statistical software (version 9.0, SAS, Cary, NC). A sample of 20 patients provided 80% power to detect a 50% change in urinary phosphate and in urinary oxalate.13
RESULTS
Overall 22 unique patients were recruited. One patient with CaOx stones withdrew prior to randomization due to an unrelated foot injury. Characteristics of the remaining 21 patients (10 CaOx and 11 CaP) including baseline 24-hour urine collections for SS profile are summarized in Table 1. Mean age was 50±14 years and 38% were men. Baseline kidney function was normal (serum creatinine 0.9±0.2 mg/dL and eGFR 87±18 ml/min/1.73 m2). Of the 21 patients 3 (14%) were maintained on citrate and 10 (48%) on thiazides (6 on long acting thiazides).
Table 1.
Demographic and clinical characteristics
| Parametera | Total (n=21) | Calcium Oxalate (n=10) | Calcium Phosphate (n= 11) |
|---|---|---|---|
| Age (year) | 50±14 | 56±11 | 45±14 |
| Male sex (n, %) | 8 (38) | 6 (60) | 2 (18) |
| Race, Caucasian (n, %) | 19 (90) | 9 (90) | 10 (91) |
| BMI (kg/m2) | 29±7 | 30±6 | 27±8 |
| Hypertension (n, %) | 5 (24) | 4 (40) | 1 (9) |
| Diabetes mellitus (n, %) | 2 (10) | 2 (20) | 0 (0) |
| Baseline Creatinine (mg/dL) | 0.9±0.2 | 0.9±0.2 | 0.8±0.2 |
| eGFR (ml/min/1.73m2) | 87±18 | 82±13 | 91±22 |
| Thiazide use (n, %) | 10 (48) | 5 (50) | 5 (45) |
| Citrate use (n, %) | 3 (14) | 2 (20) | 1 (9) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
All values presented as mean±s.d., unless otherwise specified.
Urinary SS and Concentration of Calcium Oxalate and Calcium Phosphate SS
Ten patients initially received tolvaptan and 11 placebo. One patient withdrew after the placebo phase due to a possible acute stone event. Urine samples from one study visit of two patients were not received by the laboratory. A test for treatment order effects found no statistically significant carryover effect on urine SS elements (P>0.10 for all study outcomes).
No trial related adverse events were observed in either the tolvaptan or placebo time periods. Serum sodium increased slightly on tolvaptan (142±3 vs 141±2 mEq/L, P = 0.005). Other electrolytes did not significantly change (Table 2).
Table 2.
Comparison of Blood Chemistry Tests and Urinary Analyte Excretion
| Parametera | Placebo (n= 20) | Tolvaptan (n= 19) | P* |
|---|---|---|---|
| Serum Na (mEq/L) | 141±2 | 142±3 | 0.005 |
| Serum K (mEq/L) | 4.1±0.5 | 4.0±0.5 | 0.34 |
| Serum Cl (mEq/L) | 102±3 | 102±3 | 0.33 |
| Serum Bicarb (mEq/L) | 28±3 | 27±3 | 0.29 |
| Serum BUN (mg/dL) | 15±4 | 14±5 | 0.27 |
| Serum Cr (mg/dL) | 0.9±0.2 | 0.9±0.2 | 0.21 |
| Urine volume (ml/24h) | 1833±900 | 4794±2866 | <0.001 |
| Urine pH | 6.3±0.6 | 6.5±0.4 | 0.34 |
| Osmolarity, mOsm/kg | 529±213 | 204±96 | <0.001 |
| Ammonia (mmol/24h) | 33±38 | 26±15 | 0.93 |
| Calcium (mg/24h) | 148±106 | 106±55 | 0.25 |
| Chloride (mmol/24h) | 108±72 | 91±44 | 0.39 |
| Citrate (mg/24h) | 413±267 | 391±209 | 0.73 |
| Creatinine (mg/24h) | 1096±1088 | 838±321 | 0.80 |
| Magnesium (mg/spec) | 76±53 | 87±42 | 0.26 |
| Oxalate (mg/24h) | 15±8 | 23±8 | 0.009 |
| Phosphate (mg/24h) | 714±814 | 560±306 | 0.77 |
| Potassium (mmol/24h) | 46±46 | 51±31 | 0.28 |
| Sodium (mmol/24h) | 111±56 | 97±55 | 0.35 |
| Uric acid (mg/24h) | 473±372 | 384±218 | 0.47 |
| Calcium oxalate SS (DG) | 0.95±0.87 | −0.01±1.14 | <0.001 |
| Calcium phosphate (BR) SS (DG) | −0.13±1.02 | −1.66±1.17 | <0.001 |
| Uric acid SS (DG) | −2.05±4.05 | −5.24±3.12 | 0.04 |
Abbreviations: DG, delta Gibbs; SS, supersaturation.
All values presented as mean±s.d., unless otherwise specified.
P values were analyzed using wilcoxon matched-pairs signed-ranks tests in 19 patients who completed crossover study.
Urine SS profiles on placebo were comparable to those at baseline. After tolvaptan treatment urine osmolality fell and volume increased (Figure 2a–2b and Table 2). In general, urinary solute excretions did not change (Table 2). Thus urinary CaOx, CaP and UA SS all fell significantly (Table 2). An increase in urinary oxalate excretion (23±8 vs 15±8 mg/24h, P = 0.009) was also noted while patients were on tolvaptan (Table 2).
Figure 2. Urine osmolality and volume response to treatment.
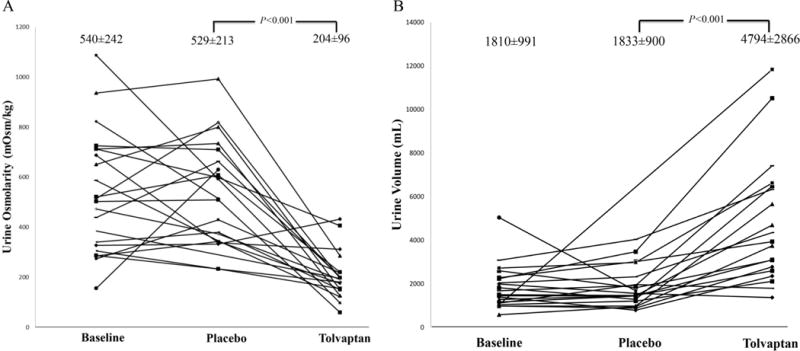
Urine osmolality (Panel A) and volume (Panel B) at baseline, on placebo and on tolvaptan. Each line represents a single patient.
There were no statistically significant interactions between treatment group and type of stone (CaOx vs CaP) and their relationship with urinary CaOx SS, CaP SS, UA SS and all other urine SS components (P> 0.05 for all interactions).
DISCUSSION
In this study short term use of the oral V2 receptor antagonist tolvaptan significantly increased urine volume and decreased urinary osmolality in a cohort of calcium stone formers, while solute excretions remained largely unchanged. The net result was a significant fall in urinary CaOx, CaP, and UA SS. These data support the commonly held belief that interventions that increase oral free water intake can decrease urinary SS. The study also suggests that oral tolvaptan is a potential therapy to achieve lower urinary SS among those stone formers unable to regularly increase fluid intake despite counseling. It is also possible, although not tested in this study, that a short course of tolvaptan could be used to train selected patients how much fluid they need to ingest in order to effectively dilute their urine for long term stone prevention.
Risk factors for urinary stones are both genetic (e.g., urinary calcium excretion14) and environmental (e.g., dietary oxalate, purine, or water intake15). Despite available interventions16, 17 a large number of patients still suffer from recurrent stone episodes.1, 2 In a previous study 6 stone-formers and 2 controls were administered distilled water to increase their urine volume from 1.023 to 2.383 L/day, which decreased the urinary activity product ratio for CaP, CaOx, and UA, and increased the minimum SS needed to elicit spontaneous CaOx nucleation (formation product ratio).18 Those limited data provided important in vivo evidence that drinking water can diminish urinary SS. Recently, the American Urological Association (AUA) and American College of Physicians (ACP) both published guidelines that recommended sufficient fluid intake to achieve a urine volume at or above 2.0–2.5 L/d in order to prevent recurrent kidney stones.16, 17. However, these recommendations were graded as weak due to low-quality evidence. Our study cohort consisted of calcium stone formers, all of whom had been previously counseled to increase water intake. Nevertheless, and despite close observation, 75% of patients in the placebo phase had a urine volume less than 2.0 L. This outcome further suggests that methods to achieve increased water intake in the urinary stone population are sorely needed.
Physiologically, when net fluid intake increases plasma osmolality falls and plasma AVP decreases.19 Interestingly, patients with urinary stone disease have reduced AVP suppression while on higher fluid intake when compared to healthy individuals.20 In our study we demonstrated that tolvaptan at a constant dose of 45 mg/day can effectively increase urine volume in CaOx and CaP stone formers by an average of 3 L per day from baseline levels, and lower urine osmolality, CaOx SS and CaP SS. Previous studies have also demonstrated the aquaretic effect of tolvaptan in healthy individuals21 and patients with congestive heart failure,22 decompensated liver cirrhosis with hyponatremia,23 and ADPKD.24 Despite the increase in urine volume while subjects were on tolvaptan, the daily excretion of sodium, potassium, calcium, chloride, citrate, phosphate and magnesium did not significantly change. Similarly, in a study of heart failure patients tolvaptan did not change urinary sodium and potassium excretion,25 and in healthy volunteers an even a higher dose of tolvaptan (45–120 mg) did not change 24 hr sodium and potassium excretions21. However effects on key urinary stone risk solutes such as calcium, oxalate, phosphate, and citrate were previously not assessed.
Interestingly, urine oxalate excretion increased slightly on tolvaptan. The mechanism(s) for this effect are not readily apparent. It is possible that urinary oxalate reabsorption and/or secretion was affected by tolvaptan. However tolvaptan should act exclusively in the collecting duct, and it is not believed that any significant oxalate transport occurs beyond the proximal tubule. Thus the observed effect on oxalate excretion might have been indirect, perhaps due to changes in filtration fraction and/or other alterations in proximal tubular transport resulting from the diuresis. It is also conceivable that tolvaptan increased oxalate generation, via unknown mechanisms. Nevertheless, it is important to note that the ultimate net effect of tolvaptan was to greatly decrease urinary oxalate concentrations and lower CaOx SS, due to the overwhelming effect on urinary dilution.
Tolvaptan is currently approved by the Food and Drug Administration (FDA) for the treatment of hypervolemic and euvolemic hyponatremia, including patients with heart failure and the syndrome of inappropriate anti-diuretic hormone (SIADH). Single doses of tolvaptan up to 480 mg and daily doses from 30 to 300 mg are considered safe.26 In our study of calcium kidney stone patients the use of 45 mg of tolvaptan (30 mg at 8 AM and 15 mg at 4 PM) also appeared to be safe.
It is important to note that simply increasing water intake should have the same net effect on urinary composition as use of tolvaptan. Certainly, counseling to do just that is standard practice, one recently endorsed by both the AUA and ACP.16, 17 Nevertheless, for unknown reasons it is well-recognized that many patients are simply not able to increase their water intake in a sustained manner. Thus, tolvaptan use might be a strategy for selected patients that fail conservative measures. Is it possible that shorter term use of tolvaptan could “train” patients to drink more, by demonstrating in a practical way exactly how much water they should be drinking? Possibly, but we found that those who received tolvaptan for week 1 had urine volumes that decreased to levels similar to baseline at week 3 when they received placebo. Thus, longer-term therapy may be needed, and/or active counseling of patients to observe the effects of tolvaptan on their fluid intake so that they try and duplicate this longer term. Importantly the risk of long-term tolvaptan use is currently unclear and future study that balances the benefits and harms of long-term use will be required.
This randomized, placebo-controlled study has several limitations. First, our study focused on the effect of tolvaptan in patients with calcium-based stones. However, one would expect similar changes in urine volume and SS profiles in other stone types. In a limited study of 2 patients with homozygous cystinuria27 tolvaptan also reduced urinary cystine SS. Our study also found that urinary UA SS fell, suggesting UA stone formers might also benefit. Second, the patient population in this study is predominantly white Americans of European descent. Future studies with more heterogeneous populations would be desirable to ascertain the effect of tolvaptan on urine SS profiles in other groups. Third, nearly half of the current patient cohort used thiazides prior to the enrollment. There are no current data on the aquaretic, natriuretic, and hypocalciuric effect of the combined use of these agents in stone formers. However, a study of tolvaptan in 12 healthy volunteers28 found that the agent did not alter the natriuretic effect of hydrochlorothiazide, and that hydrochlorothiazide did not alter the aquaretic effect of tolvaptan. In addition, tolvaptan alone or in combination with diuretics was well tolerated. Only a daily dose of 45 mg of tolvaptan was used, and thus it is unknown if lower doses might achieve similarly favorable effects. Fourth, urinary SS measurement was used as a surrogate for actual kidney stone symptomatic events in this pilot study. A reduction in SS is associated with decreased incidence of stone events,29 but a future study assessing the benefit and cost-effectiveness of tolvaptan on the incidence of actual stone events is required. Importantly, elevations in blood concentrations of specific liver enzymes were recently reported in a trial of long-term tolvaptan use for ADPKD.30 The clinical significance of this asymptomatic effect will require further study.
CONCLUSION
In summary, our study suggests that tolvaptan, an antidiuretic hormone antagonist, may be a useful agent to dilute urine and reduce CaOx, CaP, and UA SS levels. Thus this represents a potentially promising therapy to reduce urinary stone risk in selected patients that cannot otherwise increase water intake. A clinical trial to examine the use of antidiuretic hormone antagonists for prevention of recurrent USD resistant to conventional treatments is warranted.
Acknowledgments
Supported by the Mayo Clinic O’Brien Urology Research Center and Rare Kidney Stone Consortium, member of the National Institutes of Health Rare Diseases Clinical Research Network, and funded by the National Institute of Diabetes and Digestive, Kidney Diseases and National Center for Advancing Translational Sciences, and Otsuka America Pharmaceutical, Inc. Research work for this review was supported by Otsuka America Pharmaceutical, Inc. The investigators also acknowledge support from the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center for Advancing Translational Sciences (NCATS), and the Mayo Clinic O’Brien Urology Research Center (U54 DK100227). The authors declare no conflicts of interest in the conduct or write up of this project.
Footnotes
Disclosure
All the authors declared no competing interests.
References
- 1.Lieske JC, Pena de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006;69:760. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 2.Rule AD, Lieske JC, Li X, et al. The ROKS nomogram for predicting a second symptomatic stone episode. Journal of the American Society of Nephrology: JASN. 2014;25:2878. doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwin MS, Saigal CS. Urologic Diseases in America. p. 313.p. 2012. (NIH Publication No 12-7865). [Google Scholar]
- 4.Hesse A, Brändle E, Wilbert D, et al. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. European Urol. 2003;44:709. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Krambeck AE, Williams JC, Jr, et al. Distinguishing Characteristics of Idiopathic Calcium Oxalate Kidney Stone Formers with Low Amounts of Randall’s Plaque. Clinical journal of the American Society of Nephrology: CJASN. 2014 doi: 10.2215/CJN.01490214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coe FL, Parks JH, Bushinsky DA, et al. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int. 1988;33:1140. doi: 10.1038/ki.1988.122. [DOI] [PubMed] [Google Scholar]
- 7.Cheungpasitporn W, Rossetti S, Friend K, et al. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. J Nephrol. 2015 doi: 10.1007/s40620-015-0210-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeraveedu PT, Palaniyandi SS, Yamaguchi K, et al. Arginine vasopressin receptor antagonists (vaptans): pharmacological tools and potential therapeutic agents. Drug Discov Today. 2010;15:826. doi: 10.1016/j.drudis.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Torres V, Grantham J, Chapman A, et al. TEMPO Investigators. Phase 2 open-label study to determine safety, tolerability and efficacy of split-dose tolvaptan in ADPKD. Journal of the American Society of Nephrology: JASN. 2007:361–362A. [Google Scholar]
- 10.Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:295. doi: 10.1038/ki.2011.119. [DOI] [PubMed] [Google Scholar]
- 11.Brinkley L, Pak CYC. The metabolic balance regimen and nutritional aspects of clinical research. In: Pak CYC, Adams PM, editors. Techniques of patient-oriented research. New York: Raven Press; 1994. pp. 143–148. [Google Scholar]
- 12.Werness PG, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 13.Lieske JC, Regnier C, Dillon JJ. Use of sevelamer hydrochloride as an oxalate binder. J Urol. 2008;179:1407. doi: 10.1016/j.juro.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moe OW, Bonny O. Genetic hypercalciuria. Journal of the American Society of Nephrology: JASN. 2005;16:729. doi: 10.1681/ASN.2004100888. [DOI] [PubMed] [Google Scholar]
- 15.Coe FL, Moran E, Kavalich AG. The contribution of dietary purine over-consumption to hyperpuricosuria in calcium oxalate stone formers. J Chronic Dis. 1976;29:793. doi: 10.1016/0021-9681(76)90053-9. [DOI] [PubMed] [Google Scholar]
- 16.Qaseem A, Dallas P, Forciea MA, et al. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the american college of physicians. Ann Intern Med. 2014;161:659. doi: 10.7326/M13-2908. [DOI] [PubMed] [Google Scholar]
- 17.Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Pak CYC, Sakahee K, Crowther C, et al. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Int Med. 1980;93:36. doi: 10.7326/0003-4819-93-1-36. [DOI] [PubMed] [Google Scholar]
- 19.Robertson GL. Physiology of ADH secretion. Kidney Int Suppl. 1987;21:S20. [PubMed] [Google Scholar]
- 20.Kokot F, Klimek D, Wiecek A, et al. Atrial natriuretic peptide and arginine-vasopressin secretion in patients with active renal stone disease. Int Urol Nephrol. 1998;30:357. doi: 10.1007/BF02550323. [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, Hasunuma T, Sato O, et al. Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S5. doi: 10.1007/s10557-011-6299-3. [DOI] [PubMed] [Google Scholar]
- 22.Udelson JE, Orlandi C, Ouyang J, et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:1540. doi: 10.1016/j.jacc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Okita K, Sakaida I, Okada M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979. doi: 10.1007/s00535-010-0240-6. [DOI] [PubMed] [Google Scholar]
- 24.Chapman A, Torres V, Grantham J, et al. A Phase IIB pilot study of the safety and efficacy of tolvaptan, a vasopressin V2 receptor antagonist (V2RA) in patients with ADPKD. Journal of the American Society of Nephrology: JASN. 2005;16:68A. [Google Scholar]
- 25.Inomata T, Izumi T, Matsuzaki M, et al. Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S57. doi: 10.1007/s10557-011-6349-x. [DOI] [PubMed] [Google Scholar]
- 26.OPC-41061 Investigator’s Brochure. Rockville (MD): Otsuka Maryland Research Institute, Inc.; 2005. [Google Scholar]
- 27.de Boer H, Roelofsen A, Janssens PM. Antidiuretic hormone antagonist to reduce cystine stone formation. Ann Intern Med. 2012;157:459. doi: 10.7326/0003-4819-157-6-201209180-00023. [DOI] [PubMed] [Google Scholar]
- 28.Shoaf SE, Bramer SL, Bricmont P, et al. Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a non-peptide AVP antagonist, and furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol. 2007;50:213. doi: 10.1097/FJC.0b013e318074f934. [DOI] [PubMed] [Google Scholar]
- 29.Sakhaee K, Maalouf NM, Sinnott B. Clinical review Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97:1847. doi: 10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]


