Introduction
It is well recognized that traumatic brain injury (TBI) is a major cause of death and disability, contributing to approximately 30% of all injury deaths in the United States.1, 2 In the U.S., in 2010, approximately 2.5 million emergency department visits, hospitalizations, or deaths were associated with TBI.3 Worldwide, it is estimated that more than 10 million people are hospitalized or die from TBI annually, imposing a substantial socioeconomic burden with a majority of TBIs caused due to road traffic injuries.4, 5 According to the Global Burden of Disease Study 2013, deaths from injury worldwide increased by 10·7%, from 4·3 million deaths in 1990 to 4·8 million in 2013.6 The World Health Organization suggests that road traffic crashes are one of the three leading causes of global deaths from injuries and predicts that road traffic crashes would become the fifth leading cause of death by 2030.7 Locally derived data on TBI projections in India suggest that nearly 1.6 million individuals sustain TBI and seek hospital care annually, out of which nearly 200,000 lives are and a further 1 million persons are disabled, requiring rehabilitation services.8 The standard lifetime TBI prevalence rate among urban Indians is estimated as 9.3% (95% CI 7.0–11.5)9 with mean age of first TBI at 44.4 (SD 24.7) years. Despite the magnitude of the TBI problem in India, however, successful strategies to improve Indian TBI outcomes are lacking.10
One opportunity to improve severe TBI outcomes is to provide high quality acute care to patients hospitalized with TBI. The 2007 Brain Trauma Foundation Guidelines are a framework aimed at achieving this goal for patients with severe TBI.11 Despite publication more than 8 years ago, there are little data on the degree to which health care systems and intensive care units (ICUs) are adherent to these Guidelines and on whether ICU Guideline adherence improves TBI survival. Importantly, data supporting these Guideline recommendations were mostly derived from tertiary care centers in high income countries where TBI characteristics and care processes may vary compared to low and middle income countries (LMIC). Consequently, the relevance of western ICU Guidelines to the management of TBI in LMIC nations, such as in India, remains unknown. Addressing this gap is important because many LMIC nations have developed tertiary care health care facilities modeled after hospitals in high income countries and yet, these facilities offer care in the context of their local community, which may differ greatly from the U.S. Thus, it is important to understand the potential value of international collaborations in TBI research and TBI care. In recognition of the importance of understanding how to improve TBI outcomes in both nations, the National Institutes of Health (U.S), and the Department of Biotechnology (India) jointly funded work to examine TBI Guideline adherence and outcomes.
The two aims of this bi-institutional Indo-US collaborative project was to examine ICU TBI Guideline adherence rates and to examine the relationship between ICU Guideline adherence and in-patient mortality outcomes, and to examine long term outcomes in severe adult TBI at tertiary level institutions in India (Jai Prakash Narayan Apex Trauma Center [JPNATC], New Delhi), and the United States (Harborview Medical Center [HMC], Seattle, WA). We hypothesized that higher ICU Guideline adherence would be associated with lower in-patient mortality after severe TBI.
Materials and Methods
Study Center Selection and Data Sources
Two level 1 trauma centers affiliated with academic medical centers were included based on willingness to participate, a priori ability to contribute data from at-least 200 adult patients with severe TBI annually, and recognized expertise in the care of severe TBI patients. Study centers were the University of Washington’s HMC, Seattle, WA and All India Institute of Medical Sciences JPNATC, New Delhi, India. Both sites considered themselves adherent to the TBI Guidelines but adherence rates were unknown.
Ethics Committee/Institutional Review Board Approval
The University of Washington’s Institutional Review Board (IRB) approved the study at Harborview Medical Center (HMC). Written or verbal informed consent was not required for this study per IRB as this was a retrospective chart review. Patient records/information was anonymized and de-identified prior to analysis at HMC.
The All India Institute of Medical Sciences (AIIMS) Ethics Committee approved the study at Jai Prakash Narayan Apex Trauma Centre (JPNATC). Written informed consent was obtained from the next of kin/caregiver at JPNATC for prospective data collection, in accordance with AIIMS Ethics Committee requirements.
Institutional Organizational Structures Pertaining to TBI Care
Both JPNATC and HMC are academic and high volume (greater than 5000 trauma admissions per year) level 1 trauma centers with a dedicated neuro-intensive care unit with similar nurse patient ratios, 24 hour in person ICU physician coverage, similar automated vital sign data capture monitoring in the ICU, intracranial pressure (ICP) monitoring capacity, portable neuroimaging capacity, hospital trauma registry and institutional TBI quality improvement processes. At JPNATC, the ICU is specifically dedicated to neurotrauma patients while, at HMC, the ICU admits patients with a range of neurological conditions. There is a specific neurocritical care service at HMC whereas at JPNATC, the ICU is staffed by neurosurgeons and neuro-anesthesiologists.
Study Design
At the start of the study (2011), we intended to conduct our study using a retrospective design at both JPNATC and HMC, thereby allowing us to make direct study comparisons. Based on initial review of existing data, and concern for > 10% missing recorded data at JPNATC, we instead conducted a prospective cohort study at that site. Despite interest in conducting a prospective study at HMC, funding limitations led to retention of the retrospective study design at HMC. Thus, at HMC, electronic medical records were used to retrospectively abstract information on ICU Guideline adherence and outcomes of consecutive eligible patients who received care during the study period starting with 2011 and going backwards until required sample size of 200 patients was achieved. At JPNATC, we prospectively enrolled a consecutive sample of eligible severe TBI patients. As a result, study dates for HMC were 2009–2011 and 2012–2014 for JPNATC. Data were remotely and securely entered into a web based data entry system from each center and the UW Harborview Injury Prevention and Research Center served as the data coordinating center and maintained the web based data entry portal. Given original relational study goals and subsequent differences in study designs, our goal in this paper is to present but not compare data from both sites.
Study Population
Eligible participants were subjects 18 years or older with severe TBI, defined as having ≥ one ICD-9 discharge diagnosis code: 800.0–801.9, 803.0–804.9, 850.0–854.1, 959.01, 950.1–950.3, 995.55, consistent with the Centers for Disease Control and Prevention definition.12 Criteria for severe TBI required: a minimum head abbreviated injury severity (AIS) score ≥ 3, post-resuscitation Glasgow Coma Scale (GCS) score ≤ 8, alive with tracheal intubation in the ICU ≥ 48 hours from the time of ICU admission, trauma history, and abnormal admission head computed tomography. We included subjects who were transferred from the scene of the injury to an outside hospital before admission to the study centers as well as those with extra cranial injuries. Deaths occurring within 48 hours after ICU admission were excluded from the study as we felt that a minimum window of 48 hours is necessary to allow patients to experience adherence to the Guidelines so as to be able to examine its association with outcomes.
Center Training and Reliability Testing
Data abstraction training modules were used by the data abstractor at each site who underwent training followed by data abstraction from two test cases. A third case was used to determine baseline inter-abstractor reliability with the lead abstractor (NK). After pooled abstraction of the first 100 medical records, another two test cases were abstracted for reliability testing. A kappa of 0.8 was a priori considered adequate between center reliability.13–15 In person supplementary trainings at JPNATC were conducted for kappa values <0.8 until the goal was achieved. Both centers achieved excellent inter-rater agreement (kappa > 0.8)13–15 prior to data collection and abstraction. Investigators NK and MSV made in person site visits to JPNATC once during the study period.
Measures of Early ICU Guideline Adherence
Seventeen ICU clinical indicators were created to represent measures of adherence, consistent with Guideline recommendations (Table 1). The number and type of study indicators was determined a priori by the study group. For select indicators, such as treatment of hypoxia and hypotension, we added a time component based on time-dependent effect on patient outcomes. Each of the 17 clinical indicators in Table 1 was examined for conditionality; clinical indicators were considered relevant for patients who had underlying conditions that would have qualified for given treatments. For example, adherence rate for each patient in the ICU was calculated as the sum of the number of indicators to which care was adherent divided by the sum of number of applicable indicators for that patient. Then, the mean adherence rate for all patients during the first 72 hours of admission was calculated for each cohort at JPNATC and HMC.
Table 1.
17 Guideline indicator definitions and coding scheme for indicators for intensive care unit (ICU) management of adults with severe traumatic brain injury.
| Guideline Indicators** | Definitions and coding |
|---|---|
|
Hypoxia treated in 30 minutes after onset Condition = Hypoxia |
0 = Hypoxic and not treated with O2 0 = Hypoxic and treated with O2 >30 minutes 1 = Hypoxic but treated with O2 ≤ 30 minutes 2 = No hypoxia (All SaO2 ≥ 90% or PaO2 ≥ 60mmHg) |
|
Hypotension treated within 30 minutes after onset Condition = Hypotension |
0 = Hypotension and not treated 0 = Hypotension and treated > 30 minutes after hypotension onset 1 = Hypotension and treated in ≤ 30 minutes 2 = No hypotension (All SBP ≥ 90mmHg) |
| ICP monitor used | 0 = ICP monitor not used 1 = ICP monitor used |
|
All CPP between 50–70 mmHg Condition = ICP monitored |
0 = All CPP not between 50–70 mmHg 1 = All CPP between 50–70 mmHg 2 = ICP not monitored |
|
Any treatment given for high ICP Condition = High ICP* |
0 = Any treatment not given for high ICP 1 = Any treatment given for high ICP 2 = No high ICP |
|
Mannitol used for treatment of high ICP Condition = High ICP* |
0 = Mannitol is not used to treat high ICP 1 = Mannitol used to treat high ICP 2 = No high ICP |
|
Propofol used for treatment of high ICP Condition = High ICP* |
0 = Propofol not used to treat high ICP 1 = Propofol used to treat high ICP 2 = No high ICP |
|
Prophylactic hyperventilation¥
is not used for treatment of high ICP Condition = High ICP* |
0 = Prophylactic hyperventilation is used to treat high ICP 1 = Prophylactic hyperventilation is not used to treat high ICP 2 = No high ICP |
| Target temperatures± maintained for 48 hours (prophylactic hypothermia not used) | 0 = Target temperatures not maintained 1 = Target temperatures maintained |
| Pre-intubation antibiotics used | 0 = Pre-intubation antibiotics not used 1 = Pre-intubation antibiotics used |
| Early tracheostomyo performed | 0 = Early tracheostomy not performed 1 = Early tracheostomy performed |
| Deep Vein Thrombosis (DVT) prophylaxis used (Low molecular weight heparin or compression device) | 0 = DVT prophylaxis not used 1 = DVT prophylaxis used |
|
Treatment thresholds of SJO2 ≥ 50% or PbrO2 ≥15 mmHg used Condition = SJO2 or PbrO2 monitored |
0 = Treatment thresholds of SJO2<50% or PbrO2<15 mmHg used 1 = Treatment thresholds of SJO2≥50% or PbrO2≥15 mmHg used 2 = SJO2 or PbrO2 not monitored. |
| Prophylactic barbiturate coma not induced | 0 = Prophylactic barbiturate coma induced 1 = Prophylactic barbiturate coma not induced |
| Prophylactic antiepileptic drugs used to prevent early seizures | 0 = No prophylactic antiepileptic drugs used 1 = Prophylactic antiepileptic drugs used |
| Nutrition$ started within 72 hours | 0 = Nutrition not started 0 = Nutrition start >72 hours after ICU admission 1 = Nutrition start ≤ 72 hours after ICU admission |
| No steroids used | 0 = Steroids used 1 = Steroids not used |
Coding: Yes=1, No=0, NA=2; Total possible adherence score in ICU. = 17 [range 0–17]
based on 2007 BTF Guidelines, NA = Not applicable
High ICP defined as ICP>20mmHg if an ICP monitor is in place or clinical signs of high ICP (unequal pupils and/or hypertension with bradycardia or radiographic evidence of progression of TBI.
PaCO2 ≤ 25 mmHg
Target temperature is defined as temperature between 35–38.5°C.
Early Tracheostomy is defined as Tracheostomy within 48 hours to admission to Trauma center.
Nutrition includes enteral, parenteral or combined
Patient Outcomes
The main outcome was in-hospital mortality. We also examined post-discharge Glasgow Outcome Scale (GOS; GOS 5 = baseline, GOS 4 = minor-moderate impairment, GOS 3 = major impairment, GOS 2 = vegetative state and GOS 1 = death) scores at 3, 6 and 12 months as recorded at each site.16
At JPNATC, we collected the post discharge follow up GOS score noted by the TBI follow up clinic’s neurosurgeon during the 3, 6 and 12 month in-person TBI clinic visits, per clinical practice. To facilitate TBI follow up post discharge at JPNATC, patients and families were contacted by mobile telephones (voice/text) and reminded of clinic visits at discharge and between clinic visits. Given the retrospective nature of this portion of the study at HMC, post discharge GOS was assigned by the study team (NK and MSV) based on information in medical records of neurosurgery and rehabilitation clinic visits.
Power
At JPNATC, there are approximately 1300 severe TBI admissions annually; 240 patients were older than 18 years. At HMC, there were 1100 moderate-severe TBI admissions during the study period over 18 years old. Thus, 200 patients per year were available to participate per institution in this study. Based on a 20% difference in discharge mortality (main outcome) with Guideline adherence, we required a total of 200 patients at JPNATC and HMC each. We estimated the precision of Guideline adherence estimates and relationship between adherence and outcomes using Creative Research Systems Survey Sample Size Software based upon 2009 data from JPNATC.17 Minimal detectable relative risks were estimated using PASS 2008 software.18
Statistical Analyses
Patient and injury characteristics were examined in bivariate analyses by center using χ2 tests for categorical variables and t-tests for continuous variables. All analyses were clustered within hospital. We examined the ICU adherence rate overall for all 17 indicators, and by percentage adherence at each center. Statistical significance was defined as p value less than 0.05. All multiple regression analyses were adjusted for confounders such as age, gender, maximum head AIS, maximum non-head AIS, and motor GCS at admission. Multivariable modified Poisson regression (Poisson regression with a robust error variance to estimate the relative risk directly)19, 20 was performed to examine the effect of ICU adherence rate (using the ICU adherence rate as the independent variable and adjusting for all confounders) on in-hospital mortality. Because missing data were rare for variables used in the regression analyses, we conducted a complete case analysis. Available Glasgow outcome scale (GOS) scores were categorized GOS 1–3 = poor outcome and GOS 4–5 = favorable outcomes.16 Data were analyzed using Stata v. 13 (Stata Corp. 2013, College Station, TX) and SAS 9.3 (SAS Institute, Cary, NC).17
Results
Clinical Characteristics
Detailed clinical characteristics of patients at each center are presented in Table 2. At JPNATC, patients were 36.0 [SD 13.9] years, male (85%), sustained motor vehicle crash (34%), directly transported from scene (65%), and underwent at least one surgery (59%). Less than 10% of patients had an admission GCS of 3T (intubated and paralyzed); mean ISS was 31.4 [SD 13.6]. One hundred and forty eight (74%) patients had polytrauma and 13% had signs of high ICP (clinical symptoms or radiographic signs or ICP monitoring measurements > 20 mmHg). At HMC, patients were 44 [SD 18.1] years, male (73%), sustained motor vehicle crash (28%), directly transported from scene (67%), and underwent at least one surgery (60%). Over 50% had admission GCS of 3T (intubated and paralyzed); mean ISS was 38.5 [SD 11.6]. Most (169; 84%) patients had polytrauma and 48% had signs of high ICP (clinical symptoms or radiographic signs or ICP monitoring measurements > 20 mmHg) in the ICU during first 72 hours of hospital admission.
Table 2.
Clinical characteristics of 400 adults with severe traumatic brain injury across two study centers (Jai Prakash Narayan Apex Trauma Center, New Delhi, India and Harborview Medical Center, Seattle, WA). GCS = Glasgow Coma Scale. ISS = Injury Severity Score. AIS = Abbreviated Injury Scale.
| Clinical Characteristics | JPNATC (N=200) N (%) |
HMC (N=200) N (%) |
|---|---|---|
| Age (years) mean[SD] | 36.0 [13.9] | 44.1 [18.1] |
| Gender | ||
| Male | 169 (84.5) | 145 (72.5) |
| Injury mechanism | ||
| Fall | 32 (16.0) | 57 (28.5) |
| Motor vehicle crash | 68 (34.0) | 56 (28.0) |
| Self-inflicted | 0 (0.0) | 3 (1.5) |
| Struck by vehicle | 50 (25.0) | 21 (10.5) |
| Gunshot/assault | 6 (3.0) | 16 (8.0) |
| Sport | 0 (0.0) | 6 (3.0) |
| Auto rickshaw/bus crash | 9 (4.5) | 0 (0.0) |
| Motorcycle crash | 21 (10.5) | 24 (12.0) |
| Other | 14 (7.0) | 17 (8.5) |
|
Transportation to trauma center JPNATC:N = 200; HMC:N =195* |
||
| Ambulance only | 107 (53.5) | 93 (47.7) |
| Flight and ambulance | 0 | 102 (52.3) |
| Private vehicle | 27 (13.5) | 0 |
| Police/government vehicle | 56 (28.0) | 0 |
| Other | 10 (5.0) | 0 |
| Direct from scene | 130 (65.0) | 133 (66.5) |
| Admission GCS score | ||
| 3 | 13 (6.5) | 176 (88.0) |
| 4 | 38 (19.0) | 4 (2.0) |
| 5 | 15 (7.5) | 1 (0.5) |
| 6 | 38 (19.0) | 7 (3.5) |
| 7 | 74 (37.0) | 9 (4.5) |
| 8 | 22 (11.0) | 3 (1.5) |
| GCS score (admit motor) | ||
| 1, pharmacologically paralyzed | 1 (0.5) | 176 (88.0) |
| 1, not pharmacologically paralyzed | 12 (6.0) | 3 (1.5) |
| 2 | 26 (13.0) | 5 (2.5 |
| 3 | 14 (7.0) | 2 (1.0) |
| 4 | 34 (17.0) | 4 (2.0) |
| 5 | 97 (48.5) | 9 (4.5) |
| 6 | 16 (8.0) | 1 (0.5) |
| ISS mean[SD] | 31.4 [13.2] | 38.5 [11.6] |
| Head AIS score | ||
| 3 | 32 (16.0) | 1 (0.5) |
| 4 | 133 (66.5) | 47 (23.5) |
| 5 | 35 (17.5) | 148 (74.0) |
| 6 | 0 (0.0) | 4 (2.0) |
| Non head max AIS | ||
| 0 | 37 (18.5) | 8 (4.0) |
| 1 | 10 (5.0) | 25 (12.5) |
| 2 | 111 (55.5) | 31 (15.5) |
| 3 | 39 (19.5) | 68 (34.0) |
| 4 | 1 (0.5) | 48 (24.0) |
| 5 | 1 (0.5) | 20 (10.0) |
| 6 | 1 (0.5) | 0 (0.0) |
| All head computed tomography diagnoses | ||
| Epidural hematoma | 33 (16.5) | 36 (18.0) |
| Subdural hematoma | 95 (47.5) | 148 (74.0) |
| Subarachnoid hemorrhage | 35 (17.5) | 158 (79.0) |
| Intracerebral hemorrhage | 26 (13.0) | 83 (41.5) |
| Intraventricular hemorrhage | 12 (6.0) | 71 (35.5) |
| Cerebral edema | 32 (16.0) | 99 (49.5) |
| Cerebral infarction | 6 (3.0) | 20 (10.0) |
| Contusion | 134 (67.0) | 97 (48.5) |
| Diffuse axonal injury | 15 (7.5) | 69 (34.5) |
| Extracranial injury | 52 (26.0) | 169 (84.5) |
| Extracranial injury location | ||
| Face | 43 (21.5) | 100 (50.0) |
| Neck | 15 (7.5) | 2 (1.0) |
| Thorax | 15 (7.5) | 106 (53.0) |
| Abdomen | 10 (5.0) | 68 (34.0) |
| Spine | 5 (2.5) | 55 (27.5) |
| Extremities | 17 (8.5) | 120 (60.0) |
| Any surgery in 72h | 117 (58.5) | 119 (59.5) |
Transportation to Trauma Center at HMC: Data missing for 5 patients.
Discharge Outcomes and Disposition
Table 3 shows hospital and ICU LOS, GOS and discharge disposition. At JPNATC, inhospital mortality was 24% and among survivors, the most common (50%) discharge GOS score was 4 and 8% returned to baseline level of functioning. At JPNATC, discharge disposition were largely bimodal: 24% died in-hospital and 72% of patients were discharged home. No patients received in-patient rehabilitation care or were discharged to a skilled nursing facility. Approximately 4% of patients received outpatient rehabilitation care. At HMC, in-hospital mortality was 26.5% and among survivors, the most common (34%) discharge GOS score was 4 and 21% of patients returned to baseline level of functioning at discharge. Sixteen percent were discharged home, 27% of patients were discharged to an inpatient rehabilitation facility and 26% of TBI patients were discharged to a skilled nursing facility.
Table 3.
Outcome characteristics of 400 adults with severe traumatic brain injury across two study centers (Jai Prakash Narayan Apex Trauma Center, New Delhi, India and Harborview Medical Center, Seattle, WA). LOS = Length of Stay. GOS = Glasgow Outcome Scale. Data are cumulative at each time point and N = 200 subjects at each site.
| Outcome Characteristics | JPNATC (N=200) N (%) |
HMC (N=200) N (%) |
|---|---|---|
| ICU LOS (days) mean[SD] | 6.1 [2.1] | 13.5 [9.9] |
| Hospital LOS (days) mean[SD] | 18.2 [21.5] | 28.2 [24.3] |
| Discharge Disposition | ||
| In-Hospital death | 48 (24.0) | 53 (26.5) |
| Home | 143 (71.5) | 31 (15.5) |
| Outpatient rehabilitation | 7 (3.5) | 0 |
| Inpatient rehabilitation | 0 (0.0) | 53 (26.5) |
| Skilled Nursing Facility | 0 (0.0) | 51 (25.5) |
| Another facility | 2 (1.0) | 12 (6.0) |
| Discharge GOS score | ||
| In-Hospital death | 48 (24.0) | 53 (26.5) |
| Vegetative state | 11 (5.5) | 3 (1.5) |
| Major impairment | 26 (13.0) | 36 (18.0) |
| Minor /moderate impairment | 99 (49.5) | 67 (33.5) |
| Baseline functioning | 16 (8.0) | 41(20.5) |
| 3 month GOS score | ||
| Death | 58 (29.0) | 55 (27.5) |
| Vegetative state | 29 (14.5) | 1 (0.5) |
| Major impairment | 26 (13.0) | 6 (3.0 ) |
| Minor /moderate impairment | 39 (19.5) | 25 (12.5 ) |
| Baseline functioning | 46 (23.0) | 78 (39.0 ) |
| Lost to follow up | 2(1.0) | 35 (17.5) |
| 6 month GOS score | ||
| Death | 67 (33.5) | 55 (27.5) |
| Vegetative state | 6 (3.0) | 1(0.5) |
| Major impairment | 20 (1.0) | 5 (2.5) |
| Minor /moderate impairment | 47 (23.5) | 17 (8.5) |
| Baseline functioning | 58 (29.0) | 66 (33.0) |
| Lost to follow up | 2 (1.0) | 56 (28.0) |
| 12 month GOS score | ||
| Death | 71 (35.5) | 55 (27.5) |
| Vegetative state | 1 (0.5) | 1 (0.5) |
| Major impairment | 2 (1.0) | 5 (2.5) |
| Minor /moderate impairment | 25 (12.5) | 8 (4.0) |
| Baseline functioning | 99 (49.5) | 51 (25.5) |
| Lost to follow up | 2(1.0) | 80 (40.0) |
ICU Guideline Adherence
Adherence rates for the acute care clinical indicators are given in Table 4. At JPNATC, the overall ICU adherence rate was 74.9 % [SD 11.0], and the following indicators had adherence rates greater than 90%: achieving target temperature, not using prophylactic barbiturates, timely start of nutritional support, and avoidance of intravenous steroids. Intracranial pressure (ICP) monitors were placed in 63% of patients, 52% of patients with intracranial hypertension received some form of ICP reduction treatment and among patients with ICP monitoring, 94% of patients had all cerebral perfusion pressures 50–70mmHg. Ninety-nine percent of patients received prophylactic antiepileptic medications. At HMC, the overall ICU adherence rate was 71.6 % [SD 10.4] and the following indicators had adherence rates greater than 90%: achieving target temperature, not using prophylactic barbiturates, timely start of nutritional support, and avoidance of intravenous steroids. ICP monitors were placed in 84% of patients and 98% of patients with intracranial hypertension received some form of ICP reduction treatment. Among patients with ICP monitoring, 63% of patients had all cerebral perfusion pressures 50–70mmHg. Forty two percent of patients received prophylactic antiepileptic medications.
Table 4.
Adherence to intensive care unit (ICU) Guideline indicators in 400 patients with severe traumatic brain injury at Jai Prakash Narayan Apex Trauma Center, New Delhi, India (N = 200) and Harborview Medical Center, Seattle, WA (N = 200).
| Indicators | JPNATC N (%) |
HMC N (%) |
|---|---|---|
| Adherence rate mean [SD] | 74.9 [11.0] | 71.6 [10.4] |
| 1) Hypoxia treated within 30 minutes after onset (Hypoxia - JPNATC [N = 2]; HMC [N = 10]) | 1 (50.0) | 7 (70.0) |
| 2) Hypotension treated within 30 minutes after onset (Hypotension - JPNATC [N = 2]; HMC [N = 82]) | 1 (50.0) | 55 (67.1) |
| 3) Intracranial pressure (ICP) monitor used | 126 (63.0) | 167 (83.5) |
| 4) All cerebral perfusion pressure between 50–70mmHg (Among those with ICP monitoring - JPNATC [N = 126]; HMC [N = 167]) | 119 (94.4) | 105 (62.9) |
| 5) Any treatment given for high ICP (clinical or by ICP monitors) (High ICP - JPNATC [N = 25]; HMC [N = 96]) | 13 (52.0) | 94 (97.9) |
| 6) Mannitol used for treatment of high ICP (High ICP - JPNATC [N = 25]; HMC [N = 96]) | 10 (40.0) | 52 (54.2) |
| 7) Propofol used for treatment of high ICP (High ICP - JPNATC [N = 25]; HMC [N = 96]) | 6 (24.0) | 91 (94.8) |
| 8) Prophylactic hyperventilation is not used for treatment of high ICP (High ICP - JPNATC [N = 25]; HMC [N = 96]) | 22 (88.0) | 96 (100) |
| 9) Target temperatures maintained (T 35–38.5°C) | 195(97.5) | 200 (100) |
| 10) Pre-intubation antibiotics used | 136 (68.0)* | 3 (1.5) |
| 11) Early tracheostomy performed | 5 (2.5) | 40 (20.0) |
| 12) Deep Vein Thrombosis (DVT) prophylaxis used | 110 (55.0) | 197 (98.5) |
| 13) Treatment thresholds of SJO2 ≥ 50% or PbrO2 ≥ 15 mmHg used (SJO2 or PbrO2 monitored - JPNATC [N = 3]; HMC [N = 64]) | 3 (100.0) | 37 (57.8) |
| 14) Prophylactic barbiturate coma not induced | 190 (95.0) | 194 (97.0) |
| 15) Prophylactic antiepileptic drugs used to prevent early seizures | 198 (99.0) | 83 (41.5) |
| 16) Nutrition† started within 72 hours | 188 (94.0) | 187 (93.5) |
| 17) No steroids used | 198 (99.0) | 199 (99.5) |
At JPNATC, 68% patients received antibiotics in the Emergency Department around the time of tracheal intubation.
Nutrition includes enteral, parenteral or combined.
ICU Guideline Adherence and In-Patient Mortality (Main Analysis)
Table 5 shows the association between early ICU Guideline adherence rate and mortality (overall and by percent adherence). At JPNATC, an increase in ICU Guideline adherence rate by 1% was associated with 3% lower in-patient mortality (aRR 0.97; 95% CI 0.95, 0.99; Table 5). Less than 65% ICU Guideline adherence was significantly associated with nearly 2 fold higher in-hospital mortality. There was no relationship between ICU Guideline adherence rate and in-patient mortality at HMC (aRR 1.00; 95% CI 0.97, 1.02).
Table 5.
Association between intensive care unit (ICU) Guideline adherence rate and inpatient mortality in patients with severe traumatic brain injury at Jai Prakash Narayan Apex Trauma Center, New Delhi, India (N = 200) and at Harborview Medical Center, Seattle, WA, U.S (N = 200).
| JPNATC† Discharge aRR (95% CI)* |
HMC† Discharge aRR (95% CI)* |
|||
|---|---|---|---|---|
| Adherence Rate (per percentage point) | 0.97 (0.95, 0.99) | 1.00 (0.97, 1.02) | ||
| Adherence Rate (%) | ||||
| < 65% | N = 32 | 1.92 (1.11, 3.33) | N = 48 | 1.11 (0.65, 1.90) |
| 65% to < 75% | N = 59 | 1.49 (0.83, 2.67) | N = 77 | 0.73 (0.43, 1.23) |
| 75% to 100% | N = 109 | Ref | N = 75 | Ref |
Risk estimates are adjusted Relative Risk (aRR) for in-hospital mortality.
Model includes all 17 ICU indicators. Model is adjusted for age, gender, head Abbreviated Injury Score (AIS), maximum non-head AIS, and Glasgow Coma Scale score motor.
Post Discharge Outcomes
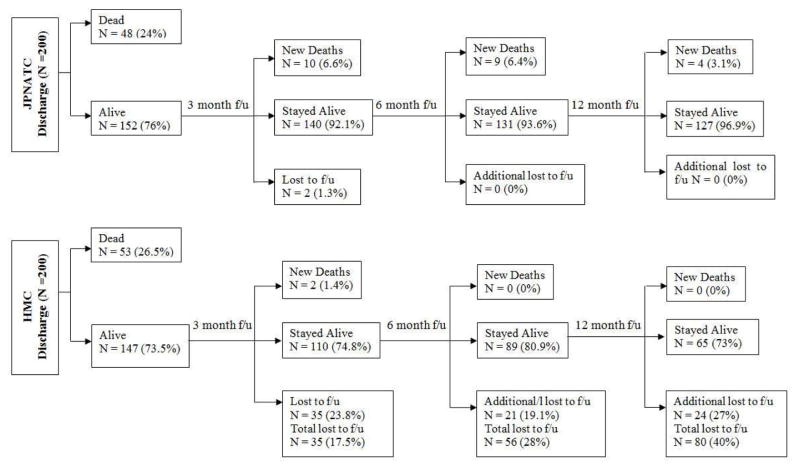
Fig. 1 shows that at JPNATC, overall TBI clinic follow up rates (including deaths) were 99% at 3, 6 and 12 months. At HMC, overall TBI follow up rates (N=200) were 83%, 72% and 60% at 3, 6 and 12 months. For discharge survivors post HMC discharge (N=147), loss to follow up at 3, 6 and 12 months was 24%, 38% and 54%, resulting in follow up rates of 76%, 62%, and 46%.
Figure 1. Post discharge follow up.
Post discharge follow up (f/u) of survivors with severe traumatic brain injury (TBI) at Jai Prakash Narayan Apex Trauma Center, New Delhi, India (N = 200 enrolled; over 98% f/u rate at JPNATC), and Harborview Medical Center (HMC), Seattle, WA, U.S. (N = 200). At JPNATC, TBI clinic follow up rates were 99% at 3, 6 and 12 months. At HMC, total (deaths + survivors) follow up rates from discharge were 83%% at 3 months, 72% at 6 months and 60% at 12 months. New deaths at 3, 6 and 12 months are post discharge deaths between time points.
Mortality
At JPNATC, the number of patients who died increased from 24% at discharge to 29% at 3 months, to 34% at 6 months and to 36% at 12 months. Among survivors at each beginning time point, there were 6.6% additional deaths from discharge to 3 months, 6.4% additional deaths from 3 to 6 months and 3.1% additional from 6 to 12 months among survivors at the earlier time point. There was only one new known death among HMC patients post discharge (Table 3, Fig. 1).
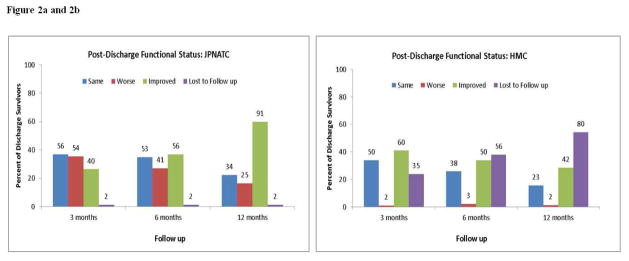
Functional Status
At discharge, 8% of JPNATC patients and 21% of HMC patients returned to baseline functional status (Table 3). Fig. 2a shows that compared to the time the 152 patients were discharged alive from JPNATC, 36%, 27% and 16% had lower functional status (GOS) at 3, 6 and 12 months. Compared to discharge, 37%, 35% and 22% had the same functional status at 3, 6 and 12 months and 26%, 37% and 60% improved in their functional status at 3, 6, and 12 months, respectively. Fig. 2b shows that of the 147 patients discharged alive from HMC with known follow ups, 1%, 2% and 1% worsened at 3, 6 and 12 months and 34%, 26% and 16% had the same functional status at 3, 6 and 12 months, respectively. Compared to discharge, 41%, 34% and 29% improved in their functional status at 3, 6, and 12 months, respectively.
Figure 2. Figure 2a and 2b: Post discharge functional status.
Post discharge functional status (Glasgow Outcome Scale score category) of 152 survivors at Jai Prakash Narayan Apex Trauma Center (JPNATC), New Delhi, India and 147 survivors at Harborview Medical Center (HMC), Seattle, WA, U.S. at each time point (3, 6 and 12 months post discharge). Data label above each bar indicates the N (number of patients with same, worse or improved outcomes) at each time point.
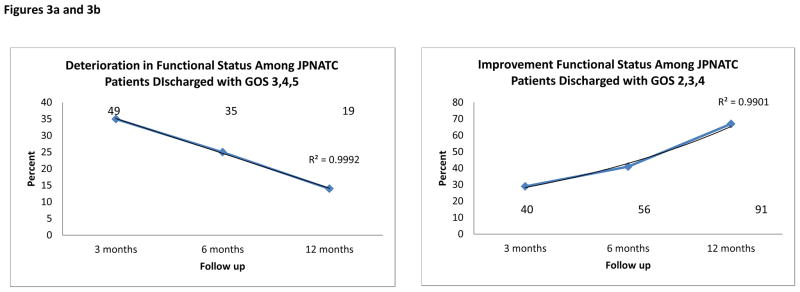
Despite the overall 60% observed overall improvement among JPNATC patients at 12 months, Table 6 shows that of the JPNATC patients discharged with favorable functional status (GOS 4–5), 37%, 24% and 12% deteriorated to a lower GOS at 3, 6 and 12 months. Table 6 also shows that for JPNATC patients discharged with the potential to improve GOS (GOS 2, 3 or 4), 29%, 41% and 67% improved to a higher GOS at 3, 6 and 12 months (R2=0.991; p< 0.01)). The rate of improvement post discharge was greater between 6 and 12 months than between 3 and 6 months post discharge. The best outcomes occurred at 12 months.
Table 6.
Glasgow Outcome Scale (GOS) score trajectory of 400 patients with severe traumatic brain injury by discharge GOS score category at Jai Prakash Narayan Apex Trauma Center (JPNATC; N=200), New Delhi, India and at Harborview Medical Center (HMC; N=200), Seattle, WA, U.S.
Data are cumulative from discharge.
| JPNATC | 3 month GOS (%) | 6 month GOS (%) | 12 month GOS (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge GOS | 1 | 2 | 3 | 4 | 5 | 9 | 1 | 2 | 3 | 4 | 5 | 9 | 1 | 2 | 3 | 4 | 5 | 9 |
| 1 (N=48) | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| 2 (N=11) | 45.5 | 0 | 18.2 | 9.1 | 27.3 | 0 | 54.6 | 0 | 0 | 27.3 | 18.2 | 0 | 54.6 | 0 | 9.1 | 9.1 | 27.3 | 0 |
| 3 (N=26) | 0 | 23.1 | 38.5 | 23.1 | 15.4 | 0 | 11.5 | 15.4 | 26.9 | 23.1 | 23.1 | 0 | 19.2 | 0 | 0 | 23.1 | 57.7 | 0 |
| 4 (N=99) | 5.1 | 23.2 | 14.1 | 31.3 | 24.2 | 2 | 10.1 | 2 | 11.1 | 35.4 | 39.4 | 2 | 12.1 | 1 | 1 | 18.2 | 65.7 | 2 |
| 5(N=16) | 0 | 0 | 0 | 6.3 | 93.7 | 0 | 0 | 0 | 12.5 | 18.8 | 68.8 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| HMC | 3 month GOS (%) | 6 month GOS (%) | 12 month GOS (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge GOS | 1 | 2 | 3 | 4 | 5 | 9 | 1 | 2 | 3 | 4 | 5 | 9 | 1 | 2 | 3 | 4 | 5 | 9 |
| 1 (N=53) | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| 2 (N=3) | 0 | 33.3 | 33.3 | 0 | 0 | 33.4 | 0 | 33.3 | 33.3 | 0 | 0 | 33.4 | 0 | 33.3 | 33.3 | 0 | 0 | 33.4 |
| 3 (N=36) | 5.6 | 0 | 13.9 | 41.7 | 2.8 | 36.1 | 5.6 | 0 | 11.1 | 27.8 | 5.6 | 50 | 5.6 | 0 | 11.1 | 16.7 | 8.3 | 58.3 |
| 4 (N=67) | 0 | 0 | 0 | 14.9 | 64.2 | 20.9 | 0 | 0 | 0 | 9 | 55.2 | 35.8 | 0 | 0 | 0 | 3 | 47.8 | 49.3 |
| 5 (N=41) | 0 | 0 | 0 | 0 | 82.9 | 17.1 | 0 | 0 | 0 | 2.4 | 65.9 | 31.7 | 0 | 0 | 0 | 0 | 39 | 61 |
Discussion
In this joint Indo-U.S. study, we examined the association between early ICU Guideline adherence and in-patient mortality among severe TBI patients. The main findings of our study are that for patients who received ICU care at JPNATC: 1) Early ICU Guideline adherence was associated with lower in-patient mortality, 2) Greater than 65% ICU Guideline adherence rate was associated with nearly 2 fold lower in-patient mortality, and 3) Although 60% of patients improved in functional status from discharge to 12 months, post discharge deaths occurred and GOS often deteriorated at home even among patients discharged with favorable functional status.
In this study, we document that ICU TBI Guideline adherence, as a bundled treatment, may benefit discharge survival in adult patients with severe TBI at JPNATC but not at HMC. These considerations may explain this observation: 1) We had more accurate and real time data collection with the overall stronger prospective study design at JPNATC, 2) A Hawthorne effect may have occurred, with aspects of care outside of Guideline adherence (such as equipment, staffing, QI processes, information systems) contributing to improving outcomes at JPNATC, 3) JPNATC patients had mostly isolated TBI whereas at HMC, 85% had polytrauma whose outcomes may have been affected by other complications that we could not capture, and /or 4) Guideline adherence is likely important everywhere, but it may be even more important (and thus should be stressed even more) in LMICs. These considerations precluded a direct comparison of patients or outcomes or generalization of JPNATC findings to HMC. Chesnut and colleagues recently examined ICP monitoring among severe TBI patients in Latin American centers, similarly raising questions around generalizability of study findings to the U.S. [21]. Both the studies suggest that differences in TBI admission characteristics, trauma care center contexts should be considered in evaluating generalizability in TBI. However, differences among severe TBI patients also exist in the U.S. and final common pathways in TBI may converge around avoidance of second insults and provision of optimal cerebral physiology, consistent with those recommended in the BTF Guidelines, and regardless of where TBI occurs. Hence, there may be value in considering the global landscape when researching TBI and also in an international examination of the BTF Guidelines even though Guideline recommendations are based primarily on data derived from the U.S. Consequently, given the original intent of this bi- institutional collaborative partnership, we decided it was important and advantageous to present data from both centers side by side.
In addition to an overall favorable Guideline adherence effect, our data show an association between achieving greater than 65% ICU Guideline adherence and a nearly 2 fold higher discharge survival. Although we empirically categorized ICU adherence rates into the designated percentage categories, determining a threshold effect was exploratory. However, this observation that a dose effect might exist suggests that the beneficial effect of ICU Guideline adherence may not be an all or none phenomenon and that in addition to incremental adherence effects, achieving a minimum adherence rate might also be impactful. While we did not examine the relative impact of adherence to various Guideline indicators on in-hospital mortality, it is noteworthy that only about half the patients at JPNATC received ICP monitoring. This might indicate that overall guideline adherence may be effective in reducing early mortality even in the absence of routine ICP monitoring or ICP monitoring guided therapy. In this study, JPNATC ICU Guideline adherence exceeded 70%, demonstrating the feasibility of achieving this threshold at this type of trauma center. Further work may be needed to further examine local implementation strategies that increase and maintain ICU TBI Guideline adherence, and to determine the needed threshold to achieve the best discharge outcomes in severe TBI. While adherence rates to specific clinical indicators comprising the Guidelines varied, as a whole, Guideline adherence was associated with discharge survival even after adjustments for injury severity. This observation is important because ICU Guideline adherence as a treatment may be a feasible and cost effective strategy to improving outcomes in other LMIC nations where tertiary trauma care is provided. This study supports adoption and implementation of evidence based Guidelines in the care of patients with severe TBI.
Although patients enrolled represent a consecutive sample of severe TBI patients at JPNATC, the excellent 3, 6 and 12 month clinical follow up rates not only allowed us to examine longitudinal TBI functional outcomes but also facilitated examining the feasibility of conducting TBI outcome research studies at JPNATC. In this study, mobile technology was used to facilitate continued engagement with TBI patients and their families. While we did not test the effectiveness on mobile technology on follow up rates, this experience suggests an effective modality to retain severe TBI patients for both clinical follow and for future research studies at JPNATC and in other settings where mobile technology is commonly used and has been associated with improving health outcomes.22, 23
Although we had access to long term follow up data at JPNATC, we specifically chose to examine the effect of early ICU Guideline adherence on in-hospital mortality given the not only plausible but likely benefit of acute care treatments such as the ICU Guideline bundle on near term outcomes such as in-patient mortality. Moreover, as this study suggests other factors which we did not capture such as nutritional support, infection and or rehabilitation services affect TBI outcomes post discharge also affect long term outcomes. Furthermore, the majority of deaths in this sample occurred in-hospital. This study suggests that that tertiary trauma centers such as JPNATC in India can achieve ICU Guideline adherence and discharge survival rates similar to that of level 1 trauma centers in the U.S.
We observed that JPNATC discharge patterns were bimodal and resulted in survivors primarily being discharged home. Less than 5% of admitted patients received any rehabilitation and no patients received in-patient rehabilitation care nor were transferred to a skilled nursing facility. At HMC, patients were commonly discharged to these facilities, suggesting an unmet TBI recovery need for patients who received initial care at JPNATC, especially given the evidence supporting the use of cognitive and behavioral rehabilitation strategies for individuals with TBI in countries including India.24, 25 Although a small study, Gupta and Taly reported improvements in Disability Rating Sale among 44 Indian patients with severe TBI who initiated rehabilitation services 3 months post TBI for one month.25 Similarly, Agrawal and colleagues recently reported continued improvement to 6 months post TBI among an Indian cohort of 58 TBI patients who were followed to 12 months, and Sinha and colleagues reported 58–61% good cognitive, functional, and psychosocial outcomes after severe TBI in 77 severe TBI patients.24, 26 The present study, which is the largest cohort of TBI patients followed to 12 months in India documents a greater increase in good outcomes between 6 and 12 months in the absence of rehabilitation care. Differences in time of peaking of best outcomes between the current work and other studies may be explained by differences in provision of early ICU Guideline adherence, discharge GOS status and/or continued post discharge TBI care. Moreover, 12% of patients discharged with favorable discharge GOS worsened and 33% of patients with the potential to improve failed to improve at 12 months. Brooks and Colleagues have shown that life expectancy of persons with TBI is lower than that of the general population but worsening of GOS post discharge may be preventable.27 Overall, while we are not able to examine the effect of start or duration of rehabilitation services on outcomes, primarily due to the small number of patients receiving rehabilitation care post JPNATC discharge, the two published works from India and the present study highlight the critical and unmet need to prevent the observed GOS deterioration and to improve post discharge and long term functional recovery post TBI discharge. Achieving this goal might reduce any disparities between LMIC and high income nations in the rate and achievement of recovery in TBI patients. In India, which has almost no rehabilitation facilities, while there is an overall improvement in post discharge TBI outcomes, many patients deteriorate at home during the follow up. These findings suggest while most patients improve despite the lack of organized rehabilitation services, outcomes may be further improved with systematic rehabilitation. Therefore, resource allocation for early rehabilitation post TBI is warranted.
Since outcomes are similar between JPNATC and HMC, we may ask the question as to whether western world pre-hospital transport practice is necessary. However, this would not be a valid question because, many injured patients in the developing world die before reaching the hospital and the prehospital injury death denominator is not known. It is also possible that only the patients likely to survive reached hospital and the duration of prehospital period may not have been long. Additionally, at JPN, we included only those patients who were expected to survive > 48 hours because we felt it was important to give the opportunity for exposure (guideline adherence) which might have favored survivors. Finally, there are differences in ISS scores as well as injury patterns between the two institutions showing that the JPNATC cohort may in-fact be slightly less severely injured than the HMC partner. Thus, firm conclusions regarding the role of prehospital care cannot be made. It is also difficult to argue if one phase of TBI care is more important than the other. Importantly, our data demonstrate that in a system with limited to no prehospital care, where adherence to prehospital care guidelines is almost nonexistent, the adherence to the ICU guidelines is entirely feasible and is able to impact patient outcomes.
There are some study limitations. We operationalized some clinical indicators based on feasibility of data collection and on best available evidence, and could not evaluate all ICU clinical indicators, or treatment paradigms that may be important to patient outcomes. There may still be residual confounding by patient or treatment factors despite adjustment for potential confounders. The pre-hospital transportation and intubation/paralytic administration patterns may also explain differences in findings in the two centers. The Indian center may have included self-selection of those who were more grievously injured (and thus inclined to die within 48 hours) due to differences in pre-hospital transport. We could not identify those subjects who did not receive the protective indicator care due to intangibles such as perceptions of futility for whom indicators could not be delivered or achieved. The adherence rates at JPNATC may be affected by the fact that this was a prospective observational study and the ICU staff was aware of the ongoing data collection. Finally, we cannot conclude which protective indicator is more effective than another nor can we generalize these findings to other levels of trauma care (such as first or second level hospitals). We only had robust data from the ICU because pre-hospital and emergency department or operating room data were not as complete as needed for this analysis. Despite these limitations, this study provides evidence that early ICU Guideline based care may improve survival in severe TBI patients who receive care in tertiary trauma centers.
In summary, in the largest prospective study of severe TBI with 12 month outcome follow up at a tertiary care trauma center in India, we show that achieving early ICU Guideline adherence above 65% adherence rate is feasible and improves discharge survival. We also report that despite achieving survival with ICU Guideline adherence, and while long term outcomes generally improved, patients discharged with favorable GOS often deteriorated. This suggests a critical and unmet need to strengthen post discharge services and optimize long term outcomes after TBI. Clinical care pathways that consider both acute care Guideline adherence and transitions of care for hospitalized TBI may benefit short and long term outcomes for these patients.
Figure 3. Figures 3a and 3b: Change in functional status.
Change in functional status (GOS) post discharge among subset of 141 patients with favorable discharge GOS (3–5) and among subset of 136 patients discharged with room for GOS improvement (GOS 2–4) at Jai Prakash Narayan Apex Trauma Center (JPNATC), New Delhi, India at each time point (3, 6 and 12 months post discharge). Data label above each point indicates the N (number of patients).
Highlights.
For severe TBI patients treated at JPNATC, achieving early ICU adherence to Guideline indicators was feasible and associated with significantly lower in-hospital mortality.
Although the ICP monitoring rates varied, in-hospitals deaths were similar between the two Institutions.
While long term outcomes generally improved, patients discharged with favorable GOS often deteriorated at home.
Use of TBI guidelines may improve TBI care and outcomes in the developing
Acknowledgments
Funding:
Jointly funded by the National Institutes of Health (U.S) NINDS 5R21NS077444-02 and the Department of Biotechnology (India) DBT N-1349
Tanya Goetz at the Harborview Injury Prevention and Research Center for the final preparation and submission of manuscript
Abbreviations
- BTF
Brain Trauma Foundation
- HMC
Harborview Medical Center
- ICU
Intensive Care Unit
- JPNATC
Jay Prakash Narayan Apex Trauma Center
- LMIC
Low middle income country
- TBI
Traumatic brain injury
Footnotes
Conflicts of interest: none
The work was performed at the Harborview Injury Prevention and Research Center (HIPRC) at the University of Washington, Seattle, WA (lead and data coordinating center); and Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Deepak Gupta, Email: drdeepakgupta@gmail.com.
Deepak Sharma, Email: dsharma@uw.edu.
Nithya Kannan, Email: nithyak@uw.edu.
Suchada Prapruettham, Email: moh_yok@yahoo.com.
Charles Mock, Email: cmock@uw.edu.
Jin Wang, Email: wangji@uw.edu.
Qian Qiu, Email: qqiu@uw.edu.
R.M Pandey, Email: rmpandey@yahoo.com.
Ashok Mahapatra, Email: akmahapatra22000@gmail.com.
H.H. Dash, Email: dr.harihardash@gmail.com.
James G. Hecker, Email: heckerj@uw.edu.
Frederick P. Rivara, Email: -fpr@uw.edu.
Ali Rowhani-Rahbar, Email: rowhani@uw.edu.
Monica S. Vavilala, Email: vavilala@uw.edu.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Langlois JA, Sattin RW. Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC) J Head Trauma Rehabil. 2005;20(3):187–8. doi: 10.1097/00001199-200505000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention. Traumatic Brain Injury in the United States: Fact Sheet. 2015 Jan; Available at: http://www.cdc.gov/traumaticbraininjury/get_the_facts.html.
- 4.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22( 5):341–53. [PubMed] [Google Scholar]
- 5.Cole TB. Global road safety crisis remedy sought: 1·2 million killed, 50 million injured annually. JAMA. 2004;291(21):2531–32. doi: 10.1001/jama.291.21.2531. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385( 9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. Epub 2014 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Violence and Injury Prevention: Injuries and violence: The facts. 2015 Available at: http://www.who.int/violence_injury_prevention/key_facts/en/
- 8.Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24–28. doi: 10.1179/016164102101199503. [DOI] [PubMed] [Google Scholar]
- 9.Khan A, Prince M, Brayne C, Prina AM. Lifetime Prevalence and Factors Associated with Head Injury among Older People in Low and Middle Income Countries: A 10/66 Study. PLOS one. 2015 Jul 6;10(7):e0132229. doi: 10.1371/journal.pone.0132229. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal A, Coronado VG, Bell JM, Baisakhiya N, Kakani A, Galwankar S, et al. Characteristics of patients who died from traumatic brain injury in two rural hospital emergency departments in Maharashtra, India, 2007–2009. Int J Crit Illn Inj Sci. 2014 Oct-Dec;4(4):293–7. doi: 10.4103/2229-5151.147521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bratton SL, Bullock MR, Carney N, Chesnut RM, Coplin W, Ghajar J, et al. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons: Guidelines for the management of severe traumatic brain injury- 3rd edition. J Neurotrauma. 2007;24( Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 12.Buck CJ. International Classification of Diseases 9th Revision Clinical Modification for Physicians. St. Louis, MO: Saunders/Elsevier, American Medical Association; 2010. [Google Scholar]
- 13.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. [Google Scholar]
- 14.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 15.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3. Hoboken, NH: John Wiley & Sons; 2003. [Google Scholar]
- 16.Jennett B, Teasdale G, Braakman R, et al. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 17.Survey Sample Size Software. Petaluma CA: Creative Research Systems; 2008. [Google Scholar]
- 18.Hintze J. PASS 2008. Kaysville, UT: NCSS; 2008. [Google Scholar]
- 19.Zhou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. Global Neurotrauma Research Group. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012 Dec 27;367(26):2471–81. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas R, Joshi A, Joshi R, Kaufman T, Peterson C, Sturmberg JP, et al. Revitalizing primary health care and family medicine/primary care in India--disruptive innovation? J Eval Clin Pract. 2009 Oct;15(5):873–80. doi: 10.1111/j.1365-2753.2009.01271.x. [DOI] [PubMed] [Google Scholar]
- 23.Sahu M, Grover A, Joshi A. Role of mobile phone technology in health education in Asian and African countries: a systematic review. Int J Electron Healthc. 2014;7(4):269–86. doi: 10.1504/IJEH.2014.064327. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal M, Joshi M. Impact of rehabilitation on functional outcome during the first year of moderate and severe traumatic brain injury. Brain Inj. 2014;28(3):292–7. doi: 10.3109/02699052.2013.865266. Epub 2013 Dec 30. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Taly AB. Functional outcome following rehabilitation in chronic severe traumatic brain injury patients: A prospective study. Ann Indian Acad Neurol. 2012 Apr;15(2):120–4. doi: 10.4103/0972-2327.94995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha S, Gunawat P, Nehra A, Sharma BS. Cognitive, functional, and psychosocial outcome after severe traumatic brain injury: a cross-sectional study at a tertiary care trauma center. Neurol India. 2013 Sep-Oct;61(5):501–6. doi: 10.4103/0028-3886.121920. [DOI] [PubMed] [Google Scholar]
- 27.Brooks JC, Shavelle RM, Strauss DJ, Hammond FM, Harrison-Felix CL. Long-term survival after traumatic brain injury Part II: Life Expectancy. Arch Phys Med Rehabil. 2015 Jun;96( 6):1000–5. doi: 10.1016/j.apmr.2015.02.0. [DOI] [PubMed] [Google Scholar]