Abstract
Zinc is an essential micronutrient for cellular homeostasis. Initially proposed to only contribute to cellular viability through structural roles and non-redox catalysis, advances in quantifying changes in nM and pM quantities of Zn2+ have elucidated increasing functions as an important signaling molecule. This includes Zn2+-mediated regulation of transcription factors and subsequent protein expression, storage and release of intracellular compartments of zinc quanta into the extracellular space which modulates plasma membrane protein function, as well as intracellular signaling pathways which contribute to the immune response. This review highlights some recent advances in our understanding of zinc signaling.
Introduction
Zinc is essential for cellular homeostasis. More specifically, ten percent of all genes code for zinc-binding motifs [1]. Zinc dyshomeostasis can lead to cell death. Once believed to be essential solely for structural and catalytic functions in proteins and enzymes, zinc recently has been recognized as a signaling agent. Specific stimuli are hypothesized to trigger a transient increase in free zinc (Zn2+), which subsequently interacts with target molecules in a signal transduction process. In contrast to alkali and alkali earth metal-mediated signaling, the term free zinc does not necessarily denote hydrated metal ions, which adds complexity to these events. Instead Zn2+ exists in a weakly bound form that is more accessible than protein-bound zinc [2]. Transient Zn2+ increases can be generated by efflux from vesicles (zincosomes) or changes in cellular redox potential mediated by cytosolic proteins [3]. Metal ion buffering and muffling are the two critical parameters that determine zinc availability and therefore the nature of signaling process [4].
Estimates place the eukaryotic resting free Zn2+ concentrations in the picomolar range depending on the cell type [5-7], although quantifying these exceedingly low concentrations remains a significant technical challenge [8,9]. Since the total concentrations of zinc in cells is between 200-300 μM [10], maintaining homeostasis requires complex cellular machinery. This includes transcription factors which are turned on or off by small changes in the cellular concentration of Zn2+ as well as proteins that are marked for degradation through the ubiquitination pathway when cellular zinc levels increase for example [11,12]. Both thermodynamic and kinetic models of homeostasis are necessary to understand zinc maintenance. In the thermodynamic model pZn describes the physiological zinc potential [5]. Analogous to pH, the value of pZn is determined by the Kd of the buffering ligand(s) (pZn = −log[Zn2+]). Strongly buffered zinc implies that high affinity ligands maintain low free Zn2+ concentrations. Under these conditions, a transient increase in Zn2+ concentration will return to resting concentrations quickly. When buffering capacity is weak, cells reach a new steady-state Zn2+ concentration. To produce lasting Zn2+ signals, buffering capacity must be lower since signals would be terminated quickly at high pZn.
Muffling denotes the time-dependent redistribution of internalized Zn2+ under non-steady state conditions, and the subsequent return to resting cytosolic concentrations [10,13]. Removing surplus zinc from the cytoplasm by muffling enables cells to increase zinc uptake, despite a lack of buffering capacity. The temporal component of muffling process, as well as the ability for cells to temporarily tolerate non-steady state Zn2+concentrations are important factors in signaling.
Cellular Zinc Entry
Two classes of zinc transporters control the concentration of zinc in the cytosol as well as in intracellular compartments. Zinc Transport (ZnT) proteins function to decrease the cytosolic concentrations, while Zinc- or Iron-regulated transport Proteins (ZIP) increase the cytosolic concentrations [14]. While ZIP transporters are members of the cation diffusion facilitator class of proteins, ZnT proteins are not. Comparison of the crystal structure of YiiP, a bacterial ZnT protein with an ab initio model of the human (h) ZIP4 transporter suggests that both protein families have a similar transmembrane metal coordination site [15,16]. Both ZnT and ZIP proteins are expressed on the plasma membrane as well as on the membranes of intracellular organelles. Therefore, both ZnT and ZIP proteins control zinc trafficking between cellular compartments and zinc availability. One of these proposed intracellular compartments, the zincosomes, are loaded with Zn2+, usually by ZnT3. Furthermore, zincosomes may interact with receptors on cell membranes [17,18].
In addition to zinc-specific routes of entry mediated by ZnT and ZIP transport proteins, several ion channels may conduct Zn2+ under specific conditions. Under normal resting conditions blood plasma has sub-nM free Zn2+ [19]. However, transient increases in extracellular free Zn2+ have been observed, most commonly in the synaptic cleft. Under these conditions, some ion channels can use the electrochemical gradient to conduct Zn2+. For example, voltage-gated calcium channels control Ca2+ cell entry in response to membrane potential changes [20]. More specifically, the dihydropyridine-sensitive L-type calcium channel (LTCC) contributes to Zn2+ import in cortical neurons [21], β-cells in pancreas islet [22], and Zn2+ release from the endoplasmic reticulum in mast cells [23]. Ionotropic glutamate receptors (GluRs) are ligand-gated ion channels expressed mainly in the central nervous system (CNS) that mediate most excitatory synaptic transmission in mammalian brains [24]. Various GluR subunits may provide Zn2+ entry routes in postsynaptic neurons and astrocytes during neuronal excitation [7,25,26]. In addition, both acetylcholine receptors (AChR) [27] and transient receptor potential (TRP) channels have been shown to conduct Zn2+ [25].
Zinc-Mediated Modulation of Membrane Protein Function
Glutamate-gated NMDA receptors play a crucial role in neuronal communication, synaptic plasticity and memory formation [28,29]. The NMDA receptor is a dimer (GluN1A and GluN2B) where each subunit consists of an amino-terminal domain (ATD), a ligand-binding domain (LBD) and transmembrane domains (TMD) [30]. Upon the requisite stimulus, zinc quanta can be released from zincosomes within presynaptic terminals into the synaptic cleft (Figure 1). The exposed ATD can bind allosterically active ligands including Zn2+ found in the extracellular space. The inhibitory role of Zn2+ on NMDA receptors was recognized when micromolar Zn2+ in cultured hippocampal neurons reduced the amplitude of excitatory postsynaptic potentials by 50% compared to controls [31]. Subsequent analysis revealed that Zn2+ inhibits NMDA directly by two different mechanisms. High-affinity Zn2+ binding to the ATD of GluN2A subunit stabilized closed-cleft conformations, which reduced channel open probability. Low-affinity binding to the TMD is voltage-dependent and transiently blocks current flow through open channel [32].
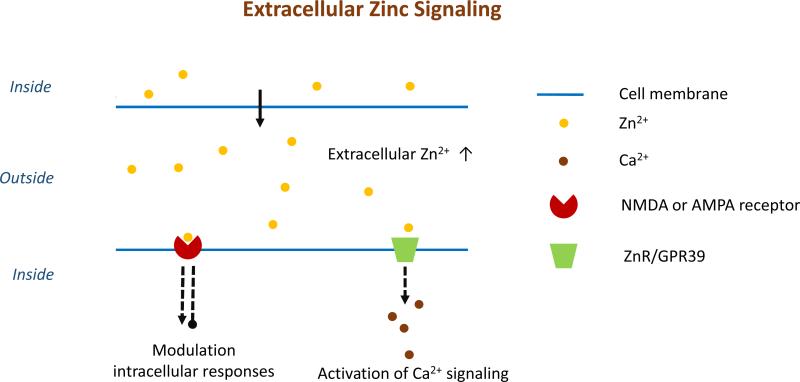
Figure 1.
A representation of extracellular Zn2+ signaling pathways. Zn2+ released into the extracellular space between cells can interact with proteins on the surface of another cell. Zn2+-binding can initiate a variety of responses including the release of Ca2+, another signaling metal ion. For example, signaling between neurons occurs when Zn2+ is released from vesicles in the presynaptic neuron into the synapse. Interactions between Zn2+ and protein receptors on the postsynaptic neuron are the next step in the signaling cascade.
The G-protein-coupled zinc sensing receptor (ZnR/GPR39) is expressed in the pancreas, gastrointestinal tract, liver, adipose tissue and the central nervous system. ZnR signaling contributes to cell proliferation, differentiation and/or death. [33-35] Unlike the inhibitory effect on the NMDA receptors upon zinc coordination, ZnR/GPR39 is an activator. Zn2+ coordination is mediated through a tridentate Zn2+-binding site on the extracellular domain which is comprised of two histidine and one aspartic acid residue [36]. Zn2+ coordination to ZnR triggers Ca2+ release via the IP3 signaling pathway [37].
Protein-Mediated Intracellular Zn2+ Release
In contrast to extracellular Zn2+ signaling which is initiated upon zinc release from an intracellular compartment into the extracellular space, intracellular Zn2+ signaling can be triggered by metal ion release from cytosolic proteins. A transient increase in intracellular Zn2+ is dependent upon oxidation of protein ligands since the metal ion is redox-inactive under physiological conditions. Consequently, a redox signal is converted into a Zn2+ signal. As an example, metallothioneins (MTs) bind up to seven Zn2+ in two thiolate-rich sites. The α-domain (C-terminal), which binds four metal ions, has a higher affinity for Zn2+, while the β-domain (N-terminal) coordinates up to three more labile metal ions [38]. At least four different MTs have been identified including MT-3 that is widely distributed in the CNS [39-41]. Nitric oxide, which has also been identified as an important signaling molecule, can oxidize the cysteine thiolate ligands, releasing a zinc signal [42].
Metal-responsive transcription factor-1 (MTF-1) is a zinc-finger transcription protein which regulates metal-responsive gene expression [43,44]. MTF-1 is the main activator for MT expression as well as select ZnTs [45,46]. MTF-1 contains six Cys2His2 zinc fingers (ZFs) which recognize and bind to DNA metal responsive elements (MRE) [47,48]. Zn2+, which can be acquired directly from mobile pools in the cytosol or indirectly by Zn2+ release from MTs by either cadmium or oxidative stress, activates MTF-1 upon coordination to ZFs [49,50]. Activated MTF-1 then localizes to the nucleus, binds to the MRE and alters protein expression levels on the transcriptional level [51].
Representative Signaling Events Trigger by Intracellular Zn2+ Release
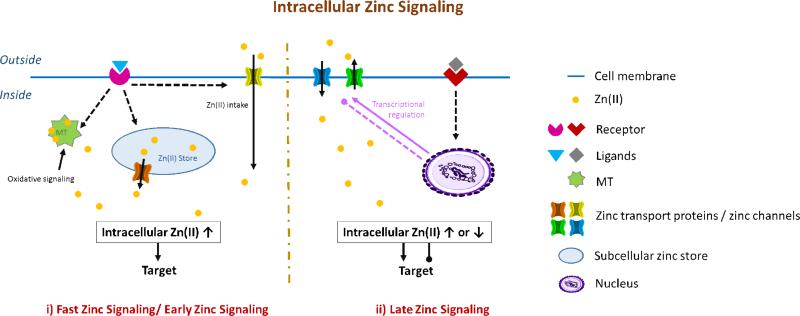
Extracellular stimuli such as hormones, growth factors and cytokines can trigger zinc transients in the cytosol [18]. Transient changes in the intracellular concentration of Zn2+ initiate second messenger pathways through early (fast) zinc signaling (EZS) or late zinc signaling (LZS, Figure 2i) [52]. EZS is transcription-independent where the timescale ranges from seconds to minutes. In contrast, LZS requires the transcription of zinc transport proteins with longer lasting effects (hours, Figure 2ii).
Figure 2.
A representation of various intracellular Zn2+ signals. In fast Zn2+ signa ling (i), binding of an extracellular ligand can trigger the release of Zn2+ from intracellular stores, liberation of Zn2+ from MTs by oxidative processes or flow of Zn2+ through permeable channels. Interaction of Zn2+ with target receptors that occurs before homeostasis is restored, constitutes a signaling pathway. Alternatively, in late Zn2+ signaling (ii), import and export by proteins changes the intracellular concentrations, which triggers transcriptional regulation of the homeostasis machinery.
Toll-like receptors (TLRs) contribute to the innate immune response by recognizing microbes [53]. TLRs are widely expressed on the surface of immune cells such as dendritic cells and macrophages, as well as on non-immune cells such as fibroblasts and epithelial cells [54-56]. When TLR-4 is activated it oligomerizes and recruits Toll/IL-1 receptor domain-containing adaptors to initiate signal transduction pathways that culminate in the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ΚB) and mitogen-activated protein kinases (MAPKs) [57-60]. NF-ΚB is a transcription factor which contributes to multiple physiological and pathological conditions, including the apoptosis, carcinogenesis, immune response and inflammation. MAPKs function to phosphorylate serine, threonine and tyrosine. Phosphorylation mediated by MAPKs contributes to initiating the immune response.
Zn2+ produces a dual effect on TLR-4 induced cytokine secretion [61]. Stimulation of human leukocytes with lipopolysaccharide (LPS) results in a moderate cytosolic Zn2+ increase. Trapping Zn2+ with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) suppresses MAPK stimulation. Zn2+-induced MAPK activation occurs within 2 min [62]. LPS can also decrease labile Zn2+, and high Zn2+ concentrations inhibit TLR-4 signals [63-66]. Zn2+ may be involved in TLR-4 signaling, regulating myeloid differentiation primary response gene 88 via EZS, and TIR-domain-containing adapter-inducing interferon-β signaling by modulating basal Zn2+ concentrations [67]; however, there is no direct evidence Zn2+ activates MAPK. While the kinase might not be the molecular target for Zn2+, Zn2+ inhibits MAPK phosphatases (MKPs) [68], so the specifics of the putative Zn2+ signaling mechanism have yet to be elucidated.
Fcε receptor I (FcεRI) belongs to the family of multi-chain immune recognition receptors (MIRRs) [69], and is found in mast cells, basophils and eosinophils [70,71]. Upon extracellular stimulus of FcεRI by immunoglobulin E, a rapid increase in intracellular Zn2+, or zinc wave is observed [72]. This zinc wave regulates downstream signaling pathways and enhances the transcription of cytokines and affects several processes, including tyrosine phosphatase activity [18].
Zinc Sparks
Cell cycle arrest at metaphase II (MII) of meiosis is a key step in mammalian oogenesis. Upon fertilization, a series of Ca2+ oscillations activate downstream molecular targets [73,74]. The impact of Zn2+ on the eukaryotic cell cycle was first recognized three decades ago [75,76], but recently, O'Halloran reported Zn2+ uptake by mouse oocytes during maturation [77]. After the onset of Ca2+ oscillations, Zn2+-enriched oocytes eject Zn2+ into the extracellular milieu in a series of coordinated zinc sparks. The process lasts for hours and facilitates cell cycle resumption. Quantitative maps of the fluxes reveal that zinc sparks originate from vesicles. These vesicles undergo dynamic movement during oocyte maturation and exocytosis upon fertilization [78]. Two zinc transport proteins, ZIP6 and ZIP10, are expressed in the cortex and have been proposed to be responsible for Zn2+ accumulation [79]. Cumulus cells also may contribute to the zinc transport network by forming cumulus-oocyte complex [80].
Zinc sparks mediate egg-to embryo transitions [65,77]. Equally, Zn2+ may exert a concentration-dependent regulation of meiosis through an interaction with the zinc-binding EM12, a central component of the cytostatic factor (CSF) [81]. EM12 contains a C-terminal zinc-binding region that inhibits the anaphase promoting complex/cyclosome [82-84]. When intracellular Zn2+ reaches a threshold concentration, coordination to the zinc binding region activates APC/C inhibitor EM12, which leads to CSF-mediated MII arrest. After activation, zinc sparks reduce both cellular zinc and EM12 activity, which drives egg activation [81].
Conclusions
Zn2+ signaling is mediated by transient changes in free zinc. Extracellular Zn2+ signaling can take place in extracellular environment such as the synaptic cleft where Zn2+ released from the presynaptic cleft modulates protein function on postsynaptic neurons. Intracellular Zn2+ signaling has been observed in many cell types and is generated in the cytosol from the extracellular space or from intracellular Zn2+ stores. Cytosolic proteins such as metallothioneins may also contribute to the Zn2+ signals through redox chemistry. The import, export and trafficking of Zn2+ by proteins all contribute to a cells capacity to generated, utilize and terminate signals. The ongoing efforts to study these various processes are vital to mapping signal transduction pathways mediated by Zn2+.
Highlights to be used.
Zinc transport proteins decrease cytosolic concentrations
Iron-regulated transport proteins increase cytosolic zinc concentrations
Extracellular zinc release is involved in a variety of signaling processes
Intracellular zinc release can be involved in signaling by binding to receptors or regulate protein at the transcriptional level
Zinc sparks can control egg to embryo transitions
Acknowledgements
This work was supported by NSF Grant CHE-0955361 and NIH grant GM105964.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 2•.Wellenreuther G, Cianci M, Tucoulou R, Meyer-Klaucke W, Haase H. The ligand environment of zinc stored in vesicles. Biochem Biophys Res Commun. 2009;380:198–203. doi: 10.1016/j.bbrc.2009.01.074. [X-ray absorption techniques are utilized to characterize the nature of vesicular (often called free) Zn2+. Measurements indicate a coordination environment including nitrogen, oxygen and sulfur donors.] [DOI] [PubMed] [Google Scholar]
- 3.Fukada T, Kambe T. Zinc Signals in Cellular Functions and Disorders. Springer; 2014. [Google Scholar]
- 4.Wang Z, Tymianski M, Jones OT, Nedergaard M. Impact of cytoplasmic calcium buffering on the spatial and temporal characteristics of intercellular calcium signals in astrocytes. J Neurosci. 1997;17:7359–7371. doi: 10.1523/JNEUROSCI.17-19-07359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Krężel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [A detailed picture of Zn2+ concentrations and binding sites in human colon cancer cells is provided. The findings highlight the importance of metallothionein as a buffer for intracellular Zn2+.] [DOI] [PubMed] [Google Scholar]
- 6.Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011;24:411–418. doi: 10.1007/s10534-010-9406-1. [DOI] [PubMed] [Google Scholar]
- 7.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh J-Y, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JG, Qin Y, Galati DF, Palmer AE. New sensors for quantitative measurement of mitochondrial Zn2+. ACS Chem Biol. 2012;7:1636–1640. doi: 10.1021/cb300171p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Qin Y, Miranda JG, Stoddard CI, Dean KM, Galati DF, Palmer AE. Direct comparison of a genetically encoded sensor and small molecule indicator: implications for quantification of cytosolic Zn2+. ACS Chem Biol. 2013;8:2366–2371. doi: 10.1021/cb4003859. [Small molecule fluorescent probes are popular for measuring intracellular metal ion concentrations. Comparison with protein-based sensors highlights some of the challenges of using small molecule probes that can perturb actual behavior.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maret W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics. 2015;7:202–211. doi: 10.1039/c4mt00230j. [DOI] [PubMed] [Google Scholar]
- 11.Mao X, Kim B-E, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 12••.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [Seminal study demonstrating the extremely tight regulation of intracellular Zn2+ in E. coli. The investigation contradicts the previous metalloregulatory dogma that proteins acquire Zn2+ from a pool of free metal ions.] [DOI] [PubMed] [Google Scholar]
- 13•.Colvin RA, Holmes WR, Fontaine CP, Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [An important primer on the thermodynamic and kinetic principles governing Zn2+ homeostasis and signaling.] [DOI] [PubMed] [Google Scholar]
- 14.Dempski RE. The cation selectivity of the ZIP transporters. Curr Top Membr. 2012;69:221–245. doi: 10.1016/B978-0-12-394390-3.00009-4. [DOI] [PubMed] [Google Scholar]
- 15.Antala S, Ovchinnikov S, Kamisetty H, Baker D, Dempski RE. Computational modeling and functional studies provide a structural scaffold for the zinc transporter hZIP4. J Biol Chem. 2015:jbc. M114. 617613. doi: 10.1074/jbc.M114.617613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 17.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [An extracellular stimulus leads to an increase in intracellular Zn2+. The generation of this “zinc wave” has the hallmarks of second messengers, and establishes Zn2+ as an important signaling agent.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magneson G, Puvathingal J, Ray W. The concentrations of free Mg2+ and free Zn2+ in equine blood plasma. J Biol Chem. 1987;262:11140–11148. [PubMed] [Google Scholar]
- 20.Van Petegem F, Minor D., Jr The structural biology of voltage-gated calcium channel function and regulation. Biochem Soc Trans. 2006;34:887. doi: 10.1042/BST0340887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol. 2000;528:39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priel T, Aricha-Tamir B, Sekler I. Clioquinol attenuates zinc-dependent β-cell death and the onset of insulitis and hyperglycemia associated with experimental type I diabetes in mice. Eur J Pharmacol. 2007;565:232–239. doi: 10.1016/j.ejphar.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki S, Hasegawa A, Hojyo S, Ohashi W, Fukada T, Nishida K, Hirano T. A novel role of the L-type calcium channel alpha1D subunit as a gatekeeper for intracellular zinc signaling: zinc wave. PLoS One. 2012;7:e39654. doi: 10.1371/journal.pone.0039654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacological reviews. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouron A, Oberwinkler J. Contribution of calcium-conducting channels to the transport of zinc ions. Pflugers Arch EJP. 2014;466:381–387. doi: 10.1007/s00424-013-1295-z. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JH, Sensi SL. Ca2+–Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 2000;23:365–371. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- 27.Ragozzino D, Giovannelli A, Degasperi V, Eusebi F, Grassi F. Zinc permeates mouse muscle ACh receptor channels expressed in BOSC 23 cells and affects channel function. J Physiol. 2000;529:83–91. doi: 10.1111/j.1469-7793.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danysz W, Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. British J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 30.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344:992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer M, Vyklicky L. The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK. Zinc effects on NMDA receptor gating kinetics. Biophys J. 2011;100:1910–1918. doi: 10.1016/j.bpj.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen L, Azriel-Tamir H, Arotsker N, Sekler I, Hershfinkel M. Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS One. 2012;e35482 doi: 10.1371/journal.pone.0035482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen L, Sekler I, Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014;5:e1307. doi: 10.1038/cddis.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popovics P, Stewart AJ. GPR39: a Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol Life Sci. 2011;68:85–95. doi: 10.1007/s00018-010-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storjohann L, Holst B, Schwartz TW. Molecular mechanism of Zn2+ agonism in the extracellular domain of GPR39. FEBS Lett. 2008;582:2583–2588. doi: 10.1016/j.febslet.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Asraf H, Salomon S, Nevo A, Sekler I, Mayer D, Hershfinkel M. The ZnR/GPR39 interacts with the CaSR to enhance signaling in prostate and salivary epithelia. J Cell Physiol. 2014;229:868–877. doi: 10.1002/jcp.24514. [DOI] [PubMed] [Google Scholar]
- 38•.Babu CS, Lee Y-M, Dudev T, Lim C. Modeling Zn2+ Release From Metallothionein. J Phys Chem A. 2014;118:9244–9252. doi: 10.1021/jp503189v. [Modeling techniques are introduced as a powerful methodology to understand how Zn2+ can be liberated from differential binding sites in metallothionein. These processes are important to maintaining homeostasis and possibly to generating Zn2+ signals.] [DOI] [PubMed] [Google Scholar]
- 39.Garrett SH, Sens MA, Todd JH, Somji S, Sens DA. Expression of MT-3 protein in the human kidney. Toxicol Lett. 1999;105:207–214. doi: 10.1016/s0378-4274(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 40.Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 41.Sallinen P, Saarela S, Ilves M, Vakkuri O, Leppäluoto J. The expression of MT 1 and MT 2 melatonin receptor mRNA in several rat tissues. Life Sci. 2005;76:1123–1134. doi: 10.1016/j.lfs.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Thambiayya K, Wasserloos KJ, Pitt BR. Central role for metallothionein (MT) in Zn dependent nitric oxide (NO) mediated resistance to LPS-induced apoptosis in sheep pulmonary artery endothelial cells (SPAEC). FASEB J. 2011;25:1100.1102. [Google Scholar]
- 43.Giedroc DP, Chen X, Apuy JL. Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxid Redox Signal. 2001;3:577–596. doi: 10.1089/15230860152542943. [DOI] [PubMed] [Google Scholar]
- 44.Saydam N, Georgiev O, Nakano MY, Greber UF, Schaffner W. Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J Biol Chem. 2001;276:25487–25495. doi: 10.1074/jbc.M009154200. [DOI] [PubMed] [Google Scholar]
- 45.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–34809. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y-J, Liu Y-C, Lin M-C, Chen Y-T, Lin L-Y. Coordinative modulation of human zinc transporter 2 gene expression through active and suppressive regulators. J Nutr Biochem. 2015;26351-359 doi: 10.1016/j.jnutbio.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Dalton TP, Bittel D, Andrews GK. Reversible activation of mouse metal response element-binding transcription factor 1 DNA binding involves zinc interaction with the zinc finger domain. Mol Cell Biol. 1997;17:2781–2789. doi: 10.1128/mcb.17.5.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koizumi S, Suzuki K, Ogra Y, Gong P, Otuska F. Roles of zinc fingers and other regions of the transcription factor human MTF-1 in zinc-regulated DNA binding. J Cell Physiol. 2000;185:464–472. doi: 10.1002/1097-4652(200012)185:3<464::AID-JCP18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Dalton TP, Li Q, Bittel D, Liang L, Andrews GK. Oxidative Stress Activates Metal-responsive Transcription Factor-1 Binding Activity. J Biol Chem. 1996;271:26233–26241. doi: 10.1074/jbc.271.42.26233. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Georgiev O, Hagmann M, Günes Ç , Cramer M, Faller P, Vasák M, Schaffner W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol Cell Biol. 2003;23:8471–8485. doi: 10.1128/MCB.23.23.8471-8485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Günther V, Lindert U, Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta. 2012;1823:1416–1425. doi: 10.1016/j.bbamcr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 54.Kim K-W, Cho M-L, Lee S-H, Oh H-J, Kang C-M, Ju JH, Min S-Y, Cho Y-G, Park S-H, Kim H-Y. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110:54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu Y-J, Rea TH. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M. β-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 57.Huang H, Zhang Z, Cao C, Wang N, Liu F, Peng J, Ren X, Qian J. The TLR4/NF-κB signaling pathway mediates the growth of colon cancer. Eur Rev Med Pharmacol Sci. 2014;18:3834–3843. [PubMed] [Google Scholar]
- 58.Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Saitoh Si, Akashi S, Yamada T, Tanimura N, Kobayashi M, Konno K, Matsumoto F, Fukase K, Kusumoto S, Nagai Y. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Yin P, Wan C, Chong X, Liu M, Cheng P, Chen J, Liu F, Xu J. Punicalagin inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation. 2014;37:956–965. doi: 10.1007/s10753-014-9816-2. [DOI] [PubMed] [Google Scholar]
- 61.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Ann Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 62.Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 63.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1- induced NF-κB activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 64.Jin J, Zeng H, Schmid KW, Toetsch M, Uhlig S, Möröy T. The zinc finger protein Gfi1 acts upstream of TNF to attenuate endotoxin-mediated inflammatory responses in the lung. Eur J Immunol. 2006;36:421–430. doi: 10.1002/eji.200535155. [DOI] [PubMed] [Google Scholar]
- 65.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Thambiayya K, Wasserloos KJ, Huang Z, Kagan VE, Croix CMS, Pitt BR. LPS-induced decrease in intracellular labile zinc,[Zn]i, contributes to apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L624–L632. doi: 10.1152/ajplung.00376.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brieger A, Rink L, Haase H. Differential regulation of TLR-dependent MyD88 and TRIF signaling pathways by free zinc ions. J Immunol. 2013;191:1808–1817. doi: 10.4049/jimmunol.1301261. [DOI] [PubMed] [Google Scholar]
- 68.Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of MAPK phosphatases by zinc accounts for ERK1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcεRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eiseman JBB. Engagement of the high-affinity IgE receptor activates src protein- related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 71.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (FcϵRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: Relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 72.Turner H, Kinet J-P. Signalling through the high-affinity IgE receptor Fcε RI. Nature. 1999;402:24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 73.Kinsey WH. Intersecting roles of protein tyrosine kinase and calcium signaling during fertilization. Cell Calcium. 2013;53:32–40. doi: 10.1016/j.ceca.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miao Y-L, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powell M, Davies M, Francis D. The Influence of Zinc on the Cell Cycle in the Root Meristem of a Zinc-Tolerant and a Non-Tolerant Cultivar of Festuca Rubra L. New Phytol. 1986;102419-428 doi: 10.1111/j.1469-8137.1986.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 76.Powell M, Davies M, Francis D. Effects of Zinc on Cell, Nuclear and Nucleolar Size, and on RNA and Protein Content in the Roor Meristem of a Zinc Tollerant and a Non-Tolerant Cultivar of Festuca Rubra L. New Phytol. 1986;104:671–679. doi: 10.1111/j.1469-8137.1986.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 77.Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [A combination of techniques are utilized to elucidate the origins of Zn2+ sparks, which are essential to the conversion of a fertilized egg into an embryo. Zn2+ was found to be compartmentalized in vesicles that undergo exocytosis responsible for the observed sparks.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kong B, Duncan F, Que E, Kim A, O'Halloran T, Woodruff T. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod. 2014:gau066. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lisle RS, Anthony K, Randall M, Diaz F. Oocyte–cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction. 2013;145:381–390. doi: 10.1530/REP-12-0338. [DOI] [PubMed] [Google Scholar]
- 81.Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod. 2012;86:114. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009;4:2. doi: 10.1186/1747-1028-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shoji S, Muto Y, Ikeda M, He F, Tsuda K, Ohsawa N, Akasaka R, Terada T, Wakiyama M, Shirouzu M. The zinc-binding region (ZBR) fragment of Emi2 can inhibit APC/C by targeting its association with the coactivator Cdc20 and UBE2C-mediated ubiquitylation. FEBS Open Bio. 2014;4:689–703. doi: 10.1016/j.fob.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]