Abstract
The DA transporter (DAT), a phosphoprotein, controls extracellular dopamine (DA) levels in the central nervous system through transport or reverse transport (efflux). Multiple lines of evidence support the claim that PKC significantly contributes to amphetamine-induced DA efflux. Other signaling pathways, involving CaMKII and ERK, have also been shown to regulate DAT mediated efflux. Here we assessed the contribution of putative PKC residues (S4, S7, S13) in the N-terminal of the DAT to amphetamine-induced DA efflux by transfecting DATs containing different serine to alanine (S-A) point mutations into DA pre-loaded HEK-293 cells and incubating these cells in amphetamine (2 µM). The effects of a S-A mutation at the non-PKC residue S12 and a threonine to alanine (T-A) mutation at the ERK T53 residue were also assessed for comparison. WT-DATs were used as controls. In an initial experiment, we confirmed that inhibiting PKC with Go6976 (130nM) significantly reduced amphetamine-induced DA efflux. In subsequent experiments, cells transfected with the S4A, S12A, S13A, T53A and S4,7,13A mutants showed a reduction in amphetamine-induced DA efflux similar to that observed with Go6976. Interestingly, cells transfected with the S7A mutant, identified by some as a PKC-PKA residue, showed unperturbed WT-DAT levels of amphetamine-induced DA efflux. These results indicate that phosphorylation by PKC of select residues in the DAT N-terminal can regulate amphetamine-induced efflux. PKC can act either independently or in concert with other kinases such as ERK to produce this effect.
Keywords: Amphetamine, CaMKII, Dopamine release, Dopamine transporter, ERK, Phosphorylation, PKC
1. Introduction
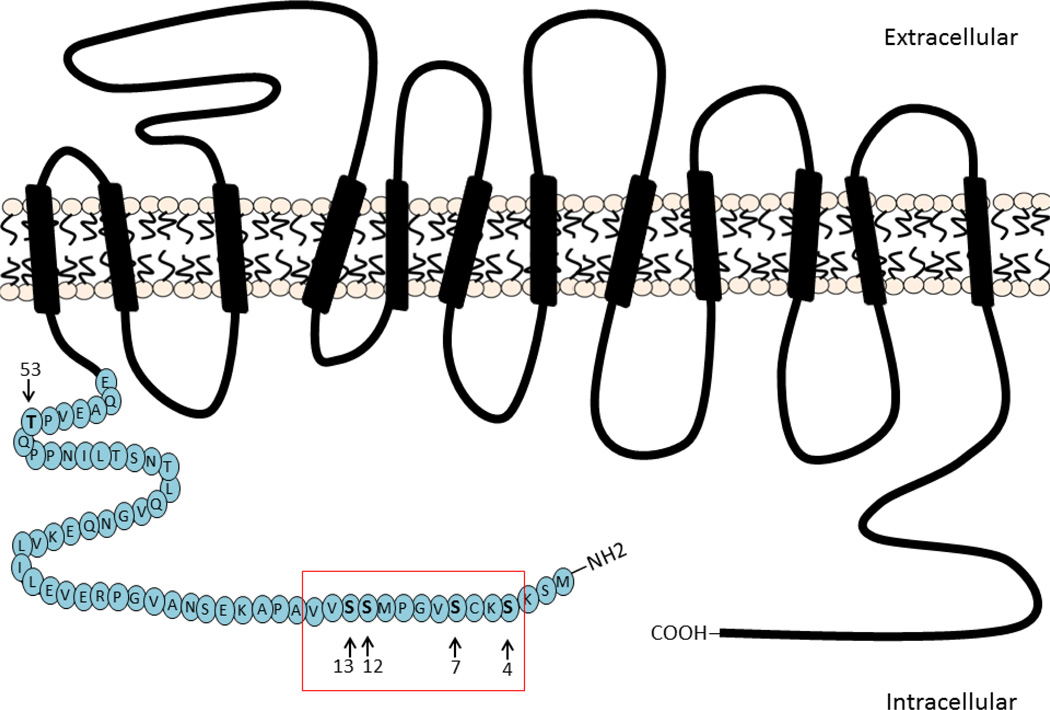
The dopamine transporter (DAT) plays an important role in the extracellular clearance of dopamine (DA) by mediating its re-uptake into the presynaptic terminal [1;2]. It is also the primary target for psychostimulants, such as cocaine, amphetamine, and methamphetamine [2]. Cocaine exerts its psychostimulant properties by binding to DAT and blocking DA re-uptake, resulting in elevated extracellular DA levels [1]. Amphetamine, on the other hand, acts as a substrate for DAT and also subsequently promotes reverse transport of DA (DA efflux) [3;4]. DA efflux is regulated by several kinases, including PKC, calcium-calmodulin-dependent protein kinase II (CaMKII), and extracellular signal-regulated kinase (ERK) [5;6]. It has been shown that the PKC inhibitor Ro31-8220 blocks amphetamine-mediated DA release in rat striatal slices [7], as well as amphetamine-induced DA overflow in the shell and core of rat nucleus accumbens in vivo [8]. Moreover, PKCβ isoforms have been shown to co-immunoprecipitate with DAT [9], and overexpressing PKCβII enhances [9] while knockout of PKCβ reduces [10] amphetamine-induced DA efflux. These data indicate that PKC plays an important role in mediating amphetamine-induced DA efflux. The N-terminal of DAT has been demonstrated to contain the primary sites for PKC activation-induced phosphorylation [11]. There is also evidence suggesting that other protein kinases such as CaMKII [12–14] and ERK [15;16] may also regulate DAT by phosphorylating residues in its N-terminal. The results of in vitro experiments using a recombinant N-terminal peptide of DAT [11,17] indicate that PKC phosphorylates the S4, S7, and S13 residues, that the S7 and S13 residues are also phosphorylated by PKA and CaMKII respectively, and that the T53 residue is phosphorylated by ERK1/2 (Figure 1). In the present study, we evaluated the potential contribution of phosphorylation at these sites to amphetamine-induced DA efflux by generating DATs containing different serine/threonine to alanine mutations (S/T-A) and transfecting them into DA pre-loaded HEK-293 cells. Effects were compared to that produced by the PKC inhibitor Go6976.
Figure 1.
Schematic representation of the DAT showing tested residues in its cytoplasmic N-terminal (arrows). Red box highlights the serine residues tested.
2. Materials and Methods
The rat DAT constructs (pcDNA3-DAT, pcDNA3-DAT-S4A, pcDNA3-DAT-S7A, pcDNA3-DAT-S12A and pcDNA3-DAT-S13A) were generously provided by Dr. Randy D. Blakely (Vanderbilt University Medical Center, Nashville, TN, USA). Other constructs (pcDNA3-DAT-S4,7,13A and pcDNA3-DAT-T53A) were generated with the GeneArt® Site-Directed Mutagenesis Kit (Invitrogen Grand Island, NY, USA) using primers as follows: DAT-S4,7,13A-Forward: CTA CCC ATG AGT AAG GCC AAA TGC GCC GTG GGA CCA ATG TCT; DAT-S4,7,13A-Reverse: AGA CAT TGG TCC CAC GGC GCA TTT GGC CTT ACT CAT GGG TAG; DAT-T53A-Forward: ATC AAC CCG CCA CAG GCA CCA GTG GAG GCT CAA; DAT-T53A-Reverse: TTG AGC CTC CAC TGG TGC CTG TGG CGG GTT GAT. The wild type (WT) or mutant DAT cDNA fragments were released from pcDNA3 vectors by EcoRI restriction digestion and then sub-cloned into a pSMPUW-IRES-GFP vector (a lentiviral vector from Cell Biolabs Inc, San Diego, CA, USA). The insertion site was downstream of an EF1 promoter.
Using procedures described elsewhere [19] with some modifications, HEK-293 cells were grown in DMEM medium supplemented with 10% fetal bovine serum. One day before transfection, the cells were placed in 6-well plates and upon 50% confluency, they were transfected either with pSMPUW-DAT (WT) or the mutant DAT constructs using the TransIT-293 Transfection Reagent system (Mirus Bio LLC, Madison, WI, USA) according to the manufacture’s instruction. The transfection efficiency (>85%) and the expression of DAT protein were confirmed by verifying GFP expression under florescence microscopy. Forty-eight hours after transfection, the cells were washed twice with KRH buffer (25 mM HEPES, pH 7.4, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgSO4, and 10 mM glucose) and preloaded at 37°C with 15 µM DA for 30 min. Cells were then again washed three times with KRH and DA efflux was subsequently assessed with an incubation procedure. Consistent with previous reports [19], there was no evidence that uptake of DA (determined as total DA content achieved in the cells) was systematically decreased relative to WT by the different mutations. Following a 5 minute equilibration period in the KRH incubation medium, five fractions (350µl) were collected every 2.5 minutes (Figure 2A). Amphetamine (2µM) was added to fraction 2. This concentration is in the low range when used in perfusion experiments [19] but was found to be preferable to 10µM which produced high background DA levels in our pilot experiments with the incubation procedure. In the case of the PKC inhibitor, the same experimental procedures were used except that 130nM of Go6976 [9] was added to the KRH incubation medium during equilibration through to fraction 2. Go6976 is a potent inhibitor of the classical Ca2+-dependent PKC isoforms [9]. Samples were immediately frozen and stored at −80°C until biochemical analysis by HPLC with electrochemical detection [18]. Experiments were conducted in triplicate. Data were calculated as % of total DA (DA released/total DA released + DA remaining in the cells) and analyzed statistically by one-way ANOVA followed by Tukey HSD post hoc tests.
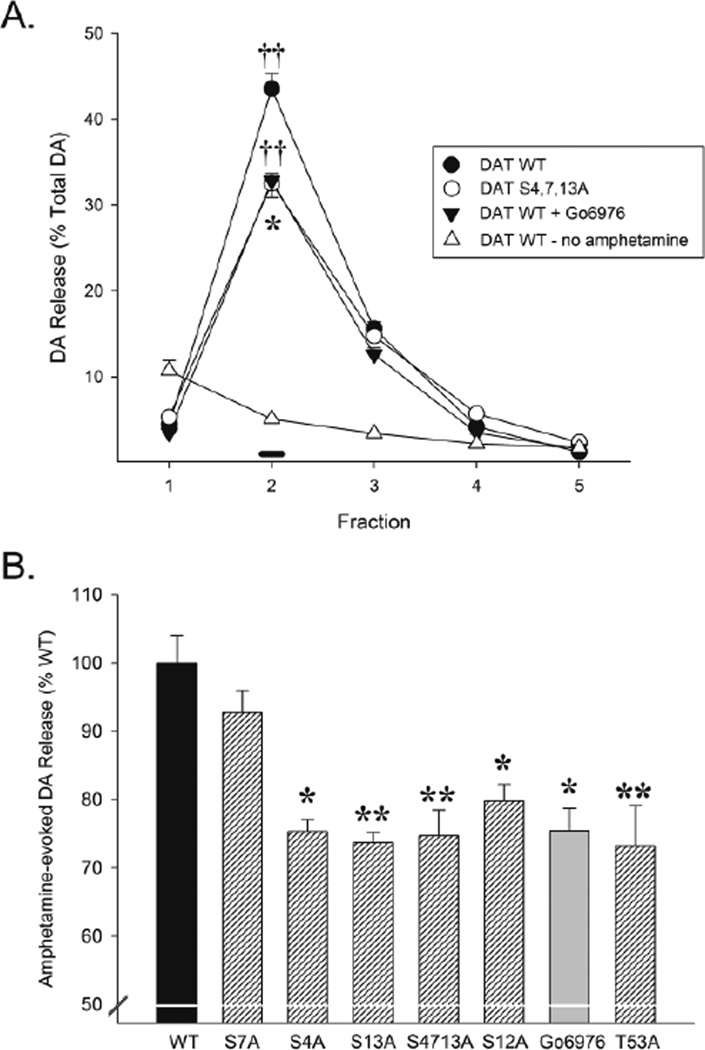
Figure 2.
Preventing phosphorylation with the PKC inhibitor Go6976 and with S/T-A mutations at PKC (S4A, S13A, S4/7/13), non-PKC (S12A), and ERK1/2 (T53A) residues in the DAT N-terminal reduces amphetamine-induced DA efflux. A. Example of the time course of amphetamine-evoked DA efflux from HEK-293 cells transfected with WT DAT or the combined PKC mutant DAT-S4,7,13A or WT DAT transfected cells in the presence of the PKC inhibitor Go6976 (130nM). Also shown is DA efflux from cells transfected with WT DAT but not exposed to amphetamine. Black bar at abscissa indicates incubation with amphetamine (2µM) at fraction 2 for the first three groups. Data are shown as mean (± SEM) DA released as % of total DA. One-way ANOVA at fraction 2 revealed a significant effect of groups [F3,11=166.05, p<0.001]. *, p<0.01, significantly different from DAT WT; ††, p<0.001, significantly different from DAT WT – no amphetamine as determined by the Tukey HSD post hoc test. B. Summary of amphetamine-evoked DA release observed at fraction 2 in the different groups. Data are shown as mean (+ SEM) amphetamine-evoked DA release expressed as % of WT DAT. One-way ANOVA revealed a significant effect of groups [F7,23=9.27, p<0.001]. *, p<0.01, **, p<0.001, significantly different from WT as determined by the Tukey HSD post hoc test.
3. Results
To confirm that PKC can regulate amphetamine-induced DA efflux, HEK-293 cells transfected with WT DAT were preloaded with DA and incubated in 2µM amphetamine with or without 130 nM Go6976. Amphetamine produced a large increase in DA release and this was significantly reduced by Go6976 (Figure 2), indicating that PKC contributes to amphetamine-induced efflux.
Since PKC has been shown in vitro to phosphorylate multiple serine residues, including S4, S7, and S13 [9], we next tested whether preventing phosphorylation by PKC at these sites with S-A mutations can inhibit amphetamine-induced DA efflux. We generated a DAT containing S-A mutations at all three residues (DAT-S4,7,13A) and transfected it into HEK-293 cells. Amphetamine-induced DA release was significantly reduced in these cells compared to WT DAT transfected cells (Figure 2). The magnitude of the reduction was almost identical to that observed with the PKC inhibitor Go6976, indicating that these serine residues are likely targets for PKC phosphorylation that regulate amphetamine-induced DA efflux.
We further assessed the three putative PKC sites individually (S4, S7, and S13) and for comparison, the ERK1/2 residue T53 [16] as well as S12, a non-PKC residue adjacent to the S13 PKC/CaMKII phosphorylation site [9]. Using S/T-A point mutations at these residues, we tested whether preventing phosphorylation at these sites individually inhibits amphetamine-induced DA release. As shown in Figure 2, not all serine residues appear to contribute to amphetamine-induced DA efflux. While the S4A and S13A mutants significantly reduced amphetamine-induced release, the S7A mutant failed to produce a significant reduction. Notably, the effects observed with S4A and S13A were similar to that observed with the S4,7,13A mutant and the PKC inhibitor Go6976. Interestingly, the T-A mutation of the ERK1/2 T53 residue significantly reduced amphetamine-induced efflux as did the S12A mutant, although in the latter case to a somewhat lesser extent. When these non-PKC mutations were combined with pharmacological inhibition of PKC by Go6976, amphetamine-induced DA efflux was further reduced. This was not observed when Go6976 was combined with the mutation of a PKC residue (Table 1). Together, these results show that PKC can act alone or in combination with other kinases to regulate amphetamine-evoked DA efflux.
Table 1.
The PKC inhibitor, Go6976, further reduces amphetamine-induced DA efflux when administered with S/T-A mutations at non-PKC residues (S12A and T53A) but has no effect when administered with a S-A mutation at a PKC residue (S13A).
| Mutation | Amphetamine evoked DA release |
% WT | Mutation alone vs Mutation + Go6976 |
|---|---|---|---|
| WT | 43.57 ± 0.89 | 100% | |
| S13A | 32.10 ± 0.63 | 73.7% | |
| S13A + Go6976 | 32.93 ± 0.40 | 75.6% | NS |
| S12A | 34.74 ± 1.07 | 79.7% | |
| S12A + Go6976 | 27.36 ± 0.47 | 62.8% | p<0.01 |
| T53A | 31.90 ± 2.53 | 73.2% | |
| T53A + Go6976 | 23.28 ± 0.61 | 53.4% | p<0.01 |
Data are shown as mean ± SEM amphetamine-evoked DA released as % of total DA. Percentages indicate reduction of DA release as % of WT. One-way ANOVA revealed a significant effect of groups [F6,20=29.29, p<0.001]. Probability levels indicate significant differences between the mutation alone and the mutation + Go conditions for each mutation as determined by the Tukey HSD post hoc test.
NS, not significant.
4. Discussion
In this study, we found that preventing phosphorylation at specific serine or threonine residues in the N-terminal of the DAT can significantly reduce amphetamine-induced DA efflux. Using the PKC inhibitor Go6976, we confirmed that PKC plays an important role in amphetamine-induced DA release in HEK-293 cells that harbor WT DAT. Further, simultaneous S-A mutation of three putative PKC residues S4, 7 and 13 [11,17,19] similarly inhibited amphetamine-induced DA efflux, supporting an important role for PKC phosphorylation of the N-terminal of the DAT in the DA releasing effects of amphetamine. These serine residues, however, did not contribute equally. While DAT-S4A and S13A reduced amphetamine-induced DA release to levels observed with DAT-4,7,13A and the PKC inhibitor Go6976, the DAT-S7A mutant failed to produce a significant reduction even though it has been identified as a PKC residue [17]. Finally, preventing phosphorylation at the non-PKC residue S12 [17] and the ERK1/2 residue T53 [15] also inhibited amphetamine-induced DA efflux. Together, these results indicate that phosphorylation by PKC of select residues in the DAT N-terminal can regulate amphetamine-induced efflux and that PKC can act either independently or in concert with other kinases such as ERK to exert this effect. This conclusion is supported by the additional finding that the PKC inhibitor Go6976 further reduced amphetamine-evoked DA efflux when administered to non-PKC DAT mutant bearing cells but was without effect when administered to cells harboring a PKC DAT mutant.
In the present experiments, preventing PKC phosphorylation of DAT either with Go6976 or by mutation of single or grouped PKC residues resulted in approximately 25% inhibition of amphetamine-induced DA efflux. Similarly, reverse dialysis of the PKC inhibitor Ro31-8220 was found to not entirely suppress amphetamine-mediated DA overflow in the nucleus accumbens in vivo, producing a 50–70% reduction in the shell and core subnuclei respectively [8]. While a number of differences exist between the different experiments, these levels are substantially lower than the still incomplete 80–90% reductions reported following truncation of the first 22 amino acids [19] or simultaneous S-A mutations of the first 5 serine residues of the DAT N-terminal [11,19]. Together, these findings suggest that PKC is not the only protein kinase that regulates amphetamine-induced DA efflux and, importantly, that it may function in concert with others at multiple residues in the N-terminal of the DAT to fully regulate its function. Indeed, DA efflux is regulated by several kinases in addition to PKC, including CaMKII and ERK1/2 [5,6], and all are capable of regulating the DAT by phosphorylating residues in its N-terminal [11–15,17]. Again, supporting this possibility, combining the PKC inhibitor Go6976 with non-PKC DAT mutations in the present experiments further reduced amphetamine-evoked DA efflux.
The DAT S13 residue is phosphorylated by PKC and CaMKII [17]. As some but not all findings indicate that CaMKII contributes to acute amphetamine-induced DA efflux and behaviors [12–14; cf, 20], it remains possible that the inhibitory effect of the DAT-S13A mutant on DA efflux observed in the present study might in part reflect an action of CaMKII or at least an interaction between it and PKC. This possibility is unlikely, however, as combining the PKC inhibitor Go6976 with the S13A mutation in the present experiments did not further reduce amphetamine-evoked DA efflux. In addition, in the present experiments, S/T-A mutation of the non-PKC residue S12 and the ERK1/2 residue T53 were each found to reduce amphetamine-induced DA efflux by approximately 25% as well. The latter finding with DAT-T53A is consistent with a recent report showing near total loss of amphetamine-induced release of a [3H]1-methyl-4-phenylpyridinium (MPP+) substrate in DAT-T53A transfected LLCPK1 cells [15]. The difference in magnitude of inhibition between the two studies may be due to the use of MPP+ as a release substrate in the latter report [15].
The findings obtained in the present study with DAT-S4A, S7A and S13A (S4 and S13, but not S7, contribute to amphetamine-induced DA efflux) contrast with the role proposed by others [19] for these residues (S7, but not S4 or S13, contributes). However, important differences between these two studies make comparison of the results obtained difficult. In the present study, effects of these S/A mutations were assessed directly on amphetamine-induced DA efflux. On the other hand, in the study of Khoshbouei and colleagues [19], the contributions of S4, S7 and S13 were assessed with S/D mutants on an S/A background and amphetamine-induced efflux determined amperometrically and defined as the current recorded with amphetamine present minus the current recorded after the addition of cocaine to the amphetamine. This approach may have introduced different ways in which effects of phosphorylation state at S7 and perhaps other residues could have influenced the measurement of amphetamine-induced DA efflux. Indeed, the lack of inhibition of amphetamine-induced DA efflux observed in the present study with the DAT-S7A mutant may reflect the integration at S7 of antagonistic signaling by PKC and PKA pathways as this residue is phosphorylated by both kinases [17]. Perhaps more importantly, however, preventing phosphorylation at S7 has been shown to reduce the affinity of the rat DAT for the cocaine analog (−)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane [17], indicating that this residue may play a more important role in regulating uptake by drugs like cocaine.
5. Conclusion
In conclusion, here we show that PKC phosphorylation of select residues in the N-terminal of the DAT contributes to amphetamine-induced DA efflux. Given the relatively low magnitude of inhibition observed, it is likely that PKC interacts with other kinases such as ERK1/2 as well as other unidentified enzymes capable of phosphorylating residues at multiple sites to fully regulate DAT function.
Highlights.
-
-
The DAT regulates the uptake and release of DA by psychostimulants like amphetamine.
-
-
The N-terminal of the DAT contains residues targeted by PKC and other kinases.
-
-
Phosphorylation by PKC at some of these residues contributes to amphetamine-induced DA efflux.
Acknowledgments
This work was supported by National Institutes of Health grant DA-09397 (P.V.).
Abbreviations
- A
alanine
- CaMKII
calcium-calmodulin protein kinase II
- D
aspartate
- DA
dopamine
- DAT
DA transporter
- ERK
extracellular signal-regulated kinase
- GFP
green fluorescent protein
- MPP+
[3H]1-methyl-4-phenylpyridinium
- PKA
protein kinase A
- PKC
protein kinase C
- S
serine
- T
threonine
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Sora I, Li B, Igari M, Hall FS, Ikeda K. Transgenic mice in the study of drug addiction and the effects of psychostimulant drugs. Ann. N. Y. Acad. Sci. 2010;1187:218–246. doi: 10.1111/j.1749-6632.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the DA and norepinephrine transporters. Mol. Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur. J. Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the DA transporter by phosphorylation. Handb. Exp. Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- 6.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol. Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated DA release in rat striatal slices. J. Pharmacol. Exp. Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- 8.Loweth JA, Svoboda R, Austin JD, Guillory AM, Vezina P. The PKC inhibitor Ro31-8220 blocks acute amphetamine-induced DA overflow in the nucleus accumbens. Neurosci. Lett. 2009;455:88–92. doi: 10.1016/j.neulet.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J. Biol. Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of DA transporter trafficking and regulates the behavioral response to amphetamine in mice. J. Pharmacol. Exp. Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster JD, Pananusorn B, Vaughan RA. DA transporters are phosphorylated on N-terminal serines in rat striatum. J. Biol. Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- 12.Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the DA transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, Javitch JA. Amphetamine-induced behavior requires CaMKII-dependent DA transporter phosphorylation. Mol. Psychiatry. 2014;19:279–281. doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinkellner T, Yang JW, Montgomery TR, Chen WQ, Winkler MT, Sucic S, Lubec G, Freissmuth M, Elgersma Y, Sitte HH, Kudlacek O. Ca(2+)/calmodulin-dependent protein kinase IIalpha (alphaCaMKII) controls the activity of the DA transporter: implications for Angelman syndrome. J. Biol. Chem. 2012;287:29627–29635. doi: 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA. DA transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J. Biol. Chem. 2012;287:29702–29712. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the DA transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA. Phosphorylation of DA transporter serine 7 modulates cocaine analog binding. J. Biol. Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker JB, Castaneda E, Robinson TE, Beer ME. A simple in vitro technique to measure the release of endogenous DA and dihydroxyphenylacetic acid from striatal tissue using high performance liquid chromatography with electrochemical detection. J. Neurosci. Methods. 1984;11:19–28. doi: 10.1016/0165-0270(84)90004-9. [DOI] [PubMed] [Google Scholar]
- 19.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the DA transporter is required for amphetamine-induced efflux. PLoS. Biol. 2004;2:387–393. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantor L, Keikilani Hewlett GH, Gnegy ME. Enhanced amphetamine- and K+-mediated dopamine release in rat striatum after repeated amphetamine: Differential requirements for Ca2+- and calmodulin-dependent phsophorylation and synaptic vesicles. J. Neurosci. 1999;19:3801–3808. doi: 10.1523/JNEUROSCI.19-10-03801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]