Abstract
Despite the importance of altruism in an individual’s participation in genetic counseling and testing, little research has explored the change in altruistic motivations to test over time. This study analyzed altruistic motivations to test and change in altruistic motivations after genetic counseling and testing among individuals (N=120) at elevated risk for BRCA1/2 mutations. The perceived benefits of genetic testing were assessed and utilized in a mixed-methods, repeated measures design at three time points: pre-counseling, counseling and post-genetic testing, along with transcripts of genetic counseling sessions. Qualitative analysis using an immersion/crystallization method resulted in six common perceived benefits of testing: cancer prevention, awareness, family’s survival, relief from anxiety, for science, and future planning. Perceived benefits were then coded into three categories according to Hamilton’s kin selection theory: altruistic motivation, personal motivation, and motivation for mutual benefit. At pre-counseling, those with a personal cancer history (p=0.003) and those with one or more children (p=.013), were significantly more likely to cite altruistic motivations to test. Altruistic motivations significantly increased post-counseling (p=0.01) but declined post-testing (p<0.001). Labov’s narrative analysis further elucidated the context of altruistic and personal motivations. The possibility of a positive test result might have led those with personal history of cancer to have altruistic motivations for testing. Genetic counseling may have increased altruistic motivations to help family and may be a prime opportunity to discuss other forms of altruism.
Keywords: altruism, BRCA1/2, genetic counseling, genetic testing, motivation to test
In a State of the Union address, the Obama Administration and the National Institutes of Health launched the Precision Medicine Initiative, an initiative that focuses on caring for individuals based on their own genetic material (Collins & Varmus, 2015). Familial cancer syndromes, such as hereditary breast-ovarian cancer (associated with the BRCA1/2 mutations), are included in this mission. In BRCA1 gene mutation carriers, the risk of breast cancer in women is about 55-65% and 39% for ovarian cancer, while the risk for BRCA2 mutation carriers is 45% for breast cancer in women, 11-17% for ovarian cancer, and 6% for breast cancer in men (Chen & Parmigiani, 2007; Howlader et al., 2015; Kirchhoff et al., 2004). These mutations increase the risk of other cancers as well (Chen & Parmigiani, 2007) and are autosomal dominant, meaning that first degree relatives have a 50% chance of having the mutation.
Motivations to have genetic testing for BRCA1/2 mutations differ, and to date, research evaluating an individual's motivations has focused only on a single time point, which is typically before undergoing testing (e.g., Durfy, Bowen, McTiernan, Sporleder, & Burke, 1999; Lehmann, Weeks, Klar, & Garber, 2002; Phillips et al., 2000). We identified no study that examined the impact of genetic counseling and testing on altruistic motivations for genetic testing. Thus, understanding how individuals undergoing genetic counseling perceive the benefits of testing (whether for self or for others) and how these motivations may change over time is critical.
Genetic counseling for BRCA1/2 mutations is recommended for at-risk individuals to manage their risk of cancer as well as cope with psychological distress (Riley et al., 2012). Factors such as getting better estimates of their family's risk of cancer, contributing to the advancement of research, and helping other women (e.g., Brandt, Hartmann, Ali, Tucci, & Gilman, 2002; d'Agincourt-Canning, 2006; Geller, Doksum, Bernhardt, & Metz, 1999; Hallowell et al., 2010; Phillips et al., 2000) were some of the altruistic motivations identified in previous studies. These motivations to provide better risk estimates for family members and to advance research have been found to be significant factors associated with genetic testing for other conditions such as colorectal cancer (Esplen et al., 2001) and Alzheimer’s disease (Roberts et al., 2003). These motivations underpin altruistic behavior, or behavior that is performed to benefit others at a cost to the actor.
A number of theories have endeavored to explain why people engage in altruistic behavior. One of the oldest and most commonly used theories (e.g., Smith, 2014; Walker & Bailey, 2014), which includes a genetic component to explain altruistic behavior, is Hamilton’s kin selection theory. According to this theory, an act of altruism increases the reproductive fitness of individuals and their genes by benefitting their related kin (Foster, Wenseleers, & Ratnieks, 2006). Hamilton’s kin selection theory predicts that an altruistic action takes place when the perceived cost to a person is less than the expected benefit to a relative of that person. He had four classifications of actions: (a) altruism: an action that is costly to the actor, but beneficial to the recipient; (b) selfishness: an action that is beneficial to the actor, but costly to the recipient; (c) spite: an action that is costly for both; and (d) mutual benefit: an action beneficial to both (West, Griffin, & Gardner, 2007). These classifications may be important motivators of genetic testing which have not been examined in existing research.
Previous studies (e.g., Boudreault et al., 2010; Claes, Denayer, Evers-Kiebooms, Boogaerts, & Legius, 2004; Erskine et al., 2014) examining an individual’s motivation to undergo genetic testing did not examine the change in altruistic motivations over time. In addition, the associations of other factors such as personal history of cancer and demographic characteristics with altruistic motivations to test for BRCA1/2 mutations have not been well studied. As predicted by Hamilton’s kin selection theory, for those with a personal history of cancer, it is likely that they have already taken actions to decrease their risk of developing cancer again, such as surgical removal of the breast and ovaries (McDonnell et al., 2001). Thus, it is more likely that their actions would have altruistic motivations to test than those with only a family history of cancer. Those with only a family history of cancer have the potential to take additional health actions to decrease their personal risk, such as increased screening and prophylactic surgery (Isaacs et al., 2002), as well as to test for the benefit of other family members and society. In addition, the testing sequence focuses first on identifying the individual in the family most likely to have a mutation, which would be an individual with a prior history of cancer (Riley et al., 2012). This is another reason why those with a personal history of cancer may have altruistic motivations, as they may be the key to identifying a genetic mutation responsible for cancer in the family.
Further, receipt of genetic counseling and testing may impact the participant’s altruistic motivations to test. Genetic counselors typically explore the benefits and risks of testing (Riley et al., 2012), which may provide additional information about benefits to themselves or others that may not have been previously considered. If the individual decides to have genetic testing, possible results include: a positive result indicating the presence of a mutation increasing the risk of cancer for him/her and potentially for his/her children; an informative negative result indicating the absence of a mutation known to cause cancer in the family; or an uninformative negative test result indicating that a mutation has not been identified in the family and another unknown/untested mutation may still be present. Those receiving uninformative negative results would be less likely to have altruistic motivations, as their results have less potential benefit for other family members. Positive results have the most potential benefit to other family members.
Purpose of the Study
For this exploratory, longitudinal study on altruistic motivations, we assessed the perceived benefits of genetic testing among men and women using questionnaires at three key time points in the genetic counseling and testing process to understand altruistic motivations to test. We examined the impact of demographics, cancer history, genetic counseling, and genetic testing upon altruistic motivations. The quantitative data were augmented with examples of genetic counseling transcripts to provide a more complete context for altruistic motivations to have genetic testing.
Method
Participants
The participants were recruited through letters to local physicians, breast cancer support groups, and Jewish community groups as part of a larger longitudinal study, which offered genetic counseling and genetic testing for BRCA1/2 mutations (Kelly et al., 2014). Eligible individuals were Ashkenazi Jewish, aged 18 years or older, and had a personal (e.g., breast cancer before age 50, ovarian cancer, male breast cancer) or family history of cancer (e.g., maternal first degree or paternal second degree relative with male breast cancer, breast cancer before age 50, ovarian cancer) or BRCA mutation suggestive of elevated risk of carrying BRCA1/2 mutations (Berry, Parmigiani, Sanchez, Schildkraut, & Winer, 1997). The Ashkenazi Jewish population was selected as they have been found to be at a higher risk of carrying BRCA1/2 mutations as compared to the general population leading to higher rates of inherited breast and other cancers and easier mutation identification (Kirchhoff et al., 2004).
Of the 142 eligible individuals, 120 participants from 70 families completed the pre-counseling questionnaire and presented for genetic counseling (85% completion rate). Study participants were mostly women (89.2%), married (78.3%), ranged in age from 18 to 83 (M = 49.7, SD = 13.2), and had a high education level (M = 16.8 years, SD = 2.4). Nearly half (46.2%) reported a family income of above $100,000 (35.9% had income between $50,000 and $100,000). About half had a personal history of cancer (47.5%), and a majority (89.2%) underwent genetic testing. Most participants (68.2%) received uninformative negative results; 22.4% received positive and 9.4% received informative negative results for BRCA1/2 mutations.
Design and Procedure
This study had a repeated measures design with participants completing questionnaires at three time points: before genetic counseling (T1), one to two days after counseling (T2), and after about six months of the receipt of genetic test results (T3), which was augmented with transcripts of genetic counseling sessions. The study was reviewed and approved by the institutional review board of Rutgers University, New Jersey Medical School, and our institution in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The detailed procedure can be found elsewhere (Kelly et al., 2014). Eligibility was determined by a board-eligible or board certified genetic counselor, and eligible individuals were mailed a T1 questionnaire, which they brought to the first genetic counseling session. Genetic counseling was conducted by board-certified or board-eligible genetic counselors (N=3), consistent with guidelines (National Society of Genetic Counselors, 2004). Illustrated by a flipbook, genetic counseling included a review of personal and family cancer history, risk factors for breast and ovarian cancer, mechanisms of cancer inheritance, meaning of positive and negative test results, and the risks and benefits of genetic testing. Counseling sessions were recorded, transcribed, and confirmed to include each of the aforementioned genetic counseling topics.
One to two days after counseling, a telephone interview (T2) was conducted by a person affiliated with the research study other than the genetic counselor. One day after the telephone interview, the genetic counselor telephoned the participants to get their decision to have genetic testing. For those proceeding to test, blood was drawn and DNA was analyzed for the common BRCA1 and BRCA2 mutations found in the Ashkenazi Jewish population. A follow-up questionnaire was mailed to all participants approximately six months after receiving the genetic test results (T3) to assess the long term impact of the genetic counseling and genetic testing on the participants’ perceived benefits of genetic testing.
Measures
Demographics
We developed a questionnaire to collect demographic information including age, gender, education, family income, marital status, and participant’s personal history of cancer at T1. Medical records were used to confirm family and personal history of cancer. Number of children (0 or ≥1) and number of siblings (0 or ≥1) were recorded from BRCAPRO pedigree analyses (Euhus et al., 2002).
Testing
The decision to test was disclosed to the genetic counselor in a telephone call. Possible responses were yes, proceed with testing; or no, no testing within the study time frame. Three test results were possible: positive (a mutation was detected); uninformative negative (a mutation was not detected, and we do not know the cause of cancer in the family, or informative negative (a mutation was identified in the family, but the individuals did not have it).
Perceived benefits of genetic testing
In our questionnaire, we included an open ended question to assess the participants’ perceived benefits of genetic testing: “In your opinion, what are the positives of genetic testing?” at all the three study time points (T1, T2, and T3).
Data Analysis
Our mixed methods approach facilitated understanding of altruistic motivations in the context of genetic counseling and testing. Quantitative and qualitative data obtained from questionnaires were analyzed at multiple time points, with the addition of narrative analysis of transcripts from genetic counseling sessions, giving our analysis both concurrent and sequential elements (Hesse-Biber, 2010). Mixed methods allow for methodological triangulation and complementarity of qualitative and quantitative data. Our approach has advantages of (a) achieving a greater depth of understanding of the experience of those undergoing counseling and testing, and (b) converging the results from two different methods, providing greater support for our conclusions than from each of these methods alone.
Two types of qualitative analysis were conducted: one on open-ended items from the questionnaire and one on the transcripts of genetic counseling sessions. These two analyses were guided by merging two different qualitative approaches to understand altruism in the context of genetic counseling, including immersion/crystallization (Borkan, 1999) and Labov’s narrative analysis (Labov & Waletzky, 1997). Data from questionnaires yielded better to open, thematic coding guided by immersion/crystallization, while genetic counseling transcripts yielded better to Labov’s narrative analysis.
Qualitative analysis of questionnaire responses
We used immersion/crystallization because it has the advantage of being useful with a pre-existing theory (Borkan, 1999). We assigned codes to participants’ questionnaire responses to the perceived benefits of genetic testing and these codes were categorized into recurrent broad themes. We then classified these according to Hamilton's kin selection theory (West et al., 2007), and we used this formulation for further quantitative analysis: (a) altruistic motivation (perceived benefits to others), (b) personal motivation (benefits to self; we used the term “personal motivations” rather than selfishness, as selfishness seemed pejorative to individuals in this context), and (c) mutual benefit (both personal and altruistic motivations). The “spite” category was not used, as none of the participants’ responses indicated they sought genetic testing to harm themselves and others.
Quantitative analysis of questionnaire responses
Descriptive statistics (i.e., means, standard deviations, frequencies, and percentages) were calculated to provide a picture of the data. Chi-Square and Fisher’s Exact tests (FET) were used to examine the baseline associations between an individual's altruistic motivation (i.e., altruism, personal, and mutual benefit), demographic variables (e.g., gender, income, marital status, personal cancer history, children, siblings), and decision to undergo genetic testing. T-tests were also calculated to analyze the associations of altruistic motivation with age and education level.
We categorized the participants according to changes in their altruistic motivations to test at the three study time points (T1; T2 and T3). Changes in participants’ altruistic motivations were analyzed using a McNemar-Bowker test. A list wise deletion method for missing values was used in repeated measures analysis. Statistical significance was set at .05.
Qualitative analysis of genetic counseling sessions
Once individuals were categorized into altruistic, personal, or mutual benefit at each time point, we then examined the change in motivations of consultands to identify the four most common trajectories over time (i.e., T1 Personal, T2 Personal, T3 Personal; T1 Personal, T2 Mutual, T3 Personal; T1 Mutual, T2 Mutual, T3 Mutual; and T1 Mutual, T2 Mutual, T3 Personal). From these, we identified participants representing the diversity of genetic counseling sessions (e.g., male breast cancer, with/without personal history, positive/negative test result), a form of theoretical sampling (Coyne, 1997).
We then initiated the second type of qualitative analysis, a narrative analysis guided by principles from Labov (Labov & Waletzky, 1997; Labov, 2003). Narrative analysis was chosen for its unique suitability to genetic counseling, an ideal method for analysis of self-disclosure and the telling of the consultand’s unique story. Labov’s narrative model has been utilized on a diversity of forms of narrative analysis, including dyadic communication in a dramaturgical context, making it suitable for the analysis of genetic counseling sessions (Labov & Waletzky, 1997; Labov, 2003; Stubbs, 1983). A key tenet is that when individuals tell stories about themselves, four components (or codes) of their stories tend to emerge: abstract (beginning, what is this about?), orientation (what is the context?), complication (what is the difficulty?), evaluation (what is the meaning?).
Results
Perceived Benefits of Genetic Testing
Most responded to the question asking about perceived benefits of genetic testing at T1 (93.3%), T2 (98.3%), and T3 (77.5%). Table 1 illustrates the six common perceived benefits of genetic testing identified from our study participants’ responses: (a) for cancer prevention, (b) to acquire knowledge, (c) for family, (d) to alleviate anxiety, (e) for science and research, and (f) for future planning. At T1, cancer prevention was reported by most participants (64.3%), indicating that they would take preventive actions if found to have one of the gene mutations. Increased frequency of cancer screenings, prophylactic mastectomy, or increased vigilance for early symptoms of cancer were some of the potential measures mentioned by the individuals. Awareness of their gene status and having knowledge of their risk of getting cancer was the second most cited (42.0%) personal benefit of genetic testing. It gave them a sense of control over their health by getting knowledge from genetic testing as opposed to being unaware and leaving it up to chance.
Table 1.
Common Themes and Representative Quotations Reflecting Perceived Benefits of Genetic Testing at 3 Time Points
| Themes | Representative Quotations | ||
|---|---|---|---|
| Personal Motivation | |||
| Time 1 (n= 112) | Time 2 (n= 118) | Time 3 (n= 93) | |
| 1. For cancer prevention | “I will take better care of myself and seek my check-ups on a more regular schedule.” (36 yr. old married woman with no cancer history); n= 72 |
“Protect myself, be on top of it.” (28 yr. old single woman with no cancer history and positive test result); n= 73 |
“Taking action, if any- i.e. removal of ovaries, etc.” (44 yr. old married woman with no cancer history and uninformative negative test result); n= 48 |
| 2. To acquire knowledge | “Curiosity, my need to know will be satisfied.” (37 yr. old single woman with history of breast cancer ); n= 47 |
“Gaining as much info as possible” (49 yr. old married woman with no cancer history and positive test result); n= 53 |
“Part of personal informational knowledge” (63 yr. old married man with history of both skin and breast cancer and uninformative negative test result); n= 47 |
| 3. To alleviate anxiety | “Peace of mind” (46 yr. old married woman with history of breast cancer); n= 18 |
“Know for sure, not have to worry continuously” (46 yr. old married woman with no cancer history and positive test result); n= 15 |
“It let me breathe for a little bit.” (37 yr. old single woman with history of breast cancer and uninformative negative test result); n= 12 |
| 4. For future planning | “Being able to take appropriate measures like early child bearing” (28 yr. old single woman with no cancer history); n= 6 |
“Have kids earlier” (57 yr. old married woman with history of breast cancer and positive test result); n= 5 |
“Get your affairs in order. Make plans” (40 yr. old married woman with no cancer history and uninformative negative result); n= 6 |
| Altruistic Motivation | |||
| 1. For family’s sake | “I want to be tested so my daughters and granddaughters can be aware and be watched closely to prevent them from having breast and/or ovarian cancer.” (Married woman with history of breast and ovarian cancer); n= 28 |
“Nothing for me, maybe my daughter, grandchildren” (54 yr. old widowed woman with history of breast cancer and positive test result); n= 57 |
“Family members can take preventive measures” (63 yr. old married woman with history of breast cancer and positive test result); n= 30 |
| 2. For science and research | “Mainly for the good of genetic advances in understanding cancer” (57 yr. old married woman with history of breast cancer); n= 15 |
“Cancer research, future cure” (42 yr. old married woman with no cancer history and declined testing); n= 24 |
“Mostly, I did it for science. No benefits for me, but I didn’t expect any.” (47 yr. old married woman with history of breast cancer and uninformative negative test result); n= 6 |
T1: Pre-Counseling; T2: Post-Counseling; T3:Post-Genetic Testing.
Altruistic motivations for the family's welfare were important reasons for about 25.0% of the participants to undergo genetic testing. They wanted to make their children aware of any potential risk of cancer and alert them to take preventive measures. Many participants (16.1%) reported elevated anxiety due to their risk of getting cancer. Genetic testing was a means of getting relief from this fear and having a sense of satisfaction. Contribution to science and research was a potential altruistic benefit of undergoing genetic testing for about 13.4% of participants. The ensuing benefit to other women in the future and to society was an important motivating factor for them. Future planning in terms of either having an early pregnancy or choosing an appropriate life partner was another reason that was cited as a benefit of genetic testing by about 5.4% of study participants.
Altruistic Motivations to Test at Pre-Counseling
At T1, personal motivations to test were identified by the greatest number of participants (51.8%). Fewer indicated the mutual benefit of testing (33.9%) and only a small percentage reported altruistic motivations exclusively (14.3%) (Table 2). Those having a personal history of cancer were significantly more likely to perceive the benefits of testing to be for altruism compared to those with no personal cancer history, X2(2, 112) = 11.56, p = .003, Cramer’s V = 0.32. Also, those having one or more children were significantly more likely to perceive benefits of testing to be for altruistic reasons compared to those without children, FET(109) = 8.4, p = .013, Cramer’s V = .27. There was no association found between participants’ motivation to test and their decision to undergo genetic testing.
Table 2.
Pre-counseling Associations with Motivations to Test
| Altruistic Motivations Only (n= 16) |
Motivations for Mutual Benefit (n= 38) |
Personal Motivations Only (n= 58 ) |
|
|---|---|---|---|
| Gender | |||
| Male | 3 (27.3%) | 2 (18.2%) | 6 (54.5%) |
| Female | 13 (12.9%) | 36 (35.6%) | 52 (51.5%) |
| Mean age in yrs. (SD) |
54.9 (12.9) | 50.7 (10.6) | 47.5 (13.9) |
| Mean education in yrs. (SD) |
16.8 (2.7) | 17.2 (2.7) | 16.6 (2.1) |
| Marital status | |||
| Single | 14 (15.9%) | 29 (33.0%) | 45 (51.1%) |
| Married | 1 (11.1%) | 2 (22.2%) | 6 (66.7%) |
| Separated/ Widowed |
1(6.6%) | 7 (46.7%) | 7 (46.7%) |
| Family income ($) | |||
| <50k | 1(5.6%) | 8 (44.4%) | 9 (50.0%) |
| 50-100k | 7 (16.6%) | 12 (28.6%) | 23 (54.8%) |
| >100k | 7 (14.3%) | 18 (36.7%) | 24 (49.0%) |
| Personal cancer history *** |
|||
| Yes | 10 (18.5%) | 25 (46.3%) | 19 (35.2%) |
| No | 6 (10.3%) | 13 (22.4%) | 39 (67.2%) |
| Decision to Test | |||
| Yes | 13 (13.0%) | 34 (34.0%) | 53 (53.0%) |
| No | 3 (25%) | 4 (33.3%) | 5 (41.7%) |
SD: Standard deviation
p < .01
Change in Altruistic Motivations to Test
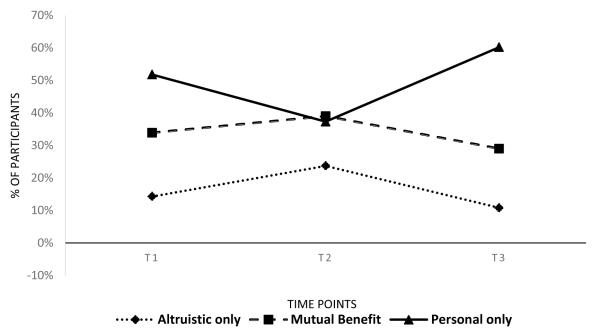
After genetic counseling (T2), 23.7% reported only altruistic motivations, 39.0% reported their motivations to be for mutual benefit, and 37.3% reported only personal motivations. Altruistic motivations and motivations for mutual benefit significantly increased, while personal motivations to test significantly decreased from T1 to T2, McNemar-Bowker test(3, 110) = 11.40, p = 0.01, Cramer’s V = .28 (Figure 1). The increase in altruistic benefits was largely due to those with one or more siblings, FET(32) = 9.02, p = 0.004, Cramer’s V = .57. The decrease in personal motivations was largely due to by those with one or more children, FET(66) = 6.39, p = 0.038, Cramer’s V = 0.32.
Figure 1.
Change in motivations to test over the 6 month study period (N=120). T1: Before genetic counseling, T2: 1-2 days after genetic counseling and T3: 6 months after the receipt of genetic test results.
At post-testing (T3), some participants (10.8%) reported only altruistic motivations, some (29.0%) reported their motivations to be for mutual benefit, and most (60.2%) reported only personal motivations. Participants’ altruistic and mutual benefit motivations decreased, while personal motivations increased from T2 to T3, McNemar-Bowker test(3, 91) = 18.11, p < 0.001, Cramer’s V = .30. There were no associations between change in motivations and demographic characteristics, cancer history, or test results. Table 3 includes the frequencies of 27 different trajectories of change in motivations at the three study time points, and Table 4 includes the characteristics of the four most common groups of participants who changed in their motivations to test out of these 27 trajectories.
Table 3.
Frequencies and Percentages of Participants’ Change in Motivations at 3 Time Points
| S. No. | Trajectories of motivations to test at T1, T2 & T3 |
n | % |
|---|---|---|---|
| 1. | T1 Altruism, T2 Altruism, T3 Altruism | 2 | 1.7 |
| 2. | T1 Altruism, T2 Altruism, T3 Mutual | 4 | 3.3 |
| 3. | T1 Altruism, T2 Altruism, T3 Personal | 2 | 1.7 |
| 4. | T1 Altruism, T2 Mutual, T3 Altruism | 1 | 0.8 |
| 5. | T1 Altruism, T2 Mutual, T3 Mutual | 0 | 0 |
| 6. | T1 Altruism, T2 Mutual, T3 Personal | 3 | 2.5 |
| 7. | T1 Altruism, T2 Personal, T3 Altruism | 1 | 0.8 |
| 8. | T1 Altruism, T2 Personal, T3 Mutual | 0 | 0 |
| 9. | T1 Altruism, T3 Personal, T3 Personal | 1 | 0.8 |
| 10. | T1 Mutual, T2 Altruism, T3 Altruism | 2 | 1.7 |
| 11. | T1 Mutual, T2 Altruism, T3 Mutual | 3 | 2.5 |
| 12. | T1 Mutual, T2 Altruism, T3 Personal | 3 | 2.5 |
| 13. | T1 Mutual, T2 Mutual, T3 Altruism | 2 | 1.7 |
| 14. | T1 Mutual, T2 Mutual, T3 Mutual | 6 | 5.0 |
| 15. | T1 Mutual, T2 Mutual, T3 Personal | 6 | 5.0 |
| 16. | T1 Mutual, T2 Personal, T3 Altruism | 0 | 0 |
| 17. | T1 Mutual, T2 Personal, T3 Mutual | 0 | 0 |
| 18. | T1 Mutual, T3 Personal, T3 Personal | 5 | 4.2 |
| 19. | T1 Personal, T2 Altruism, T3 Altruism | 2 | 1.7 |
| 20. | T1 Personal, T2 Altruism, T3 Mutual | 2 | 1.7 |
| 21. | T1 Personal, T2 Altruism, T3 Personal | 2 | 1.7 |
| 22. | T1 Personal, T2 Mutual, T3 Altruism | 0 | 0 |
| 23. | T1 Personal, T2 Mutual, T3 Mutual | 5 | 4.2 |
| 24. | T1 Personal, T2 Mutual, T3 Personal | 12 | 10.0 |
| 25. | T1 Personal, T2 Personal, T3 Altruism | 0 | 0 |
| 26. | T1 Personal, T2 Personal, T3 Mutual | 4 | 3.3 |
| 27. | T1 Personal, T3 Personal, T3 Personal | 18 | 15.0 |
| Missing | 34 | 28.3 | |
| Total | 120 | 100.0 |
Table 4.
Description of Participants whose Altruistic Motivations to Test Changed over the Study Time Period*
| T1 Personal, T2 Personal, T3 Personal |
T1 Personal, T2 Mutual, T3 Personal |
T1 Mutual, T2 Mutual, T3 Mutual |
T1 Mutual, T2 Mutual, T3 Personal |
|
|---|---|---|---|---|
|
| ||||
| Total | 18 (15.0%) | 12 (10.0%) | 6 (5.0%) | 6 (5.0%) |
| Gender | ||||
| Male | 0 | 2 | 1 | 0 |
| Female | 18 | 10 | 5 | 6 |
| Genetic test result | ||||
| Positive | 4 | 4 | 1 | 3 |
| Informative Negative |
2 | 0 | 0 | 1 |
| Uninformative Negative |
10 | 7 | 5 | 2 |
| Declined | 2 | 1 | 0 | 0 |
| Personal history of cancer |
||||
| Yes | 3 | 2 | 5 | 2 |
| No | 15 | 10 | 1 | 4 |
| Mean age in years (SD) |
39.2 (10.8) | 49.4 (13.3) | 57.2 (8.9) | 44.0 (12.3) |
Reported for groups of participants with n ≥ 6. T1: Pre-Counseling; T2: Post-Counseling; T3:Post-Genetic Testing. SD: Standard deviation.
Qualitative Analysis of Genetic Counseling Sessions
Group I: T1 Personal, T2 Personal, T3 Personal (the largest group)
Participant #149 Abstract: Participant #149 was a 39 year old woman with no personal history of cancer. She had a positive result. Orientation: Genetic counselor and participant. Complication: She had an adolescent daughter and older sisters, most of whom were diagnosed with breast and/or ovarian cancer. She also had a paternal history of prostate cancer, and her mother had breast cancer. Due to concerns about her strong family history of cancer, she did not opt for fertility treatment. Evaluation: She was concerned about the implications of her strong family history for her own risk of cancer, and she regularly went for cancer screening: “Given my family history, my line has always been, you know, I have very aggressive screening.” Being the youngest sibling and the only one with no personal history of cancer, she had a strong motivation to undergo genetic testing for herself.
Group II: T1 Personal, T2 Mutual, T3 Personal
Participant #42 Abstract: Participant #42 was a 60 year old woman with a prior history of breast cancer who tested uninformative negative. Orientation: Social worker, genetic counselor, and participant. Complication: She had a currently unknown cancer type, possibly leukemia. She had a daughter who was 28, an estranged son who may not want test results, a sister, and many cousins and was concerned about the risk for the next generation. She was surprised about her potentially elevated risk of ovarian cancer, a presenting concern. Very active in Hadassah, she requested that a physician from the cancer center speak to her group about the STAR trial, to learn more about Evista. Evaluation: When asked in genetic counseling about her primary concern, she explained: “I’m not the one I’m concerned about. My children, my nieces, my nephew, my cousins, you know, and the thing is, my feeling is also that if it helps through research, identifying other things you’re identifying, absolutely.”
Group III: T1 Mutual, T2 Mutual, T3 Mutual
Participant #118 Abstract: Participant #118 was a 72 year old male with a personal history of breast cancer who received an uninformative negative result. Orientation: Genetic counselor and participant. Complication: He had a mastectomy of the left breast, had been taking Tamoxifen, and had a cousin with melanoma. He had three children, and his wife had passed away. Recently, a member of his cancer support group had taken a turn for the worse, and the participant was concerned about his own health. He was a scientist, and although not a geneticist, he was trying to learn more about genetics and had been doing his own research, noting “…being involved with this has already had a salutary effect as….I finally buckled down and read through the files that I’d got. Both about my own cancer, and some of the literature I’d assembled.” Evaluation: He was anxious about future cancer episodes for himself and was also motivated to test to encourage him to stay aware, and “to reinforce the screening and watching.” He was seeking information for his children, as well.
Group IV: T1 Mutual, T2 Mutual, T3 Personal
Participant #148 Abstract: Participant #148 was a 47 year old female with no personal history of cancer who tested positive. Orientation: Genetic counselor, the participant, and her husband. Complication: Her mother and sister died of metastatic breast cancer, and her mother had been psychologically unstable. The participant had two children. There were multiple miscarriages in the family. Recently, she experienced irregular periods and was diagnosed with a small abnormality after ultrasound, “and nothing came out in the tests.” She had a prophylactic mastectomy because her sister died of breast cancer and was concerned about starting estrogen replacement therapy due to her history of miscarriages. Evaluation: When asked about her motivation for undergoing genetic testing, she said “Absolutely my children… Wouldn’t want them to have to go through it…” She was also contemplating prophylactic oophorectomy for herself: “I would never even question taking my ovaries out.”
Discussion
This study investigated the associations of demographics, cancer history, and decision to test with pre-counseling altruistic motivations to test and changes in these motivations after receiving genetic counseling and genetic test results. We utilized a mixed methods approach by qualitatively and quantitatively analyzing the participants’ responses to questionnaires asking about perceived benefits of genetic testing and further elucidated these perceived benefits by adding narrative analyses of genetic counseling sessions.
Overall, about one-tenth of participants cited only altruistic motivations to have testing, but most did indicate that they would test for altruistic reasons. Among altruistic reasons to test, helping family members was the most cited benefit of genetic testing. Further, we found that participants who had children were significantly more altruistic as compared to those without any children, and this theme was also evident in selected counseling sessions. These findings are consistent with Hamilton’s kin selection theory (Foster et al., 2006) which asserts that altruistic behavior is more likely to be exhibited towards family members. However, many participants considered testing to be important for science and research. Thus, other theoretical models of altruism which do not rely upon genetic relatedness, such as reciprocal altruism (Trivers, 1971) and indirect reciprocity (Nowak & Sigmund, 1998) may help in explaining this altruistic behavior.
More participants in our study reported the benefits of genetic testing to be for personal benefit than for altruistic reasons before counseling, which is inconsistent with a previous study by Phillips et al. (2000). They found that the two most important motivations to undergo genetic testing were altruistic in nature, including a desire to advance research (87%) and benefit to other family members (78%). The Phillips et al. (2000) study was conducted among the Ashkenazi Jewish population in Canada which has a universal healthcare system, while in the United States, healthcare is largely operated by private insurance companies. Concerns about cost might have led our study participants’ motivations to be more for personal benefit than for altruism.
Another important finding of the present study was the effect of personal cancer history on motivation to test. At pre-counseling, those with a personal history of cancer were more likely to report altruistic or mutual benefits of genetic testing as compared to those without personal cancer history. In our narrative analysis, one woman specifically noted that her own personal experience coping with cancer led her to be concerned for the welfare of her children and other family members, indicating convergence of our qualitative and quantitative data and deepening our understanding of the larger context of medical decision making. The testing sequence often recommends that the individual who had cancer previously test first to provide information to additional family members. Thus, if the reason for cancer is identified in the family (association with a known hereditary mutation) then, family members who are unaffected by cancer can receive a true negative result. In fact, our narrative analysis indicates that this is occurring; in the case of one of our participants, the potential of a positive result is almost presented as a gift to be given to other family members. Further, other unaffected family members may be actively encouraging those with a prior cancer diagnosis to test to provide information in hopes of receiving an informative negative result.
The altruistic and mutual benefit motivations increased while personal benefit motivations decreased after receiving genetic counseling. Increased understanding of benefits of genetic testing for family might have lead those with siblings to increase their altruistic motivations and those with children to decrease their personal motivations after counseling. During genetic counseling, family history, mechanism of cancer inheritance, and chances of passing gene mutations to children are discussed (National Society of Genetic Counselors, 2004). Our narrative analysis supported this extensive discussion of the meaning of genetic testing for the individual and family, beyond just children. This discussion might have led to a better understanding of implications of genetic testing for family’s cancer risk and the survival of future generations. In addition, it may have corrected some misperceptions about benefits of genetic testing to the consultand, resulting in decreased perceptions of personal benefits.
Study Limitations
Some limitations of our study should be noted. To begin, our participants were Ashkenazi Jewish, and most had high socioeconomic status. Also, those who had greater levels of altruism may have been more likely to volunteer for this study. Thus, our findings should be generalized with caution. In addition, altruistic behavior has been found to be associated with better mental and physical well-being, and to be intrinsically rewarding (Lozada et al., 2011), and these factors were beyond the scope of this study. Lack of a control group is another limitation of our study as all participants received genetic counseling. Thus, conclusions about the impact of genetic counseling on altruistic motivations cannot be drawn. Finally, although we encouraged individuals to make their own decisions, we cannot rule out family coercion, rather than altruism, when individuals reported helping family members by testing. This could explain why the largest percentage of individuals in the altruistic category cited perceived benefits for family (65%) rather than the benefit of society and science/research (35%). In spite of these limitations, this is the first study to examine novel factors associated with altruistic motivation to test and the effect of genetic counseling and testing on participants’ changing altruistic motivations for genetic testing. We analyzed both questionnaire responses and genetic counseling sessions in a mixed-methods design which is novel and provided in-depth understanding of participants’ altruistic motivations to test.
Conclusion, Practice Implications, and Research Recommendations
The benefits of genetic testing include the ability to take steps for prevention and early detection of cancer for both the person undergoing testing, as well as his or her family. In this study, we found that the majority of participants cited benefits of genetic testing to be either for their own good or for mutual benefit, rather than for purely altruistic reasons, and those having children were more likely to cite altruistic motivations to test. The altruistic motivations increased after genetic counseling, and our narrative analysis of counseling sessions indicated a thorough discussion of the implications of genetic testing for the individual and family – topics that participants may not have fully considered prior to counseling. These altruistic motivations to test were especially important factors for those with a personal history of cancer. Thus, although these motivations were previously considered as static, our data provide important insights into the opportunity to discuss the implications of genetic testing for family members, an important role of genetic counselors. The addition of a control group to future study designs could lead to more definitive conclusions regarding the impact of genetic counseling sessions on altruistic motivations to test. Future studies should explore the risk communication process and motivations to give and receive genetic information in the context of family. The examination of interactions among family members, whether within or outside of the context of genetic counseling is important, which is quite challenging with the current tools available. Further, given the increase in altruistic motivations following genetic counseling, genetic counselors may want to more routinely discuss other forms of altruism, such as the potential to improve science through the consultand’s participation in research. Also discussing being a supportive resource for others going through the process of genetic counseling could be beneficial, as genetic counseling seems to opens the door for the consideration of more general altruistic motivations, which in turn may provide long-term benefits to the consultand and his or her family.
Informed Consent.
“All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.” “I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.”
Acknowledgments
Funding: This study was supported by a grant from the National Cancer Institute (R03 CA128459-01, Kelly, PI).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Berry DA, Parmigiani G, Sanchez J, Schildkraut J, Winer E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. Journal of the National Cancer Institute. 1997;89(3):227–238. doi: 10.1093/jnci/89.3.227. [DOI] [PubMed] [Google Scholar]
- Borkan J. Immersion/crystallization. In: Crabtree BF, Miller WL, editors. Doing qualitative research. 2nd ed Sage; Thousand Oaks, CA: 1999. pp. 179–194. [Google Scholar]
- Boudreault P, Baldwin EE, Fox M, Dutton L, Tullis L, Linden J, Palmer CG. Deaf adults' reasons for genetic testing depend on cultural affiliation: Results from a prospective, longitudinal genetic counseling and testing study. Journal of Deaf Studies and Deaf Education. 2010;15(3):209–227. doi: 10.1093/deafed/enq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Hartmann E, Ali Z, Tucci R, Gilman P. Motivations and concerns of women considering genetic testing for breast cancer: A comparison between affected and at-risk probands. Genetic Testing. 2002;6(3):203–205. doi: 10.1089/109065702761403360. [DOI] [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes E, Denayer L, Evers-Kiebooms G, Boogaerts A, Legius E. Predictive testing for hereditary non-polyposis colorectal cancer: Motivation, illness representations and short-term psychological impact. Patient Education and Counseling. 2004;55(2):265–274. doi: 10.1016/j.pec.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. New England Journal of Medicine. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne IT. Sampling in qualitative research. Purposeful and theoretical sampling; merging or clear boundaries? Journal of Advanced Nursing. 1997;26(3):623–630. doi: 10.1046/j.1365-2648.1997.t01-25-00999.x. [DOI] [PubMed] [Google Scholar]
- d'Agincourt-Canning L. Genetic testing for hereditary breast and ovarian cancer: Responsibility and choice. Qualitative Health Research. 2006;16(1):97–118. doi: 10.1177/1049732305284002. [DOI] [PubMed] [Google Scholar]
- Durfy SJ, Bowen DJ, McTiernan A, Sporleder J, Burke W. Attitudes and interest in genetic testing for breast and ovarian cancer susceptibility in diverse groups of women in western Washington. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 1999;8(4):369–375. [PubMed] [Google Scholar]
- Erskine KE, Hidayatallah NZ, Walsh CA, McDonald TV, Cohen L, Marion RW, Dolan SM. Motivation to pursue genetic testing in individuals with a personal or family history of cardiac events or sudden cardiac death. Journal of Genetic Counseling. 2014;23(5):849–859. doi: 10.1007/s10897-014-9707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplen MJ, Madlensky L, Butler K, McKinnon W, Bapat B, Wong J, Gallinger S. Motivations and psychosocial impact of genetic testing for HNPCC. American Journal of Medical Genetics. 2001;103(1):9–15. [PubMed] [Google Scholar]
- Euhus DM, Smith KC, Robinson L, Stucky A, Olopade OI, Cummings S, Tomlinson G. Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. Journal of the National Cancer Institute. 2002;94(11):844–851. doi: 10.1093/jnci/94.11.844. [DOI] [PubMed] [Google Scholar]
- Foster KR, Wenseleers T, Ratnieks FLW. Kin selection is the key to altruism. Trends in Ecology & Evolution. 2006;21(2):57–60. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Geller G, Doksum T, Bernhardt BA, Metz SA. Participation in breast cancer susceptibility testing protocols: Influence of recruitment source, altruism, and family involvement on women's decisions. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 1999;8(4):377–383. [PubMed] [Google Scholar]
- Hallowell N, Cooke S, Crawford G, Lucassen A, Parker M, Snowdon C. An investigation of patients' motivations for their participation in genetics-related research. Journal of Medical Ethics. 2010;36(1):37–45. doi: 10.1136/jme.2009.029264. doi:10.1136/jme.2009.029264. [DOI] [PubMed] [Google Scholar]
- Hesse-Biber SN. Mixed methods research: Merging theory with practice. Guilford Press; New York: 2010. [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Cronin KA. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute; Bethesda, MD: Apr, 2015. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site. [Google Scholar]
- Isaacs C, Peshkin BN, Schwartz M, Demarco TA, Main D, Lerman C. Breast and ovarian cancer screening practices in healthy women with a strong family history of breast or ovarian cancer. Breast Cancer Research and Treatment. 2002;71(2):103–112. doi: 10.1023/a:1013800409238. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Ellington L, Schoenberg N, Jackson T, Dickinson S, Porter K, Andrykowski M. Genetic counseling content: How does it impact behavior? Journal of Behavioral Medicine. 2014:1–11. doi: 10.1007/s10865-014-9613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff T, Kauff ND, Mitra N, Nafa K, Huang H, Palmer C, Offit K. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2004;10(9):2918–2921. doi: 10.1158/1078-0432.ccr-03-0604. [DOI] [PubMed] [Google Scholar]
- Labov W. Uncovering the event structure of narrative. Georgetown University Round Table; 2003. pp. 63–83. [Google Scholar]
- Labov W, Waletzky J. Narrative analysis: Oral versions of personal experience. Journal of Narrative & Life History. 1997;7(1-4):3–38. [Google Scholar]
- Lehmann LS, Weeks JC, Klar N, Garber JE. A population-based study of Ashkenazi Jewish women's attitudes toward genetic discrimination and BRCA1/2 testing. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2002;4(5):346–352. doi: 10.1097/00125817-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychology. 1991;10(4):259. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- Lozada M, D'Adamo P, Fuentes MA. Beneficial effects of human altruism. Journal of Theoretical Biology. 2011;289:12–16. doi: 10.1016/j.jtbi.2011.08.016. doi:10.1016/j.jtbi.2011.08.016. [DOI] [PubMed] [Google Scholar]
- McDonnell SK, Schaid DJ, Myers JL, Grant CS, Donohue JH, Woods JE, Hartmann LC. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2001;19(19):3938–3943. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- National Society of Genetic Counselors [Retrieved 12/5/2008];Familial cancer risk counseling: standard protocol. 2004 from www.nsgc.org.
- Nowak MA, Sigmund K. Evolution of indirect reciprocity by image scoring. Nature. 1998;393(6685):573–577. doi: 10.1038/31225. [DOI] [PubMed] [Google Scholar]
- Parmigiani G, Berry DA, Iversen E, Jr, Müller P, Schildkraut J, Winer EP. Modeling risk of breast cancer and decisions about genetic testing. Springer; 1999. [Google Scholar]
- Phillips KA, Warner E, Meschino WS, Hunter J, Abdolell M, Glendon G, Goodwin PJ. Perceptions of Ashkenazi Jewish breast cancer patients on genetic testing for mutations in BRCA1 and BRCA2. Clinical Genetics. 2000;57(5):376–383. doi: 10.1034/j.1399-0004.2000.570508.x. [DOI] [PubMed] [Google Scholar]
- Reilly PR, Boshar MF, Holtzman SH. Ethical issues in genetic research: Disclosure and informed consent. Nature Genetics. 1997;15(1):16–20. doi: 10.1038/ng0197-16. [DOI] [PubMed] [Google Scholar]
- Riley BD, Culver JO, Skrzynia C, Senter LA, Peters JA, Costalas JW, Trepanier AM. Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling. 2012;21(2):151–161. doi: 10.1007/s10897-011-9462-x. doi:10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- Roberts JS, LaRusse SA, Katzen H, Whitehouse PJ, Barber M, Post SG, Green RC. Reasons for seeking genetic susceptibility testing among first-degree relatives of people with Alzheimer disease. Alzheimer Disease and Associated Disorders. 2003;17(2):86–93. doi: 10.1097/00002093-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Smith JE. Hamilton's legacy: Kinship, cooperation and social tolerance in mammalian groups. Animal Behaviour. 2014;92:291–304. [Google Scholar]
- Stubbs M. Discourse analysis: The sociolinguistic analysis of natural language. University of Chicago Press; 1983. [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. The Quarterly Review of Biology. 1971;46(1):35–57. [Google Scholar]
- Walker RS, Bailey DH. Marrying kin in small-scale societies. American Journal of Human Biology. 2014;26(3):384–388. doi: 10.1002/ajhb.22527. [DOI] [PubMed] [Google Scholar]
- West SA, Griffin AS, Gardner A. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. Journal of Evolutionary Biology. 2007;20(2):415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]