Abstract
Purpose
While bladder outlet obstruction (BOO) is well-established to elicit an inflammatory reaction in the bladder that leads to overactive bladder and fibrosis, little is known about the mechanism by which this is initiated. Nod-Like Receptors (NLRs) and the structures they form (inflammasomes) have been identified as sensors of cellular damage (including pressure-induced damage) and triggers of inflammation. Recently, we identified these structures in the urothelium. In this study we assess the role of the NLRP3 inflammasome in the bladder dysfunction resulting from BOO.
Materials and Methods
BOO was created in female rats by insertion of a 1 mm (o.d.) transurethral catheter, tying a silk ligature around the urethra and removing the catheter. Untreated and sham-operated rats served as controls. BOO rats were given vehicle (10% ethanol) or 10 mg/kg of glyburide (an NLRP3 inhibitor; daily for 12 days, p.o.). Inflammasome activity, bladder hypertrophy, inflammation and bladder function (urodynamics) were assessed. Results: BOO increased urothelial inflammasome activity, bladder hypertrophy, and inflammation and reduced voiding volume. Glyburide blocked inflammasome activation, reduced hypertrophy and prevented inflammation. The reduction in void volume was also attenuated by glyburide, mechanistically by an increase in the duration of detrusor contraction and voiding period.
Conclusion
The results suggest the importance of the NLRP3 inflammasome in the induction of inflammation and bladder dysfunction secondary to BOO. Arresting these processes with NLRP3 inhibitors may prove useful in treating the symptoms they produce.
Keywords: Inflammasomes, urinary bladder neck obstruction, inflammation, cystitis, innate immunity
Introduction
Bladder outlet obstruction (BOO) results from numerous conditions (e.g. stones, organ prolapse, posterior urethral valves) although the most prevalent cause is benign prostatic hyperplasia (BPH).1 Pharmacotherapy (alpha blockers, 5-alpha reductase inhibitors) treats moderate symptoms but does not completely eliminate high intravesical pressures. Persistent pressure provokes detrusor hypertrophy and may be transmited to the upper tracts, leading to renal failure. Recent studies demonstrate that elevated pressures produce a chronic inflammatory state in the bladder2, 3 leading to irritative symptoms such as frequency, urgency and urge incontinence. These often are more devastating to quality of life than the original obstructive symptoms. Chronic bladder inflammation eventually leads to fibrosis and decompensation2, 4 with poor success rates using current therapies.5 Thus, treating underlying inflammation could prevent or delay the progression of bladder dysfunction in BOO.
Sterile inflammation in BOO is likely triggered through the innate immune system by patterns in molecules released from pressure-stressed or dying cells. In other tissues, these Danger Associated Molecular Patterns (DAMPs) are recognized by Nod-like receptors (NLRs) that form multimeric structures called inflammasomes that promote the maturation and release of the pro-inflammatory cytokine IL-1β (and IL-18). We have recently shown the presence of NLRs in the urothelium6, 7 and their ability to respond to DAMPs such as ATP and monosodium urate that are common components of urine.7 We hypothesize that during BOO NLRP3 (the best-studied NLR and the one known to respond to DAMPs) is activated in the urothelium by high pressures, triggering inflammation, irritative symptoms and eventually fibrosis and decompensation. To begin to address this hypothesis we have examined NLRP3 activation during BOO and assessed the ability of an NLRP3 inhibitor (glyburide (Gly), a.k.a. glybenclamide)8 to block inflammation and voiding dysfunction at an early stage (day 12) of BOO in rats. The results strongly support a causative role for NLRP3 in this disorder.
Materials and Methods
Animals, Surgery and Pharmacological Treatments
Rats (Sprague Dawley, female, ≈200 g, ≈49 days old, Harlan, Prattville AL) were used in all studies. Female rats are the standard for BOO studies based on the ease of surgery, lack of tortuosity and short length of the urethra, and complications arising from ducts associated with the prostatic gland and seminal vesicles.9 Protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. In preparation for all surgeries, rats were anesthetized with ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg) (i.p.).
For BOO and Sham rats, a catheter (P50 tubing; O.D. 1 mm) was inserted transurethrally. The abdominal cavity was opened and a 5-0 silk suture passed around the urethra and tied (securely for BOO, loosely for Sham). The catheter was then removed.
For urodynamics a suprapubic catheter (PE-50 tubing with a flared end) was implanted at the time of BOO (with the exception of 3 rats as described below). A piece of silicon tubing (3–4 mm) placed under the flare served as a spacer between the bladder wall and the catheter opening, preventing urothelial growth over the orifice and occlusion.10 Before this technical improvement, clogging catheters necessitated separate BOO and catheter placement, the later occurring 24 h prior to urodynamics (i.e. day 11). In the present study three rats (two in the BOO + vehicle group and one in the BOO + glyburide group) were subjected to the multiple surgeries and data collected. Thereafter, we used a spacer which allowed for a single surgery. Upon final analysis, all urodynamics parameters of the three rats subjected to the multiple surgeries were well within 2 standard deviations of the other animals in their respective groups and thus were included for statistical analysis. Upon placement, the catheter was secured with a purse string suture (6-0 prolene), tunneled to the back of the neck and secured to interscapular tissue (5-0 silk). The abdominal wall and skin were closed separately (5-0 PGA) and the catheters fitted to a Quick Connect Harness (SAI Infusion Technologies, Lake Villa, IL).
With the first dose given during post-operative recovery, BOO animals were given daily glyburide (10 mg/kg in 1 ml, p.o.) or vehicle (40% ethanol). This dose is common in the literature11, 12 and is considerably less than the 500 mg/kg/day used in the original assessment of glyburide’s ability to inhibit NLRP3.8 The glyburide was prepared by suspending 5 mg/ml in 100% ethanol at 56 °C with occasional vortexing until dissolved. It was diluted (2:3) with PBS and immediately administered.
Caspase-1 Assay
As described,7, 13 bladder wall was scraped, urothelial cells pelleted and resuspended in 25 μl of 10 mM MgCl2, 0.25% Igepal CA-630 and added to 25 μl of 40 mM Hepes (pH 7.4), 20 mM NaCl, 2 mM EDTA, 20% Glycerol. Extract (20 μl) was combined with 50 μl assay buffer (25 mm HEPES, 5% sucrose, 0.05% CHAPS (pH 7.5), 10 μl 100 mM dithiothreitol and 20 μl 1 mM N-Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarin (Ac-YVAD-AFC) in blacked-walled 96 well plates. Fluorescence (Excitation 400 nm, Emission 505 nm) was measured every 30 sec for 15 min and the slope determined. Protein concentrations were assessed and a standard curve of fluorescence versus free AFC was used to calculate specific activity.
Bladder Hypertrophy
At sacrifice, bladders used for caspase-1 and Evan’s blue extravasation studies were weighed and results of each experimental group combined.
Evan’s Blue Extravasation
Rats were injected with 25 mg/kg Evan’s Blue (i.v.).14 One hour later bladders were weighed and placed in formamide (1 ml; overnight; 56 °C). Absorbance (620 nM) was measured and the results calculated from a standard curve.
Histology/Immunocytochemistry
Bladders were fixed (overnight, 10% neutral buffered formalin, 4°C) and paraffin-embedded. Sections (5 μm) were stained with hematoxylin and eosin or subjected to immunocytochemistry using standard techniques. (antigen retrieval-citrate pH 6.0; Santa Cruz anti-NLRP3 (cat# SC-66846) and isotype control (SC-2027) ; Vector Laboratories ImmPRES HRP Anti-Rabbit IgG (cat# MP-7401) and DAB substrate kit (cat# SK-4100).
Urodynamics
On post-operative day 12 rats Harnesses were removed and the rats placed into a restrainer above an analytical balance inside of a Small Animal Cystometry Lab Station (Catamount, Inc., St. Albans, VT).7, 10, 14 Sterile saline was infused at 80 μl/min. Intravesicular pressure (measured with an in-line transducer) and the voided volume were measured for 60–120 min (Med-CMG software; Catamount). One micturition cycle was defined as the time intravesicular pressure returned to baseline after a previous void until it returned to baseline following the next void. During each cycle, voiding pressure was recorded as the peak intravesical pressure, void volume as the amount of urine voided and the intercontraction interval (ICI) as the time between successive peaks in voiding pressure. The duration of contraction is the time in which the intravesicular pressure exceeded threshold during a void. The duration of the void is the time from whence the reading changed on the scale to the time it stabilized. The flow rate is the void volume divided by the void time. For each rat and each parameter, the average of the recorded micturition cycles was used in statistical analysis
Statistical analysis
All assays and parameters were assessed by a one-way analysis of variance followed by a Tukey’s post-hoc analysis using GraphPad InStat software (La Jolla, CA).
Results
BOO activates NLRP3 in urothelium
Rats that underwent sham surgery did not show significant changes in urothelial caspase-1 compared to control (Figure 1). In contrast, BOO rats treated with vehicle increased the activity of this enzyme nearly 3-fold. This increase was prevented by daily administration of glyburide.
Figure 1.

Glyburide blocks BOO-induced caspase-1 activation in the urothelium. Animals were subjected to no surgery (Con), sham surgery (Sham) or BOO as described and given vehicle (Veh) or glyburide (Gly) as described. On day 12, caspase-1 activity in the urothelium was analyzed as described. Bars: mean±SEM. Stats: ANOVA and Tukey’s. * p<0.05. Any difference not marked was not significant. n=4, 5, 5 and 3, respectively.
NLRP3 mediates approximately 50% of BOO-induced bladder hypertrophy
As shown in Figure 2, bladder weight was greatly increased in the BOO + vehicle rats compared to control or sham and this increase was reduced ≈50% with glyburide.
Figure 2.

BOO-induced bladder hypertrophy is reduced by glyburide. Experimental groups are the same groups described in Figure 1. Bladders from animals used for caspase-1 activity and Evan’s blue extravasation studies were weighed at sacrifice and those belonging to a given group combined. Bars: mean ± SEM. Stats: ANOVA and Tukeys. *p<0.05; ***p<0.00. Any difference not marked was not significant. n=11, 16, 27, 17, respectively.
NLRP3 mediates BOO-induced bladder inflammation
As shown in Figure 3 bladders from Sham rats had a slight but non-significant increase in Evan’s blue dye extravasation compared to control whereas BOO + vehicle rats displayed a large increase. Glyburide reduced dye extravasation to levels not significantly different from Control or Sham rats.
Figure 3.

Glyburide prevents BOO-induced Evan’s blue extravasation. Experimental groups are the same groups described in Figure 1. Bars: mean ± SEM. Stats: ANOVA and Tukeys. *p<0.05 vs. Con. Any difference not marked was not significant. n= 4, 4, 6, 6, respectively.
Glyburide prevents most of the histological changes associated with BOO but does not affect tissue distribution of NLRP3
As shown in Figure 4, control urothelium is predominantly 3–4 cell layers thick with underlying back-to-back smooth muscle fascicles and no significant inflammation. In contrast, the obstructed bladder urothelium ranges from 3–10 cell layers thick. The obstructed bladder also shows marked acute inflammation, mostly involving the urothelium and subepithelial connective tissue. The inflammatory cells infiltrate between smooth muscle fascicles, where there is associated collagen deposition, thickening the bladder wall. Outside of the smooth muscle layer is a mild to moderate chronic inflammatory infiltrate. Glyburide treated rats demonstrate decreased urothelial thickness and inflammation, with a scant to mild infiltrate of acute and chronic inflammatory cells involving the urothelium and subepithelial connective tissue. There is a mild chronic inflammatory infiltrate in the connective tissue surrounding the smooth muscle layer. The sham specimens appear histologically similar to the control specimens.
Figure 4.
Glyburide reverses histological changes associated with BOO. Experimental groups are the same groups described in Figure 1. At the end of the experiment, bladders were removed, fixed, sectioned, and stained with hematoxylin and eosin. The arrow indicates an inflammatory infiltrate while the brackets indicate the urothelial layer.
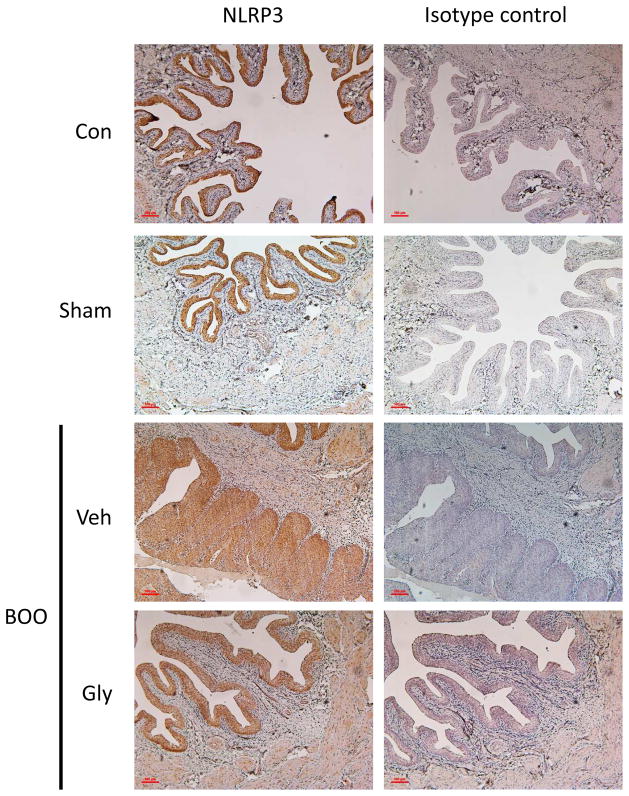
Immunocytochemistry for NLRP3 (Figure 5) show expression localized to the urothelia with little or no expression in other tissues. BOO nor glyburide appeared to change the tissue distribution.
Figure 5.
Tissue localization of NLRP3 remains unchanged by BOO or glyburide. Experimental groups are the same groups described in Figure 1. At the end of the experiment, bladders were removed, fixed, sectioned, and stained with antibody to NLRP3 or an isotype control. Development was through HRP-DAB so that the positive staining appears brown.
Glyburide increases voiding volume by increasing the length of contraction and length of the void
To assess the role of NLRP3 in bladder dysfunction associated with BOO, urodynamics was performed. Figure 6A shows a representative tracing of intravesicular pressure, whereas 6B depicts the tracing derived from the scale during a representative void from the various groups. Figures 7A and B compare the voiding pressure and flow rate, respectively, from the four groups. Voiding pressure in Sham rats was not significantly different from control. However, BOO rats treated with vehicle showed a highly significant increase in voiding pressure, consistent with physical obstruction. Glyburide decreased voiding pressure slightly, but significantly, relative to vehicle-treated animals. Importantly, voiding pressure remained elevated above sham and controls indicating that these animals were obstructed. Further evidence of obstruction is shown in Figure 6B by the flow rate measurements. Flow rate was drastically reduced in the vehicle and glyburide-treated BOO rats, compared to control and sham-operated rats, with no difference between the two.
Figure 6.
Representative urodynamic pressure and scale tracings. Experimental groups are the same groups described in Figure 1. (A.) Intravesicular pressure tracings as measured through several micturition cycles. Tracings illustrate the voiding pressures and the length between voids (the ICI) which is highly related to the voiding volume. (B.) Continuous tracing from the scale representing a single void recorded during urodynamics. Tracings illustrate the total voided volume as well at the time required to record the void (i.e. the flow rate).
Figure 7.
Average voiding pressure (A.) and urine flow rate (B.) recorded during urodynamics of the 4 experimental groups described in Figure 1. *p<0.05; ***p<0.00. Any difference not marked was not significant. n=8, 6, 9, 5, respectively.
The parameters most affected by glyburide were voided volume and the highly related intercontraction interval (ICI). As shown in Figure 8, vehicle-treated BOO rats displayed a significant (≈50%) decrease in voiding volume, an effect that was completely reversed in the glyburide-treated animals. Likewise, a virtually identical pattern is seen with the ICI (Figure 7B).
Figure 8.
Average voiding volume (A.) and intercontraction interval (B.) recorded during urodynamics of the 4 experimental groups described in Figure 1. *p<0.05. Any difference not marked was not significant. n=8, 6, 9, 5, respectively.
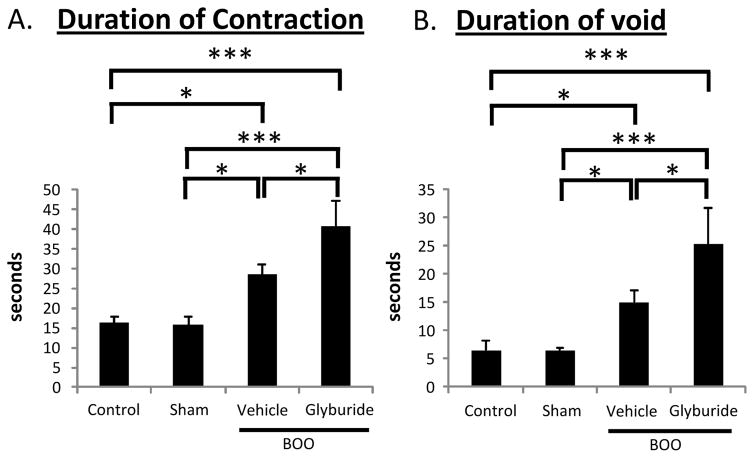
As shown in Figure 9A, vehicle-treated rats showed an increased contraction time, which is necessary to push the urine past the obstruction. This was also evident in the duration of the void (Figure 9B). However, glyburide significantly increased the duration of contraction and voiding time.
Figure 9.
Average duration of detrusor contraction (A.) and void (B.) recorded during urodynamics of the 4 experimental groups described in Figure 1. *p<0.05; ***p<0.00. Any difference not marked was not significant. n=8, 6, 9, 5, respectively.
Discussion
BOO restricts urethral flow leading to high voiding pressures as the detrusor contracts with greater force to overcome the obstruction. Although there is a consensus that heightened pressure triggers inflammation,2 little is understood of the underlying mechanism. Here we suggest a central role for NLRP3 in stimulating inflammation by demonstrating that inhibition of NLRP3 alleviates at least one major index of bladder dysfunction.
Previous studies implicated an inflammatory response early in the progression of BOO with Kanno, et. al.15 showing that IL-1β is particularly relevant in this phase. The functional component of the NLRP3 inflammasome is caspase-1 which cleaves pro-IL-1β to its active form. Our results show a four-fold increase in caspase-1 activity 12 days following the creation of BOO whereas caspase-1 activity in the sham-operated animals was not significantly elevated. BOO animals that received the NLRP3 antagonist, glyburide, had caspase-1 levels similar to the sham and control groups, demonstrating that this major pathway to inflammation was effectively blocked.
BOO also initiates increases in bladder weight (>5-fold) with this parameter inhibited only 50% with glyburide, despite the fact that Evan’s Blue extravasation was completely inhibited. While the reason for this remaining increase in bladder weight is unknown it may be due to detrusor hypertrophy in response to increased load, fibrosis or non-NLRP3 mediated inflammation independent of extravasation. Similar to the changes in Evan’s Blue extravasation, histological changes were mostly reversed with glyburide. Immunocytochemistry indicated primarily urothelial localization, as previously shown 6, 7, with no effect of BOO or glyburide.
Functional bladder analysis (cystometry) demonstrated classic parameters that clinically define BOO; elevated voiding pressure and decreased flow rate. Moreover, voided volume was decreased in obstructed animals given vehicle, a result consistent with the vast majority of the literature.16–20 There are at least two possible reasons for this observation. The first is that the time point being assessed is beyond the “compensatory” phase where the bladder is capable of generating sufficient pressure to overcome increased resistance, maintaining normal voiding efficiency, and instead we are observing the “decompensated” phase. If this were the case, serial measurements over time would likely show a peak in voiding pressures, followed by decline, and constant voided volumes would also be followed by decline. Since we did not perform serial measurements to document the temporal appearance of each phase, it is not possible to completely rule out this possibility. However, as stated above we are working with a time frame that would be considered early in rat BOO,16–22 making it unlikely these bladders have decompensated. The more likely explanation for the decreased void volumes is that the procedure we used to create obstruction produces a severe enough increase in resistance that the bladder cannot generate sufficient force to overcome it, even in the early phase. We did not measure post void residuals in this study as doing so creates unacceptable amounts of artifacts when performing these measurements on non-anesthetized animals. However, the observed increase in voiding frequency, coupled with the low volumes, suggests that we are witnessing a decrease in efficiency. In this model of partial obstruction, the contractile pressure was sufficient to generate urinary flow, but it was not sustained long enough to maintain a normal voiding volume.
Blockade of NLRP3 did nto effect the elevated voiding pressure and flow rate, as expected since the severity of obstruction remained unchanged. However, the voided volumes and urinary frequency in the glyburide-treated animals were close to control values. It is well-known that inflamed muscle tissue fatigues more rapidly compared to unaffected muscle tissue.23 Therefore, preventing NLRP3 activation likely inhibits the inflammatory response and subsequently preserves the functional capability of the detrusor. The results suggest that NLRP3 inhibitors could serve clinically to prevent inflammation-induced bladder deterioration over the long term and ameliorate lower urinary tract symptoms in earlier phases.
This study provides strong evidence that BOO activates the NLRP3 inflammasome, thus initiating an inflammatory response that contributes to voiding dysfunction. The mechanism by which NLRP3 is activated, however, remains unclear but is likely to be multifactorial. One of the most prevalent means by which NLRP3 is activated is by oxidative stress 24–26 and there are several sources by which this can occur in BOO. Severe and/or prolonged bladder distension can cause hypoperfusion and cells undergoing cycles of relative ischemia followed by reperfusion produce bursts of reactive oxygen species (ROS).27 Furthermore, the increased load on the detrusor muscle requires a higher rate of metabolism, providing another source of ROS. Beyond the contribution of oxidative stress, urothelial cells subjected to elevated pressure are known to produce ATP, 28 a well-established DAMP. In other fields of study, such as orthopedics and periodontics, pressure is known to cause necrosis and/or apoptosis. Necrosis is well-established to release DAMPs 29 while apoptosis, classically considered non-inflammatory, can also be pro-inflammatory under certain circumstances.30 Once inflammasomes are activated, the mature Il-1β is released by a death process called pyroptosis which is similar to necrosis and itself releases intracellular DAMPs, thus propagating the resulting inflammation.25 While each of these possibilities begs further investigation, not only for elucidating the true mechanism but also exploring potential targets for intervention, it is apparent that activation of NLRP3 plays a role in urinary dysfunction associated with BOO.
Conclusions
The results suggest the importance of the NLRP3 inflammasome in the induction of inflammation and bladder dysfunction secondary to BOO. Excitingly, inhibiting NLRP3 with the FDA-approved drug glyburide ameliorates inflammation and improves voiding dysfunction, even in the continued presence of the obstruction. These observations suggest that glyburide, or a next-generation NLRP3 antagonist, may be useful in combating the long-term degeneration of bladder function associated with obstructive uropathies.
Acknowledgments
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R01DK103534.
This work was also supported in part by intradepartmental funds from the Medical University of South Carolina Department of Urology.
Abbreviations and Acronyms
- BOO
Bladder Outlet Obstruction
- NLR
NOD-like Receptor
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- BPH
benign prostatic hyperplasia
- DAMPs
Danger Associated Molecular Patterns
- ROS
reactive oxygen species (ROS)
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173:1309. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe PD, Wang J, Jiao H, et al. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;106:1686. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 3.Oka M, Fukui T, Ueda M, et al. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol. 2009;182:382. doi: 10.1016/j.juro.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 4.Deveaud CM, Macarak EJ, Kucich U, et al. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518. [PubMed] [Google Scholar]
- 5.Jock M, Leggett RE, Schuler C, et al. Effect of partial bladder outlet obstruction and reversal on rabbit bladder physiology and biochemistry: duration of recovery period and severity of function. BJU Int. 2014;114:946. doi: 10.1111/bju.12687. [DOI] [PubMed] [Google Scholar]
- 6.Hughes FM, Jr, Turner DP, Purves JT. The Potential Repertoire of the Innate Immune System in the Bladder: Expression of Pattern Recognition Receptors in the Rat Bladder and a Rat Urothelial Cell Line (MYP3 cells) Int Urol Nephrol. 2015 doi: 10.1007/s11255-015-1126-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes FM, Jr, Vivar NP, Kennis JG, et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal Physiol. 2014;306:F299. doi: 10.1152/ajprenal.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steers WD, De Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988;140:864. doi: 10.1016/s0022-5347(17)41846-5. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MP, Hughes FM, Jr, Engmann AK, et al. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015;115:8. doi: 10.1111/bju.13039. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Liu L, Li J, et al. Combined contributions of impaired hepatic CYP2C11 and intestinal breast cancer resistance protein activities and expression to increased oral glibenclamide exposure in rats with streptozotocin-induced diabetes mellitus. Drug Metab Dispos. 2012;40:1104. doi: 10.1124/dmd.111.043513. [DOI] [PubMed] [Google Scholar]
- 12.Silawat N, Gupta VB. Chebulic acid attenuates ischemia reperfusion induced biochemical alteration in diabetic rats. Pharm Biol. 2013;51:23. doi: 10.3109/13880209.2012.698288. [DOI] [PubMed] [Google Scholar]
- 13.Hughes FM, Jr, Corn AG, Nimmich AR, et al. Cyclophosphamide Induces an Early Wave of Acrolein-Independent Apoptosis in the Urothelium. Adv Biosci Biotechnol. 2013;4 doi: 10.4236/abb.2013.48A2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes FM, Jr, McKeithan P, Ellett J, et al. Simvastatin suppresses cyclophosphamide-induced changes in urodynamics and bladder inflammation. Urology. 2013;81:209, e9. doi: 10.1016/j.urology.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Kanno Y, Mitsui T, Kitta T, et al. The inflammatory cytokine IL-1beta is involved in bladder remodeling after bladder outlet obstruction in mice. Neurourol Urodyn. 2015 doi: 10.1002/nau.22721. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa K, Sakai T, Ishibashi K, et al. Involvement of angiotensin II type 1 receptor on pathological remodeling and dysfunction in obstructed bladder. Int J Urol. 2012;19:457. doi: 10.1111/j.1442-2042.2012.02965.x. [DOI] [PubMed] [Google Scholar]
- 17.Fullhase C, Hennenberg M, Sandner P, et al. Reduction of obstruction related bladder overactivity by the guanylyl cyclase modulators BAY 41-2272 and BAY 60-2770 alone or in combination with a phosphodiesterase type 5 inhibitor. Neurourol Urodyn. 2014 doi: 10.1002/nau.22665. [DOI] [PubMed] [Google Scholar]
- 18.Fullhase C, Soler R, Gratzke C, et al. Urodynamic evaluation of fesoterodine metabolite, doxazosin and their combination in a rat model of partial urethral obstruction. BJU Int. 2010;106:287. doi: 10.1111/j.1464-410X.2009.09008.x. [DOI] [PubMed] [Google Scholar]
- 19.Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology. 2011;78:721, e1. doi: 10.1016/j.urology.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 20.Kang YJ, Jin LH, Park CS, et al. Early sequential changes in bladder function after partial bladder outlet obstruction in awake sprague-dawley rats: focus on the decompensated bladder. Korean J Urol. 2011;52:835. doi: 10.4111/kju.2011.52.12.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KJ, Park EY, Kim HS, et al. Expression of transient receptor potential vanilloid 4 and effects of ruthenium red on detrusor overactivity associated with bladder outlet obstruction in rats. World J Urol. 2014;32:677. doi: 10.1007/s00345-013-1099-y. [DOI] [PubMed] [Google Scholar]
- 22.Bisogni S, Ferreira FT, Amstalden Neto A, et al. Influence of oxidative stress on inducing micturition dysfunction following chronic infravesical obstruction and the protective role of an antioxidant diet - association of in vivo and in vitro studies in rats. Int Braz J Urol. 2012;38:552. doi: 10.1590/s1677-55382012000400016. [DOI] [PubMed] [Google Scholar]
- 23.Straub RH. Evolutionary medicine and chronic inflammatory state--known and new concepts in pathophysiology. J Mol Med (Berl) 2012;90:523. doi: 10.1007/s00109-012-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4C:296. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi O, Nomiya M, Andersson KE. Functional consequences of chronic bladder ischemia. Neurourol Urodyn. 2014;33:54. doi: 10.1002/nau.22517. [DOI] [PubMed] [Google Scholar]
- 28.Olsen SM, Stover JD, Nagatomi J. Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng. 2011;39:688. doi: 10.1007/s10439-010-0203-3. [DOI] [PubMed] [Google Scholar]
- 29.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidovich P, Kearney CJ, Martin SJ. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem. 2014;395:1163. doi: 10.1515/hsz-2014-0164. [DOI] [PubMed] [Google Scholar]