Abstract
Early adverse events have been shown to increase the incidence of interstitial cystitis/painful bladder syndrome in adulthood. Despite high clinical relevance and reports of stress-related symptom exacerbation, animal models investigating the contribution of early life stress to female urological pain are lacking. We examined the impact of neonatal maternal separation (NMS) on bladder sensitivity and visceral neuroimmune status both prior-to, and following, water avoidance stress (WAS) in adult female mice. The visceromotor response to urinary bladder distension was increased at baseline and 8d post-WAS in NMS mice, while colorectal sensitivity was transiently increased 1d post-WAS only in naïve mice. Bladder micturition rate and output, but not fecal output, were also significantly increased following WAS in NMS mice. Changes in gene expression involved in regulating the stress response system were observed at baseline and following WAS in NMS mice, and WAS reduced serum corticosterone levels. Cytokine and growth factor mRNA levels in the bladder, and to a lesser extent in the colon, were significantly impacted by NMS and WAS. Peripheral mRNA levels of stress-responsive receptors were differentially influenced by early life and adult stress in bladder, but not colon, of naïve and NMS mice. Histological evidence of mast cell degranulation was increased in NMS bladder, while protein levels of protease activated receptor 2 (PAR2) and transient receptor potential ankyrin 1 (TRPA1) were increased by WAS. Together, this study provides new insight into mechanisms contributing to stress associated symptom onset or exacerbation in patients exposed to early life stress.
Keywords: maternal separation, early life stress, pain, interstitial cystitis, hippocampus, hypothalamic-pituitary-adrenal axis
1. Introduction
Interstitial cystitis (IC), which is associated with painful bladder syndrome (PBS), is characterized by recurrent pain in the bladder or surrounding region that is often associated with increased voiding and urgency (Offiah et al., 2013). Population prevalence of IC/PBS is nine times more common in women than in men (Berry et al., 2011; Link et al., 2008), affecting 3.3 million women in the U.S. alone. Mood disorders, such as depression, anxiety, and panic disorder, are common among chronic pelvic pain patients due to altered functioning of corticotropin releasing factor (CRF)-responsive regions of the brain, including the hypothalamic-pituitary-adrenal (HPA) axis, which regulates stress response and influences the perception of pain (Heim et al., 1998; Hubbard et al., 2011; Mayson and Teichman, 2009). Exposure to early life stress or trauma is a significant risk factor for developing HPA abnormalities and associated chronic pain syndromes (Maniam et al., 2014). As such, a significant proportion of IC/PBS patients report a history of adverse childhood events (Carrico et al., 2009; Jones et al., 2009; Mayson and Teichman, 2009; Peters et al., 2009; Seth and Teichman, 2008; Tietjen et al., 2010).
Regulation of the stress response occurs through CRF and glucocorticoid receptors located at each site along the HPA axis and on higher, regulatory limbic structures, such as the hippocampus, amygdala, and frontal cortex (Ulrich-Lai and Herman, 2009). The two CRF receptors (CRF1 and CRF2) serve opposing roles, as pharmacological antagonism or genetic deletion of CRF1 has been shown to be anxiolytic and pharmacological blockade or genetic deletion of CRF2 is anxiogenic (Bale et al., 2002). Binding at the glucocorticoid (GR) and mineralocorticoid (MR) receptors is largely anxiogenic; however, exposure to chronic stress decreases hippocampal GR expression, reducing the extent of descending inhibition onto the hypothalamus and thereby increasing CRF release and glucocorticoid production (Herman et al., 2005; Ulrich-Lai and Herman, 2009). Downstream propagation of neurogenic inflammation, as a result of improper HPA axis output, has been implicated in other chronic pain disorders (Black, 2002) and may underlie exacerbation of dormant IC/PBS symptoms during periods of high stress (Lutgendorf et al., 2000).
One of the primary peripheral targets of downstream HPA axis output is the mast cell, which is a highly granulated, stem cell-derived immune cell that expresses functional CRF receptors and can respond to and release CRF and related neuropeptides (Black, 2002; Cao et al., 2005; Kempuraj et al., 2004). Multiple independent studies have confirmed that mast cell infiltration is increased in biopsies from IC/PBS patients (Christmas and Rode, 1991; Kastrup et al., 1983; Larsen et al., 2008; Peeker et al., 2000; Spanos et al., 1997; Tomaszewski et al., 2001). These observations have been correlated with increased release of granular contents (Theoharides et al., 1995), elevated nerve growth factor (NGF), histamine, and pro-inflammatory cytokine protein levels in patient serum (Jiang et al., 2013) and urine (Corcoran et al., 2013; Jacobs et al., 2010; Lotz et al., 1994; Yun et al., 1992) samples, and increased density of substance P (SP)-immunopositive nerve fibers and juxtaposition to mast cells in patient biopsies (Pang et al., 1995). Tryptase, a major component of mast cell granules, can bind to and activate protease activated receptor-2 (PAR2) located on adjacent sensory nerve endings (Cenac et al., 2002; Cenac et al., 2007; Sipe et al., 2008). Activation of PAR2 has been shown to sensitize transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1) in vivo (Chen et al., 2011; Sipe et al., 2008), two channels that have been implicated in the development of inflammatory-induced visceral hyperalgesia (Birder et al., 2002; Brierley et al., 2009; Jones et al., 2005).
Despite its involvement in stress-related pathologies and immunomodulatory effects, dysregulation of the HPA axis has not been investigated in early life stress-induced urinary bladder hypersensitivity and dysfunction. Using a model of neonatal maternal separation (NMS), we investigated the impact of early life stress on urinary bladder sensitivity and function both prior-to, and following, exposure to water avoidance stress (WAS) in adulthood. To determine whether the effects of NMS were specific to the bladder, we also evaluated colorectal sensitivity and output prior to and following WAS exposure. Output of the HPA axis and relevant gene expression in the hypothalamus and hippocampus were examined in NMS and naïve mice with or without exposure to WAS. Cytokine, growth factor, CRF1, and CRF2, mRNA levels; histological evidence of mast cell degranulation; and protein levels of TRPA1 and PAR2 were assayed in the bladder and colon. Together, these results increase our understanding of how early life stress predisposes an individual to developing stress-associated bladder pain syndromes during adulthood.
2. Results
2.1 Neonatal and adult stress exposure differentially increase urinary bladder and colorectal sensitivity
Previous studies in our laboratory have determined that NMS impacts adult anxiety-like behaviors and vaginal sensitivity with associated molecular changes in the pelvic viscera and HPA axis of female mice (Pierce et al., 2014). The purpose of the current study is to determine the impact of acute adult stress exposure on urinary bladder and colorectal sensitivity and associated measurements of HPA axis output and regulation in the same model of female NMS mice. Estrous cycle stage was estimated following physiological or behavioral testing or at the time of sacrifice and no significant effect of apparent cycle stage was observed for any of the reported results (data not shown).
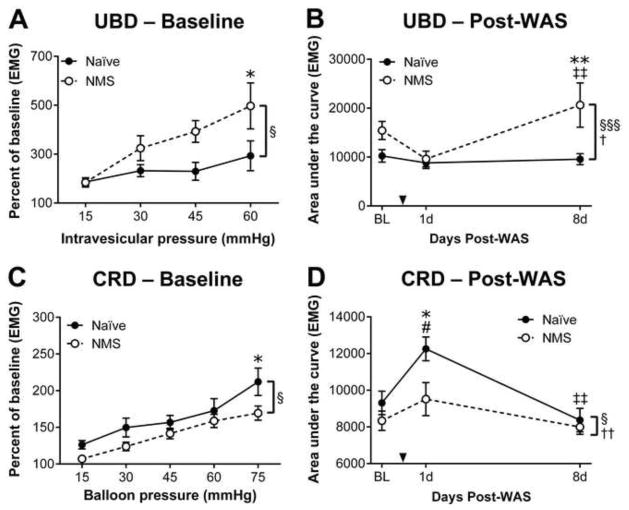
The visceromotor response (VMR) during urinary bladder distension (UBD) or colorectal distension (CRD) was recorded in naïve and NMS mice to evaluate changes in pelvic organ sensitivity prior to additional stress exposure. In all mice, the VMR during either UBD (Figure 1A) or CRD (Figure 1C) significantly increased in response to greater intravesicular or balloon pressure (p < 0.0001, two-way RM ANOVA), respectively, confirming a physiological response to organ distension. At baseline, the VMR of NMS mice during UBD was significantly higher than that of naïve mice over the entire distension series (p < 0.05, two-way RM ANOVA) and at the highest pressure applied (p < 0.05, Bonferroni’s multiple comparisons test; Figure 1A). In contrast, NMS mice displayed significantly decreased VMR during CRD, both over the entire distension series (p < 0.05, two-way RM ANOVA) and at the highest applied pressure (p < 0.05, Bonferroni’s multiple comparisons test; Figure 1C). Mice were then exposed to WAS and the VMR was reassessed during either UBD or CRD at 1 day (1d) and 8 days (8d) post-WAS. Naïve mice exhibited no change in VMR during UBD at either 1d or 8d post-WAS; however, NMS mice displayed a transient decrease in VMR during UBD at 1d and a significant increase in VMR at 8d post-WAS, when compared to both their 1d post-WAS measurements and their naïve counterparts (p < 0.01, Bonferroni’s multiple comparisons test; Figure 1B). Exposure to WAS transiently and significantly increased VMR during CRD only in naïve mice at 1d post-WAS, compared to their baseline measurements and their NMS counterparts (p < 0.05, Bonferroni’s multiple comparisons test; Figure 1D). The VMR during CRD was unaffected by WAS in NMS mice at both the 1d and 8d time points (Figure 1D).
Figure 1.
The visceromotor response (VMR) during urinary bladder distension (UBD) or colorectal distension (CRD) was measured to determine the impact of neonatal maternal separation (NMS) and water avoidance stress (WAS) on pelvic organ sensitivity. A) At baseline (BL), NMS mice (n=9) displayed significantly greater VMR during UBD across the entire distension series, and at the highest intravesicular pressure, compared to naïve mice (n=8). B) The area under the curve (AUC) was measured for VMR during UBD at BL, 1d, and 8d post-WAS in naïve and NMS mice and revealed a significant effect of both NMS and WAS on bladder sensitivity, particularly at the 8d post-WAS time point. C) At baseline, NMS mice (n=14) had a slight, but significant reduction in VMR during CRD compared to naïve mice (n=13), particularly at the highest intraballoon pressure applied. D) The AUC was measured for VMR during CRD in naïve (n=5) and NMS (n=5) mice at BL, 1d, and 8d post-WAS and revealed a significant effect of both NMS and WAS on colorectal sensitivity, particularly at the 1d post-WAS time point when naïve mice had a significant increase in VMR that was not observed in NMS mice. Brackets indicate a significant effect of NMS (§,§§§ p < 0.05, 0.001) or WAS (†,††, p < 0.05, two-way RM ANOVA (A, C) or two-way ANOVA (B, D); *, ** p < 0.05, 0.01 vs. naïve, # p < 0.05 vs. BL, ‡‡ p <0.01 vs. 1d post-WAS, Bonferroni’s multiple comparisons test.
2.2 Neonatal and adult stress exposure significantly impact urinary bladder output
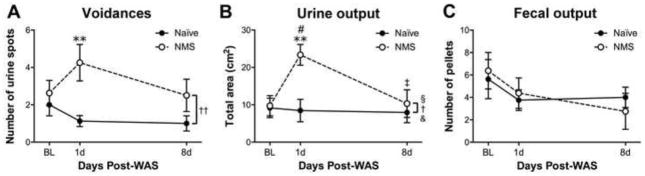
The functional output of the bladder and colon was characterized in NMS and naïve mice prior-to and following WAS to determine the impact of early life and adult stress on micturition and defecation rates. The number of voidances and total urine output during a 1h testing period were not different between naïve and NMS mice at baseline (Figure 2A–B). However, at 1d post-WAS, the number of voidances was significantly increased in NMS mice, compared to naïve mice (p < 0.01, Bonferroni’s multiple comparisons test; Figure 2A), and total urine output was significantly higher in NMS mice compared to both their own baseline measurements (p < 0.05, Bonferroni’s multiple comparisons test) and naïve mice (p; < 0.01, Bonferroni’s multiple comparisons test; Figure 2B). Voidance rate and total urine output were returned to baseline levels by 8d post-WAS in NMS mice. Fecal output was also measured pre- and post-WAS and neither NMS nor WAS had a significant impact (Figure 2C).
Figure 2.
Micturition frequency, and urine and fecal output were measured over a 1 h testing period to determine changes in bladder and gastrointestinal function resulting from neonatal maternal separation (NMS) and water avoidance stress (WAS). A) Void frequency, measured as the number of urine spots measured following a 1 h test period, was differentially affected by WAS in the naïve and NMS mice. At 1d post-WAS, voidance rate in NMS mice was significantly higher than in naïve mice, and had returned to BL levels by 8d post-WAS. B) The total urine output during the 1 h test period was significantly impacted by NMS, WAS, and an NMS/WAS interaction. At 1d post-WAS, NMS mice had a significantly higher output than both their naïve counterparts and their BL measurements. Total output from NMS mice had returned to BL levels at 8d post-WAS. C) Neither NMS nor WAS significantly impacted fecal output. Brackets indicate a significant effect of NMS (§ p < 0.05), WAS (†,†† p < 0.05, 0.01), or an NMS/WAS interaction (& p < 0.05), two-way ANOVA; * p < 0.05 vs. naïve, Bonferroni’s multiple comparisons test. BL and 1d, N=8 for each group; 8d, N=4 for each group.
2.3 Neonatal and adult stress alter HPA axis output and regulation
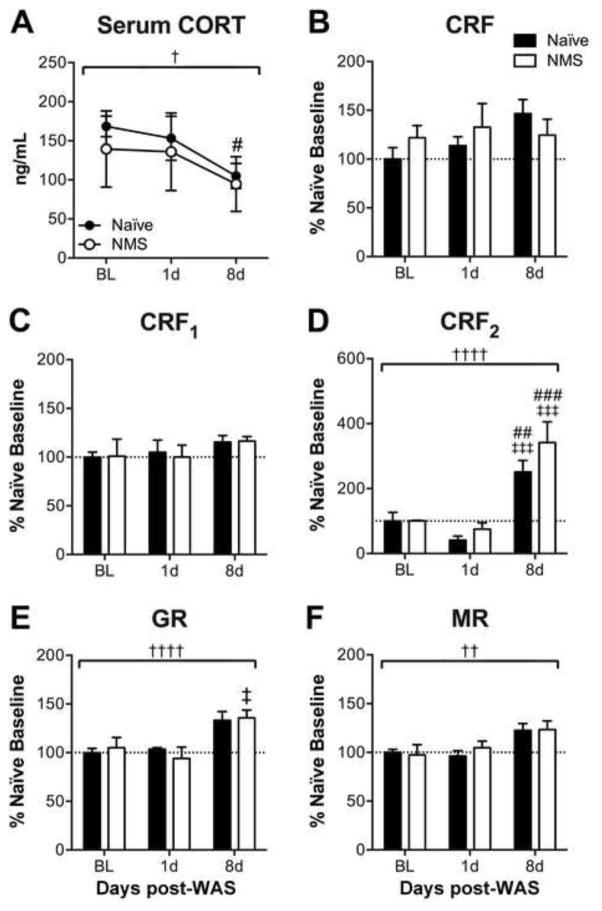
To determine the effect of neonatal and adult stress exposure on the output and regulation of the HPA axis, resting serum corticosterone (CORT) and gene transcript levels were measured in hypothalamus and hippocampus from NMS and naïve mice at 1 and 8d post-WAS exposure and compared to baseline levels. Early life stress exposure did not significantly impact serum CORT levels; however, exposure to WAS had a significant overall effect of decreasing serum CORT (p < 0.05, two-way RM ANOVA), specifically at 8d post-WAS (p < 0.05, Bonferroni’s multiple comparisons test; Figure 3A). Real-time PCR was performed to measure mRNA levels in the hypothalamus. No significant effect of NMS or WAS was observed on either CRF or CRF1 mRNA levels in the hypothalamus (Figure 3B–C). In contrast, WAS significantly increased the mRNA levels of CRF2 (p < 0.0001, two-way ANOVA), GR (p < 0.001, 0.01, two-way ANOVA), and MR (p < 0.01, two-way ANOVA) in the hypothalamus of both naïve and NMS mice, particularly CRF2 and GR at 8d post-WAS (p < 0.05, Bonferroni’s multiple comparisons test; Figure 3D–F).
Figure 3.
To evaluate HPA axis output, serum corticosterone (CORT) and mRNA levels of receptors involved in HPA axis regulation in the hypothalamus were evaluated in response to neonatal maternal separation (NMS) and water avoidance stress (WAS). A) Repeated tail vein serum analysis revealed a significant effect of WAS on serum CORT in both naïve and NMS mice, in particular at 8d post-WAS in naïve mice, compared to baseline (BL) measurements. No significant effect of NMS or WAS was observed on hypothalamic mRNA levels of corticotropin-releasing factor (CRF, B) or its receptor, CRF1 (C). Exposure to WAS significantly increased mRNA levels of CRF2 (D), glucocorticoid receptor (GR, E), and mineralocorticoid receptor (MR, F) in both NMS and naïve hypothalamus. Brackets indicate a significant effect of WAS (†,††,†††† p < 0.05, 0.01, 0.0001), two-way RM ANOVA (A) or two-way ANOVA (B–F); #, ##, ### p < 0.05, 0.01, 0.001 vs. BL; ‡, ‡‡‡ p <0.05, 0.001 vs. 1d post-WAS; Bonferroni’s multiple comparisons test. N=3–5 for all groups/time points.
Hippocampal mRNA levels were measured to assess potential changes in limbic regulation of the HPA axis resulting from early life or adult stress. The mRNA levels of CRF2 were significantly reduced by WAS exposure (p < 0.05, two-way ANOVA), particularly in NMS mice at both 1d and 8d post-WAS when compared to baseline measurements (p < 0.05, Bonferroni’s multiple comparisons test; Figure 4A). Hippocampal CRF2 mRNA levels were significantly impacted by both NMS (p < 0.05, two-way ANOVA) and WAS (p < 0.0001, two-way ANOVA), such that at 1d post-WAS CRF2 mRNA levels were significantly lower in NMS compared to naïve (p < 0.05, Bonferroni’s multiple comparisons test) and at 8d post-WAS both NMS and naïve had significantly higher CRF2 mRNA levels than either their baseline or 1d post-WAS measurements (p < 0.001, Bonferroni’s multiple comparisons test; Figure 4B). Hippocampal GR mRNA levels were significantly impacted by NMS and an NMS/WAS interaction (p < 0.05, two-way ANOVA); only 1d post-WAS measurements were significantly different between naïve and NMS mice (p < 0.05, Bonferroni’s multiple comparisons test; Figure 3C). Hippocampal MR mRNA levels were significantly impacted by NMS and WAS (p < 0.05, two-way ANOVA) such that 1d post-WAS measurements in NMS hippocampus was significantly lower than baseline and naïve (p < 0.05, Bonferroni’s multiple comparisons test) and had recovered to near-baseline levels at 8d post-WAS (Figure 4D). A non-significant trend (p = 0.06, two-way ANOVA) was observed for reduced brain-derived neurotrophic factor (BDNF) mRNA levels in NMS hippocampus (Figure 4E).
Figure 4.
Hippocampal mRNA levels of receptors involved in negative regulation of the HPA axis were evaluated in response to neonatal maternal separation (NMS) and water avoidance stress (WAS). A) Exposure to WAS significantly decreased the hippocampal mRNA level of corticotropin-releasing factor receptor 1 (CRF1) overall, as well as in NMS mice at 1d and 8d post-WAS compared to baseline (BL) levels. B) NMS similarly decreased overall hippocampal mRNA levels of CRF receptor 2 (CRF2), however, WAS exposure caused a transient and significant decrease in in NMS hippocampus at 1d post-WAS, compared to naïve, and a significant increase in CRF2 mRNA levels in both naïve and NMS hippocampus at 8d post-WAS compared to their respective BL and 1d post-WAS levels. C) The hippocampal mRNA levels of glucocorticoid receptor (GR) was significantly impacted by NMS and an NMS/WAS interaction, particularly at 1d post-WAS. D) Mineralocorticoid receptor (MR) mRNA levels were significantly affected by WAS and an NMS/WAS interaction, resulting in a transient decrease in expression at 1d post-WAS in NMS mice and a return to BL levels at 8d post-WAS. E) Despite a slight reduction at 1d post-WAS in NMS mice, no significant effects of NMS or WAS were observed on the hippocampal mRNA levels of brain-derived neurotrophic factor (BDNF). Brackets indicate a significant effect of NMS (§ p < 0.05), WAS (†, †††† p < 0.05, 0.0001), or NMS/WAS interaction (& p < 0.05), two-way ANOVA; *, ** p < 0.05, 0.01 vs. naïve; #, ##, ###, #### p < 0.05, 0.01, 0.001, 0.0001 vs. BL; ‡‡, ‡‡‡, ‡‡‡‡ p < 0.01, 0.001, 0.0001 vs. 1d post-WAS; Bonferroni’s multiple comparisons test. N=3–5 for all groups/time points.
2.4 Neonatal and adult stress exposure impact peripheral measures of HPA axis output
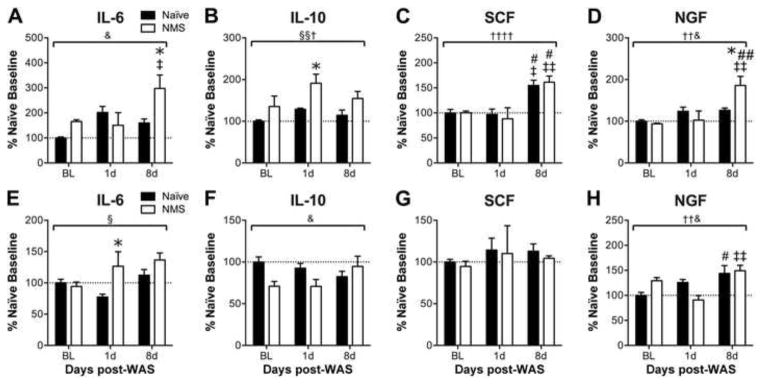
Real-time PCR was performed to characterize changes in pelvic organ gene expression, related to altered peripheral output of the HPA axis, resulting from early life and adult stress exposure. The mRNA level of interleukin-6 (IL6) was significantly impacted by a NMS/WAS interaction effect in the bladder (p < 0.05, two-way ANOVA), resulting in a significant increase in NMS bladder at 8d post-WAS compared to 1d post-WAS NMS and naïve counterparts (p < 0.05, Bonferroni’s multiple comparisons test; Figure 5A). Colonic IL6 mRNA levels were significantly increased by NMS (p < 0.05, two-way ANOVA), particularly at 1d post-WAS (p < 0.05, Bonferroni’s multiple comparisons test; Figure 5E). The mRNA level of interleukin-10 (IL10) was differentially impacted by NMS and WAS in the two organs, as NMS and WAS both significantly increased IL10 expression in the bladder (p < 0.05, two-way ANOVA; Figure 5B), yet had a significant interaction effect on reducing IL10 expression in the colon (p < 0.05; Figure 5F). Stem cell factor (SCF), a mast cell signaling cytokine and growth factor, was significantly increased by WAS in bladder (p < 0.0001, two-way ANOVA), particularly at the 8d post-WAS time point compared to baseline and 1d post-WAS measurements in both naïve and NMS mice (p < 0.05, Bonferroni’s multiple comparisons test; Figure 5C), but no effects of NMS or WAS were observed on SCF mRNA levels in colon (Figure 5G). The mRNA levels of NGF were significantly impacted by WAS and a NMS/WAS interaction effect in both bladder (p < 0.05, two-way ANOVA; Figure 5D) and colon (p < 0.05, two-way ANOVA; Figure 5H), but with differential patterns in the NMS mice: NGF expression was significantly increased in NMS bladder at 8d post-WAS compared to baseline, 1d post-WAS, and naïve measurements (p < 0.05, Bonferroni’s multiple comparisons test; Figure 5D), whereas colonic NGF mRNA at 8d post-WAS was significantly increased in naïve compared to baseline and in NMS compared to 1d post-WAS measurements (p < 0.05, Bonferroni’s multiple comparisons test; Figure 5H).
Figure 5.
The mRNA levels of cytokines and growth factors involved in neurogenic inflammation were measured in the bladder (A–D) and colon (E–H) to determine alterations in downstream activation of the HPA axis in response to neonatal maternal separation (NMS) and water avoidance stress (WAS). A) A significant interaction effect of NMS and WAS was observed on mRNA levels of interleukin (IL) 6 in the bladder. Both NMS and WAS separately and significantly increased IL10 mRNA levels in the bladder (B), whereas WAS alone and/or a NMS/WAS interaction significantly increased stem cell factor (SCF, C) and nerve growth factor (NGF, D) mRNA levels in the bladder, respectively. The mRNA level of IL6 in the colon was significantly increased in NMS mice overall (E). A significant NMS/WAS interaction effect was observed for both IL-10 (F) and NGF (H) mRNA levels in the colon, with NGF mRNA levels being significantly elevated at 8d post-WAS in both naïve and NMS colon. The mRNA levels of SCF were unaffected by NMS or WAS in the colon. Brackets indicate a significant effect of NMS (§, §§ p < 0.05, 0.01), WAS (†,††, †††† p < 0.05, 0.01, 0.0001), or NMS/WAS interaction (& p < 0.05), two-way ANOVA; * p < 0.05 vs. naïve, #, ## p < 0.05, 0.01 vs. BL, ‡, ‡‡ p <0.05, 0.01 vs. 1d post-WAS; Bonferroni’s multiple comparisons test. N=3–5 for all groups/time points.
The mRNA levels of CRF1 and CRF2 were also evaluated in the bladder and colon of NMS and naïve mice at baseline and following WAS. Bladder CRF1 mRNA levels were significantly lower in NMS mice (p < 0.05, two-way ANOVA; Figure 6A), whereas WAS exposure significantly reduced CRF2 mRNA levels in the bladder (p < 0.01, two-way ANOVA; Figure 6B), with a transient decrease in expression in naïve bladder at 1d post-WAS and increased expression in both naïve and NMS bladder at 8d post-WAS compared to 1d post-WAS measurements (p < 0.05, Bonferroni’s multiple comparisons test; Figure 6B). No significant effect of NMS or WAS on CRF1 or CRF2 mRNA levels was observed in the colon (Figure 6C–D).
Figure 6.
Peripheral expression of receptors involved in stress signaling was evaluated in response to neonatal maternal separation (NMS) and water avoidance stress (WAS). A significant effect of NMS was observed on reducing corticotropin-releasing factor receptor 1 (CRF1) mRNA levels in bladder (A), but no effects of NMS or WAS were observed in colon (C). A significant effect of WAS was observed on mRNA levels of CRF receptor 2 (CRF2) in bladder, with a transient reduction at 1d post-WAS followed by a recovery to BL levels at 8d post-WAS (B), but no effects of NMS or WAS were observed in colon (D). Brackets indicate a significant effect of NMS (§ p < 0.05) or WAS (†† p < 0.01), two-way ANOVA; # p < 0.05 vs. BL, ‡ p <0.05 vs. 1d post-WAS; Bonferroni’s multiple comparisons test. N=3–5 for all groups/time points.
2.5 Neonatal and adult stress exposure significantly increase mast cell degranulation and protein expression of associated pain-signaling receptors in the bladder
To determine if mast cell activation could be contributing towards the bladder-specific effects observed in NMS mice following WAS, bladder tissue from NMS and naïve mice was processed for mast cell visualization and Western blotting. Mast cells were visualized using acidified toluidine blue and analyzed for state of degranulation (Figure 7B–C′). Bladders from NMS mice contained a significantly larger percentage of mast cells exhibiting evidence of degranulation than did bladders from naïve mice (p < 0.001, two-way ANOVA; Figure 7A). Exposure to WAS also had an overall effect of increasing mast cell degranulation (p < 0.05, two-way ANOVA), primarily driven by a significant increase in the percentage of degranulated mast cells in naïve bladder at 1d post-WAS (p < 0.05, Bonferroni’s multiple comparisons test; Figure 7A). Evidence of mast cell degranulation in colon sections from the same mice did not reveal any differences in percent degranulation between naïve and NMS mice, either at baseline or following WAS exposure (data not shown). Protein levels of PAR2 and TRPA1 were significantly increased following WAS exposure in both naïve and NMS bladder (p < 0.05, two-way ANOVA; Figure 7D–E).
Figure 7.
Histological evidence of mast cell degranulation and protein levels of the tryptase receptor, protease-activated receptor 2 (PAR2), and transient receptor potential ankyrin 1 (TRPA1) were measured in the bladder to determine the potential role of the mast cell-nerve axis in bladder hypersensitivity resulting from neonatal maternal separation (NMS) and water avoidance stress (WAS). A) Both NMS and WAS has significant effects on increasing mast cell degranulation in the bladder. B–C) Photomicrographs show intact (arrow) and degranulated (arrowhead) mast cells visualized in bladders from NMS and naïve mice. Higher magnification shows distinct morphology of intact (B′, red arrow B) and degranulated (C′, red arrowhead C) mast cells from naïve and NMS bladder, respectively. D–E) Representative Western blots are shown for PAR2, TRPA1, and corresponding GAPDH protein expression with bands at 32, 127, and 35kD, respectively. WAS significantly increased protein levels of both PAR2 (D) and TRPA1 (E) in the bladder from naïve and NMS mice. Brackets indicate a significant effect of NMS (§§§ p < 0.001) or WAS († p < 0.05), two-way ANOVA; *, *** p < 0.05, 0.001 vs. naïve, Bonferroni’s multiple comparisons test. Scale bars represent 100″m (B, C) and 10″m (B′, C′). N=3–5 for all groups/time points.
3. Discussion
Patients suffering from chronic pelvic pain syndromes commonly report symptom onset or worsening during times of stress. Experiencing stress or trauma early in life increases the likelihood of developing chronic pelvic pain later in life, as well as comorbid mood disorders (Peters et al., 2009). Here we have provided the first evidence of early life stress-induced bladder hypersensitivity in mice. Exposure to adult stress further exacerbated urinary bladder sensitivity in NMS mice and transiently increased colorectal sensitivity in naïve mice. Enhanced neuroimmune profiles in the bladder of NMS mice suggest that early life stress contributes to a painful phenotype precipitated by an aberrant response to acute stress in adulthood.
Increased colorectal (Coutinho et al., 2002; Moloney et al., 2012; O’Malley et al., 2010; Zhang et al., 2009) or vaginal (Pierce et al., 2014) hypersensitivity has previously been shown to manifest in rodent models of NMS and this study provides the first evidence of urinary bladder hypersensitivity following NMS in female mice. Baseline bladder hypersensitivity in NMS mice was further exacerbated, in a delayed and prolonged fashion, by exposure to WAS, while a more immediate increase in colorectal sensitivity was observed only in naïve mice. The difference in VMR at 1d post-WAS, both in terms of NMS- and organ-effect, likely reflects alterations in the neuroimmune response in NMS mice rather than stress-induced hypoalgesia, considering that naïve mice showed no change in VMR during UBD and an increase in VMR during CRD at the same time point. Similarly, changes in gene expression of stress-signaling receptors were not observed in the colon of naïve or NMS mice. The current study revealed an overall negative impact of NMS on colorectal sensitivity, both at baseline and following WAS, which is contradictory to previous studies using a 14d-long NMS protocol in either rats (Coutinho et al., 2002; O’Malley et al., 2011; Wu et al., 2009) or mice (Moloney et al., 2012) and suggests that a three-week long NMS protocol in female mice may generate a hypersensitive phenotype that, among the pelvic viscera, is restricted to the urogenital organs (Pierce et al., 2014). This observation highlights the importance of noting the duration of stress and the strain and sex of the animal used across differing NMS protocols. No correlations were observed between stage of estrous cycle and behavior, serum CORT, molecular analysis, or mast cell degranulation. While estrous cycle has been shown to have an impact on VMR to organ distension (Berkley et al., 2001; Cason et al., 2003; Nagabukuro and Berkley, 2007), experimental treatment within such studies was large enough to negate estrous cycle effects. This suggests the effect of NMS and WAS presented in the current study was large enough to overcome relatively minor fluctuations in organ sensitivity across the estrous cycle.
Subsets of IC/PBS patients that report histories of early life stress, such as sexual and physical abuse, frequently present with increased voiding and urgency (Carrico et al., 2009; Mayson and Teichman, 2009; Seth and Teichman, 2008), and individuals that experienced multiple types of maltreatment are more likely to develop comorbid pain disorders (Tietjen et al., 2010). Furthermore, patients with bladder dysfunction, in the absence of pain, are more likely to report early life abuse than the general population (Kudo et al., 2012). Increased expression of CRF and CRF2 was observed in the urothelium and associated innervation of the bladder following cyclophosphamide-induced bladder inflammation in rats (LaBerge et al., 2006), and micturition retention has been reported in mice exposed to social defeat stress (Chang et al., 2009; Wood et al., 2009), suggesting a behavioral relationship between stress and bladder dysfunction. Single or chronic WAS exposure has been shown to increase micturition rates in female (Smith et al., 2011) and male (McGonagle et al., 2012) rats, but with sex-dichotomous outcomes resulting in decreased and increased void volumes, respectively. In the present study, WAS exposure induced a transient, yet significant, increase in micturition frequency and total urine output only in NMS mice. Strikingly, neither NMS nor WAS had effects on fecal output, increased rates of which have been reported in animal models of IBS in males, a phenotype sensitive to CRF antagonism (Buckley et al., 2014; Kim et al., 2014; Rho et al., 2014; Suda et al., 2013).
In our previous study, we demonstrated that performing NMS throughout the 3 week pre-weaning period resulted in a stronger phenotype than the standard 14d-long separation period, which we attributed to disruption of proper limbic structure maturation during the third week of life, particularly within the hippocampus (Pierce et al., 2014). Here, we show that although WAS-induced changes in mRNA levels were observed in both the hypothalamus and hippocampus, only in the latter were NMS-associated changes denoted. Exposure to WAS significantly, and selectively, decreased the mRNA levels of MR and GR only in the NMS hippocampus at the 1d time point and had a longer-lasting effect on CRF1 and CRF2 mRNA levels. This is opposite what has previously been reported in rat NMS models following acute stress exposure, however, this is likely due to variations in NMS-duration, species, and the nature of the acute stressor (O’Malley et al., 2011). The transient decrease in mRNA levels returned to naïve-like levels by 8d post-WAS, suggesting that negative regulation from the NMS hippocampus was acutely, but not chronically, impaired following WAS exposure. Interestingly, WAS-induced changes in mRNA levels in the hypothalamus did not differ between naïve and NMS mice and also were not observed until the 8d post-WAS time point. The delayed increase of expression in genes involved in negative regulation of the HPA axis including CRF2, GR, and MR suggest that changes in gene expression within the hypothalamus may have been driven by the transiently reduced negative regulation from the hippocampus, such as due to a BDNF-induced change in long term potentiation (LTP) (Datson et al., 2012; Suri and Vaidya, 2013). Exposure to chronic stress disrupts LTP, which can be rapidly reversed by GR antagonist treatment (Krugers et al., 2006), and exposure to acute and chronic stress, or treatment with exogenous glucocorticoids, significantly downregulates BDNF mRNA and protein levels in the rodent hippocampus (Suri and Vaidya, 2013). Our observation of a concurrent decrease in hippocampal CRF2, GR, and MR mRNA levels only in the NMS mice at 1d post-WAS further suggests that the hippocampal response to adult stress is dysregulated following neonatal stress exposure and likely contributes to the downstream molecular changes within the hypothalamus. The decrease in serum CORT at 8d post-WAS, particularly in naïve mice, provides further evidence of a latent increase in negative regulation on the HPA axis. Evidence of hypocortisolism has been observed in healthy individuals under ongoing stress (Caplan et al., 1979), as well as a variety of stress-related chronic pain disorders, including chronic pelvic pain (Heim et al., 1998), (Petrelluzzi et al., 2008) or fibromyalgia (Griep et al., 1998), and animal models of stress-induced hyperalgesia (Zhang et al., 2012).
The specific contributions of peripherally active CRF1 and CRF2 towards visceral sensitivity, inflammatory-state, and permeability have largely been shown to be organ system-dependent. CRF1 has been implicated in WAS-induced colorectal hypersensitivity (Larauche et al., 2008) and gut-barrier dysfunction (Barreau et al., 2004; Schwetz et al., 2005); however, CRF2 antagonism blocked the release of the proinflammatory cytokine IL6 from cultured cardiomyocytes (Huang et al., 2009) and expression of IL10 was increased in cytotrophoblast cells by a CRF2-mediated mechanism (Novembri et al., 2011). Stress-induced bladder hypersensitivity and vascular permeability has also been shown to be CRF2-mediated (Boucher et al., 2010; Robbins and Ness, 2008). Here, CRF1 mRNA levels were significantly lower in NMS bladder, compared to naïve, with no impact of WAS observed in either group. In comparison, CRF2 mRNA levels were decreased at baseline in NMS bladder and significantly increased at 8d post-WAS, compared to 1d post-WAS levels. The relative decrease in CRF1 decrease and increase in CRF2 in NMS bladder may promote a local CRF2-dominant signaling environment, thereby driving the observed increase in VMR and micturition output post-WAS. A similar, but reversed, phenomenon has been proposed to contribute to IBS-like phenotypes (Nozu and Okumura, 2015). The lack of a NMS or WAS effect on peripheral CRF receptors in the colon may contribute to the minimal behavioral manifestations in colorectal sensitivity and dysfunction in the present study.
The role of mast cells in driving neurogenic inflammation and visceral afferent sensitization in mucosal diseases such as IBS and asthma is well recognized (Beunk et al., 2013; Buckinx et al., 2011). Degranulated mediators from mast cells, including tryptase and histamine, bind to receptors on nearby visceral afferents and can drive stress-mediated sensitization, a process thought to contribute to chronic functional pain (Barbara et al., 2004; Steinhoff et al., 2000). This nerve-mast cell axis is perpetuated by the chemoattraction of mast cells to antidromic release of peptidergic molecules (de Garavilla et al., 2001; Yano et al., 1989), which, in turn, has been shown to increase mast cell cytokine expression (Niizeki et al., 1997). In the bladder, mast cells, rather than T-regulatory cells or macrophages, are the major source of IL10 (Chan et al., 2013) and increased tryptase, histamine, IL6, and NGF have all been reported in the urine of IC/PBS patients (Boucher et al., 1995). Here we observed histological evidence of a significant increase in mast cell degranulation in NMS bladder compared to naïve. Although both naïve and NMS mice had similar increases in the mRNA levels of the mast cell signaling cytokine, SCF, both IL6 and NGF were specifically increased in NMS bladder at 8d post-WAS indicating greater neuroimmune activation in this tissue. Indeed, mast cell activation, histamine release, NGF expression, and associated pelvic organ hypersensitivity have all been shown to be increased by stress exposure (Merrill et al., 2013); however, rats deficient in mast cells did not exhibit a requisite increase in NGF, histamine, or PAR2 following cold restraint stress (Yang et al., 2012), and treatment with mast-cell stabilizers can prevent, but not reverse, stress-induced pelvic hypersensitivity (Barreau et al., 2007; van den Wijngaard et al., 2009).
Neonatal stress or visceral injury has been shown to alter expression of TRP channels, which have been implicated in driving chronic pain phenotypes (Al-Chaer et al., 2000; Barreau et al., 2004; Chen et al., 2011; Christianson et al., 2010; Gold and Gebhart, 2010; Randich et al., 2006; Sipe et al., 2008). Here, we report that acute stress increased protein levels of PAR2 and TRPA1 in the bladder, findings that, when combined with elevated mast cell degranulation and NGF mRNA levels in the bladder, further indicate a heightened presence of neuroimmune signaling in NMS mice, particularly at 8d post-WAS when VMR to bladder distension was highest. TRPA1 and its family member TRPV1 have been shown to be sensitized in vivo by downstream signaling from PAR receptors (Chen et al., 2011; Sipe et al., 2008) and likely contribute to chronic functional pain (Barbara et al., 2004; Steinhoff et al., 2000). Furthermore, early life stress has been shown to increase TRPA1 protein levels in the bladder (Pierce et al., 2014) and experimental cystitis using cyclophosphamide induced a TRPA1-, but not TRPV1-, dependent bladder hyperalgesia (DeBerry, 2014).
3.1 Conclusion
This study provides evidence that early life stress in female mice increased bladder hypersensitivity and dysfunction in adulthood, which was exacerbated following exposure to WAS. Molecular findings from NMS mice suggest that hippocampal regulation of the HPA axis was diminished, particularly immediately following WAS exposure, leading to compensatory changes in hypothalamic gene expression at a later time point. Peripheral evidence of neurogenic inflammation in the bladder of NMS mice, including increased mast cell degranulation, pro-inflammatory gene expression, and imbalanced CRF receptor levels, suggest central and peripheral dysregulation of the HPA axis may drive the specific urological hypersensitivity. Together with previous work demonstrating psychological abnormalities and pelvic organ sensitivities associated with NMS, this study provides new insight into mechanisms that may contribute to stress-associated symptom onset and exacerbation in a population of patients exposed to early life stress.
4. Experimental Procedure
4.1 Animals
Experiments were performed on female C57Bl/6 mice (Charles River, Wilmington, MA) born and housed in the Research Support Facility at the University of Kansas Medical Center at the indicated ages in Table 1. Mice were housed on a 12-h light cycle from 600 to 1800h and received water and food ad libitum. All research performed conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals in accordance with the guidelines specified by the University of Kansas Medical Center Animal Care and Use Protocols.
Table 1.
Age of mice in weeks at experimental time points
| Baseline | 1d post-WAS | 8d post-WAS | |
|---|---|---|---|
| Behavioral testing | |||
| UBD | 16 | 17 | 18 |
| CRD | 21 | 22 | 23 |
| Micturition | 11, 18 | 11, 18 | 19 |
| In vitro analysis | |||
| Serum | 8 | 8 | 9 |
| mRNA/protein | 12 | ||
| 12 | |||
| 9 | |||
| Mast cells | 28 | ||
| 11 | |||
Mice underwent behavioral testing or were sacrificed at the above ages noted in weeks. Each row represents separate cohorts of animals that were repeatedly exposed to the stated behavioral test (urinary bladder distension [UBD], colorectal distension [CRD], or micturition analysis), repeated blood draw (serum corticosterone [CORT]), or were sacrificed prior to (baseline) or following exposure to water avoidance stress (WAS). Micturition was analyzed in two separate groups of mice beginning at either 11 or 18 weeks of age. In vitro analysis was performed on tissues from naïve and NMS mice that were exposed to WAS, but in the absence of any behavioral testing.
4.2 Neonatal Maternal Separation
All mice used in this study were born in house from pregnant dams (Charles River, Wilmington, MA) delivered to the animal facility during the last week of gestation. The separation procedure was performed as previously described (Fuentes et al., 2015). Day of birth was designated as postnatal day (P) 0 and from P1 until P21 individual litters were removed daily and placed en masse into clean glass beakers containing a small amount of home cage bedding to maintain scent. Pups were held at 34°C and 50% humidity from 1100 to 1400 hours. Fresh gloves were rubbed with home cage bedding before handling each litter to avoid rejection by the dam. Corresponding naïve mice were born, housed, and weaned during the same time frame to avoid potential complications arising from variations in prenatal shipping conditions, housing environment, and normal husbandry procedures. All mice were weaned on P22.
4.3 Water avoidance stress
Water avoidance stress (WAS) was performed for one hour, within the first six hours of the light cycle. Mice were placed individually on a round platform (5 cm diameter) centrally affixed to the bottom of a container (36 cm length × 31 cm width × 27 cm height) filled with room temperature tap water up to 1 cm below the top of the platform.
4.4 Urinary bladder distension
Under inhaled isoflurane (4% induction, 2% maintenance), the bare ends of two Teflon-coated stainless steel electrode wires (0.003″ diameter; Grass Technologies, West Warwick, RI) were acutely implanted into the left and right abdominal musculature using a 26-gauge needle. A 24-gauge angiocatheter was inserted intravesically via the urethra and secured in place with tape. Anesthesia was lowered until hindlimb reflexes, but not escape behaviors, were present (approximately 1% isoflurane). A custom-made distension control device (The University of Iowa Medical Instruments, Iowa City, IA) was used to control the gas flow from a compressed nitrogen tank equipped with a dual-stage low delivery pressure regulator (Matheson-Linweld, Kansas City, MO) and a separate pressure monitor (World Precision Instruments, Sarasota, FL) was used to regulate the pressure within the bladder. Following three 60mmHg distensions to establish stable responses, each pressure (15, 30, 45, 60mmHg) was applied in triplicate for 20 seconds with a 2-minute rest period in between. Electromyographic (EMG) activity was amplified, filtered, and recorded using Spike 2 software (Cambridge Electronic Design, Cambridge, UK) on a personal computer and analyzed off-line. The VMR was quantified by measuring the area under the curve of the entire distension period divided by the duration of the distension and expressed as a percent of baseline EMG activity.
4.5 Colorectal distension
Electrode implantation was performed as previously described (Christianson and Gebhart, 2007). Under inhaled isoflurane (4% induction, 2% maintenance) and aseptic conditions, the bare ends of two Teflon-coated stainless steel electrode wires (0.003″ diameter; Grass Technologies) were surgically implanted into the right abdominal musculature, secured via 5-0 prolene sutures, tunneled subcutaneously to a small incision made in the nape of the neck, and externalized for access during testing. Skin incisions were closed using 5-0 silk suture. Following recovery from anesthesia, mice were housed singly and allowed to recover for a minimum of 4 days before undergoing testing.
To facilitate balloon insertion and maintain proper restraint during testing, mice were briefly sedated with inhaled isoflurane and a custom-made polyethylene plastic balloon (length, 1.5 cm; diameter, 0.8 cm) was inserted into the distal colon, 0.5cm past the anal verge, and secured to the base of the tail with tape. The mouse was then placed into a Broome-style rodent restraint (Kent Scientific, Torrington, CT), the free ends of the electrode wires were attached to a differential amplifier (Model 1700, A-M Systems, Sequim, WA), and the mice were allowed to recover from anesthesia for 30 minutes. The balloon was inflated with air from a compressed nitrogen tank equipped with a dual-stage low delivery pressure regulator (Matheson-Linweld) and a separate pressure monitor (World Precision Instruments) was used to regulate the pressure inside the balloon. Each pressure (15, 30, 45, 60, 75mmHg) was applied in triplicate for 20 seconds with a 4-minute rest period in between. A custom-made distension control device (The University of Iowa Medical Instruments) was used to control the gas flow through the system. The EMG activity was amplified, filtered, and recorded off-line as described for UBD in section 2.5.
4.6 Micturition analysis
Mice were placed on a piece of Bio-rad Model 583 Gel Dryer filter paper (Bio-Rad Laboratories, Hercules, CA) and covered by a clear plexiglass container (36 cm length × 20 cm width × 13 cm height) for 1 h. The number and size of urine spots were measured using Image J following visualization with ultraviolet light. Micturition frequency and total fecal output were determined as the total number of individual urine spots or fecal pellets, respectively. Total urine output was determined by quantifying the total area of urine spots during the testing period.
4.7 Mast cell infiltration and degranulation
Mice were overdosed with inhaled isoflurane (>5%) and transcardially perfused with ice-cold 4% paraformaldehyde. Urinary bladder and distal colon were dissected, post-fixed in 4% paraformaldehyde for 1h at room temperature, cryopreserved in 30% sucrose at 4°C overnight, and then separately mounted in Tissue-Tek OCT mounting media (Sakura Finetek, Torrance, CA) and cut transversely into thin sections (7 μm) using a cryostat. Slides were stained for 10 minutes with a 1% toluidine blue solution acidified with 1M HCl to a pH less than 1.0, dried overnight, washed and coverslipped with 1xPBS for analysis. Using light microscopy (Nikon eclipse 90i, Nikon Instruments, Inc., Melville, NY), digital images were captured (QIClick digital CCD Camera, QImaging, Surrey, BC, Canada) and the total number of non-degranulated mast cells (dense metachromasia with no or faint nuclear outline and/or no granular extrusion around the cell) and degranulated mast cells (less intense metachromasia and obvious clear outline of the nucleus and/or free granules within the cytoplasm), as described in (Florenzano and Bentivoglio, 2000), were counted in at least 8 separate sections spanning the length of each tissue. The percentage of degranulated to total mast cells was calculated according to the following equation for each tissue/mouse: (degranulated mast cells/Total mast cells) x 100.
4.8 mRNA extraction and qRT-PCR
Mice were overdosed with inhaled isoflurane (>5%) and, following decapitation, whole brains were removed and frozen on dry ice. Hypothalamus and hippocampus were dissected, immediately snap frozen in liquid nitrogen, and stored at −80°C. The urinary bladder and distal 1.5cm segment of the colon were also removed and subsequently bisected longitudinally (to facilitate both mRNA and protein [section 4.9] analysis), snap frozen in liquid nitrogen, and stored at −80°C. All tissues were separately homogenized using Trizol reagent (Ambion, Austin, TX) followed by mRNA isolation using RNeasy micro kit (Quiagen, Valencia, CA), as per the manufacturer’s instructions. Sample concentration and purity was determined using a 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA) and cDNA was synthesized from total RNA (0.5μg) using the iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative, real-time PCR amplification was performed using 0.2 μg of total cDNA (from the 0.5 μg reverse-transcribed cDNA) and SsoAdvanced SYBR Green Supermix (Bio-Rad) on a Bio-Rad CFX manager 3.1 real time PCR system with indicated 20μM primers (Integrated DNA Technologies, Coralville, IA) listed in Table 2. Samples were run in triplicate and negative control reactions were run with each amplification series with β-actin (bladder and colon) or GAPDH (brain) as the housekeeping gene. To reduce variability among efficiency due to fluctuations in baseline fluorescence, the raw (i.e. non-baseline corrected) PCR data was imported to the LinRegPCR software (version 2012.3) (Ramakers et al., 2003; Ruijter et al., 2009; Tuomi et al., 2010) and PCR efficiency values were derived for each individual sample by fitting a regression line to a subset of data points within the sample’s log-linear phase. Threshold cycle (Ct) values were subtracted from that of the selected housekeeping gene and the percentage of fold change over naïve controls was calculated using the Pfaffl method (Pfaffl, 2001).
Table 2.
Primers used for real-time PCR analysis
| Gene | Forward (5′ – 3′) | Reverse (3′ – 5′) | Expected amplicon size (bp) |
|---|---|---|---|
| IL6 | CTGCCAGAGACTTCCATCCAGTT | GAAGTAGGGAAGGCCGTGG | 70 |
| IL10 | GCTGGACAACATACTGCTAACC | ATTTCCGATAAGGCTTGGCAA | 78 |
| SCF | CCCTGAAGACTCGGGCTTA | CAATTACAAGCGAAATGAGAGCC | 64 |
| NGF | ACACTCTGATCACTGCGTTTTTG | CCTTCTGGGACATTGCTATCTGT | 69 |
| CRF | CCTCAGCCGGTTCTGATCC | GCGGAGGAAGTATTCTTCACCC | 44 |
| CRF1 | CCCTGCCTTTTTCTACGGTGT | TTCCCGGTAGCCATTGTTTGT | 54 |
| CRF2 | CCTGTGGACACTTTTGGAGCA | TGTTGCAGTAGGTGTAGGGAC | 76 |
| GR | GACTCCAAAGAATCCTTAGCTCC | CTCCACCCCTCAGGGTTTTAT | 108 |
| MR | GAAAGGCGCTGGAGTCAAGT | CCATGTAGCTGTTCTCATTGGT | 85 |
| BDNF | CAGGTTCGAGAGGTCTGACGA | CGCGTCCTTATGGTTTTCTTCG | 115 |
| GAPDH | ATGTGTCCGTCGTGGATCTGA | ATGCCTGCTTCACCACCTTCTT | 164 |
| β-actin | AGTGTGACGTTGACATCCGTA | GCCAGAGCAGTAATCTCCTTCT | 112 |
4.9 Protein analysis
Total protein was isolated using Cell Extraction Buffer containing Halt protease and phosphatase inhibitors (ThermoFisher Scientific, Waltham MA) and Na3VO4. Protein concentrations were determined using a Dc protein assay (ThermoFisher Scientific). Samples were reduced by heating to 95°C for 5 minutes in the presence of 2-mercaptoethanol, subjected to SDS-PAGE (Criterion 4% to 12% Bis-Tris gels; Bio-Rad Laboratories), and transferred to Nitrocellulose transfer membrane (Whatman GmbH, Dassel, Germany) by Criterion Blotter wet transfer (Bio-Rad). The membranes were blocked for 1 hour at room temperature in 5% milk in Tris-buffered saline with Tween-20 then incubated overnight at 4°C with PAR2 (1:1000; Abcam), TRPA1 (1:1000; Aviva) or GAPDH (1:2000; Cell Signaling) antisera. Membranes were washed with Tris-buffered saline with Tween-20 and incubated for 1 hour with anti-rabbit secondary antibody (1:10,000; Cell Signaling). Densitometry was performed using Quantity One 4.6.9 software (Bio-Rad Laboratories).
4.10 Serum corticosterone
Blood was collected from the tail vein during the early half of the light-cycle (0800-1100 hrs), allowed to clot on ice for 1 hour and centrifuged at 10,000 rpm for 10 minutes. Serum (clear supernatant) was collected and stored at −20°C until analysis. Serum corticosterone (CORT) was quantified using ELISA kit according to manufacturer’s instructions (ALPCO, Salem, NH).
4.11 Vaginal lavage and estrous cycle estimation
The perineum was dried from urine and 100 μl of phosphate buffered saline (PBS) was gently expelled into the vaginal canal 3 times with a transfer pipette without penetrating the vaginal orifice. The collected fluid was placed into a single well of a 96-well plate and immediately examined with light microscopy. The stage of estrous cycle was estimated by determining the ratios of the following cell types: nucleated epithelial cells, cornified epithelial cells, and leukocytes. Vaginal lavage was performed only once immediately following physiological or behavioral analysis to avoid inducing additional stress (Caligioni, 2009) or potentially sensitizing the vaginal canal (Yano et al., 2010), which would have compromised the observed behavioral outcomes.
4.12 Statistics
Calculations were performed using Microsoft Excel and statistical analysis was performed using two-way (with or without repeated measures) analysis of variance (ANOVA) and followed by Bonferroni’s multiple comparisons test (GraphPad Prism 6, GraphPad Software, Inc, La Jolla, CA) as denoted in the results/figures. All data are expressed as mean ± SEM. A p value of less than 0.05 was considered significant.
Highlights.
Neonatal maternal separation increases bladder sensitivity in adult female mice.
Water avoidance stress exacerbates early life stress-induced bladder sensitivity.
Hippocampal regulation of the HPA axis is disrupted by early life and adult stress.
Early life and adult stress increase mast cell degranulation in the bladder.
The colorectum is largely unaffected by early life and adult stress in mice.
Acknowledgments
This work was supported by NIH grants R01 DK099611 (JAC), R01 DK103872 (JAC), Center of Biomedical Research Excellence (COBRE) grant P20 GM104936 (JAC), start-up funds and core support from the Kansas Institutional Development Award (IDeA) P20 GM103418, core support from the Kansas IDDRC P30 HD002528, and The Madison and Lila Self Fellowship Program (ANP). We would like to thank Natalie Walker and Isabella Fuentes for technical assistance.
Abbreviations
- NMS
Neonatal maternal separation
- WAS
water avoidance stress
- PAR2
protease activated receptor 2
- TRPA1
transient receptor potential ankyrin 1
- IC/PBS
interstitial cystitis/painful bladder syndrome
- CRF
corticotropin releasing factor
- HPA
hypothalamic-pituitary-adrenal
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- NGF
nerve growth factor
- SP
substance P
- TRPV1
transient receptor potential vanilloid 1
- VMR
visceromotor response
- UBD
urinary bladder distension
- CRD
colorectal distension
- CORT
corticosterone
- BDNF
brain-derived neurotrophic factor
- IL6
interleukin-6
- IL10
interleukin-10
- SCF
stem cell factor
- LTP
long term potentiation
- EMG
electromyographic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–85. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–9. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–6. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. The Journal of Physiology. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001;306:185–8. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. Journal of Urology. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beunk L, Verwoerd A, van Overveld FJ, Rijkers GT. Role of mast cells in mucosal diseases: current concepts and strategies for treatment. Expert Rev Clin Immunol. 2013;9:53–63. doi: 10.1586/eci.12.82. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–60. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–53. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Boucher W, El-Mansoury M, Pang X, Sant GR, Theoharides TC. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. British Journal of Urology. 1995;76:94–100. doi: 10.1111/j.1464-410x.1995.tb07840.x. [DOI] [PubMed] [Google Scholar]
- Boucher W, Kempuraj D, Michaelian M, Theoharides TC. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010;106:1394–9. doi: 10.1111/j.1464-410X.2010.09237.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095 e3. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckinx R, Adriaensen D, Nassauw LV, Timmermans JP. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci. 2011;5:54. doi: 10.3389/fnins.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235–50. doi: 10.1113/jphysiol.2014.279968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Caplan RD, Cobb S, French JR., Jr White collar work load and cortisol: disruption of a circadian rhythm by job stress? J Psychosom Res. 1979;23:181–92. doi: 10.1016/0022-3999(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Carrico DJ, Sherer KL, Peters KM. The relationship of interstitial cystitis/painful bladder syndrome to vulvodynia. Urol Nurs. 2009;29:233–8. [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–31. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–15. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–47. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–59. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297:F1101–8. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–51. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–9. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas TJ, Rode J. Characteristics of mast cells in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol. 1991;68:473–8. doi: 10.1111/j.1464-410x.1991.tb15388.x. [DOI] [PubMed] [Google Scholar]
- Corcoran AT, Yoshimura N, Tyagi V, Jacobs B, Leng W, Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31:241–6. doi: 10.1007/s00345-012-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Datson NA, Speksnijder N, Mayer JL, Steenbergen PJ, Korobko O, Goeman J, de Kloet ER, Joels M, Lucassen PJ. The transcriptional response to chronic stress and glucocorticoid receptor blockade in the hippocampal dentate gyrus. Hippocampus. 2012;22:359–71. doi: 10.1002/hipo.20905. [DOI] [PubMed] [Google Scholar]
- de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, D’Andrea MR, Mayer EA, Wallace JL, Hollenberg MD, Andrade-Gordon P, Bunnett NW. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001;133:975–87. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerry JJ. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain (Amsterdam) 2014 doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–69. [PubMed] [Google Scholar]
- Fuentes IM, Pierce AN, O’Neil PT, Christianson JA. Assessment of Perigenital Sensitivity and Prostatic Mast Cell Activation in a Mouse Model of Neonatal Maternal Separation. J Vis Exp. 2015 doi: 10.3791/53181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep EN, Boersma JW, Lentjes EG, Prins AP, van der Korst JK, de Kloet ER. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. J Rheumatol. 1998;25:1374–81. [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med. 1998;60:309–18. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, Theoharides TC. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J Mol Endocrinol. 2009;42:397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Smaldone MC, Tyagi V, Philips BJ, Jackman SV, Leng WW, Tyagi P. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. Can J Urol. 2010;17:4989–94. [PubMed] [Google Scholar]
- Jiang YH, Peng CH, Liu HT, Kuo HC. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One. 2013;8:e76779. doi: 10.1371/journal.pone.0076779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–6. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–9. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup J, Hald T, Larsen S, Nielsen VG. Histamine content and mast cell count of detrusor muscle in patients with interstitial cystitis and other types of chronic cystitis. Br J Urol. 1983;55:495–500. doi: 10.1111/j.1464-410x.1983.tb03356.x. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–8. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- Kim YS, Lee MY, Ryu HS, Choi ES, Oh JT, Yun KJ, Choi SC. Regional Differences in Chronic Stress-induced Alterations in Mast Cell and Protease-activated Receptor-2-positive Cell Numbers in the Colon of Ws/Ws Rats. J Neurogastroenterol Motil. 2014;20:54–63. doi: 10.5056/jnm.2014.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Goltstein PM, van der Linden S, Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci. 2006;23:3051–5. doi: 10.1111/j.1460-9568.2006.04842.x. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three Types of Neurochemical Projection from the Bed Nucleus of the Stria Terminalis to the Ventral Tegmental Area in Adult Mice. The Journal of Neuroscience. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- Larauche M, Bradesi S, Million M, McLean P, Tache Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1033–40. doi: 10.1152/ajpgi.00507.2007. [DOI] [PubMed] [Google Scholar]
- Larsen MS, Mortensen S, Nordling J, Horn T. Quantifying mast cells in bladder pain syndrome by immunohistochemical analysis. BJU Int. 2008;102:204–7. doi: 10.1111/j.1464-410X.2008.07576.x. discussion 207. [DOI] [PubMed] [Google Scholar]
- Link CL, Pulliam SJ, Hanno PM, Hall SA, Eggers PW, Kusek JW, McKinlay JB. Prevalence and psychosocial correlates of symptoms suggestive of painful bladder syndrome: Results from the Boston Area Community Health Survey. Journal of Urology. 2008;180:599–606. doi: 10.1016/j.juro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Villiger P, Hugli T, Koziol J, Zuraw BL. Interleukin-6 and interstitial cystitis. J Urol. 1994;152:869–73. doi: 10.1016/s0022-5347(17)32594-6. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol. 2000;164:1265–9. [PubMed] [Google Scholar]
- Maniam J, Antoniadis C, Morris MJ. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front Endocrinol (Lausanne) 2014;5:73. doi: 10.3389/fendo.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441–7. doi: 10.1007/s11934-009-0070-3. [DOI] [PubMed] [Google Scholar]
- McGonagle E, Smith A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic SA. Water avoidance stress results in an altered voiding phenotype in male mice. Neurourol Urodyn. 2012;31:1185–9. doi: 10.1002/nau.22207. [DOI] [PubMed] [Google Scholar]
- Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol. 2013;305:R147–56. doi: 10.1152/ajpregu.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, O’Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early-life stress induces visceral hypersensitivity in mice. Neurosci Lett. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2007;132(Suppl 1):S96–103. doi: 10.1016/j.pain.2007.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizeki H, Alard P, Streilein JW. Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol. 1997;159:5183–6. [PubMed] [Google Scholar]
- Novembri R, Torricelli M, Bloise E, Conti N, Galeazzi LR, Severi FM, Petraglia F. Effects of urocortin 2 and urocortin 3 on IL-10 and TNF-alpha expression and secretion from human trophoblast explants. Placenta. 2011;32:969–74. doi: 10.1016/j.placenta.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol. 2015;50:819–30. doi: 10.1007/s00535-015-1086-8. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Dinan TG, Cryan JF. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides. 2010;31:662–70. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2011;214:221–9. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- Offiah I, McMahon SB, O’Reilly BA. Interstitial cystitis/bladder pain syndrome: diagnosis and management. Int Urogynecol J. 2013;24:1243–56. doi: 10.1007/s00192-013-2057-3. [DOI] [PubMed] [Google Scholar]
- Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744–50. doi: 10.1111/j.1464-410x.1995.tb07384.x. [DOI] [PubMed] [Google Scholar]
- Peeker R, Enerback L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol. 2000;163:1009–15. [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258–62. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Petrelluzzi KF, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11:390–7. doi: 10.1080/10253890701840610. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AN, Ryals JM, Wang R, Christianson JA. Vaginal hypersensitivity and hypothalamic-pituitary-adrenal axis dysfunction as a result of neonatal maternal separation in female mice. Neuroscience. 2014;263:216–30. doi: 10.1016/j.neuroscience.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–6. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–79. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Rho SG, Kim YS, Choi SC, Lee MY. Sweet food improves chronic stress-induced irritable bowel syndrome-like symptoms in rats. World J Gastroenterol. 2014;20:2365–73. doi: 10.3748/wjg.v20.i9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. J Pain. 2008;9:991–8. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Tache Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G704–12. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Seth A, Teichman JM. Differences in the clinical presentation of interstitial cystitis/painful bladder syndrome in patients with or without sexual abuse history. J Urol. 2008;180:2029–33. doi: 10.1016/j.juro.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–98. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Golovatscka V, Pothoulakis C, Bradesi S, Mayer EA, Rodríguez LV. The Effects of Acute and Chronic Psychological Stress on Bladder Function in a Rodent Model. Urology. 2011;78:967.e1–967.e7. doi: 10.1016/j.urology.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos C, Pang X, Ligris K, Letourneau R, Alferes L, Alexacos N, Sant GR, Theoharides TC. Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol. 1997;157:669–72. [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Suda K, Setoyama H, Nanno M, Matsumoto S, Kawai M. Involvement of parasympathetic pelvic efferent pathway in psychological stress-induced defecation. World J Gastroenterol. 2013;19:1200–9. doi: 10.3748/wjg.v19.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol. 1995;153:629–36. doi: 10.1097/00005392-199503000-00021. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA. Childhood maltreatment and migraine (part III). Association with comorbid pain conditions. Headache. 2010;50:42–51. doi: 10.1111/j.1526-4610.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- Tuomi JM, Voorbraak F, Jones DL, Ruijter JM. Bias in the Cq value observed with hydrolysis probe based quantitative PCR can be corrected with the estimated PCR efficiency value. Methods. 2010;50:313–22. doi: 10.1016/j.ymeth.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der Coelen D, Bulmer DC, Peeters PJ, Aerssens J, de Hoogt R, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2009;21:1107–e94. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1671–8. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang R, Zhou C, Xu Y, Guan X, Hu J, Xu Y, Li S. Enhanced expression of vascular cell adhesion molecule-1 by corticotrophin-releasing hormone contributes to progression of atherosclerosis in LDL receptor-deficient mice. Atherosclerosis. 2009;203:360–70. doi: 10.1016/j.atherosclerosis.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Yang CQ, Wei YY, Zhong CJ, Duan LP. Essential role of mast cells in the visceral hyperalgesia induced by T. spiralis infection and stress in rats. Mediators Inflamm. 2012;2012:294070. doi: 10.1155/2012/294070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–86. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Lilly E, Barousse M, Fidel PL., Jr Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–37. doi: 10.1128/IAI.00388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SK, Laub DJ, Weese DL, Lad PM, Leach GE, Zimmern PE. Stimulated release of urine histamine in interstitial cystitis. J Urol. 1992;148:1145–8. doi: 10.1016/s0022-5347(17)36844-1. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Li Z, Chung EK, Zhang HQ, Xu HX, Sung JJ, Bian ZX. Activation of extracellular signal-regulated protein kinase is associated with colorectal distension-induced spinal and supraspinal neuronal response and neonatal maternal separation-induced visceral hyperalgesia in rats. J Mol Neurosci. 2009;37:274–87. doi: 10.1007/s12031-008-9134-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gandhi PR, Standifer KM. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol Pain. 2012;8:76. doi: 10.1186/1744-8069-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]