Data from a number of studies have strongly implicated the dopamine (DA) system in the pathophysiology of schizophrenia. This is particularly robust for the positive or the psychotic symptoms of schizophrenia, which can be mimicked by DA agonists and attenuated by D2 antagonist antipsychotic drugs. Nonetheless, there is little evidence for a major dysfunction within the dopamine system itself; instead, current research has focused on a disruption in the regulation of the DA system. One region in particular that has shown correlations with DA dysfunction is the limbic portion of the hippocampus, which comprises the ventralmost segment in rats analogous to the anterior aspect in humans. Thus, studies in schizophrenia patients have shown hyperactivity in the hippocampus that correlates with psychosis, as well as a loss of parvalbumin GABAergic inhibitory neurons (1). In order to examine the pathophysiology of schizophrenia, we have employed a developmental disruption model that uses the mitotoxin methylazoxymethanol acetate (MAM). This drug is administered to pregnant rats at gestational day 17 to mimic the second trimester in humans, during which insults have a higher impact on inducing schizophrenia births. The rats are then examined peripubertally for developmental changes, and as adults to test for dysfunctions that correspond to schizophrenia in humans.
The adult offspring of MAM-treated pregnant rats display a number of characteristics consistent with schizophrenia (2,3), including neuroanatomical (thinning of limbic cortices with an increase in cell packing density, loss of parvalbumin interneurons), behavioral deficits (prepulse inhibition of startle, reversal learning, extradimensional shift, latent inhibition, social interaction), and pharmacological responses (hyper-responsivity to PCP, increased locomotion to amphetamine). Furthermore, as in humans, there is hyperactivity in the ventral hippocampus (vHipp) and a disruption of rhythmic activity including delta and gamma rhythms (3). There was also a substantial increase in DA neuron population activity.
Dopamine neuron regulation and its impact on information processing
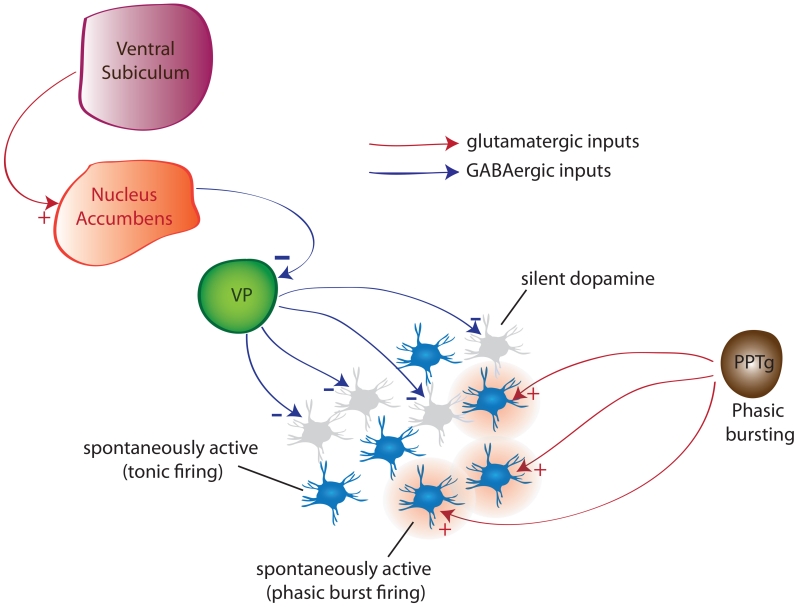
DA neurons in both anesthetized and awake rats exhibit several activity states that are regulated by different systems and differentially impact system function (Fig. 1). In the basal state, DA neurons discharge in a slow, irregular tonic firing pattern. However, if the organism is exposed to a behaviorally salient stimulus, DA neurons transition to a rapid burst-firing mode. Thus, burst firing is considered to be the behaviorally relevant phasic response to stimuli. Burst firing is driven by a glutamatergic input arising primarily from the brainstem pedunculopontine tegmentum (PPTg) acting on NMDA receptors(1). However, for glutamate to activate NMDA receptors, the neurons must be in a depolarized, spontaneously firing state; otherwise there is a magnesium block of the NMDA channel. Therefore, only neurons that are firing are capable of transitioning to burst firing. The number of DA neurons firing (i.e., population activity) is controlled by a potent GABAergic input from the ventral pallidum (VP) that holds a subset of neurons in a nonfiring state. Therefore the PPTg drives the phasic burst firing, but the VP controls the number of DA neurons that can be driven to burst firing, or in essence, the level of amplification of the phasic signal. The VP itself is potently modulated by a circuit originating in the vHipp to the ventral striatum. When the vHipp is activated, it drives the ventral striatum, inhibiting the VP and releasing the DA neurons from inhibition to increase population activity. The vHipp itself has been associated with context-dependency, or the ability to adjust behavioral responses depending on the context or setting. Therefore, the gain of the DA system is dependent on the behavioral context, with the more DA neurons firing, the greater the behavioral activation (1). In a benign context, the vHipp drives a low DA population activity; therefore, when a behaviorally salient stimulus activates the PPTg, only a small number of DA neurons transition to burst firing and the phasic signal is low in amplitude. However, when in a highly threatening context, the vHipp drives large increases in DA population activity such that the same stimulus causing the same PPTg activation now causes a massive DA phasic response in order to effectively deal with the potentially threatening event.
Figure 1.
Dopamine neurons exist in distinct states of activity; baseline tonic population activity (i.e., proportion firing spontaneously), and rapid, salience-driven phasic burst firing In the normal rat, approximately half are firing spontaneously, with the other half in an inhibited, non-firing state. This inhibition is maintained by a potent GABAergic inhibitory input from the ventral pallidum. This state is regulated by an input from the hippocampus ventral subiculum. Activation of the subiculum excites the nucleus accumbens, which then inhibits the ventral pallidum to release dopamine neurons from inhibition. The number of dopamine neurons active is an important variable, in that it sets the amplitude of the rapid, burst firing-mediated phasic dopamine response driven by the pedunculopontine tegmentum. Since only spontaneously firing dopamine neurons can be driven to burst fire, changing the number of neurons active will affect the amplitude of the phasic dopamine response.
In the MAM rat, there is a near doubling in the number of DA neurons spontaneously firing compared to control, and this occurs in the lateral VTA that projects to the associative striatum (4). This can be reversed by inactivating the vHipp. Moreover, vHipp inactivation also normalizes the increased locomotor response to amphetamine in MAM rats. The increase in DA neuron population activity is consistent with the increased fluorodopa uptake observed in schizophrenia patients (5); increased flurodopa uptake indicates more DA terminals active, and is therefore consistent with more DA neurons firing. Therefore in the MAM rat or the schizophrenia patient, any stimulus whether it’s salient or not will cause a massive DA system response, causing the individual to be unable to selectively filter between threatening or neutral stimuli. This increased DA system responsivity is also consistent with studies showing increased amphetamine-induced DA release in schizophrenia patients that correlates with worsening of psychosis (6).
Hippocampal hyperactivity and therapeutic approaches
Why is the vHipp hyperactive in schizophrenia and in MAM rats? A robust finding in schizophrenia brains is a loss of parvalbumin-containing inhibitory GABAergic neurons in both the prefrontal cortex and hippocampus, which correlates in both subjects with a loss of parvalbumin-dependent evoked gamma rhythmic activity. This loss of parvalbumin occurs early in development, with neuronal loss in the hippocampus emerging in the young adult (7). Therefore, we propose that the loss of parvalbumin inhibition in the vHipp drives the increased vHipp activity, which in turn increases DA neuron responsivity, leading to psychosis (Fig. 2). Current treatments for schizophrenia depend on reversing the overstimulation of DA receptors in the striatum using DA antagonists. Indeed, our studies show that DA receptor blockade by antipsychotic drugs leads to an overdrive of the DA system in MAM rats, thereby decreasing DA neuron population activity via depolarization block-induced inactivation of firing. Given that depolarization block depends on a baseline hyperactive state, this could account for why antipsychotic drugs show the most rapid onset in the most psychotic patients, presumably due to the higher baseline population activity. However, this is not restoring the system to normal; it is instead acting at a site that we believe is at least 5 synapses downstream from the deficit in the hippocampus. A more effective approach would be to restore inhibition in the hippocampus. For this, we used a GABA-A alpha 5 positive allosteric modulator, since the alpha 5 subunit is selectively concentrated in the hippocampus. We found that this drug selectively restored normal hippocampal firing, normal DA neuron population activity, and normalized the behavioral response to amphetamine in the MAM rat(8). One issue however is that other drugs that should work on this circuit have been tested clinically, and despite encouraging preclinical results have failed to show efficacy. However, there is an important difference between the preclinical and clinical trials; in the clinic, the patients have already been exposed to many years of antipsychotic treatment, and are withdrawn for only a week before testing the novel compound. We found that pretreating the MAM rats with haloperidol for only 3 weeks and withdrawing them for a week completely prevented the actions of our novel compound (9). Therefore, we propose that pre-exposure to an antipsychotic drug changes the DA system from a hippocampal-overdriven DA system to a postsynaptic supersensitive DA system, such that upon withdrawal only another DA antagonist would be effective. This demonstrates the need to select patient populations carefully when evaluating drugs with novel actions.
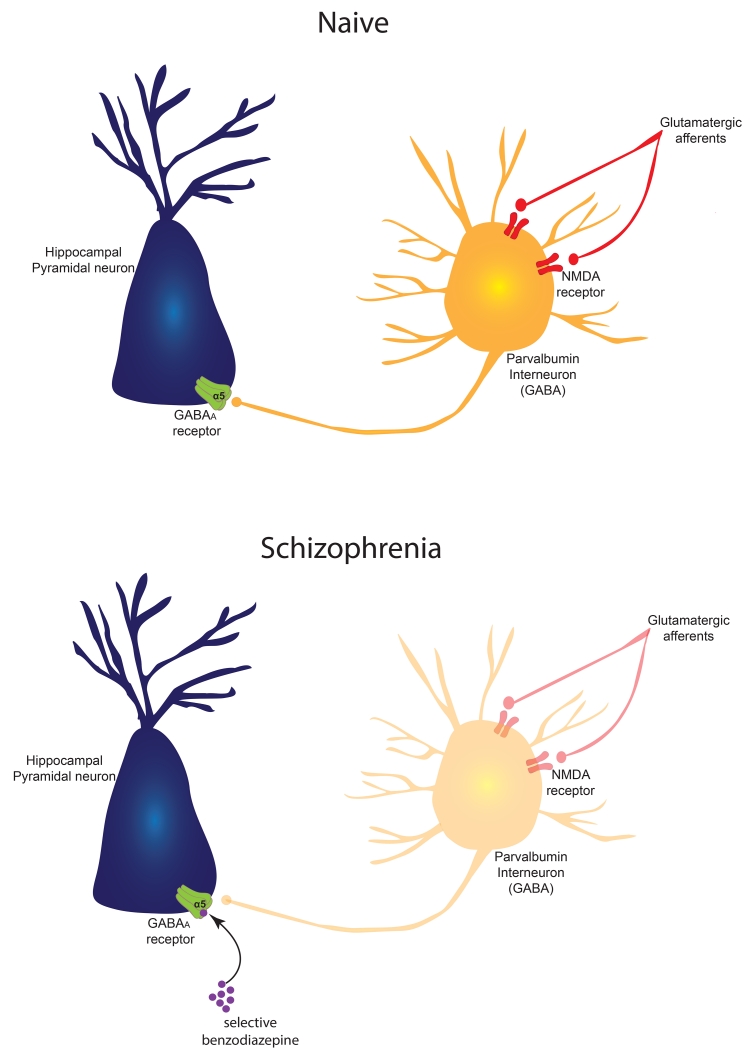
Figure 2.
(top) In the naïve, untreated animal, hippocampal pyramidal neuron activity is potently modulated by an interneuron network. One neuron type that has a significant effect on modulating pyramidal neuron activity is the parvalbumin-containing GABAergic interneuron. This neuron is driven by glutamatergic inputs acting on NMDA receptors, and inhibits the pyramidal neuron via activation of a GABA A receptor subtype, containing the alpha-5 subunit which is selectively concentrated in the hippocampus. If the glutamatergic drive of the parvalbumin interneuron is disrupted, e.g., by the NMDA channel blocker PCP, the resultant diminished drive of the parvalbumin interneuron would lead to hippocampal neuron overactivity and disruption of gamma rhythms.
(bottom) In the MAM rat or the schizophrenia patient, there is a selective loss of parvalbumin interneurons that is most substantial in the ventral subiculum/anterior hippocampus, respectively. As a result, the pyramidal output neurons are hyperactive and dysrhythmic. This state can be normalized by a GABA potentiating drug, such as a benzodiazepine, which binds to the alpha subunit to increase the affinity of the receptor for GABA, thereby compensating for the loss of parvalbumin GABA neurons. This could be made selective for the hippocampus by a benzodiazepine-like drug that is selective for the GABA A alpha 5 subunit.
Prevention of schizophrenia
While these studies show a potentially more effective therapeutic target, a better approach would be to prevent the transition to schizophrenia in susceptible individuals. A major risk factor in schizophrenia is early life stress. Indeed, studies have shown that not only will early life stress predispose one to schizophrenia, but that children at risk for schizophrenia that show higher stress responsivity are more likely to transition to schizophrenia. We found that MAM rats examined peripubertally prior to parvalbumin neuron loss show higher anxiety and stress responsivity compared to controls. Therefore, we tested whether attenuating stress at this critical period would impact the adult MAM rat. We found that anxiolytic doses of diazepam administered peripubertally for 10 days effectively prevented both the increase in DA population activity and increased behavioral response to amphetamine in the adult without impacting controls (10). Therefore, if we could identify susceptible individuals based on stress responsivity early in life and treat the stress, we may prevent the transition to psychosis. Importantly, this also shows that MAM treatment does not “cause schizophrenia,” but instead makes the animals more susceptible to the deleterious effects of stress. If the same thing is happening in humans with respect to genetic risk factors, this suggests that the genes also do not “cause” schizophrenia the way that huntington genes cause Huntington’s disease. Instead, the genetic predisposition may cause the individual to be more susceptible to environmental factors such as stress.
Acknowledgements
This work was supported by USPHS MH57440, MH19118 and MH104320. I have received support/honoraria from: Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen
References
- 1.Grace AA. Dopamine system dysregulation by the hippocampus: Implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. Journal of Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodge DJ, Grace AA. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–8. doi: 10.1017/S1461145711000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, Valli I, Day F, McGuire PK. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophrenia bulletin. 2012;38:1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 7.Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:1609–1619. doi: 10.1017/S146114571400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABAAR positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophrenia bulletin. 2014;40:341–350. doi: 10.1093/schbul/sbt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1881–1888. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]