Abstract
Objective
Cervical cancer is the most common cancer among women in Malawi. National guidelines recommend screening women aged 30–45 years every five years; however, no specific recommendations exist for women with HIV. We aimed to assess the frequency of high-grade dysplasia (CIN 2 or CIN3) and cervical cancer among women in central Malawi and to examine associations with CIN2+ (CIN2/3 or cancer).
Methods
We extracted cervical pap smear, biopsy, loop electrosurgical excision procedure and uterine specimen reports from a hospital pathology database from November 2012 to November 2013. We used logistic regression to estimate associations with CIN2+.
Results
We reviewed specimens from 824 women; we excluded 194 with unknown HIV status, leaving 630 in the analytic sample. Twelve percent had high-grade dysplasia and 109 women (17%) had cancer. Twenty-five percent of high-grade dysplasia cases and 35% of cancers occurred among women outside recommended screening ages. The odds of having CIN2+ were 6.55 times (95% CI 4.44–9.67) greater for HIV+ women.
Conclusions
High-grade dysplasia and cervical cancer are very common among Malawian women, especially HIV+ women. HIV infection was strongly associated with CIN2+. Expanding screening to women not covered by current guidelines could avert a substantial proportion of cervical cancer cases in Malawi.
Keywords: Cervical cancer, high-grade dysplasia, HIV, Malawi, sub-Saharan Africa
Introduction
Cervical cancer is the second most common cancer and the leading cause of cancer death among women in Africa.1 Although incidence varies, many countries in sub-Saharan Africa have rates of cervical cancer which are among the highest in the world.2 In the Malawi national cancer registry, cervical cancer accounts for over 45% of female cancer cases.3 More than 70% of Malawian women with cervical cancer are diagnosed with inoperable, advanced disease.4,5 In addition to high incidence of cervical cancer, Malawian women have one of the highest rates of cervical cancer mortality.6,7
Cervical cancer can be prevented by screening for and treating precancerous lesions. Cervical intraepithelial neoplasia (CIN) is a precancerous lesion that can progress to cervical cancer if left untreated. Progression to invasive cancer may require many years after the initial oncogenic human papillomavirus (HPV) infection, with low-grade cervical dysplasia (CIN1) progressing slowly and high-grade dysplasia (CIN2 or CIN3) progressing more rapidly to invasive cancer.8
Malawi implemented a visual inspection with acetic acid (VIA) screening campaign in 2004, to detect treatable precancerous lesions. Current reproductive health guidelines recommend screening women aged 30–45 years who are not pregnant, have no personal history of cervical cancer, have not undergone a total hysterectomy or have a sexually transmitted infection with abnormal discharge.9 However, a 2011 report from the Ministry of Health indicated that 12% of women with cervical cancer were between 20 and 29 years old, and 32% were older than 50 years, suggesting that women outside the current VIA guidelines were interested in and might also benefit from screening.10
Additionally, Malawi has no specific recommendations for cervical cancer screening for women with HIV, even though HIV-infected women are at increased risk. Data from various settings demonstrate that HIV increases the risk of cervical cancer by 2–22 fold.11 This may reflect women with HIV having higher prevalence and persistence of co-infection with oncogenic HPV and also having diverse high-risk HPV types as previous studies have shown.12–15 HIV infection may also allow CIN to progress more rapidly to invasive cancer.16,17 As a result, specifically targeting HIV-positive women who are at high risk may be crucial for national cervical cancer prevention efforts in Malawi.
Approximately 11% of Malawi’s population is HIV-positive, and women account for 59% of HIV-infected individuals in the country.18 Understanding associations between HIV and cervical cancer in Malawi is therefore important for cancer control efforts as well as comprehensive HIV care. Additionally, whether current VIA screening guidelines are adequate to avert most cervical cancer cases in Malawi is unknown. We therefore conducted a retrospective analysis of data from the Kamuzu Central Hospital (KCH) pathology laboratory database comparing pathologic diagnoses between women with and without HIV to assess associations between HIV status and high-grade cervical dysplasia and cancer.
Methods
Study population
KCH is one of the two national teaching hospitals in Malawi; KCH serves the entire central region with an estimated catchment area of five million people. The KCH pathology laboratory was initiated in July 2011 and has been previously described.19 Ongoing quality control procedures include weekly telepathology conferences with US collaborators using a virtual microscopy system.20 The laboratory provides services to all KCH departments and also processes specimens from other health facilities throughout the central region of Malawi. Specimens from seven health facilities were included in the study.
In Malawi, VIA screening is performed at district level health centres as well as public and private clinics. Women with positive VIA results and lesions amenable to cryotherapy may receive cryotherapy the same day if such services are available at that facility. Otherwise, women with positive results are referred to another facility for cryotherapy or further evaluation in the weekly colposcopy clinic conducted by consultant gynaecologists at KCH. Because our study relied on pathology laboratory data, the study population represents women who were likely VIA-positive, had a lesion not amenable to cryotherapy, or were clinically suspected to have cervical cancer, and were therefore referred for cervical biopsy. Cervical cytology was done for women whose repeat VIA was negative or colposcopy was normal at KCH colposcopy clinic. During the same appointment at the colposcopy clinic or after histological confirmation, abnormalities were treated with loop electrosurgical excision procedure (LEEP), cone biopsy or hysterectomy as needed.
Data
This cross-sectional analysis used electronic pathology reports from 1037 specimens from women undergoing cervical cytology, cervical biopsy, LEEP or hysterectomy for diagnostic evaluation or treatment of suspicious cervical lesions. We included all cervical, endometrial and uterine specimens collected from November 2012 to November 2013. The institutional pathology database was created for clinical purposes and does not include extensive data on patient demographics or risk factors for cervical cancer. Histology and cytology data as well as patient age and HIV status were extracted from pathology laboratory requisition forms.
Conventional cytology and colposcopy-directed biopsy services often resulted in multiple samples being submitted for the same women during diagnosis, treatment and follow-up. We conducted an individual patient-level analysis and only included one report per woman during the study period. For women with multiple specimens, we included the report with the most advanced diagnosis in the main analysis and also conducted sensitivity analysis using the largest specimen received (i.e. pathology results based on a larger specimen may be more accurate than a cytology smear).
Permission to conduct this research was approved under the parent study approval covering the KCH cancer registry and pathology databases. The need for informed consent was exempted from the University of North Carolina-Chapel Hill institutional review board due to the nature of the study, which involved secondary analysis of routinely collected data.
Outcome variables
The diagnoses of CIN and cervical cancer were determined by a KCH pathologist. Histological and cytological results were categorised as no dysplasia/normal, CIN1, CIN2, CIN3 and invasive cancer. We defined high-grade dysplasia as CIN2 or CIN3 (CIN2/3). The main outcome in the logistic regression model was confirmed CIN2+, which included CIN2/3 and invasive cervical cancer.
Statistical approach
Patient and specimen characteristics were summarised descriptively to determine differences in proportions by HIV status. We compared the statistical significance of categorical variables using Chi-squared tests and t tests for age as a continuous variable. We estimated logistic regression models to assess the associations of HIV and age with CIN2+. We conducted two sensitivity analyses: (1) estimating models which included patients with unknown HIV status and (2) estimating results using the largest specimen for women with multiple laboratory reports. All statistical analyses were performed in STATA 12 using a two-sided alpha value of 0.05 to assess statistical significance.
Results
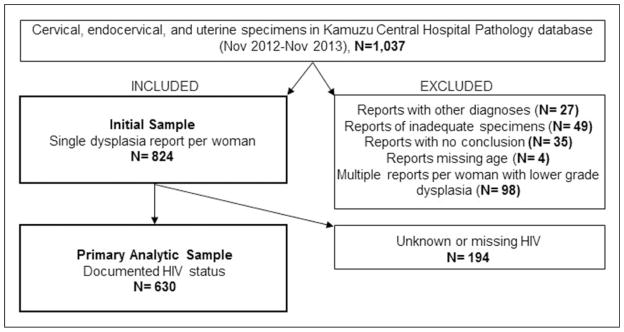
We extracted 1037 pathology reports from the KCH pathology database from patients undergoing pap test, cervical biopsy, LEEP or hysterectomy. We excluded reports with other diagnoses (e.g. leiomyoma and choriocarcinoma), inadequate specimens, conclusions without comments on dysplasia and those missing age. Details of the exclusion criteria are displayed in Figure 1.
Figure 1.
Inclusion and exclusion criteria applied to identify the analytic sample.
Table 1 describes the results of 824 unique women after including only one report per woman. The mean age of women was 39.6 years (SD =12.0), and 20% of the sample was younger than the recommended starting age for screening (30 years). Of all women, 425 (52%) were within the recommended age range for VIA screening (30–45 years). Histology specimens accounted for 62% of all samples, and 86% of specimens were from within KCH departments, such as the colposcopy clinic. HIV status was missing for 194 (23%) of reports.
Table 1.
Characteristics of cervical specimens from KCH pathology database.
| HIV-negative, 404 (49.0%) | HIV-positive, 226 (27.4%) | Unknown, 194 (23.5%) | Total, 824 | p-value | |
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| <30 | 115 (28.5) | 36 (15.9) | 17 (8.8) | 168 (20.4) | |
| 30–34 | 61 (15.1) | 56 (24.8) | 29 (15.0) | 146 (17.7) | |
| 35–39 | 58 (14.3) | 60 (26.5) | 30 (15.5) | 148 (18.0) | |
| 40–44 | 40 (9.9) | 38 (16.8) | 25 (12.9) | 103 (12.5) | |
| 45–49 | 45 (11.1) | 22 (9.7) | 25 (12.9) | 92 (11.2) | |
| ≥50 | 85 (21.0) | 14 (6.2) | 68 (35.0) | 167 (20.3) | |
| Specimen type | |||||
| Pap test | 217 (53.7) | 57 (25.2) | 39 (20.1) | 313 (38.0) | <0.001 |
| Biopsy | 155 (38.4) | 117 (51.8) | 116 (59.8) | 388 (47.1) | <0.001 |
| LEEP | 16 (4.0) | 28 (12.4) | 9 (4.6) | 53 (6.4) | <0.001 |
| Uterus | 20 (4.9) | 26 (11.5) | 34 (17.5) | 80 (9.7) | <0.001 |
| Health facility | 0.005 | ||||
| KCH | 332 (82.2) | 207 (91.6) | 168 (86.6) | 707 (85.8) | |
| Other facility | 72 (17.8) | 19 (8.4) | 26 (13.4) | 117 (14.2) | |
| Dysplasia outcome | |||||
| No dysplasia | 319 (79.0) | 84 (37.2) | 113 (58.2) | 516 (62.6) | <0.001 |
| CIN1 | 18 (4.4) | 22 (9.7) | 7 (3.6) | 47 (5.7) | 0.008 |
| CIN2/3 | 23 (5.7) | 55 (24.3) | 9 (4.6) | 87 (10.5) | <0.001 |
| Cancer | 44 (10.9) | 65 (28.8) | 65 (33.1) | 174 (21.1) | <0.001 |
KCH: Kamuzu Central Hospital; LEEP: loop electrosurgical excision procedure; CIN: cervical intraepithelial neoplasia.
For the main analysis, we excluded 194 women with no documented HIV status leaving 630 women for the primary analytic sample. HIV prevalence among women with documented status was 36% (226/630). Among women with documented HIV status, 64% (403/630) had normal findings or no dysplasia, 6% (40) had CIN1, 12% (78) had CIN2/3 and 17% (109) had invasive cervical cancer. HIV-negative women more frequently had normal findings or no dysplasia than HIV-positive women (79% vs. 37% respectively, p <0.001). Twenty-four per cent (55/226) of the HIV-positive women had CIN2/3 compared to 6% (23) of HIV-negative women (p <0.001). Similarly, HIV-positive women were more likely to be diagnosed with cervical cancer (29% vs. 11%, p <0.001). HIV-positive women with cancer were significantly younger than HIV-negative women with cancer (38.4 vs. 49.1 years, p <0.001).
Figure 2 displays the frequency of cervical abnormalities and seroprevalence by age group. The majority of women with cancer were 35–39 years old, whereas the majority of women with CIN1 were younger than 30 years old. Although HIV prevalence was lower in older age groups, CIN2/3 and cervical cancer were still common. Thirty-five per cent (38/109) of women diagnosed with cancer were not within the recommended screening age group (30–45 years). Thirty per cent of cancers were among women over 45 years old, and 5% of cervical cancers were diagnosed among women younger than 30 years old. High-grade dysplasia was also commonly diagnosed among women outside of the targeted screening ages; 14% of women with CIN2/3 were younger and 11% older than the recommended screening age range.
Figure 2.
Frequency of cervical abnormalities among women with documented HIV status (n =630).
The results of logistic regression modeling on the outcome of CIN2+ are displayed in Table 2. HIV significantly increased the likelihood of having CIN2+ (adjusted odds ratio [OR] 6.55, 95%CI 4.43–9.67), after controlling for age. Similarly when controlling for HIV status, each additional year of age increased the odds of having CIN2+ by 4%. Adjusted ORs for HIV infection and age were not different in sensitivity analyses which included women with positive, negative and unknown HIV status (adjusted OR for HIV infection 6.41, 95%CI 4.37–9.40 and adjusted OR for age 1.04 95%CI 1.02–1.05).
Table 2.
Estimation results from logistic regression modela.
Includes only women with documented HIV status.
CIN2/3 or cervical cancer
CIN: cervical intraepithelial neoplasia; OR: odds ratio; CI: confidence interval.
p <0.001.
For additional sensitivity analyses, we analysed the largest sample instead of the highest grade conclusion among women with multiple specimens. Overall, 21 outcomes changed, such that there were fewer cases of CIN2/3 (79/824) and fewer cervical cancer cases (166/824). Over half of the women with different outcomes were HIV-positive (13/21) and four were indeterminate because they were undocumented and recorded as positive on a follow-up sample. Upon further examination of these women with different outcomes, eight uterine specimens were diagnosed with lower grade dysplasia than a previous biopsy, but were determined to be from cutting poor tissue sections. Nonetheless, using the largest specimen, we estimated similar associations with CIN2+ (adjusted OR for HIV infection 6.16, 95%CI 4.14–9.14, adjusted OR for age 1.04, 95%CI 1.02–1.06).
Discussion
In this study of a pathology database at a national teaching hospital in Malawi, high-grade dysplasia and cervical cancer were common and were significantly associated with HIV infection and increasing age. The frequency of cervical cancer among all women with submitted gynecologic specimens was 17%, and notably, over 25% of high-grade dysplasia cases and 35% of cancer cases occurred among women outside currently recommended screening ages. Our sample consisted only of women with specimens submitted to the pathology laboratory, and also had a high HIV seroprevalence of 36%, more than three times the generalised seroprevalence in Malawi of 11%. These factors likely explain the high frequency of cervical dysplasia and cancer in the study population.
We found that HIV infection was strongly associated with CIN2+ among Malawian women. The significant increase in high-grade dysplasia and cervical cancer among women with HIV is consistent with other studies.16 Similar to other reports from sub-Saharan Africa,17,21 we found that HIV-positive women were diagnosed with cancer at younger ages, approximately a decade before HIV-negative women. This may suggest HIV-positive women are at risk of developing cancer at younger ages and have more rapid progression to cancer from the time of oncogenic HPV acquisition. However, this might also reflect differences in age structure between the HIV-positive and HIV-negative populations.22 Regardless, our data suggest that policy makers in Malawi should consider updating cervical cancer screening guidelines to expand screening for HIV-positive women. This may be particularly important to reduce morbidity and mortality from cervical cancer, given that more than half of new HIV infections occur among young Malawians aged 15–24 years who often have early sexual debut and engage in high-risk sex behaviours.23 For comparison, World Health Organization cervical cancer screening guidelines already recommend screening for women aged 30 years and older, and for all women or girls as soon as they have tested positive for HIV.24
We also found that older age was associated with CIN2+. Women older than 45 years were commonly diagnosed with cancer in this study. These women may have been diagnosed and treated at younger ages if screening was more widely implemented in Malawi thus preventing progression of pre-existing lesions. These data suggest that older women may also benefit from screening as cervical cancer prevention efforts continue to expand, and that potential cervical cancer cases can be averted by screening women older than current recommendations. A robust evidence base to guide screening policies for older women will become especially important in the coming decades, given the demographic transitions and aging of populations that are anticipated to occur with expanded access to anti-retroviral treatment (ART) in most sub-Saharan African countries including Malawi.
Similar to a 2011 Ministry of Health report, a large proportion of this sample diagnosed with cancer was outside of the recommended screening age range.10 This suggests the current screening guidelines are missing women at risk of developing cervical cancer. One-third of cancer cases occurred among women older than 45 years, who may not be encouraged to attend screening. Additionally 14% of women diagnosed with high-grade dysplasia were young women in their 20s, who potentially might benefit from decades of normal life expectancy if cervical cancer was effectively prevented. Given the continued high burden of cervical cancer and a decade of experience now with VIA screening in place in Malawi, identifying optimal strategies to increase screening coverage for women at risk is an urgent public health priority. Formal cost-effectiveness evaluations may help determine how broadly or narrowly VIA screening should be targeted under conditions of resource constraints, providing metrics that can be directly compared with other competing public health interventions.
Our study has several strengths. This secondary analysis of routinely collected pathology data is highly reflective of clinical practice at a national teaching hospital in a resource-limited country in sub-Saharan Africa. Additionally, we had pathologically confirmed diagnoses for all cases, which is notable in a region where the extreme scarcity of diagnostic pathology services has been frequently described.25 Our study included a variety of specimen types from women undergoing different screening and treatment procedures, with only 38% of specimens included being cytology. This is notable given that VIA rather than cytology has emerged as the principal mode for cervical cancer screening in many sub-Saharan African settings, in part due to pathology limitations, and it may serve to make our study findings more generalisable to the region.
The main limitation of our study is the lack of access to extensive demographic or clinical data, including health and behavioural risk factors and presence of high-risk HPV types. For HIV-infected women, data regarding ART status and CD4 count were not available for analysis. Although we relied on provider reports of HIV status, it seems unlikely that a large proportion of women would have had HIV status mis-classified by their referring provider. Furthermore, findings were consistent when we included women with unknown HIV status in the analysis. Another important note is that the sample includes women referred to KCH for follow-up of abnormal screening, and the results may represent a subset of women at higher risk for unidentified reasons. Women were referred from various area health facilities (e.g. ART and sexually transmitted infection clinics) which see specific patient populations and use different screening procedures (e.g. screening younger women with risk factors or older symptomatic women). Finally, our data source was the institutional database for the main pathology laboratory serving the northern and central regions of Malawi. As such, there is referral bias in that only women who managed to have a hospital visit and had specimens submitted to the laboratory would have been included in our study. Results may therefore not reflect broader VIA screening programmes, where many women with normal VIA findings would never have subsequent specimens taken. In addition, biopsies were not always taken on women found to have advanced, inoperable cervical cancer on exam given that chemotherapy and radiation were not available treatment options during the study period.
In conclusion, we found that high-grade cervical dysplasia and cancer were very common among gynaecologic specimens submitted to a pathology laboratory at a national teaching hospital in Malawi, and these were significantly associated with HIV infection and increasing age. A substantial fraction of cases occurred outside the current age range recommended to receive cervical cancer screening in Malawi. These findings have significant implications for national cancer control efforts, given high morbidity and mortality which continue to result from cervical cancer in our setting. With VIA now well established in Malawi, strong consideration should be given to extend screening to HIV-infected women and sexually active women outside currently recommended screening ages, including older women who may never have been screened. Opportunities to integrate HIV testing and cervical cancer screening could also be explored. Evidence-based expansion of cervical cancer screening that is informed by current and future research is needed to continue reducing burden from this common and often fatal disease, such that Malawian women can reap benefits from cancer screening analogous to what has been achieved in resource-rich settings over the last many decades.
Acknowledgments
Funding
Dr. Kohler is supported by the UNC Cancer Care Quality and Training Program funded by the National Cancer Institute (R25 CA116339) and the UJMT Fogarty Global Health Fellows Program funded by the Fogarty International Center (R25 TW009340). Dr. Tang is supported by the Fogarty International Center at the National Institutes of Health (K01 TW009657-01). Dr. Gopal is supported by the Fogarty International Center (K01 TW009488), National Cancer Institute (R21 CA180815), AIDS Malignancy Consortium (U01 CA121947) and Program in Global Oncology of the UNC Lineberger Comprehensive Cancer Center (P30 CA016086). Dr. Liomba is supported by the Medical Education Partnership Initiative (R24 TW008927-01).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
These data were presented as an abstract at the fifth Annual Consortium of Universities for Global Health in Washington, DC, on 10 May 2014.
References
- 1.Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H, Bray F, et al. GLOBOCAN 2008 v2.0, cancer incidence and mortality worldwide. Lyon, France: International Agency for Research on Cancer; 2010. [accessed 6 June 2015]. http://globocan.iarc.fr. [Google Scholar]
- 3.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadza E, Chirwa E, Maluwa A, et al. Factors that contribute to delay in seeking cervical cancer diagnosis and treatment among women in Malawi. Health. 2012;4:1015–1022. [Google Scholar]
- 5.WHO. Cervical cancer screening in developing countries. Geneva: World Health Organization, Programme on Cancer Control DoRHaR; 2002. [Google Scholar]
- 6.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. The Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 7. [accessed 6 June 2015];GLOBOCAN 2012 v1.0., cancer incidence and mortality worldwide: IARC cancer base no. 11. 2013 http://globocan.iarc.fr.
- 8.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health. Malawi National reproductive Health Service Delivery Guidelines. Chapter 13. Lilongwe, Malawi: Ministry of Health; 2009. pp. 135–150. [Google Scholar]
- 10.Malawi Ministry of Health. VIA programme report. Jun, 2011. [Google Scholar]
- 11.Denny LA, Franceschi S, de Sanjosé S, et al. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5 0):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Denny L, Boa R, Williamson A-L, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol. 2008;111:1380–1387. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- 13.Firnhaber C, Zungu K, Levin S, et al. Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus–infected women in Johannesburg, South Africa. Acta Cytol. 2011;53:10–17. doi: 10.1159/000325079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clifford GM, Goncalves MA, Franceschi S. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20:2337–2344. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]
- 15.De Vuyst H, Alemany L, Lacey C, et al. The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine. 2013;31(Suppl 5 0):F32–F46. doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS. 2013 doi: 10.1177/0956462413491735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bogaert LJJ. Age at diagnosis of preinvasive and invasive cervical neoplasia in South Africa: HIV-positive versus HIV-negative women. Int J Gynecol Can. 2011;21:363–366. doi: 10.1097/IGC.0b013e3182094d78. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS. [accessed March 2014];Malawi country overview. 2013 Available at: http://www.unaids.org/en/regionscountries/countries/malawi/
- 19.Gopal S, Krysiak R, Liomba G. Building a pathology laboratory in Malawi. The Lancet Oncol. 2013;14:291–292. doi: 10.1016/S1470-2045(13)70109-8. [DOI] [PubMed] [Google Scholar]
- 20.Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PloS One. 2013;8:e70361. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moodley M, Moodley J, Kleinschmidt I. Invasive cervical cancer and human immunodeficiency virus (HIV) infection: a South African perspective. Int J Gynecol Can. 2001;11:194–197. doi: 10.1046/j.1525-1438.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 22.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Int Med. 2010;153:452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Statistical Office and ICF Macro. Malawi demographics and health survey 2010. Zomba, Malawi and Calverton, Maryland: NSO and ICF Macro; 2011. [Google Scholar]
- 24.WHO. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. South Africa: World Health Organization; 2013. [PubMed] [Google Scholar]
- 25.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. The Lancet Oncol. 2013;14:e152–e157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]