Abstract
Background
Data regarding unselected patients with metastatic clear-cell renal cell carcinoma (ccRCC) treated with first-line pazopanib are limited.
Patients and Methods
We reviewed records of patients with metastatic ccRCC treated with first-line pazopanib during 11/09-11/12. Cox models were fitted to evaluate the association of progression-free survival (PFS) and overall survival (OS) with patient co-variables.
Results
Eighty-eight patients were identified; 74 were evaluable for response: 2 (3%) had complete response, 27 (36%) had partial response, 36 (49%) had stable disease, and 9 (12%) had progressive disease. Median PFS was 13.7 months (95% CI: 8.7 – 18.3). PFS was correlated with Karnofsky performance score < 80 (HR = 3.26, p < 0.0001) and serum lactate dehydrogenase >1.5 ULN (HR = 3.25, p = 0.0135). Median OS was 29.1 months (95% CI: 20.2 – NA). OS was correlated with brain metastasis (HR = 2.55, p = 0.0089), neutrophilia (HR = 1.179, p = 0.0178), and anemia (HR = 3.51, p = 0.0001). No treatment-related deaths occurred. Fifty-three patients received second-line therapy (VEGFR-TKI [22], mTORi [22], others [9]); median PFS was 8.6 months (95% CI: 3.3 – 25.7) with VEGFR-TKI and 5 months (95% CI: 3.5 – 15.2) with mTORi, p = 0.41; median OS was 19.9 months (95% CI: 12.9 – NA) and 14.2 months (95% CI: 8.1 – NA), from initiation of second-line VEGFR-TKI or mTORi, respectively, p = 0.37.
Conclusions
In this retrospective study, first-line pazopanib confirmed its efficacy in metastatic ccRCC. Trends for longer PFS and OS were observed with VEGFR-TKI than mTORi after first-line pazopanib.
Keywords: renal cell carcinoma, pazopanib, targeted therapy, tyrosine-kinase inhibitor, mTOR inhibitor, angiogenesis
Introduction
Approximately one-third of patients with renal cell carcinoma (RCC) present with metastatic disease at initial diagnosis, and 25% develop metastases after nephrectomy.1,2 Although metastasectomy may be curative in select patients, for the vast majority of patients, metastatic disease is incurable, and patients require systemic therapy to control symptoms and prolong survival.3 During the past decade, the anti–vascular endothelial growth factor (VEGF) agents and mammalian target of rapamycin (mTOR) inhibitors have supplanted cytokines (interleukin-2, interferon-alfa) as the mainstay therapy in metastatic RCC. These agents have led to prolongation of progression-free survival (PFS) and, in some patients, overall survival (OS).4Currently, four VEGF receptor tyrosine kinase inhibitors (VEGFR-TKIs) are approved in the United States and Europe: sorafenib, sunitinib, pazopanib, and axitinib. Several other novel agents are in preclinical development and clinical trials.5
Pazopanib inhibits angiogenesis by targeting VEGF receptors, platelet-derived growth factor receptors, and c-Kit (CD117). It was approved for metastatic RCC in the United States in November 2009 and in Europe in June 2010. The safety and efficacy of pazopanib were evaluated in a randomized, double-blind, placebo-controlled phase III trial in treatment-naïve and cytokine-pretreated patients.6 Pazopanib demonstrated prolonged PFS compared to placebo (9.2 vs 4.2 months, P < 0.0001) and produced a higher objective response rate (30% vs 3%, P < 0.001). Results from a large randomized phase III trial in the first-line therapy setting of metastatic clear-cell RCC (the COMPARZ trial) showed non-inferiority in efficacy of pazopanib compared to sunitinib, with a differentiated safety profile favoring pazopanib.7 In the randomized, double-blind PISCES study, which had patient preference as primary endpoint, 70% of patients preferred pazopanib, compared to 22% of patients who preferred sunitinib, mostly due to less fatigue with pazopanib.8 The National Comprehensive Cancer Network treatment guidelines currently recommend pazopanib (category I) in the first-line setting and after cytokine therapy.
We sought to explore the efficacy and safety of pazopanib in a real-world setting in unselected patients, especially those with compromised performance status or brain metastasis, who would not be eligible to participate in clinical trials. Another aim of this study was to obtain data on outcomes of patients treated with salvage targeted therapy after first-line pazopanib therapy.
Patients and Methods
In this retrospective study, we included consecutive patients with metastatic clear-cell RCC who were treated in the first-line setting with pazopanib from November 1, 2009, through November 1, 2012 in the Genitourinary Medical Oncology Clinic at The University of Texas, MD Anderson Cancer Center (MDACC). Inclusion criteria required adequate follow-up, defined as at least one clinic visit to MDACC every 3 months while receiving pazopanib. Patients who received prior chemotherapy or cytokines were excluded. Radiographic evaluation consisted of computed tomography scans of the chest, abdomen, and pelvis every 3 months, with brain magnetic resonance imaging and bone scans obtained as clinically indicated. Complete blood counts and serum chemistries were obtained initially every 3 weeks for 9 weeks, then every 6 weeks.
The study was approved by the MDACC Institutional Review Board. Clinical data were collected from the institution's electronic medical records system. Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events, version 4.0. Radiographic response to first-line pazopanib and second-line VEGF-TKI and mTORi was assessed by two blinded radiologists (BT, CD), using the Response Evaluation Criteria in Solid Tumors (RECIST), v.1.1. Progression-free survival in first-line was defined as the time from initiation of pazopanib therapy to the date of disease progression or death from any cause. Progression-free survival with second-line targeted therapy was defined as the time from initiation of VEGFR-TKI or mTORi to the date of disease progression or death from any cause. Overall survival was defined as the time from initiation of pazopanib therapy to the date of death from any cause.
The Kaplan-Meier method was used to estimate PFS and OS times. Univariable and multivariable Cox proportional hazards models were fitted to evaluate the association of PFS and OS with clinical co-variables. Variables with P values <0.15 in the univariable analysis were included in the multivariable analysis. The backward selection procedure was used for model selection. Variables with P values <0.05 were considered statistically significant.
Results
Patient' Characteristics
Eighty-eight consecutive patients with metastatic clear-cell RCC, previously untreated with systemic therapy, met the study's inclusion criteria and constitute the patient cohort in the analysis. Table 1 lists patients' characteristics. Median age was 65 years; 69% of patients were men; 78% had prior nephrectomy; 23% had Karnofsky performance score (KPS) <80%; and 31% had favorable-risk, 57% had intermediate-risk, and 12% had poor-risk disease by Memorial Sloan-Kettering Cancer Center (MSKCC) criteria.9 The median number of metastatic sites was 1 (range, 1-7).
Table 1. Patients' Characteristics (N=88).
| Characteristic | Number (%)a |
|---|---|
| Median age, years (range) | 65 (34-90) |
| Sex | |
| Male | 61 (69%) |
| Female | 27 (31%) |
| Ethnic origin | |
| White | 68 (77%) |
| Hispanic | 10 (11%) |
| Black | 6 (7%) |
| Asian | 3 (3%) |
| Other | 1 (1%) |
| Prior nephrectomy | 69 (78%) |
| Prognostic feature | |
| KPS score <80 | 20 (23%) |
| Time from diagnosis to treatment <1 year | 44 (50%) |
| Low hemoglobin | 18 (20%) |
| Corrected calcium >10 mg/dL | 6 (7%) |
| Neutrophil count >ULN | 13 (15%) |
| Platelet count >ULN | 8 (9%) |
| MSKCC risk subgroup | |
| Favorable (0 risk factors) | 27 (31%) |
| Intermediate (1 risk factor) | 50 (57%) |
| Poor (2 or 3 risk factors) | 11 (12.5%) |
| IMDC risk subgroup | |
| Favorable (0 risk factors) | 35 (40%) |
| Intermediate (1-2 risk factors) | 44 (50%) |
| Poor (3-6 risk factors) | 9 (10%) |
| Sites of metastases | |
| Lung | 59 (67%) |
| Bone | 40 (46%) |
| Lymph node | 33 (38%) |
| Adrenal gland | 14 (16%) |
| Brain | 14 (16%) |
| Liver | 10 (11%) |
| Contralateral kidney | 6 (7%) |
| Pancreas | 5 (6%) |
| Otherb | 14 (16%) |
Unless otherwise indicated.
Includes muscle, soft tissue, renal fossa, pleura, thyroid.
KPS, Karnofsky performance status; ULN, upper limit of normal range; MSKCC, Memorial Sloan-Kettering Cancer Center; IMDC, International Metastatic Renal Cell Carcinoma Data Consortium
Efficacy and Safety
Among 74 evaluable patients, two (3%) patients had a complete response (CR), 27 (36%) had a partial response (PR), 36 (49%) had stable disease (SD), and nine (12%) had progressive disease (PD), as best response to first-line pazopanib therapy (Figure 1). Among the 11 patients with MSKCC poor-risk disease, five had SD as best response. Among the 14 patients with brain metastasis, five patients (36%) had demonstrable regression of their brain metastases and six patients (43%) had stabilization of their brain metastases. The median follow-up time for all patients who were alive at the time of analysis was 29.3 months (range, 25.4-33.2).
Figure 1.
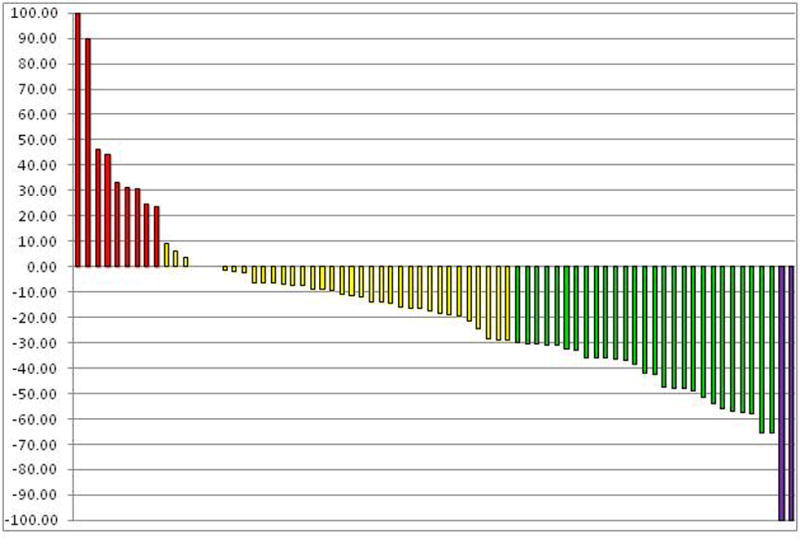
Waterfall plot of tumor response evaluated by Response Evaluation Criteria in Solid Tumors. Red bars represent patients whose best response to therapy was progressive disease, yellow bars indicate stable disease, green bars indicate partial response, and purple bars indicate complete response.
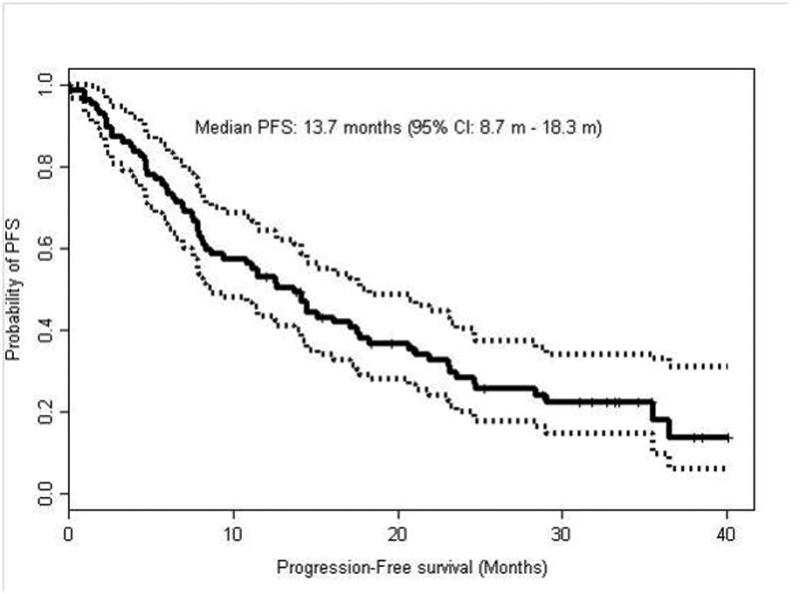
The median PFS time with first-line pazopanib (Figure 2) was 13.7 months (95% CI: 8.7-18.3). By multivariable analysis, PFS was correlated with KPS <80 (hazard ratio [HR] = 3.26, P <0.0001) and serum lactate dehydrogenase (LDH) level >1.5 × upper limit of normal [UNL] (HR = 3.25, P = 0.0135). The MSKCC and International Metastatic Renal Cell Carcinoma Data Consortium (IMDC) risk groups had prognostic implications, with patients in the good-risk group having superior outcomes compared to patients with intermediate-risk and poor-risk groups. The MSKCC good-risk group had a median PFS of 21.1 months compared to 12.6 months and 2.3 months for intermediate-risk and poor- risk groups, respectively (p=0.004). The IMDC good-risk group had a median PFS of 21.1 months compared to 9.4 and 5.9 months for intermediate-risk and poor-risk groups, respectively (p=0.015). Other variables assessed included body-mass-index (BMI) and neutrophil to lymphocyte ratio (NLR). No statistically significant difference was detected for time on first line pazopanib therapy for patients with BMI greater than 25 or 30 compared to patients who had BMI under these cut points. In addition, using a NLR cut point of 3, patients with NLR above 3 did not had a statistically different time on first line pazopanib compared to patients with a NLR below 3.
Figure 2. Kaplan-Meier curve for progression-free survival (PFS) in patients treated with pazopanib as first-line therapy.
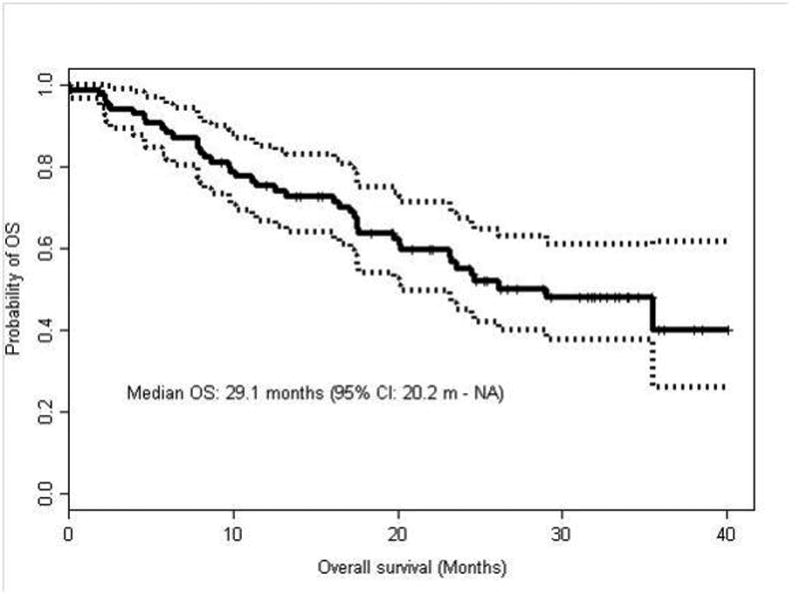
Forty-one of the 88 patients have died. The median OS time (Figure 3) for patients treated with first-line pazopanib was 29.1 months (95% CI: 20.2 - NA). Patients with MSKCC good-risk disease had a median OS of 35.4 months compared to 23.1 and 7.9 months for patients with intermediate-risk and poor-risk disease, respectively (p=0.005). Patients with IMDC good-risk disease had a median OS of 35.5 months compared to 24.6 and 23.2 months for patients with intermediate-risk and poor-risk disease, respectively (p=0.078). By multivariable analysis, OS was correlated with brain metastasis (HR = 2.55; 95% CI: 1.264-5.145; P = 0.0089), neutrophil count >ULN (HR = 1.179; 95% CI: 1.029 - 1.352; P = 0.0178), and hemoglobin <12 (HR = 3.51; 95% CI: 1.838 - 6.705; P = 0.0001). Not surprisingly, patients who would typically be excluded from clinical trials due to poor performance status or brain metastasis had significantly worse outcome than patients without these risk factors. Patients who had a BMI less than or equal to 25 had an inferior OS of 17.2 months compared to 33.5 months for patients with a BMI greater than 25 (HR 1.72, 95% CI 1.05-3.23). Patients with a NLR greater than 3 had inferior survival with a median OS of 20.1 months versus 44.6 months for patients with an NLR less than 3 (HR 0.4167, 95% CI: 0.239-0.659, p=0.0004). Both BMI and NLR did not remain significant on multivariate analysis.
Figure 3. Kaplan-Meier curve for overall survival (OS) in patients treated with pazopanib as first-line therapy.
Seventy-three patients (83%) started first-line pazopanib at 800 mg daily, and 19 (26%) of these patients required dose reductions due to AEs. Seven patients started pazopanib at 600 mg daily, and three of these patients required dose reductions to 400 mg daily. Eight patients started pazopanib at 400 mg daily. At the time of analysis, 30% of patients were still receiving pazopanib, 55% had discontinued pazopanib due to PD or had died while receiving pazopanib, and 5% had discontinued for other reasons (including one patient who had CR to pazopanib and one patient who underwent complete resection of all metastatic disease following pazopanib therapy). There were no treatment-related deaths.
Nine patients (10%) discontinued pazopanib due to AEs: seven patients had liver toxicity; one patient had nausea, vomiting, and diarrhea; and one patient had anorexia and weight loss. Thirty-eight patients (43%) had exacerbations of preexisting hypertension while receiving pazopanib therapy, and five patients (6%) developed new-onset hypertension. Fourteen patients (16%) developed increased liver function tests while receiving pazopanib therapy. No grade 4 toxicity was reported. AEs are summarized in Table 2.
Table 2. Adverse Events (N=88).
| Adverse Event | All Grades | Grade I | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Fatigue | 51 (58%) | 31 (35%) | 18 (21%) | 2 (2%) |
| Diarrhea | 34 (39%) | 24 (27%) | 9 (10%) | 1 (1%) |
| HTN exacerbation | 38 (43%) | 24 (27%) | 12 (14%) | 2 (2%) |
| Anorexia/ Weight loss | 24 (27%) | 15 (17%) | 8 (9%) | 1 (1%) |
| Nausea/Vomiting | 21 (24%) | 13 (15%) | 8 (9%) | - |
| Increased LFTs | 17 (19%) | 2 (2%) | 3 (3%) | 12 (14%) |
| Hair and skin changes | 12 (14%) | 5 (6%) | 7 (8%) | - |
| Myalgias/Arthragias | 9 (10%) | 7 (8%) | 2 (2%) | - |
| Abdominal pain | 7 (8%) | 4 (5%) | 2 (2%) | 1 (1%) |
| New-onset HTN | 5 (6%) | 1 (1%) | 4 (5%) | - |
| Hypothyroidism | 5 (6%) | - | 5 (6%) | - |
There were no Grade 4 adverse events.
HTN, hypertension; LFTs, liver function tests
Of the total 88 patients, 53 (60%) patients received second-line therapies after pazopanib (while 30% continued pazopanib at the time of analysis, and 10% died or otherwise received no further therapy). Second-line therapies were associated with median PFS of 5.9 months (95% CI: 3.5 - 8.5) and median OS of 18.6 months (95% CI: 9.8 - 37.6) from the start of second-line therapy. At the time of initiation of second-line therapy, patients with an IMDC score of 0-1 had a median OS of 19.9 months (95% CI: 14.0-not reached) compared to patients with 2-5 points having a median OS of 8.1 months (95% CI: 4.6-not reached); this difference was statistically significant (p=0.025).
Twenty-two patients (25%) received a second-line VEGFR-TKI (sunitinib, sorafenib, or axitinib) following pazopanib, yielding a median PFS of 8.6 months (95% CI: 3.3 - 25.7) and a median OS of 19.9 months (95% CI: 12.9 - NA) from the start of second-line VEGFR-TKI therapy. The IMDC score prior to second-line VEGFR-TKI was 0 in 4 patients, 1-2 in 13 patients, and greater than 2 in 5 patients. Twenty-two patients (25%) received a second-line mTORi (everolimus or temsirolimus) following pazopanib, yielding a median PFS of 5 months (95% CI: 3.5 - 15.2) and a median OS of 14.2 months (95% CI: 8.1 - NA) from the start of second-line mTORi therapy. The IMDC score prior to second line mTORi therapy was 0 in 1 patient, 1-2 in 18 patients, and greater than 2 in 3 patients. Compared with second-line mTORi, second-line VEGFR-TKI following pazopanib produced longer PFS (Figure 4) and longer OS (Table 4 and Figure 5), although the differences in PFS and OS between the two classes of agents did not reach statistical significance. The nine patients who received other therapies in the second-line setting following pazopanib (including chemotherapy and investigational agents) had median PFS of 3.0 months (95% CI: 1.7 - NA). Thirty-five patients who received second-line therapies had evaluable disease by RECIST. Six of these patients (17%) achieved PR, 18 (51%) had SD, and 11 (31%) had PD, as best response. Fourteen patients (23%) receiving salvage therapy after pazopanib continued second-line therapy beyond 12 months. Twenty-nine patients (33%) received therapies in the third-line and subsequent settings. Second-line therapies and outcomes are detailed in Tables 3 and 4.
Figure 4.
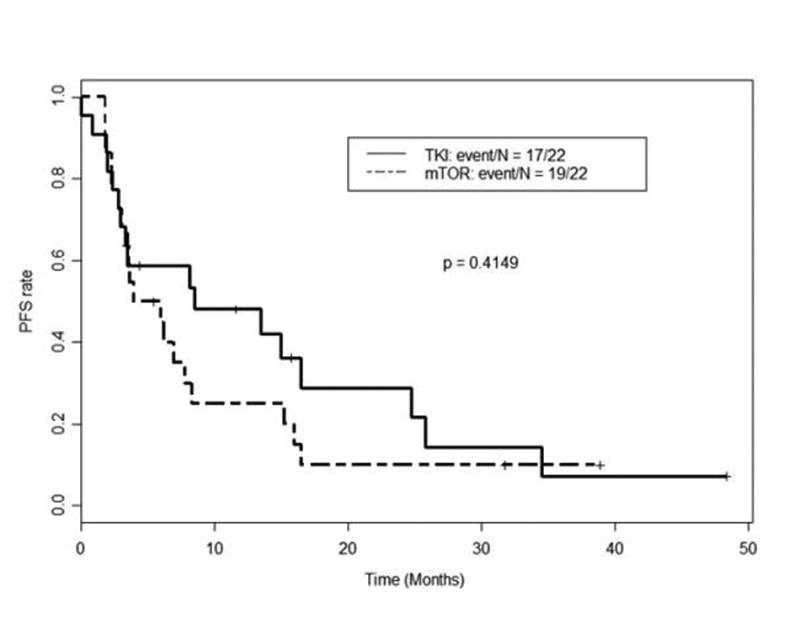
Kaplan-Meier curves for progression-free survival (PFS) as measured from time of initiation of second-line mammalian target of rapamycin inhibitors (mTORi) vs. VEGFR tyrosine kinase inhibitors (VEGFR-TKI) following first-line pazopanib
Table 4. Overall Survival (OS) for Second-Line Therapy Following First-Line Pazopanib.
| Second Line Therapy | Number of patients | Median (95% CI) | OS Rate at 1 Year (95% CI) | OS Rate at 2 Years (95% CI) | P-value (Median OS VEGFR-TKI vs mTORi) |
|---|---|---|---|---|---|
| VEGFR-TKI | 22 - (17 sunitinib/ 2 sorafenib/ 3 axitinib) | 19.9 months (95% CI: 12.9 - NA ) | 0.76 (95% CI: 0.6 - 0.97 ) | 0.45 (95% CI: 0.27 - 0.77) | 0.37 |
| mTOR inhibitor | 22 - (17 everolimus/ 5 temsirolimus) | 14.2 months (95% CI: 8.1 - NA ) | 0.5 (95% CI: 0.32 - 0.78 ) | 0.19 (95% CI: 0.04 - 0.89 ) |
Figure 5.
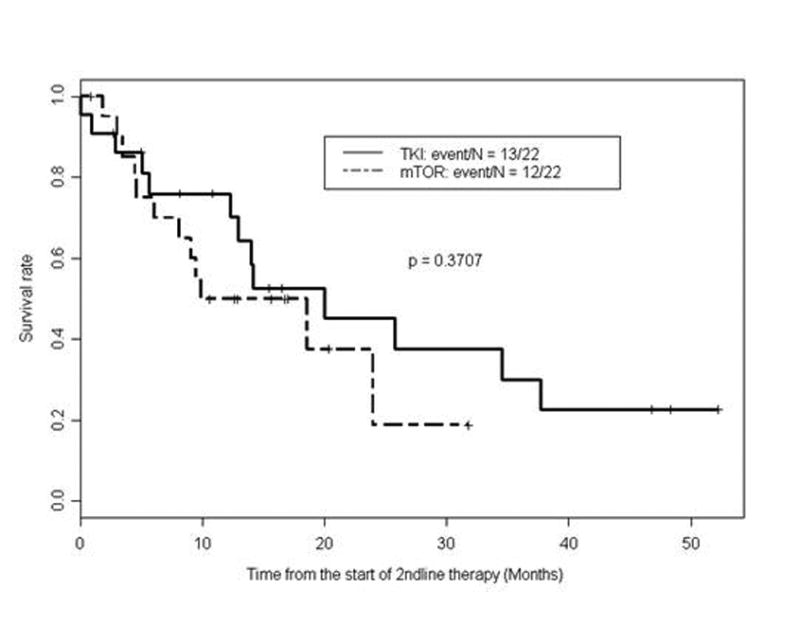
Kaplan-Meier curves for overall survival (OS) as measured from time of initiation of second-line mammalian target of rapamycin inhibitors (mTORi) vs. VEGFR tyrosine kinase inhibitors (VEGFR-TKI) following first-line pazopanib
Table 3. Progression-free Survival (PFS) and Response on Second-Line Therapy Following First-Line Pazopanib (N=88).
| Second-Line Therapy | Number of patients (%) | Median PFS of Second-Line Therapies, in months (95% CI) | Responses |
|---|---|---|---|
| None Patients censored | 35 (40%) | - | - |
| VEGFR-TKI | 22 (25%) - (17 sunitinib/ 2 sorafenib/ 3 axitinib) | 8.6 months (95% CI: 3.3-25.7) | 16 evaluable patients: 2 (12.5%) PR, 12 (75%) SD, 2 (12.5%) PD ORR – 14 (88%) |
| mTOR inhibitor | 22 (25%) - (17 everolimus/ 5 temsirolimus) | 5.0 months (95% CI: 3.5-15.2) | 13 evaluable patients: 3 (23%) PR, 6 (46%) SD, 4 (31%) PD ORR – 9 (69%) |
| Other | 9 (10%) (including chemotherapy/ investigational agents) | 3.0 months (95% CI: 1.7 - NA) | 6 evaluable patients: 1 (17%) PR, 5 (83%) PD ORR – 1 (17%) |
CI, confidence interval; mTOR, mammalian target of rapamycin; NA, not applicable; ORR, objective response rate; PR, partial response; PD, progressive disease; SD, stable disease; TKI, tyrosine kinase inhibitor.
Discussion
Randomized controlled trials have established the efficacy of pazopanib in prolonging PFS compared to placebo in treatment-naïve and cytokine-pretreated patients with metastatic clear-cell RCC.6 Studies such as COMPARZ and PISCES suggest pazopanib has a differentiated safety profile compared with sunitinib with better overall tolerability.7,8 However, there is a paucity of data regarding the efficacy and safety of pazopanib in unselected patients outside the context of randomized prospective clinical trials, especially in patients with compromised performance status and brain metastasis.
Our data confirm the efficacy and safety of pazopanib in a real-world setting in a group of unselected patients, including those who would be ineligible for clinical trials due to compromised performance status, or comorbidities. The efficacy data in our study are comparable to those reported from the pivotal phase III trial by Sternberg and colleagues.6 The median PFS of 13.7 months and the 39% objective response rate with first-line pazopanib in our retrospective study are similar to the results obtained by Sternberg and colleagues who reported a median PFS of 11.1 months in treatment-naïve patients and a 30% objective response rate. The less stringent scheduling of imaging studies in our study (scans were usually obtained at 3-month intervals and in some instances at longer intervals) may explain our slightly longer median PFS, but the median OS of 29 months in our study is remarkably comparable with the OS results of the COMPARZ trial.7
A single-institution retrospective study similar to ours was conducted by Galvis and colleagues at The Christie Hospital in Manchester, United Kingdom.10 Their study results largely confirm our data. These researchers identified 104 treatment-naïve and cytokine refractory patients who received pazopanib for metastatic RCC. The majority of their patients were treated with pazopanib off protocol, but some of them received it within the context of trials. The baseline characteristics of these patients were similar to our study population and outcomes were also comparable. Galvis and colleagues reported a median PFS of 13 months in treatment-naïve and cytokine-refractory patients compared to our PFS of 13.7 months in treatment-naïve patients only. Similarly, they concluded that a portion of their poor-risk patients appeared to benefit from pazopanib therapy.
Vogelzang and colleagues published a retrospective review of 177 patients with metastatic RCC who received pazopanib in the first-line setting11 and reported a median OS of 22 months and a median PFS of 8.5 months. They also reported a median PFS of 5.7 months in patients receiving second-line mTOR inhibitors following pazopanib, which was comparable to the median PFS of 5.9 months for patients who received second-line therapies in our study.
In our study, PFS was significantly associated with KPS <80 and elevated serum LDH. Both of these are well-known risk factors in metastatic RCC and are components of the MSKCC risk stratification model. Overall survival was significantly associated with brain metastasis, neutrophilia, and anemia. The brain is a site of metastasis known to be associated with poor outcomes.12,13 Neutrophilia and anemia are also well-known predictors of poor outcomes and are included in the IMDC risk stratification model.14
Several studies have shown benefit of VEGF-TKI therapy after disease progression with a first-line VEGFR-TKI.15,16 We sought to evaluate outcomes of patients receiving subsequent therapies after front-line pazopanib. Twenty-two patients (25%) received a second VEGF-TKI (sunitinib, sorafenib, or axitinib), and an equal number received an mTOR inhibitor (everolimus or temsirolimus). There was a trend for longer PFS with second-line VEGFR-TKI compared with mTOR inhibitors after first-line pazopanib therapy (median PFS 8.6 months versus 5.0 months). Also, there was a trend for superior OS with second-line VEGFR-TKI compared with mTOR inhibitors after first-line pazopanib therapy (median 19.9 months versus 14.2 months, and higher OS rates at 1 and 2 years). However, it would be difficult to draw firm conclusions regarding the optimal therapeutic sequence, VEGFR-TKI followed by VEGFR-TKI versus VEGFR-TKI followed by mTOR inhibitor, given the small number of patients, the different TKI and mTOR inhibitors used in the second-line setting, and the retrospective nature of our study. The most optimal sequence of therapeutic agents after first-line VEGFR-TKI is rapidly evolving as newer agents such as the anti-Programmed Death (PD)-1 antibody, nivolumab (NCT01668784 CheckMate 025 phase III clinical trial), and the VEGFR/cMet/AXL inhibitor, cabozantinib (NCT01865747 METEOR phase III clinical trial), are entering this disease state space.
Pazopanib was well tolerated in our real-world unselected patient population: 91% of AEs were mild to moderate (Grade 1 or 2), and only 10% of patients discontinued therapy due to toxicity. Only five (6%) of our patients developed new-onset hypertension, and 38 patients (43%) had an exacerbation of preexisting hypertension. The relatively low rate of new-onset hypertension observed may be due in part to the fact that many of our patients were already diagnosed with hypertension and may also be due to incomplete data capture of new-onset hypertension in a retrospective study. We also noted a lower incidence of elevated transaminases than previously reported in trials with pazopanib, likely due to underreporting of laboratory abnormalities in retrospective studies.
We are aware of the inherent limitations of this retrospective study and recognize that our conclusions are constrained by inherently inadequate and sometimes incomplete data capture in medical records. We further acknowledge the intrinsic selection biases that exist in all retrospective studies and have attempted to limit these as much as possible.
In summary, our findings confirm the efficacy and safety of pazopanib as first-line therapy for unselected, real-world patients with metastatic clear-cell RCC who are treated outside the randomized clinical trial setting. The sequencing of therapeutic agents in metastatic renal cell carcinoma is rapidly evolving and will be defined in large prospective randomized trials.
Acknowledgments
FUNDING: This work was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant [5 P30 CA016672]. Dr. Matrana was supported by NIH grant [T32CA009666-15].
RELEVANT CONFLICTS OF INTEREST: Drs. Nizar M. Tannir and Eric Jonasch have received research funding from GlaxoSmithKline and Novartis. They have also received honoraria for their participation in advisory board meetings with GlaxoSmithKline and Novartis. Dr. Marc Matrana has received travel support and speaking honoraria from GlaxoSmithKline.
References
- 1.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 2.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 3.Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–2882. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 4.Buchler T, Pavlik T, Bortlicek Z, et al. Objective response and time to progression on sequential treatment with sunitinib and sorafenib in metastatic renal cell carcinoma. Med Oncol. 2012;29:3321–3324. doi: 10.1007/s12032-012-0293-x. [DOI] [PubMed] [Google Scholar]
- 5.Matrana MR, Atkinson B, Jonasch E, Tannir NM. Emerging targeted therapies in metastatic renal cell carcinoma. Curr Clin Pharmacol. 2011;6:189–198. doi: 10.2174/157488411797189398. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. Clin Oncol. 2014;32:1412–1418. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 10.Galvis V, Chow S, Thistlethwaite FC. Clinical practice outcomes of patients treated with pazopanib for metastatic renal cell cancer (mRCC) - 6 year experience at a referral centre in Manchester, UK. Abstract 2763. European Cancer Congress; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 11.Vogelzang NJ, Hackshaw MD, Hutson TE, et al. First-line and sequential use of pazopanib followed by mammalian target of rapamycin inhibitor therapy among patients with advanced renal cell carcinoma in a US community oncology setting. Clin Genitourin Cancer. 2014 Nov 15; doi: 10.1016/j.clgc.2014.11.001. pii: S1558-7673(14)00252-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Kim JW, Chung HT, Paek SH, Kim DG, Jung HW. Brain metastasis from renal cell carcinoma. Prog Neurol Surg. 2012;25:163–175. doi: 10.1159/000331190. [DOI] [PubMed] [Google Scholar]
- 13.Nieder C, Spanne O, Nordøy T, Dalhauq A. Treatment of brain metastases from renal cell cancer. Urol Oncol. 2011;29:405–410. doi: 10.1016/j.urolonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 15.Matrana M, Atkinson B, Tannir N. Combinatorial and sequential targeted therapy in metastatic renal cell carcinoma In: Lara PN, Jonasch E, eds Kidney Cancer: Principles and Practice. New York, NY: Springer-Verlag; 2012. pp. 225–240. [Google Scholar]
- 16.Matrana MR, Duran C, Shetty A, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with pazopanib after disease progression with other targeted therapies. Eur J Cancer. 2013;49:3169–3175. doi: 10.1016/j.ejca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


