Abstract
This paper proposes a heuristic framework for an Addictions Neuroclinical Assessment (ANA) that incorporates key functional domains derived from the neurocircuitry of addiction. We review how addictive disorders (AD) are presently diagnosed, and the need for new neuroclinical measures to differentiate patients who meet clinical criteria for addiction to the same agent while differing in etiology, prognosis and treatment response. The need for a better understanding of the mechanisms provoking and maintaining addiction, as evidenced by the limitations of current treatments and within-diagnosis clinical heterogeneity, is articulated. In addition, recent changes in the nosology of AD, challenges to current classification systems, and prior attempts to subtype individuals with AD are described. Complementary initiatives, including the Research Domain Criteria (RDoC) project, which have established frameworks for the neuroscience of psychiatric disorders, are discussed. Three domains, executive function, incentive salience, and negative emotionality, tied to different phases in the cycle of addiction, form the core functional elements of AD. Measurement of these domains in epidemiologic, genetic, clinical, and treatment studies will provide the underpinnings for an understanding of cross-population and temporal variation in addictions, shared mechanisms in addictive disorders, impact of changing environmental influences, and gene identification. Finally, we show that it is practical to implement such a deep neuroclinical assessment using a combination of neuroimaging and performance measures. Neuroclinical assessment is key to reconceptualizing the nosology of AD on the basis of process and etiology, an advance that can lead to improved prevention and treatment.
Keywords: addiction, substance use, nosology, diagnosis, assessment, neuroimaging
Introduction
The problem of etiologic and functional heterogeneity among patients addicted to the same agent is not new. It has long been recognized that these common diseases are etiologically heterogeneous, and that dichotomous affected/unaffected classifications fail to capture severity and distinctiveness of addictive disorders (AD). A revolution in understanding the neurobiologic basis of addiction has not been translated into the clinic. Translation of neuroscience to practice would identify the etiologic factors and functional outcomes that unify people addicted to different agents and that differentiate people addicted to the same agent. Moreover, the lack of assessments of these neurobiologic domains in people has impeded genetic, ecologic, and clinical translational research. Changes in the classification of AD, have not, arguably, advanced this nosology appreciably in several decades.
Attempts to identify meaningful subtypes of AD have predominately focused on alcohol use disorders (AUD). Jellinek (1), Cloninger, Babor, Lesch, and others (see (2) for a comprehensive review), have clinically subclassified alcoholism. Other addictive agents, including cocaine (3, 4), opioids (5), club drugs (6), and cannabis (7, 8), have been the focus of similar efforts. Despite this work, there remains little consensus in the field regarding subtypes of various AD. We propose this lack of agreement is because classification schemes have been limited by measures available.
This review proposes a framework and rationale for an Addictions Neuroclinical Assessment (ANA). It is our aim to establish such a framework and rationale with present knowledge of the neurobiologic basis of addiction, gleaned from humans and model organisms. Three main neurofunctional domains, executive function, incentive salience, and negative emotionality, should be assessed in patients with addictions, including behavioral addictions (“process” addictions as defined by the American Society of Addiction Medicine, e.g. gambling) and in individuals at risk, for purposes of better understanding the heterogeneity of AD and eventually to improve the nosology. Other measures of exposure to addictive agents, and use (e.g., impulsive, habitual, and compulsive) and related phenomena, genetics, and agent-specific outcomes would be closely integrated with measures of neuroscience domains whose importance we hypothesize transcends any particular addictive agent.
Changes in Nosology
The diagnosis of AD has shifted over time while adhering to a focus on clinical presentation rather than etiology. This emphasis has not been without benefit. The ability to diagnose AD by clinical criteria has provided a reliable foundation for the practice of addiction medicine. It has also been a springboard for neuroscience and genetic studies and clinical trials that have yielded insights on AD, e.g., for neural mechanisms (9), genetics (10), and treatment (11).
In the most recent version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; 20) AD are grounded in clinical-life outcomes both because of the relevance of symptom-based diagnoses as indicators of impairment and need for intervention, but also because of lack of evidence-based alternatives. Properly assessed using DSM symptoms, AD have high inter-rater reliabilities (13); furthermore, factor analyses show that on a statistical basis they are internally coherent or “valid’ (14). These virtues, while important, are insufficient. A diagnosis with high inter-rater reliability is not necessarily useful if the diagnosis is heterogeneous. AD diagnosis is based on endorsement of symptoms in several domains of life impact. In contrast with most medical diagnoses, the nosology and diagnosis of AD is outcome-based rather than process-based. Such a deficiency is shared by other psychiatric disorders, as discussed (15). In identifying a research agenda for the then-under development DSM-5, Charney and colleagues outlined the need for a neuroscience-based framework to foster development of psychiatric nosology based on pathophysiology, rather than clinical presentation.
Translating etiology into clinical practice, across clinical diagnostic categories
By way of comparison, a diagnosis of cancer affecting any particular organ is diagnosed using cellular, genetic, molecular, and imaging measures, combined with clinical history. Progress in treatment and prevention, e.g., the utility of trastuzumab (a monoclonal antibody interfering with the HER2/neu receptor) in the treatment of certain breast cancers (16) or the ability of the BRCA1 (a gene producing tumor-suppressing proteins) genotype to predict enhanced risk of breast cancer (17), has occurred because of integration of these measures with clinical history. The clinical observations are irreplaceable but do not themselves replace the need for physiologic data, in the form of an imaging, genetic, or molecular measure.
Addiction diagnoses reimagined and informed by mechanistically relevant measures, whether from neuroimaging, genetics, and/or epigenetics, are at present precluded by lack of deep data on individuals with AD and others at risk. Pharmacotherapies to treat addictions provide one example of how present nosology impacts outcomes. For example, there are three FDA-approved medications to treat alcoholism: acamprosate (approved 2004), naltrexone (approved 1994), and disulfiram (approved 1951). Behavioral treatments including cognitive behavior therapy, motivational enhancement therapy, 12-step facilitated therapy, and behavioral couples/family therapies also have efficacy (18, 19). To a limited extent, these behavioral treatments and medications appear to target different neurobiological components of the addiction cycle, e.g., naltrexone is an opioid antagonist and is hypothesized to target the rewarding effects of alcohol (20, 21), while acamprosate antagonizes NMDA function and metabotropic glutamate receptors and is hypothesized to target “craving” associated with alcohol acute and protracted withdrawal (22-24). A mechanistically-informed nosology may enable identification of improved treatment options and better matching to treatments.
Cloninger’s tridimensional personality theory for AUD, with three corresponding neurofunctional systems (25), was one of the first efforts to reimagine an addictions diagnosis on the basis of process and to propose a method for measuring the relevant domains. A main limitation of Cloninger’s scheme was that only a personality questionnaire was available to access the target processes, and, as will be seen later, subsequent addictions neuroscience investigations over the past two decades have led to a somewhat different conceptualization of the neurofunctional domains involved in addiction.
The Research Domain Criteria (RDoC) (26) initiative from the National Institute of Mental Health (NIMH) is a broad framework relevant to multiple psychiatric disorders. RDoC is intended to advance the goal of a neuroscience-based research framework for psychiatric diseases (12). Recently, an RDoC framework modified for alcoholism, Alcohol Addiction Research Domain Criteria (AARDoC), was proposed (27). Both RDoC and AARDoc, like Cloninger’s tridimensional personality structure, are research frameworks within which specific functional domains can be positioned and prioritized. Building on AARDoC, we propose a clinical framework for the assessment of addictions: ANA. ANA will provide the heuristic framework for measures of neurobiologic/neuropsychologic functions in AD and begin to address the practical problem of specifying a panel of instruments that may be widely used by researchers.
The need for ANA
Addictive disorders are a public health crisis. The 2013 National Survey on Drug Use and Health (NSDUH) estimated that 20.3 million adults had a substance use disorder (SUD), approximately 8.5% of the population (28). Some 1.3 million adolescents, or 5.2% of the U.S. adolescent population, had a SUD (28). Behavioral addictions are similarly pervasive; between one and three percent of U.S. individuals engage in pathological gambling, with high rates of comorbid psychiatric disorders among those who do (29). Availability of treatments for AD is limited, e.g., approximately 80% of individuals with alcoholism (30) and close to 90% percent of individuals with pathological gambling do not receive treatment (31). While the FDA-approved medications discussed above have efficacy, less than four percent of individuals use any medication for an alcohol use disorder (32). Because of advances in technology and our understanding of neuromechanisms of addiction, meshing neuroscience-based assessments with clinical measures appears feasible and imperative. Such an approach will build upon existing treatment options to find ones that are more targeted towards the individual.
Similar Initiatives
For addictions and other psychiatric disorders, partially overlapping conceptual frameworks and approaches are in place and underway worldwide. Knowledge gained from these may be brought to bear in designing ANA. We have identified five of particular relevance: RDoC (26), Impaired Response Inhibition and Salience Attribution (iRISA) (33), IMAGEN (34), PhenX (35), and Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) (36). We discuss each briefly, and compare the initiatives in Table 1.
Table 1.
Comparison of ANA and related initiatives.
| ANA | RDoC | iRISA | IMAGEN | PhenX | CNTRICS | |
|---|---|---|---|---|---|---|
| Neuroscience domains |
✔ | ✔ | ✔ | ✔ | ✔ | |
| Standardized assessment package |
✔ | ✔ | ✔ | ✔ | ||
| Disseminate package to various settings |
✔ | ✔ | ✔ | ✔ | ||
| Identify meaningful subtypes of disorder |
✔ | ✔ | ✔ | |||
| Describe individualized treatments |
✔ | ✔ |
RDoC originated as part of the NIMH 2008 strategic plan. The goal of RDoC is to create a research framework for studying psychiatric disorders. Grounded in neuroscience research, this framework spans five domains: Negative Valence Systems, Positive Valence Systems, Cognitive Systems, Systems for Social Processes, and Arousal and Regulatory Systems. The domains are further organized by units of analysis, ranging from genes to paradigms (see http://www.nimh.nih.gov/research-priorities/rdoc/research-domain-criteria-matrix.shtml for an overview of the RDoC matrix). ANA captures information in three of five RDoC domains. A major difference between the two is that RDoC serves as a research framework rather than a clinical framework. Many publications have expanded on conceptual and methodological implications of RDoC, e.g. (26, 37-40).
iRISA, as described by Goldstein and Volkow (33, 41), identifies disruptions in neural circuits that relate to AD, with an emphasis on response inhibition and salience attribution. The iRISA model presents an addiction cycle of intoxication, bingeing, withdrawal, and craving, and identifies the underlying neural disruptions with an emphasis on neuroadaptations and maladaptations in the prefrontal cortex (PFC) associated with each phase of the process. The framework presented in iRISA, and emphasis on disruptions in PFC function for AD, are relevant to all three of the domains that will be assessed in ANA.
The IMAGEN consortium, including collaborators from multiple European nations, has as its goal the identification of neurally-based predictors of increased risk for developing AD (see: http://www.imagen-europe.com). IMAGEN has recruited approximately 2,000 adolescents, who are being longitudinally followed. The standard neuroimaging battery includes measures of reward, emotion recognition, response inhibition, and general cognition. Other measures include neuropsychological testing and blood collection for genomic analyses. Publications using the IMAGEN sample range from data analytic methods (42, 43) to imaging-genetic findings related to reward, oxytocin function and others (44-46) and behavioral findings (47, 48). Unlike RDoC, IMAGEN does not seek to establish a framework of neurobiologic domains, but identifies useful assessments.
PhenX seeks to standardize the measurement of 21 domains including environmental exposures, demographics, and substance use (http://www.phenxtoolkit.org). PhenX was launched in 2007 by RTI International, with funding from the National Human Genome Research Institute. The measures were developed with input from researchers in academia, government, and scientific organizations. The PhenX toolkit includes a group of assessments specifically focused on substance abuse and addiction (SAA), identified with support from domain experts and funded by the National Institute on Drug Abuse (NIDA). The PhenX Real world Implementation and Sharing (RISING) consortium is a significant step forward in the practical application of PhenX measures (49). PhenX publications have been largely focused on implementation of PhenX measures (35, 50-53), including a recent publication on the commonality of findings in different addictive disorders across measures of addiction (54).
CNTRICS began with the primary goal of identifying neuroscience-based treatments to improve cognitive deficits associated with schizophrenia, with principal investigators at the University of California, Davis, and University of Washington, along with a steering committee of scientists from academia, government, and AstraZeneca, a pharmaceutical company. Extensive details about CNTRICS may be found at its website: http://cntrics.ucdavis.edu/index.shtml. The constructs include working memory, long-term memory, executive control, social/emotional processing, attention, and perception. The CNTRICS group has published extensively on the construct and task selection process, e.g.(55-58). Further, the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia (CNTRACS; http://cntracs.ucdavis.edu/) consortium has grown out of CNTRICS as a way to test the practicality and applicability of the measures identified.
ANA Domains
The ANA domains are derived from a conceptual framework in which AD lead to elements of impulsivity and compulsivity dysfunction. Three functional domains, executive function, incentive salience, and negative emotionality, are involved. Changes in these domains can be staged, heuristically, as: Binge-Intoxication (reward and incentive salience, habits, representing the incentive salience domain), Withdrawal-Negative Affect (stress and negative emotional states, including but not limited to withdrawal, representing the negative emotionality domain) and Preoccupation-Anticipation (executive function) (59). It is notable that a recent review (60), identified three major domains of neurofunctional impairment related to gambling disorder, namely loss of control, craving/withdrawal, neglect of other areas of life. These domains closely parallel the three ANA domains.
Executive Function
The executive function domain broadly includes processes related to organizing behavior towards future goals (61). Although including the totality of executive functions under ANA is infeasible, certain subdomains of executive function bear particular relevance for addictions. As described (61), we focus on executive function processes related to the cross-temporal organization of behavior, including attention, response inhibition, planning, working memory, behavioral flexibility, and valuation of future events. Taken together, these processes provide a reasonably comprehensive overview of those executive function systems disrupted in addictions.
Dysfunction in these processes is well-documented among individuals addicted to various agents. Deficits in attention have been shown among individuals addicted to alcohol (62), cocaine (63), and nicotine (64). Response inhibition is impaired among heroin (65) and methamphetamine (66) addicts and in pathological gamblers (67). Further, alterations in planning are evident among those addicted to nicotine (67) and opioids (68); disruptions in working memory are evident in alcohol (62), cocaine (63), and cannabis (69) addiction. Finally, behavioral flexibility is notably impaired among those addicted to cocaine (70) and amphetamine (71), and deficits in valuation of future events are well-documented in alcohol (72) and nicotine (73) addiction.
Dysfunction in executive function, producing loss of top-down control in the frontal cortex, is etiological in driving many of these deficits, and such top-down control directly impacts on incentive salience and impulsivity in the binge-intoxication stage presumably via glutamatergic connections to the basal ganglia and impacts on negative emotional states via glutamatergic connections to the extended amygdala (9).
Incentive Salience
Alterations in incentive salience are also well-documented among individuals with AD and have been intimately linked to the circuitry of the basal ganglia. The construct of incentive salience can be defined as a psychological process that transforms the perception of stimuli, imbuing them with salience, and making them attractive. Incentive salience as a construct has its roots in incentive motivation (74) and conditioned reinforcement (75), and was hypothesized to be linked directly to phasic activation of the mesocorticolimbic dopamine system (76). A series of studies were conducted in which investigators recorded from dopamine neurons in the ventral tegmental area in primates during repeated presentation of rewards and presentation of stimuli associated with reward. Dopamine cells fired upon the first exposure to a novel reward, but repeated exposure to dopamine caused the neurons to stop firing upon reward consumption and fire instead when they were exposed to stimuli that were predictive of the reward (77).
With respect to measures of various components of incentive salience, the neural responses of addicted individuals are altered to both cue and non-cue targets (78-80), with increased craving for substances in response to related cues (81, 82), and differences in reward learning (83). Importantly, cue reactivity to addictive agents is associated with increased risk for relapse (81, 84-86), and there are strong positive correlations between cue response and attentional bias (78, 87-89).
The phasic dopaminergic activation that drives incentive salience is hypothesized to also engage habit formation and compulsive-like responding for addictive agents via activation of cortical-striatal-pallidal-thalamic loops (90, 91).
Negative Emotionality
Increases in negative emotional responses to various stimuli and overall self-reported dysphoria are found in individuals with AD (92, 93). Clinicians and researchers have long considered the notion that reduction of negative affect may be a primary driver for excessive consumption of addictive agents (described alternately as self-medication or tension-reduction). Indeed, hypohedonia is widely documented as a clinical feature of AD (94-98) and is highly associated with increased craving for drugs of abuse (99) and relapse (100), which may contribute significantly to the increased salience of cues associated with addictive agents, and loss of interest in others, e.g., (97). A complete assessment of reward constructs must include measurement of hypohedonia (101).
Another key component of the negative emotional states associated with the withdrawal-negative affect stage of the addiction cycle is the engagement of the brain stress systems including both the hypothalamic –pituitary-adrenal axis and extrahypothalamic systems (102). The brain stress systems include such neurotransmitter systems as corticotropin releasing factor, dynorphin, norepinephrine, hypocretin (orexin), substance P, and vasopressin. Equally compelling is evidence for dysregulation of the brain anti-stress systems such as neuropeptide Y, nociception, endocannabinoids and oxytocin. Increased activity in brain stress systems and decreased activity in brain anti-stress systems are hypothesized to significantly contribute to negative emotionality (102).
“Omic” information capture in ANA
ANA is focused on capture of measures of three main neurofunctional domains, however, modern “omic” technologies enable the simultaneous capture of information relevant to these domains as well as information on comprehensive genetic, molecular, or neurofunctional variation, depending on the different technologies. To analyze a gene, or given set of genes, or to study their epigenetic control, it is often more cost effective, and informative, to use an “omic” sequencing- or array-based technology.
Although individual genes contribute a small proportion of the variance in development of addictions, they may still contribute understanding of the mechanisms leading to AD. For this reason, genetic sampling should be a standard, but ancillary part of ANA. The present importance of ANA for neuroassessment of addictions should not be overestimated, but the future importance of genetics for understanding heterogeneity within AD cannot be overestimated. Identifying genetic variants underlying phenotypic differences will maximize the utility of ANA, as will collection of DNA samples and genotyping with a one million marker array or similar tool. Further, analysis of changes in transcriptome, including microRNAs, and measurements of epigenetic changes in DNA and chromatin, may be critical for understanding neuroadaptations associated with heavy substance use (103). The goal is to use such changes as indices of function of molecular networks. It would be important to assess these changes in the context of longitudinal and/or large cross-sectional studies in which exposures and correlates of molecular responses are measured.
If feasible, exome sequencing should be performed. Whole genome SNP arrays enable comprehensive analysis for effects of common alleles of moderate or large effect. Most of these SNPs will not be strong predictors of individual outcome but may be key in understanding outcome, e.g., alcohol metabolic gene variants that predict alcohol-induced flushing, alcoholism risk, and, in moderate drinkers, esophageal cancer (104). Although pharmacogenetics is in early stages of research, progress is being made in identifying variants that predict clinical success (105, 106). For example, a common OPRM1 polymorphism predicts response of alcoholic patients to naltrexone (107), and via reward (108) although the results are mixed (109). Such analyses will allow ANA datasets to be combined with other samples that may only have available the clinical diagnosis, but with similar genomic analyses.
A critical aspect of ANA is use of neuroimaging. The use of positron emission tomography (PET) scanning has been essential to elucidating the role of dopamine in various AD, e.g. (110, 111). To significantly advance the nosology and treatment of addictions, we should use neuroimaging technologies that enable multidimensional information capture to understand the mechanisms driving these disorders. ANA will include functional MRI-based domain-specific assessments, along with imaging-based measures of brain structure (e.g. volume, morphometry, white matter integrity) and function, e.g., to assess differences in resting state functional connectivity identified in alcohol dependent patients (112). The salience of neuroimaging to ANA is underscored by recent imaging-genetics findings suggesting, for example, differences in neural response to alcohol cues as a function of genotype (113) and genetic modulation of neural connectivity related to nicotine addiction (114) and of resting state functional connectivity in AUD (115).
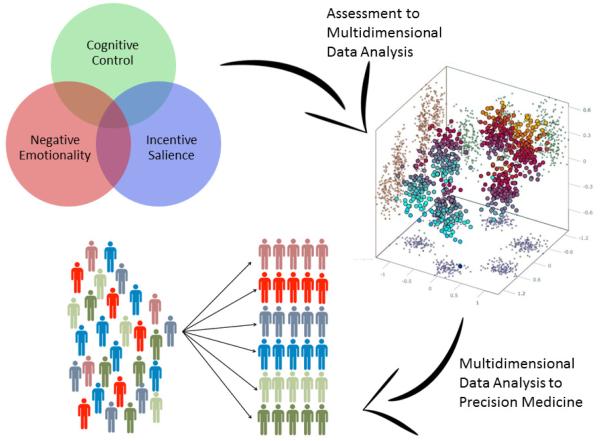
As mentioned, many measures specific to a specific addictive agent, including behavioral addictions, or to particular exposures and outcomes, would be ancillary to ANA. Guided by clinical problems, ANA should incorporate other measures of function and predisposition that are not included within the primary domains, but vital to the etiology and treatment of AD, e.g., habitual or compulsive use of an addictive agent. There are important distinctions in process and outcome between different addictive agents, and even for the same addictive agent within different individuals. A virtue of applying the same measures across different addictive disorders, including behavioral addictions, and in people with different exposures or at different points in the clinical course of addiction, is to better understand unifying mechanisms and variation at baseline and following maladaptive change. A schematic of the ANA domains and relevant ancillary assessment domains (Figure 1) illustrates the importance of core neuroassessment and the roles of other measures to improve the depth, breadth, and specificity of characterization of the individual patient. A comprehensive, although not final, list of potential measures, organized by domain, appears in Table 2. This battery would be supplemented by additional measures not included within the three domains but important for understanding AD, including features of agent use and outcomes, e.g., the Addiction Severity Index (116), Timeline Follow-Back (117), important aspects of personality, e.g., the NEO-PI-R (118), and environment, e.g., the Pittsburgh Sleep Quality Index (119), the Inventory of Socially Supportive Behaviors (120). A graphic depicting the process of multidimensional information capture to data analysis to improved diagnosis appears in Figure 2.
Figure 1.
ANA Primary Domains and Variables for Ancillary Assessment
Table 2.
Proposed Measures for ANA
| Executive Function | ||
|
| ||
| Measure | Time to Complete | Type of Task |
|
| ||
| Stop Signal Reaction Task (123) | 10 minutes | Behavioral |
| Appetitive Go-NoGo (124) | 10 minutes | Behavioral |
| Continuous Performance Test (125) | 15 minutes | Behavioral |
| Tower of London (126) | 15 minutes | Behavioral |
| Wisconsin Card Sorting Test (127) | 15 minutes | Behavioral |
| Delay Discounting (128) | 15 minutes | Behavioral |
| N-Back (129) | 10 minutes | Behavioral |
| Beads in a Jar Task (130) | 5 minutes | Behavioral |
| Barratt Impulsiveness Scale (131) | 5 minutes | Self-Report |
|
| ||
| Negative Emotionality | ||
|
| ||
| Measure | Time to Complete | Type of Task |
|
| ||
| Approach Avoidance Task (132) | 10 minutes | Behavioral |
| Cyberball (133) | 10 minutes | Behavioral |
| Trier Social Stress Test (134) | 20 minutes | Behavioral |
| Cold Pressor Task (135) | 10 minutes | Behavioral |
| Digit Span (136) | 5 minutes | Behavioral |
| Two-step Task (Model- Free Model-Based) (137) |
15 minutes | Behavioral |
| Beck Depression Inventory (138) | 5 minutes | Self-Report |
| Beck Anxiety Inventory (139) | 5 minutes | Self-Report |
| Fawcett-Clark Pleasure Scale (140) | 5 minutes | Self-Report |
| Toronto Alexithymia Scale (141) | 5 minutes | Self-Report |
| Childhood Trauma Questionnaire (142) |
5 minutes | Self-Report |
| Facial Emotion Matching Task (143) |
10 minutes | Neuroimaging |
|
| ||
| Incentive Salience | ||
|
| ||
| Measure | Time to Complete | Type of Task |
|
| ||
| Choice task (explicit version) (144) | 15 minutes | Behavioral |
| Dot-probe attentional bias task (cues) (145) | 10 minutes | Behavioral |
| Obsessive-Compulsive Drinking Scale (146) | 5 minutes | Self-Report |
| Cue Reactivity Task (80) | 10 minutes | Neuroimaging |
| Monetary Incentive Delay Task (147) |
10 minutes | Neuroimaging |
Figure 2.
Proposed ANA Process from Data Capture to Precision Medicine Implementation
Lastly, practical considerations regarding the implementation of ANA must be considered. Given the breadth of potential assessments, a comprehensive battery would take approximately 10 hours. Many of the measures could be collected in any setting with access to a laptop computer, although the MRI would require specialized facilities. We have made efforts to consider measures that may be attained free or at relatively little cost; the largest cost involved would be the use of MRI. Depending on resources, these may be obtained at a local academic or hospital setting. Additional costs include data analysis and interpretation. A range of ~3,000 to ~5,000 per individual seems feasible and, if resulting in significantly improved prognosis, well worth the investment.
ANA Summary
A few final points about these domains and their relevance for ANA bear mention. First, although we have highlighted significant positive findings in each domain, there is considerable variability in the literature. Not all individuals with AD evidence disruptions in the three primary domains. This variability is symptomatic of the need to systematically understand the heterogeneity within AD. Second, although presented independently, there is considerable overlap and interactions between domains at multiple levels of analysis. One of the most prominent examples is the relevance of PFC dysfunction for various aspects of AD (41). These disruptions underlie deficits in executive function, emotion regulation, and reward modulation, not surprising given the neurocircuitry connections (121). These domains do not comprise the totality of disturbances related to addiction, but serve as a useful starting framework for further exploration. Later studies might expand upon, known differences in alcohol response, e.g., those related to acute tolerance (122), and in responses to other drugs, whether of pharmacokinetic or pharmacodynamic origin.
Finally, several factors are challenges for application of ANA, including the magnitude of the problem of addiction, complexity of causation, and changing nature of problems that patients with AD experience over time. Furthermore, a broad combination of collaborations and partnerships in academia, government, and private industry will be needed to realize its advantages. This review has the more modest goal of providing a heuristic framework for ANA, with some evaluation of practicality. Given the multifactorial nature of AD, changing nature of exposure and response of human populations to addictive agents, anticipated development of new methods for treatment and prevention, and development of new, transformative technologies, we do not anticipate that any one functional domain or imaging or genetic predictor will resolve the heterogeneity of AD or be sufficient to characterize an individual patient. Rather, it is our goal that by collecting multidimensional information and focusing on a limited number of functional domains, our understanding of the mechanisms of addiction can be improved and prevention/treatment can be better targeted. Identifying the major domains underlying AD and how the profile of vulnerability to each domain varies among individuals, and over time, not only will be vital to understand the heterogeneity of the disorder, but will also enable us to tailor treatment more effectively to the individual.
Acknowledgements
We acknowledge the Division of Intramural Clinical and Biological Research, the Office of the Clinical Director, the Office of the Director, the Laboratory of Neurogenetics, and the Division of Treatment and Recovery Research, all at NIAAA. We thank the following individuals for their thoughtful feedback on the development of ANA: Vijay Ramchandani, Elliot Stein, Betty Jo Salmeron, Terry Goldberg, Rita Goldstein, B.J. Casey, and Valerie Voon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Jellinek EM. Alcoholism, a genus and some of its species. Canadian Medical Association journal. 1960;83:1341–1345. [PMC free article] [PubMed] [Google Scholar]
- 2.Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychology review. 2009;19:115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 3.Ball SA, Carroll KM, Babor TF, Rounsaville BJ. Subtypes of cocaine abusers: support for a type A-type B distinction. Journal of consulting and clinical psychology. 1995;63:115–124. doi: 10.1037//0022-006x.63.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Falck RS, Wang J, Carlson RG. Crack cocaine trajectories among users in a midwestern American city. Addiction. 2007;102:1421–1431. doi: 10.1111/j.1360-0443.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Bi J, Chan G, Oslin D, Farrer L, Gelernter J, et al. Improved methods to identify stable, highly heritable subtypes of opioid use and related behaviors. Addictive behaviors. 2012;37:1138–1144. doi: 10.1016/j.addbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramo DE, Grov C, Delucchi K, Kelly BC, Parsons JT. Typology of club drug use among young adults recruited using time-space sampling. Drug and alcohol dependence. 2010;107:119–127. doi: 10.1016/j.drugalcdep.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babor TF, Webb C, Burleson JA, Kaminer Y. Subtypes for classifying adolescents with marijuana use disorders: construct validity and clinical implications. Addiction. 2002;97(Suppl 1):58–69. doi: 10.1046/j.1360-0443.97.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 8.Wittchen HU, Behrendt S, Hofler M, Perkonigg A, Rehm J, Lieb R, et al. A typology of cannabis-related problems among individuals with repeated illegal drug use in the first three decades of life: Evidence for heterogeneity and different treatment needs. Drug and alcohol dependence. 2009;102:151–157. doi: 10.1016/j.drugalcdep.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 10.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 11.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of general psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 12.Association AP . Diagnostic and Statistical Manual of Mental Disorders. Fifth American Psychiatric Publishing, Incorporated; Washington, DC: 2013. [Google Scholar]
- 13.Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clinical psychology & psychotherapy. 2011;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- 14.Nelson CB, Rehm J, Ustun TB, Grant B, Chatterji S. Factor structures for DSM-IV substance disorder criteria endorsed by alcohol, cannabis, cocaine and opiate users: results from the WHO reliability and validity study. Addiction. 1999;94:843–855. doi: 10.1046/j.1360-0443.1999.9468438.x. [DOI] [PubMed] [Google Scholar]
- 15.Charney DS, Barlow DH, Botteron K, Cohen JD, Goldman D, Gur RE, et al. Neuroscience research agenda to guide development of a pathophysiologically based classification system. In: Kupfer DJ, First MB, Regier DA, editors. A research agenda for DSM—V. American Psychiatric Association; Arlington, VA, US: 2002. pp. 31–83. [Google Scholar]
- 16.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 17.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 18.Kadden R, Carbonari J, Litt M, Tonigan S, Zweben A. Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcoholism, clinical and experimental research. 1998;22:1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 19.Witkiewitz K, Marlatt A. Behavioral therapy across the spectrum. Alcohol Res Health. 2011;33:313–319. [PMC free article] [PubMed] [Google Scholar]
- 20.Ray LA, Hutchison KE, MacKillop J, Miranda R, Jr, Audette A, Swift R, et al. Effects of naltrexone during the descending limb of the blood alcohol curve. American Journal on Addictions. 2008;17:257–264. doi: 10.1080/10550490802138400. [DOI] [PubMed] [Google Scholar]
- 21.Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. American Journal of Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- 22.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate. CNS drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Scott L, Figgitt D, Keam S, Waugh J. Acamprosate. CNS Drugs. 2005;19:445–464. doi: 10.2165/00023210-200519050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Spanagel R, Putzke J, Stefferl A, Schöbitz B, Zieglgönsberger W. Acamprosate and alcohol: II. Effects on alcohol withdrawal in the rat. European journal of pharmacology. 1996;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 25.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 26.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 27.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcoholism, clinical and experimental research. 2015;39:579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- 28.Administration SAaMHS . In: 2013 National Survey on Drug Use and Health. Services HaH, editor. Washington, DC: 2013. [Google Scholar]
- 29.Lorains FK, Cowlishaw S, Thomas SA. Prevalence of comorbid disorders in problem and pathological gambling: systematic review and meta-analysis of population surveys. Addiction. 2011;106:490–498. doi: 10.1111/j.1360-0443.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 30.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slutske WS. Natural Recovery and Treatment-Seeking in Pathological Gambling: Results of Two U.S. National Surveys. American Journal of Psychiatry. 2006;163:297–302. doi: 10.1176/appi.ajp.163.2.297. [DOI] [PubMed] [Google Scholar]
- 32.Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug and alcohol dependence. 2009;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Molecular psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, et al. The PhenX Toolkit: get the most from your measures. American journal of epidemiology. 2011;174:253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophrenia bulletin. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter WT. RDoC and DSM-5: what's the fuss? Schizophrenia bulletin. 2013;39:945–946. doi: 10.1093/schbul/sbt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. The American journal of psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nymberg C, Jia T, Ruggeri B, Schumann G. Analytical strategies for large imaging genetic datasets: experiences from the IMAGEN study. Annals of the New York Academy of Sciences. 2013;1282:92–106. doi: 10.1111/nyas.12088. [DOI] [PubMed] [Google Scholar]
- 43.Thyreau B, Schwartz Y, Thirion B, Frouin V, Loth E, Vollstadt-Klein S, et al. Very large fMRI study using the IMAGEN database: sensitivity-specificity and population effect modeling in relation to the underlying anatomy. NeuroImage. 2012;61:295–303. doi: 10.1016/j.neuroimage.2012.02.083. [DOI] [PubMed] [Google Scholar]
- 44.Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, et al. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biological psychiatry. 2014;76:367–376. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 45.Muller KU, Gan G, Banaschewski T, Barker GJ, Bokde AL, Buchel C, et al. No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addiction biology. 2014 doi: 10.1111/adb.12136. [DOI] [PubMed] [Google Scholar]
- 46.Nymberg C, Banaschewski T, Bokde AL, Buchel C, Conrod P, Flor H, et al. DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2357–2365. doi: 10.1038/npp.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, et al. Aversive learning in adolescents: modulation by amygdala-prefrontal and amygdala-hippocampal connectivity and neuroticism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:875–884. doi: 10.1038/npp.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarty CA, Huggins W, Aiello AE, Bilder RM, Hariri A, Jernigan TL, et al. PhenX RISING: real world implementation and sharing of PhenX measures. BMC medical genomics. 2014;7:16. doi: 10.1186/1755-8794-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hendershot T, Pan H, Haines J, Harlan WR, Junkins HA, Ramos EM, et al. Using the PhenX Toolkit to Add Standard Measures to a Study. In: Haines Jonathan L, et al., editors. Current protocols in human genetics. 2011. Chapter 1:Unit1 21. [DOI] [PubMed] [Google Scholar]
- 51.Hitz MM, Conway PG, Palcher JA, McCarty CA. Using PhenX toolkit measures and other tools to assess urban/rural differences in health behaviors: recruitment methods and outcomes. BMC research notes. 2014;7:847. doi: 10.1186/1756-0500-7-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarty CA, Berg R, Rottscheit CM, Waudby CJ, Kitchner T, Brilliant M, et al. Validation of PhenX measures in the personalized medicine research project for use in gene/environment studies. BMC medical genomics. 2014;7:3. doi: 10.1186/1755-8794-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan H, Tryka KA, Vreeman DJ, Huggins W, Phillips MJ, Mehta JP, et al. Using PhenX measures to identify opportunities for cross-study analysis. Human mutation. 2012;33:849–857. doi: 10.1002/humu.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conway KP, Vullo GC, Kennedy AP, Finger MS, Agrawal A, Bjork JM, et al. Data compatibility in the addiction sciences: an examination of measure commonality. Drug and alcohol dependence. 2014;141:153–158. doi: 10.1016/j.drugalcdep.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barch DM, Berman MG, Engle R, Jones JH, Jonides J, Macdonald A, 3rd, et al. CNTRICS final task selection: working memory. Schizophrenia bulletin. 2009;35:136–152. doi: 10.1093/schbul/sbn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ. CNTRICS imaging biomarkers selection: Working memory. Schizophrenia bulletin. 2012;38:43–52. doi: 10.1093/schbul/sbr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter CS, Barch DM, Gur R, Gur R, Pinkham A, Ochsner K. CNTRICS final task selection: social cognitive and affective neuroscience-based measures. Schizophrenia bulletin. 2009;35:153–162. doi: 10.1093/schbul/sbn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragland JD, Cohen NJ, Cools R, Frank MJ, Hannula DE, Ranganath C. CNTRICS imaging biomarkers final task selection: Long-term memory and reinforcement learning. Schizophrenia bulletin. 2012;38:62–72. doi: 10.1093/schbul/sbr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 60.Romanczuk-Seiferth N, van den Brink W, Goudriaan AE. From Symptoms to Neurobiology: Pathological Gambling in the Light of the New Classification in DSM-5. Neuropsychobiology. 2014;70:95–102. doi: 10.1159/000362839. [DOI] [PubMed] [Google Scholar]
- 61.Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, et al. Adolescent Substance Abuse: The Effects of Alcohol and Marijuana on Neuropsychological Performance. Alcoholism: Clinical and Experimental Research. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalapatapu RK, Vadhan NP, Rubin E, Bedi G, Cheng WY, Sullivan MA, et al. A Pilot Study of Neurocognitive Function in Older and Younger Cocaine Abusers and Controls. The American Journal on Addictions. 2011;20:228–239. doi: 10.1111/j.1521-0391.2011.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yakir A, Rigbi A, Kanyas K, Pollak Y, Kahana G, Karni O, et al. Why do young women smoke? III. Attention and impulsivity as neurocognitive predisposing factors. European Neuropsychopharmacology. 2007;17:339–351. doi: 10.1016/j.euroneuro.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Pau CW, Lee TM, Shui-fun FC. The impact of heroin on frontal executive functions. Archives of Clinical Neuropsychology. 2002;17:663–670. [PubMed] [Google Scholar]
- 66.Salo R, Nordahl TE, Moore C, Waters C, Natsuaki Y, Galloway GP, et al. A dissociation in attentional control: evidence from methamphetamine dependence. Biological Psychiatry. 2005;57:310–313. doi: 10.1016/j.biopsych.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 67.Roca M, Torralva T, López P, Cetkovich M, Clark L, Manes F. Executive functions in pathologic gamblers selected in an ecologic setting. Cognitive and Behavioral Neurology. 2008;21:1–4. doi: 10.1097/WNN.0b013e3181684358. [DOI] [PubMed] [Google Scholar]
- 68.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolla KI, Brown K, Eldreth D, Tate K, Cadet J. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 70.Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. The Journal of neuropsychiatry and clinical neurosciences. 2014 doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- 71.Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of Cognitive Dysfunction in Chronic Amphetamine and Heroin Abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 72.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 73.Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Experimental and clinical psychopharmacology. 2007;15:187. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- 74.Bindra D. A motivational view of learning, performance, and behavior modification. Psychological review. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- 75.Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- 76.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research Brain research reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 77.Schultz W. Dopamine neurons and their role in reward mechanisms. Current opinion in neurobiology. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 78.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and biobehavioral reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T. Midbrain-driven emotion and reward processing in alcoholism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1844–1853. doi: 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction Biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction biology. 2012;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, et al. Role of GABRA2 in moderating subjective responses to alcohol. Alcoholism, clinical and experimental research. 2011;35:400–407. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jokisch D, Roser P, Juckel G, Daum I, Bellebaum C. Impairments in learning by monetary rewards and alcohol-associated rewards in detoxified alcoholic patients. Alcoholism, clinical and experimental research. 2014;38:1947–1954. doi: 10.1111/acer.12460. [DOI] [PubMed] [Google Scholar]
- 84.Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cueinduced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 85.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and alcohol review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 87.Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological bulletin. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 89.Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, et al. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction biology. 2012;17:807–816. doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- 90.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neuroscience and biobehavioral reviews. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Frontiers in psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinz A, Schmidt LG, Reischies FM. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients--neurobiological correlates. Pharmacopsychiatry. 1994;27(Suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- 96.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biological psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of substance abuse treatment. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, et al. Alcohol protracted withdrawal syndrome: the role of anhedonia. Substance use & misuse. 2008;43:271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- 99.Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, et al. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- 100.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 101.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry : the journal of mental science. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 102.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong CCY, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011;106:480–489. doi: 10.1111/j.1360-0443.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- 104.Brooks PJ, Goldman D, Li TK. Alleles of alcohol and acetaldehyde metabolism genes modulate susceptibility to oesophageal cancer from alcohol consumption. Human genomics. 2009;3:103–105. doi: 10.1186/1479-7364-3-2-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones JD, Comer SD, Kranzler HR. The Pharmacogenetics of Alcohol Use Disorder. Alcoholism: Clinical and Experimental Research. 2015;39:391–402. doi: 10.1111/acer.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of general psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, et al. Naltrexone vs Placebo for the Treatment of Alcohol Dependence: A Randomized Clinical Trial. JAMA psychiatry. 2015;72:430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- 110.Volkow ND, Chang L, Wang G-J, Fowler JS, Ding Y-S, Sedler M, et al. Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association With Metabolism in the Orbitofrontal Cortex. American Journal of Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 111.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, et al. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. The Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addiction biology. 2015 doi: 10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential Neural Response to Alcohol Priming and Alcohol Taste Cues Is Associated With DRD4 VNTR and OPRM1 Genotypes. Alcoholism: Clinical and Experimental Research. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proceedings of the National Academy of Sciences. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu XH, Sarah G, Dutta N, Schwandt M, Yan Jia, Hodgkinson Colin A., Cortes Carlos, Kerich Michael, Hall Samuel, Sun Hui, Phillips Monte, Momenan Reza, Lohoff Falk W. VMAT1 influences withdrawal severity and resting-state functional connectivity in alcohol dependence. Human Brain Mapping. doi: 10.1002/hbm.22951. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of substance abuse treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 117.Sobell LC, Sobell MB. Measuring alcohol consumption. Springer; 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- 118.Costa PT, McCrae RR. NEO PI-R. Professional manual Odessa, FL: Psychological Assessment Resources. 1992:3. [Google Scholar]
- 119.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 120.Stokes JP, Wilson DG. The inventory of socially supportive behaviors: Dimensionality, prediction, and gender differences. American journal of community psychology. 1984;12:53–69. doi: 10.1007/BF00896928. [DOI] [PubMed] [Google Scholar]
- 121.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- 123.Loeber S, Duka T. Acute alcohol impairs conditioning of a behavioural reward-seeking response and inhibitory control processes—implications for addictive disorders. Addiction. 2009;104:2013–2022. doi: 10.1111/j.1360-0443.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- 124.Somerville LH, Hare T, Casey B. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of cognitive neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Archives of clinical neuropsychology. 2002;17:235–272. [PubMed] [Google Scholar]
- 126.Krikorian R, Bartok J, Gay N. Tower of London procedure: a standard method and developmental data. Journal of Clinical and Experimental Neuropsychology. 1994;16:840–850. doi: 10.1080/01688639408402697. [DOI] [PubMed] [Google Scholar]
- 127.Heaton RK. Wisconsin card sorting test: computer version 2. Odessa: Psychological Assessment Resources. 1993 [Google Scholar]
- 128.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 129.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Phillips LD, Edwards W. Conservatism in a simple probability inference task. Journal of experimental psychology. 1966;72:346. doi: 10.1037/h0023653. [DOI] [PubMed] [Google Scholar]
- 131.Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 132.Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: The Approach–Avoidance Task. Behaviour research and therapy. 2007;45:2990–3001. doi: 10.1016/j.brat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 133.Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior research methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- 134.Kirschbaum C, Klauer T, Filipp S-H, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology. 1975;12:268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 136.Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sebold M, Deserno L, Nebe S, Schad DJ, Garbusow M, Högele C, et al. Model-based and model-free decisions in alcohol dependence. Neuropsychobiology. 2014;70:122–131. doi: 10.1159/000362840. [DOI] [PubMed] [Google Scholar]
- 138.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8:77–100. [Google Scholar]
- 139.Steer RA, Beck AT. Beck Anxiety Inventory. 1997 [Google Scholar]
- 140.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients: the pleasure scale. Archives of General Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 141.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale— I. Item selection and cross-validation of the factor structure. Journal of psychosomatic research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 142.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 143.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 144.Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, et al. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biological psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O'Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and alcohol dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 146.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 147.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]