Abstract
Diminished adult neurogenesis is known to play a key role in the pathogenesis of diverse neurodegenerative disorders such as HIV-associated neurological disorders (HAND). Cocaine, often abused by HIV-infected patients, has been suggested to worsen HIV-associated CNS disease. Mounting evidence also indicates that HIV-infection can lead not only to neuronal dysfunction or loss, but can also negatively impact neurogenesis, resulting in generation of fewer adult neural progenitor cells (NPCs) in the dentate gyrus of the hippocampus, brain area critical for memory and learning. The crucial role of Platelet-Derived Growth Factor-BB (PDGF-BB) in providing tropic support for the neurons as well as in promoting NPC proliferation has been demonstrated by us previously. However, whether PDGF-BB regulates neuronal differentiation especially in the context of HAND and drug abuse remains poorly understood. In this study we demonstrate that pre-treatment of rat hippocampal NPCs with PDGF-BB restored neuronal differentiation that had been impaired by HIV Tat & cocaine. To further study the intracellular mechanism(s) involved in this process, we examined the role of transient receptor potential canonical (TRPC) channels in mediating neuronal differentiation in the presence of PDGF-BB. TRPC channels are Ca2+-permeable, nonselective cationic channels that elicit a variety of physiological functions. Parallel but distinct ERK, Akt signaling pathways with downstream activation of CREB were found to be critical for neuronal differentiation. Pharmacological blocking of TRPC channels resulted in suppression of PDGF-mediated differentiation and PDGF-BB-induced activation of ERK and Akt, culminating also to inhibition of PDGF-induced activation of CREB. Taken together these findings underpin the role of TRPC channel as a novel target regulating cell differentiation mediated by PDGF-BB. This finding could have implications for development of therapeutic interventions aimed at restoration of Tat and cocaine-mediated impairment of neurogenesis in drug abusing HAND patients.
Keywords: Cocaine, HIV Tat, Neurogenesis, TRPC Channels, Neural Progenitor Cell, CREB, Ca2+
Background
Diminished adult neurogenesis is known to play a key role in the pathogenesis of diverse neurodegenerative disorders such as HIV-associated neurological disorders (HAND). In fact, HIV infection and drug abuse are emerging as interlinked epidemics. Cocaine, often abused by HIV-infected patients, has been suggested to exacerbate neurpathogenesis of HIV infection via upregualtion of virus replication [1], enhancing the breach of blood-brain barrier [2], and mediating the activation of glial cells [3, 4]. It is well documented that in response to injury, in addition to the glial cells, the neuronal progenitor cells (NPCs) that can both proliferate and differentiate leading to restoration of neuronal homeostasis, are the key players of the host protective response. Since HIV Tat and cocaine co-operatively mediate cognitive impairment, it is plausible that not only are these two agents impacting neuronal loss/injury but that they can also impair the ability of NPCs to mediate appropriate neurogenesis, leading to generation of fewer adult neural progenitor cells (NPCs) in the dentate gyrus of the hippocampus, an important region for memory and learning [5]. While our previous studies have demonstrated that HIV Tat together with cocaine can significantly inhibit the proliferation of NPCs, whether the two can also inhibit neuronal differentiation remains unexplored and is the focus of this study.
Neuronal progenitor cells are of importance in brain therapeutics owing to their capacity to differentiate into neuronal cells, thereby representing a potential source for neuronal replacement. These cells also serve as a model for studying factors controlling early stages of neuronal differentiation. Diverse family of neurotrophins such as fibroblast growth factor (FGF) & brain-derived neurotropic factors (BDNF) are known to control neurogenesis by maintaining neuronal homeostasis via a range of signaling mechanisms[6]. Studies from our lab have identified the role of platelet-derived growth factor-B chain (PDGF-B) as a critical factor that reverses neuronal toxicity mediated by HIV Tat & cocaine while also promoting proliferation of NPCs [7]. The dimeric isoform of PDGF-B chain (PDGF-BB) has also been implicated as a key factor in the developing postnatal rat brains [8]. Whether PDGF-BB can also facilitate neuronal differentiation has never been explored before and was the focus of the current study.
Similar to early neural development in vivo, several signaling pathways and related transcription factors play important roles in the regulation of cell growth and differentiation of NPCs. It has been shown that the mitogen-activated protein kinase (MAPK) pathway is of particular interest since its activation is critical for cell survival, differentiation and growth during neural development [9, 10]. Previous studies have identified the role of extracellular signal-regulated protein kinase (ERK) and phosphoinositide-3-kinase (PI3K)/Akt pathways in PDGF-BB mediated protection of NPCs against HIV Tat and cocaine toxicity[7]. Following activation with PDGF-BB, ERK is translocated to the nucleus, leading to downstream activation of the transcription factors such as the cAMP-response element-binding protein (CREB), that ultimately lead to increased neuronal survival[11]. While the role of signaling pathways in neuronal survival are well recognized, the roles of ERK and Akt pathway and the potential downstream transcription factor CREB in neuronal differentiation remains less clear. In addition, recent studies have identified that activation of transient receptor potential canonical channels (TRPC), that belong to a family of Ca2+-permeable, nonselective cation channels, is critical for neuronal development in various models [12, 13]. TRPCs are formed by homomeric or heteromeric complexes of TRP proteins that constitute at least six subfamilies: TRPC (TRP-canonical), TRP-vanilloid, TRPM (TRP-melastatin), TRP-mucolipins, TRPPs (TRP-polycystins), and TRPA1 (TRP-ankyrin transmembrane protien1). These channels are important in various physiological processes ranging from sensation to male fertility [12]. Our previous findings have demonstrated that PDGF-BB mediated protection of neurons against HIV Tat-mediated neurotoxicity and NPC proliferation involved the TRPC channels-both in vitro & in vivo [14]. We thus sought to examine whether TRPC channels were also essential for neuronal differentiation mediated by PDGF-BB and, if so, what were the downstream effectors of TRPC channel activation.
In this study we provde direct evidence that PDGF-BB reverses HIV Tat and cocaine mediated impairment of neuronal differentiation. We demonstrate distinct signaling pathways-TRPC/ERK as well as PI3K/Akt to play a role in this process. We also confirmed the activation of CREB in PDFG-BB mediated differentiation of NPCs. Our data support the possibility that PDGF signaling can be harnessed as an adjunctive therapy in HIV-infected cocaine-abusing individuals by actively promoting neurogenesis.
Results
Selective neuronal differentiation of NPCs mediated by PBDG-BB exposure
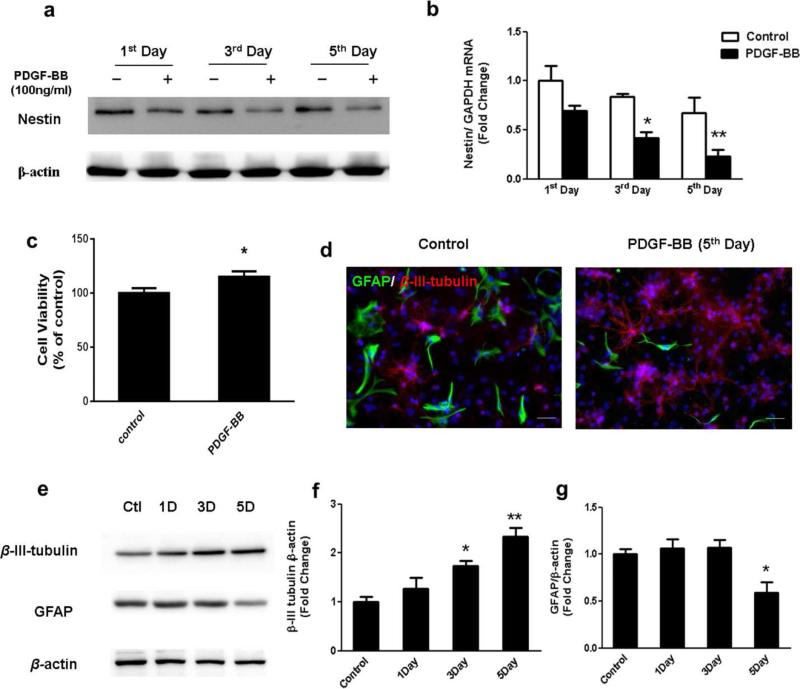
Newly generated neural cells undergo differentiation before they mature and become functional. An essential step for therapeutic and research applications of stem cells is their ability to differentiate into specific cell types. Neuronal cells are of great interest for medical treatment of neurodegenerative diseases, however, efforts to generate these cells have been met with only modest success. Having determined the role of PDGF-BB in promoting proliferation in our previous studies [15, 16], it was of interest to investigate the role of PDGF-BB (100ng/ml) in the differentiation of NPCs. Nestin, a class VI intermediate filament protein which is highly expressed in NPCs and does not express in differentiated cells, has been utilized as a biological marker to identify undifferentiated NPCs [17]. The differentiation rates of NPCs on the 1st, 3th and 5th days after PDGF-BB treatment were determined by immunoblotting as well as quantitative PCR. As shown in Fig.1a and 1b, there was significant decrease of nestin expression in PDGF-BB-exposed group on day 3 and 5. We also confirmed that the viability of the differentiated NPCs after PDGF-BB exposure were not reduced as shown in Figure 1c. These experimental results demonstrated that PDGF-BB treatment significantly enhanced neuronal differentiation of NPCs without reducing the viability of NPCs.
Figure 1. PDGF-BB promotes neuronal differentiation of rat primary NPCs.
Representative blots (a) and RT-PCR (b) showing the protein as well as gene expression level of Nestin, a NPC maker in control group and the PDGF-BB treated group after one, three, and five days culture; (c) Effect of PDGF-BB on the cell survival of rat primary NPCs using the MTT assay. (d) Representative immunofluorescence images showing the expression of neuronal maker β-III-Tubulin (Red), astrocyte maker GFAP (Green) and nuclei DAPI staining (Blue) in NPCs after 5days culture in control or PDGF-BB treated group. Scale bar: 20 μm; (e) Representative blots and quantification of the blot density (f)representative blots showed the expression pattern of β-Tubulin III and GFAP in NPCs cultured for 1day, 3 days and 5 days with PDGF-BB exposure. All the data in these figures are presented as mean±S.D. of at least three individual experiments. *P<0.05 versus control group, **P<0.01 versus control group.
The next step was to identify the specific types of cells generated from NPCs following PDGF-BB exposure. β-III-tubulin is a cytoskeletal protein whose expression increases during axonal outgrowth, and it is expressed exclusively by neurons and considered to be a specific marker for neurons. Glial Fibrillary Acidic Protein (GFAP), which is thought to maintain mechanical strength and shape of cells, is primarily expressed by astrocytes. Immunostaining of cells with the respective cell-specific markers was used to determine the phenotype of differentiated NPCs following PDGF-BB exposure for 5 days. As evident in Fig 1d, PDGF-BB exposure resulted in alterations in cellular morphology with formation of neurites and cell body extensions. This was further confirmed by immunoblotting analysis shown in Figure 1e-g, Results indicated that exposure of NPCs to PDGF-BB resulted in a time-dependent increase in the expression of the neuronal marker (β-III-tubulin), with a concomitant decrease in the expression of astrocyte marker (GFAP). These findings thus suggested that PDGF-BB stimulated neuronal differentiation while inhibiting astrocyte differentiation. Thus our results indicated that PDGF-BB treatment can effectively promote in vitro differentiation of NPCs predominantly into cells of the neuronal lineage.
PDGF-BB reverses HIV-1 Tat & cocaine-mediated impairment of neuronal differentiation
Our previous studies have illustrated the important role of PDGF-BB in ameliorating HIV Tat & cocaine-mediated reduction of NPC proliferation, so we next sought to examine the role of Tat & cocaine in mediating neuronal differentiation of NPC. To assess the effect of Tat & cocaine on neuronal differentiation, NPCs were exposed to cocaine (10 μM) & Tat (100ng/ml) and assessed for neuronal differentiation maker at various time points (1, 3 & 5 days). Both Tat and cocaine concentrations used in our study are in the physiological range as reported previously[3]. As shown in Fig.2a, exposure of NPCs with Tat & cocaine resulted in decreased expression of β-III-tubulin compared with the control groups and this effect was time-dependent. Interestingly, in the 5 day treatment group, we were unable to determine the neuronal phenotype in differentiated NPCs (Fig.2b). These findings thus suggested that both Tat & cocaine impaired NPC differentiation with decreased differentiation towards the neuronal phenotype. Since PDGF-BB can reverse NPC proliferation defect mediated by Tat & cocaine, we thus examined that whether PDGF-BB can also promote neurogenesis by enhancing differentiation of NPCs towards a neuronal lineage. We thus next sought to examine the role of PDGF-BB-mediated differentiation of NPCs exposed to HIV Tat & cocaine. NPCs were treated with or without Tat & cocaine followed with PDGF-BB (100ng/ml) treatment for 5 days and assessed for differentiation of neuronal maker β-III-tubulin. As shown in Figure 2c, immunoblotting analysis indicated that PDGF-BB significantly reversed Tat & cocaine impaired neuronal differentiation by upregulating the expression of β-III-tubulin. To confirm specific neural cell lineages, quantitative PCR was used to measure gene expression after the PDGF-BB treatment. In Fig 2d, the results showed that Tat & cocaine exposure down-regulated β-III-tubulin expression thereby indicating that the number of neuronal differentiated NPCs was much less compared to the untreated control group. PDGF-BB treatment significantly reversed Tat & cocaine mediated downregulation of gene expression for β-III-tubulin. This result was also confirmed by immunostaining as shown in Fig 2e. Taken together these finding suggested that PDGF-BB plays an important role in reversing HIV Tat & cocaine mediated impairment of neuronal differentiation.
Figure 2. HIV-Tat & Cocaine caused loss of neuronal differentiation is reversed by PDGF-BB exposure.
(a) Expression of neuronal maker β-III-Tubulin was determined from NPCs cultured for 1 day, 3 days, 5 days under the exposure to HIV Tat & cocaine by Western Blot analysis; (b) Representative immunofluorescence images showing the β-III-Tubulin expression in differentiated NPCs after HIV Tat& Cocaine treatment for 5 days. Scale bar: 20 μm; (c) Western blot analysis as well as RT-PCR analysis (d) of β-III-Tubulin expression level in cells exposed to HIV Tat & cocaine in the absence or presence of PDGF-BB; (e) Representative immunofluorescence images showing the β-III-Tubulin expression in differentiated NPCs after HIV Tat& Cocaine treatment for 5 days with or with PDGF-BB exposure. Scale bar: 20 μm. The data are presented as mean±S.D. of at least three individual experiments. **P<0.01 versus control group; ##P< 0.01 versus Tat& cocaine -treated group.
TRPC channels contribute to PDGF-BB-increased Ca2+ transients in the presence of HIV Tat & cocaine
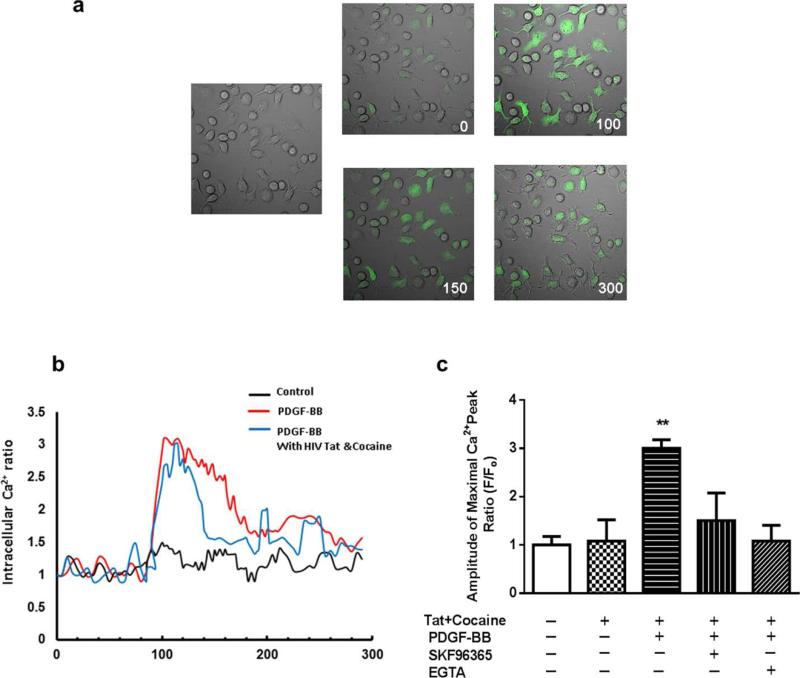
Based on the premise that Ca2+ signaling play a key role during the three critical steps of neurogenesis: proliferation, differentiation, and migration. All of these processes have also been shown to be Ca2+ dependent. A deeper digesting of how Ca2+ signaling contribute to PDGF-BB mediated neuronal differentiation in the presence of HIV Tat & cocaine is crucial for studying the underlined intracellular mechanism in this process. To study the effect of PDGF-BB on the intracellular Ca2+ level, we measured Ca2+ transients by Fluo-4 dependent Ca2+ imaging. Exposure of NPCs to PDGF-BB triggered rapid and sustained intracellular Ca2+ elevation in NPCs (Fig. 3a). This response was also observed in NPCs in the presence of HIV Tat& cocaine (Fig. 3b).Based on our previous studies that TRPC channels function as Ca2+ influx channels and they contribute to PDGF-BB mediated neuronal survival in the presence of HIV Tat & cocaine. We rationalized that PDGF-BB initiate Ca2+ transients via TRPC channels which contribute to PDGF-BB mediated neuronal differentiation in the presence of HIV Tat&cocaine in NPCs. NPCs were pretreated with the TRPC blocker SKF96365 followed by PDGF-BB exposure and subsequently assessed for Ca2+ signaling in the presence or absence of Tat & cocaine. Pretreatment of NPCs with SKF96365 resulted in failure of PDGF-BB to initiate Ca2+ transients (Fig. 3c). Additional validation of the role of extracellular Ca2+ influx in PDGF-BB mediated Ca2+ transients was supported by pretreating the cells with EGTA, which resulted in suppression of PDGF-BB mediated Ca2+ response in NPCs (Fig. 3c).
Figure 3. TRPC channels contribute to PDGF-BB-increased Ca2+ influx in the presence of HIV Tat and cocaine.
(a) NPCs loaded with Fluo-4 [Ca2+]i sensitive fluorophores exposed to PDGF-BB were recorded within a single field using a Zeiss ISM 710 confocal microscope (right panel, numbers in the panels indicate time in seconds) and differential interference contrast (left panel). (b) Changes of mean fluorescent intensity (F/Fo) in HIV Tat & cocaine exposed NPCs in the absence or presence of PDGF-BB. (c) Bars represent the Ca2+ fluorescence intensity at the maximal amplitude in NPCs exposed to Tat+cocaine &/or PDGF-BB in the absence or presence of indicated drugs (SKF96365 20 μM; EGTA: 2mM). **P<.01 versus control group.
TRPC channels are critical for PDGF-BB-mediated increase of neuronal differentiation of NPCs
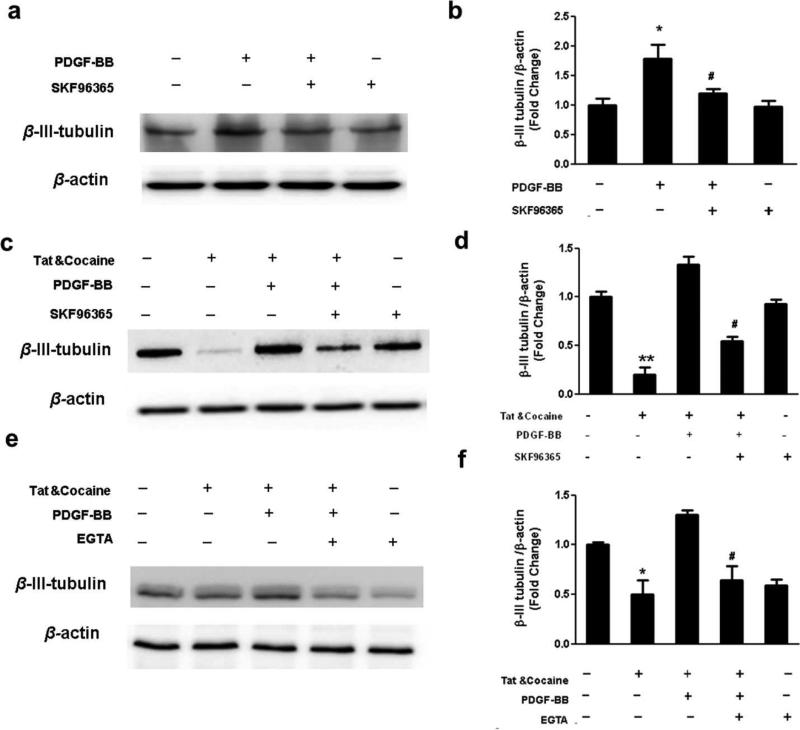
Since TRPC channels has been shown to play an important role in PDGF-BB mediated neurogenesis, we rationalized that PDGF-BB will promote neurogenesis by enhancing differentiation of NPCs towards a neuronal lineage through activation of TRPC pathway and that Ca2+ influx through TRPC channels is a prerequisite for increased neurogenesis mediated by PDGF-BB. To study the effect of TRPC channels on neuronal differentiation mediated by PDGF-BB, NPCs were pretreated with the pan TRPC inhibitor SKF96365 (20 μM) followed PDGF-BB exposure. Our results indicated that the TRPC blocker abolished PDGF-BB-mediated increase in neuronal differentiation (Fig.4a&b), thus underscoring the important role of TRPC in PDGF-BB mediated neuronal differentiation. Next, we sought to examine the role of TRPC channels in PDGF-BB mediated neuronal differentiation in the presence of Tat & cocaine. Pretreatment of NPCs with SKF96365 resulted in failure of PDGF-BB to rescue Tat & cocaine-mediated inhibition of neuronal differentiation (Fig4.c&d). Additional validation of the role of extracellular Ca2+ influx in PDGF-BB mediated neuronal differentiation of NPCs was supported by pretreating the cells with EGTA, which resulted in suppression of neuronal differentiation mediated by PDGF-BB (Fig.4e&f).
Figure 4. Ca2+ transients via activation of TRPC channels contribute to PDGF-BB mediated neuronal differentiation.
(a) Expression of neuronal maker β-III-Tubulin was determined from NPCs exposed to PDGF-BB in the absence or presence of TRPC channels inhibitor SKF96365 (20 μm) by Western Blot analysis and (b) the quantification of blot density. *P<0.05 versus control group; # P<0.01 versus PDGF-BB treated group; (c-d) Western blot analysis showing the effect of TRPC channels on PDGF-BB-mediated protective effect of neuronal differentiation from HIV-Tat & cocaine treatment in NPCs. NPCs were pretreated with TRPC channels inhibitor SKF96365 followed by assessment of β-III-Tubulin expression level. (e-f) Western blot analysis showing the effect of calcium on PDGF-BB-mediated protective effect of neuronal differentiation from HIV-Tat & cocaine treatment in NPCs. NPCs were pretreated with calcium chelator EGTA (2mM) followed by assessment of β-III-Tubulin expression level. The data are presented as mean±S.D. of three individual experiments. *P<0.05 versus control group; **P<0.01 versus control group; #P< 0.05 Versus both PDGF and HIV Tat & cocaine-treated group.
Activation of ERK/Akt signal pathways are important for PDGF-BB mediated neuronal differentiation
The MAPK and PI3K/Akt pathway has been demonstrated to play a crucial role in PDGF-BB mediated NPC and neuronal proliferation based on previous reports from others as well as us. It was therefore of interest to examine the effect of ERK and Akt in PDGF-BB mediated neuronal differentiation of NPCs. Exposure of NPCs to PDGF-BB resulted in sustained activation of ERK as well as PI3K/Akt pathways (Fig.5a). Having determined the role of TRPC channels in PDGF-BB-mediated neuronal differentiation, we next wanted to examine the role of these channels in PDGF-BB-mediated activation of ERK and Akt. Interestingly, pretreatment of NPCs with SKF96365 significantly attenuated PDGF-BB-induced ERK but not Akt phosphorylation, thereby suggesting that PDGF-BB-mediated activation of ERK rather than Akt involved TRPC signaling (Fig.5b). The functional role of PDGF-BB-induced ERK/Akt activation was further corroborated by detecting presence of neuronal markers in differentiated NPCs. In cells pretreated with either the ERK inhibitor U0126 or the PI3K/Akt inhibitor LY294002, PDGF-BB failed to exert its protective effect (Fig.5e), thereby underpinning the role of ERK and AKT in PDGF-BB –mediated neuronal differentiation. To specifically confirm the role of EKR/Akt activation in PDGF-BB-mediated neuronal differentiation in the presence of Tat & cocaine, we again sought the approach of pharmacological inhibitors. By pretreating NPCs with ERK as well as Akt inhibitors, PDGF-BB failed to restore Tat & cocaine mediated loss of neuronal differentiation (Fig.5f).
Figure 5. PDGF-induced neuronal differentiation involves activation of ERK and PI3K/Akt pathways.
(a) Representative blot of PDGF-mediated ERK and Akt activation in NPCs exposed to PDGF for 1 day, 3 days and 5 days respectively. (b) Representative blot of PDGF-mediated phosphorylation of ERK and Akt in NPCs in the presence or absence of TRPC channels inhibitors SKF96365 (20 μm); (c-d) Activation of ERK/Akt are essential for PDGF-mediated neuronal differentiation. The expression level of β-III-Tubulin was determined by western blot analysis alone with blot intensity quantification. *P<0.05 versus control group; # P<0.01 versus PDGF-BB treated group; (e-f) The expression level of β-III-Tubulin under HIV Tat & cocaine exposure & PDGF-BB in the presence or absence of PI3K inhibitor LY294002 (20 μm) was determined by western blot analysis alone with blot intensity quantification. (g-h) The expression level of β-III-Tubulin under HIV Tat & cocaine exposure & PDGF-BB in the presence or absence of ERK inhibitor U0126 (20 μm) was determined by western blot analysis alone with blot intensity quantification. The data are presented as mean±S.D. of three individual experiments. *P<0.05 versus control group; #P< 0.05 versus both PDGF and HIV Tat & cocaine-treated group.
Activation of downstream transcriptional factor CREB by PDGF-BB rescues Tat & cocaine-mediated impairment of neuronal differentiation
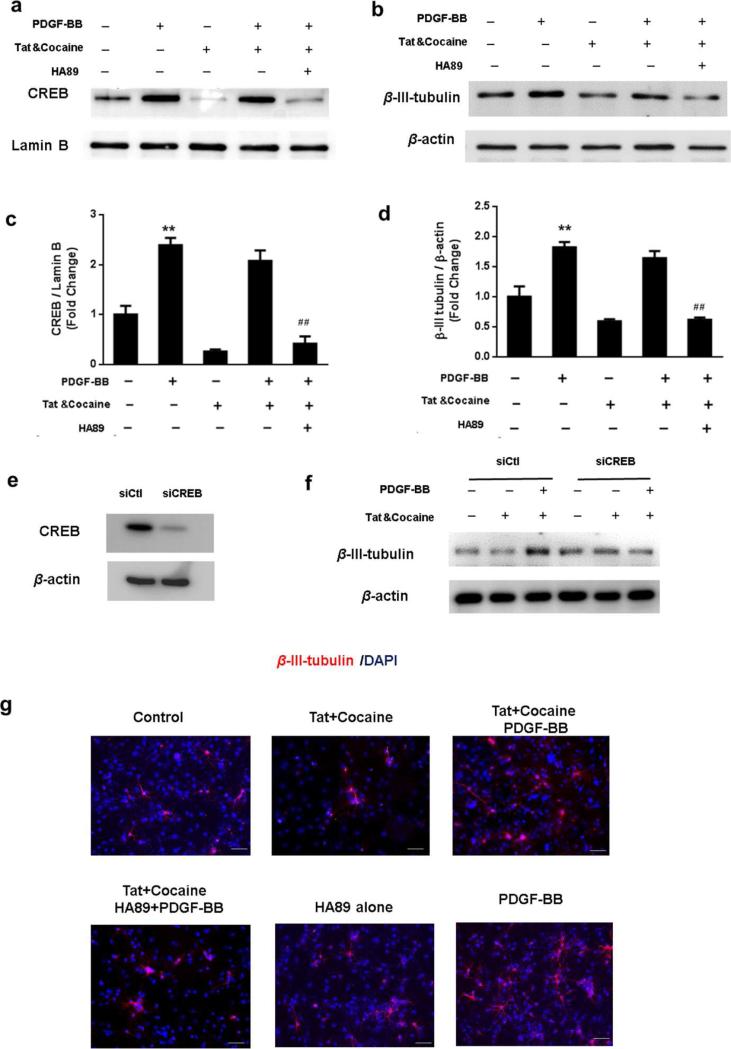
To examine the role of CREB in PDGF-BB mediated neuronal differentiation, NPCs were pretreated with the protein kinase A (PKA) inhibitor H89 (10μM) and assessed for differentiation of NPCs. As shown in Fig.6a&c, Tat & cocaine inhibited nucleus translocation of CREB, which was reversed following PDGF-BB treatment. Pre-treatment with the PKA inhibitor on the other hand, resulted in significant decrease in PDGF-BB-mediated nuclear translocation of CREB in Tat & cocaine exposed cells. The next logical step was to examine the functional relevance of CREB in PDGF-BB mediated neuronal differentiation of NPCs in the presence of Tat & cocaine. As shown in our findings, in NPCs pretreated with H89 inhibitors, PDGF-BB failed to rescue Tat & cocaine mediated loss of neuronal differentiation (Fig.6b&d). The role of CREB activation in PDGF-BB-induced neuronal differentiation was further confirmed by siRNA approach to knock down the expression of CREB in NPCs transfected with siRNA specific target on CREB (Fig.6e). From our result (Fig.6f), CREB knockdown using siCREB resulted in the similar response as the pharmacologic inhibitor H89 we used previously. We future validated our studies by immunostaining of neuronal marker in different experimental groups, which also confirmed that inhibition of CREB activity significantly blocked PDGF-BB mediated neuronal differentiation in the presence of cocaine &Tat (Fig.6g). Taken together, these data suggested that CREB pathway was involved in PDGF-BB-mediated neuronal differentiation of NPCs, and that this pathway also contributed to the PDGF-BB-mediated protection of NPCs from Tat & cocaine mediated impairment of neurogenesis.
Figure 6. CREB is essential for PDGF-BB mediated protective effect from HIV Tat& cocaine caused loss of neuronal differentiation.
(a)Representative blot of PDGF-mediated nucleus translocation of CREB in NPCs under HIV Tat & cocaine exposure with or without PDGF-BB treatment in the presence or absence of CREB pathway inhibitor protein kinase A (PKA) inhibitor H89(10μM). (b) Representative blot of PDGF-mediated neuronal differentiation in NPCs under HIV Tat & cocaine exposure with or without PDGF-BB treatment in the presence or absence of CREB pathway inhibitor H89. (c-d) Quantification analysis of CREB/ Lamin B, β-III-Tubulin/ β-actin from three individual experiments. *P<0.05; **P<0.01 versus control group; ##P<0.01 both PDGF and HIV Tat & cocaine-treated group. (e) Cultured NPCs were transfected with siRNA targeting CREB (siCREB) or a control nontargeting siRNA (siCtl). At 36 h post-transfection, the knocking down efficiency was checked by immunoblotting. (f) Representative blot of PDGF-BB-mediated neuronal differentiation in siRNA transfected NPCs followed by cocaine&Tat exposure. (g) Representative immunofluorescence images showing the β-III-Tubulin expression in differentiated NPCs under HIV Tat & cocaine exposure with or without PDGF-BB treatment in the presence or absence of CREB pathway inhibitor H89. Scale bar: 20 μm.
Discussion
Previous findings from our lab have demonstrated critical roles of the neurotropic factor- PDGF-BB in promoting NPC proliferation as well as in neuronal survival against HIV Tat & cocaine-mediated neurotoxicity [7, 14]. The present study was aimed at exploring whether PDGF-BB was robust in also modulating neuronal differentiation of NPCs in the presence of HIV Tat and cocaine in vitro. Our findings demonstrate that PDGF-BB was able to reverse the co-operative impairment of neuronal differentiation of NPCs mediated by both HIV Tat and cocaine. We identified a novel role of the Ca2+-permeable channel TRPC in PDGF-mediated neuronal differentiation of NPCs. We also demonstrated that exposure of NPCs to exogenous PDGF-BB activated TRPCs, leading in turn, to activation of downstream ERK signaling, followed by nuclear translocation of CREB, ultimately culminating in neuronal survival. Consistent with our previous studies, we also found that PDGF-mediated activation of Akt was independent of TRPC channel activation, and was also a pathway contributing to PDGF-BB mediated neuronal differentiation of NPCs.
Active neurogenesis occurs throughout life and relies upon the orchestrated processes involving proliferation, differentiation and migration and is regulated by a variety of pathological as well as physiological stimuli including neurotropic factors[18, 19]. Both HIV viral proteins as well as drugs of abuse such as cocaine can negatively affect the self-renewal capacity of the hippocampus by diminishing the proliferative rate of NPCs [5, 20]. In this study, we confirmed the effect of PDGF-BB mediated neuronal differentiation in rat primary NPCs, In addition, our results also demonstrated that HIV Tat and cocaine mediated impairment of neuronal differentiation was reversed by exposure of cells to PDGF-BB. In agreement with these studies, PDGF-BB has been previously implicated as a crucial factor for both neurogenesis as well as neuroprotection in several neurodegenerative disease models [21, 22]. Furthermore, pretreatment of PDGF-BB is known to induce striatal neurogenesis in a Parkinsonian rat model of 6-hydroxydopamine lesions [22]. It has also been reported that the expression level of PDGF-BB was significantly increased following stroke, and was closely linked to stroke-induced neurogenesis and neuronal differentiation [23].
A novel finding of this study is the role of TRPC in PDGF-BB-mediated reversal of neuronal differentiation mediated by HIV Tat and cocaine. The mammalian TRPC channel family consists of seven members, TRPC1-7, which appear to function as receptor-operated channels responsible for Ca2+ influx. Our previous reports indicated the involvement of TRPC signaling in PDGF-BB mediated proliferation of NPCs, and this involved activation of Ca2+ signaling pathway leading to downstream mediators promoting neuronal proliferation. Intriguingly, other reports have also demonstrated the protective role of TRPC1 in MPTP-induced toxicity in SH-SY5Y cells [24]. More recent studies have also shed light on the mechanisms underlying the activation of TRPC channels by another growth factor such as brain-derived neurotropic factor [25]. Ca2+ has also been known to mediate cell differentiation. In fact, TRPC channel has been known to function as the Ca2+ influx channel. As shown in this study pretreatment of cells with EGTA, an extracellular Ca2+ chelator used to exclude extracellular Ca2+, was able to successfully block PDGF-BB mediated neuronal differentiation. Our results indicated that influx of Ca2+ following activation of TRPC channel by PDGF-BB is a pre-requisite for neuronal differentiation mediated by the growth factor. This lends credence to the idea that TPRC channels can be envisioned as promising targets for promoting neurogenesis in HIV-infected cocaine-abusing individuals.
In the present study, we also demonstrated that PDGF-BB mediated reversal of neuronal differentiation involved activation of ERK as well as PI3K/Akt pathways. ERK activity was important for neuronal differentiation of NPCs, and this is in keeping with previous reports. For example studies by Colomno et al. provided evidence that insulin-like growth factor modulated NPC differentiation and growth through activation of the MAPK pathway [26]. Another finding also demonstrated that somatic NPC cultures required activation of the ERK signaling pathway to sustain proliferation and neuronal differentiation of the P19 stem cells [27]. In fact, influx of Ca2+ is known to regulate numerous physiological processes through a wide range of target proteins including ERK activation, which is essential for neuronal survival [28, 29]. Consist with these findings, our results also indicate that upon activation of the TRPC calcium channel by PDGF-BB, activation of ERK signaling was crucial for PDGF-mediated neuronal differentiation of NPCs. Furthermore, in this study we also showed that PDGF-mediated neuronal differentiation against HIV Tat & cocaine involved activation of PI3K/Akt pathway, an effect that was in agreement with previous reports demonstrating that PDGF stimulated PI3K/Akt pathways in various other cell types [29-31]. Interestingly, PDGF-mediated activation of Akt was independent of TRPC channel activation, since TRPC inhibitor failed to inhibit PDGF-mediated Akt phosphorylation.
The transcription factor CREB has emerged as a major regulatory factor for neuronal survival mediated by growth factors such as PDGF-BB [32, 33]. Our findings also unraveled the role of CREB in PDGF-BB-mediated modulation of neuronal differentiation in the presence of HIV Tat & cocaine. We detected a significant increase in CREB translocation into the nucleus in NPCs exposed to PDGF-BB compared with the cells not treated with the growth factor. Further dissection of CREB pathway was done using the pharmacologic approach wherein it was identified that the ERK and PI3K/Akt pathway were upstream of CREB and played an essential role in PDGF-BB mediated modulation of neuronal differentiation in the presence of HIV Tat & cocaine exposure. These findings were also consistent with our previous report suggesting the role of CREB in PDGF-BB mediated neuroprotection against HIV Tat [7].
Our findings have demonstrated PDGF-BB promotes neuronal differentiation and reverses the impairment mediated by HIV Tat & cocaine. Our results also illustrate the detailed molecular pathway by which PDGF-BB mediates neuronal differentiation of NPCs involving activation of TRPC channels, leading to Ca2+ influx, which in turn, triggers activation of ERK and the downstream transcription factor CREB. Our findings also implicate another independent PI3K/Akt pathway in this process. Taken together our findings suggest that PDGF-BB-mediated neuroprotection plays an important role against HIV Tat and cocaine mediated impairment of neuronal differentiation. A better understanding of these molecular pathways could be critical for the development of therapeutic strategies against HAND.
Acknowledgments
This work was supported by grants DA036157 and DA033150 from the National Institutes of Health.
Footnotes
Authorship
Contribution: L.Y. designed and performed research and wrote the manuscript; X.C. performed research; G.H.designed all primers and performed research; Y.C. and K.L performed research and S.B. planned and designed the research and wrote the manuscript.
Conflict of Interest
The authors declare no competing financial interests.
Reference
- 1.Dhillon NK, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13(6):483–95. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 2.Yao H, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–55. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, et al. Role of Sigma Receptor in Cocaine-Mediated Induction of Glial Fibrillary Acidic Protein: Implications for HAND. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao H, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–62. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan A, et al. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64(16):2120–32. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. 2010;70(5):339–49. doi: 10.1002/dneu.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao H, et al. Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci. 2012;32(29):9835–47. doi: 10.1523/JNEUROSCI.0638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smits A, et al. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88(18):8159–63. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan YW, et al. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci. 2012;32(19):6444–55. doi: 10.1523/JNEUROSCI.6076-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shioda N, Han F, Fukunaga K. Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–87. doi: 10.1016/S0074-7742(09)85026-5. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116(1):1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum Genomics. 2011;5(2):108–16. doi: 10.1186/1479-7364-5-2-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudes M, et al. Crucial role of TRPC1 and TRPC4 in cystitis-induced neuronal sprouting and bladder overactivity. PLoS One. 2013;8(7):e69550. doi: 10.1371/journal.pone.0069550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, et al. PDGF-mediated protection of SH-SY5Y cells against Tat toxin involves regulation of extracellular glutamate and intracellular calcium. Toxicol Appl Pharmacol. 2009;240(2):286–91. doi: 10.1016/j.taap.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, et al. Involvement of miR-9/MCPIP1 axis in PDGF-BB-mediated neurogenesis in neuronal progenitor cells. Cell Death Dis. 2013;4:e960. doi: 10.1038/cddis.2013.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao J, et al. Platelet-derived growth factor-BB restores HIV Tat -mediated impairment of neurogenesis: role of GSK-3beta/beta-catenin. J Neuroimmune Pharmacol. 2014;9(2):259–68. doi: 10.1007/s11481-013-9509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D, et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010;28(12):2162–71. doi: 10.1002/stem.541. [DOI] [PubMed] [Google Scholar]
- 18.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira SL, et al. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. 2013;83(1):76–89. doi: 10.1002/cyto.a.22161. [DOI] [PubMed] [Google Scholar]
- 20.Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190(2):216–26. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- 21.Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168–81. doi: 10.1007/s11481-013-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohapel P, et al. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132(3):767–76. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 23.Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6(3):238–44. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj S, et al. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122(4):1354–67. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortin DA, et al. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci. 2012;32(24):8127–37. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boney CM, Smith RM, Gruppuso PA. Modulation of insulin-like growth factor I mitogenic signaling in 3T3-L1 preadipocyte differentiation. Endocrinology. 1998;139(4):1638–44. doi: 10.1210/endo.139.4.5920. [DOI] [PubMed] [Google Scholar]
- 27.Reffas S, Schlegel W. Compartment-specific regulation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) by ERK-dependent and non-ERK-dependent inductions of MAPK phosphatase (MKP)-3 and MKP-1 in differentiating P19 cells. Biochem J. 2000;352(Pt 3):701–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Agell N, et al. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14(8):649–54. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 29.Fukuchi M, Tabuchi A, Tsuda M. Transcriptional regulation of neuronal genes and its effect on neural functions: cumulative mRNA expression of PACAP and BDNF genes controlled by calcium and cAMP signals in neurons. J Pharmacol Sci. 2005;98(3):212–8. doi: 10.1254/jphs.fmj05001x4. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, et al. Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport. 2002;13(18):2483–6. doi: 10.1097/00001756-200212200-00021. [DOI] [PubMed] [Google Scholar]
- 31.Sodhi A, Biswas SK. Monocyte chemoattractant protein-1-induced activation of p42/44 MAPK and c-Jun in murine peritoneal macrophages: a potential pathway for macrophage activation. J Interferon Cytokine Res. 2002;22(5):517–26. doi: 10.1089/10799900252981990. [DOI] [PubMed] [Google Scholar]
- 32.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 33.Kingsley-Kallesen ML, Kelly D, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 promoter by cAMP-responsive element-binding protein (CREB) and activating transcription factor-1 (ATF-1) is modulated by protein kinases and the coactivators p300 and CREB-binding protein. J Biol Chem. 1999;274(48):34020–8. doi: 10.1074/jbc.274.48.34020. [DOI] [PubMed] [Google Scholar]